Abstract
Objective
Low frequency (≤ 1 Hz) rTMS (LF-rTMS) can reduce excitability in the underlying cortex and/or promote inhibition. In patients with Parkinson’s disease (PD) several TMS elicited features of motor corticospinal physiology suggest presence of impaired inhibitory mechanisms. These include shortened silent period (SP) and steeper input-output (I-O) curve of motor evoked potential (MEP) size than in normal controls. However, studies of LF-rTMS effects on inhibitory mechanisms in PD are scarce.
In this companion paper to the clinical paper describing effects of 4 consecutive days of LF-rTMS on dyskinesia in PD (Filipović et al., 2009), we evaluate the delayed (24h) effects of the LF-rTMS treatment on the physiological measures of excitability of the motor cortex in the patients. There are very few studies of physiological follow up of daily rTMS treatments.
Methods
Nine patients with PD in Hoehn & Yahr stages 2 or 3 and prominent medication induced dyskinesia were studies. This was a placebo-controlled, crossover study, with two treatment arms, “real” rTMS and “sham” rTMS (placebo). In each of the treatment arms, the rTMS (1800 pulses; 1Hz rate; intensity of the real stimuli just-below the active motor threshold) was delivered over the motor cortex for four consecutive days. Motor cortex excitability was evaluated at the beginning of the study and the next day following each of the four-day rTMS series (real and sham) with patients first in the practically defined “off” state, following 12h withdrawal of medication, and subsequently in a typical “on” state following usual morning medication dose.
Results
The SP was significantly longer following real rTMS in comparison to both baseline and sham rTMS. The effect was independent from the effects of dopaminergic treatment. There was no difference in MEP size, rest and active motor threshold. The I-O curve, recorded from the relaxed muscle, showed a trend towards diminished slope in comparison to baseline, but the difference was not significant. There was no consistent correlation between prolongation of SP and concomitant reduction in dyskinesia following real rTMS.
Conclusions
Low-frequency rTMS delivered over several consecutive days is able to induce changes in excitability of motor cortex and promote apparent increase in inhibitory activity that can persist for at least a day after.
Significance
The results confirm the existence of a residual after-effect of consecutive daily applications of rTMS that might be relevant to the clinical effect that was observed in this group of patients and could be further exploited for potential therapeutic uses.
Keywords: Parkinson’s disease, repetitive transcranial magnetic stimulation, motor cortex, inhibition, dyskinesia
Introduction
A number of studies have shown that rTMS can modulate the excitability of the motor cortex beyond the period of stimulation. Increased excitability usually occurs if higher frequencies (above 5 Hz) are used (Pascual-Leone et al., 1994), while decrease in excitability has been shown not only in the motor cortex, but in the visual cortex as well, if low frequency (≤ 1 Hz) trains are given for 5 minutes or more (Chen et al., 1997; Boroojerdi et al., 2000; Cantello et al., 1991; Maeda et al., 2000; Muellbacher et al., 2000). The mechanism involved is not known, but the stimulation rate is similar to that producing long-term depression in animal studies (reviewed in Post et al., 1999; and Ziemann, 2004). In addition, the effects of rTMS are not restricted only to the point of stimulation, but can be also detected at distant though connected sites within the same functional circuit both at cortical and subcortical levels (Fox et al., 1997; Gerschlager et al., 2001; Siebner et al., 2003).
There are reports of a beneficial clinical effect of low-frequency rTMS (LF-rTMS) on diseases with increased cortical excitability such as focal hand dystonia (Siebner et al., 1999) and epilepsy (Tergau et al., 1999; Fregni et al., 2006). There is also evidence that the physiological effect in patients may even be stronger than that seen in healthy subjects (Siebner et al., 1999; 2003). In patients with Parkinson’s disease (PD) several TMS elicited features of motor corticospinal physiology suggest presence of impaired inhibitory mechanisms. These include shortened silent period (SP) and steeper input-output (IO) curve of motor evoked potential (MEP) size than in normal controls – changes that are typically ameliorated by levodopa/dopaminergic medication in concert with relief of symptoms of the disease (reviewed in Cantello et al., 2002; and Lefaucheur, 2005).
We have recently reported beneficial clinical effect of LF-rTMS on medication induced dyskinesia in PD (Filipović et al., 2009). As a part of that study we also recorded neurophysiological parameters of cortical excitability. This provided an opportunity to test whether in a group of patients with a condition characterized by reduced cortical inhibition, LF-rTMS applied over motor cortex for several consecutive days is able to induce a sustainable and measurable change in the excitability of the motor cortex and in particular increase in inhibition.
Methods
Design of study
This was a placebo-controlled, single-blinded, crossover study, with each treatment arm lasting one week, and each period of treatment separated by a minimum of two weeks. The two treatment arms consisted of 4 successive daily visits (from Monday to Thursday) each, when either “real” rTMS or “sham” rTMS (placebo) were delivered. The same type of rTMS was used throughout successive 4 days and the order of the treatments was randomly assigned. The time of day for treatment visits was kept constant for each patient.
The baseline evaluation session (e0) was during a week preceding the first treatment session. The treatment evaluation sessions (e1 and e2) were on the first Friday after the end of the each rTMS series (i.e. next day after the last rTMS session of each series), respectively. At each evaluation session, first, a set of clinical and neurophysiological tests was carried out with patients in so called practically defined ‘off’ state, following at least 12 hours (overnight) refrain from anti-parkinsonian medication. Second set of tests was carried out once patients achieved stable ‘on’ state, after taking their usual morning medication dose. Since the study was designed to test the effect of rTMS on medication-induced dyskinesia in Parkinson’s disease, on each evaluation visit a clinical evaluation was carried out as well. Patients were examined using Unified PD Rating Scale (UPDRS) Motor Section (Part 3). In addition, in “on” state, dyskinesias were rated off-line from videotapes using the Clinical Dyskinesia Rating Scale (CDRS) developed by Hagell and Widner (1999). The most severe involuntary movements observed are scored from 0 (none) to 4 (extreme), in each of the seven body areas: face, neck, trunk, and four extremities, separately for hyperkinesias (i.e., choreic movements) and dystonia. The results have been already published (Filipović et al., 2009). Sessions were always organised in the morning hours at the earliest convenience to the patient.
Patients
Nine right-handed, non-demented patients with idiopathic PD, satisfying United Kingdom Parkinson’s Disease Society Brain Bank criteria (Gibb and Lees, 1988), manifesting obvious dyskinesias present most of the day were studied. They were recruited through the outpatient department of the Frenchay Hospital (Bristol, UK). All patients were on the fixed dose of their usual antiparkinsonian medication for at least one month prior to starting the study until the end of the study. Informed consent was obtained from each patient according to the Declaration of Helsinki, and study protocol was approved by the Frenchay Local Research Ethics Committee. The details of patients’ characteristic are presented on Table 1. They were essentially the same patients as in Filipović et al. (2009) paper, but without one patient whose neurophysiological data had to be discarded because inability to relax adequately due to excessive dyskinesia in on phase.
Table 1.
Patients’ characteristics
| Pt | Gender | Age (years) |
PD duration (years) |
Type | UPDRS† offmed |
UPDRS† onmed |
Dyskinesia score† |
Worst side |
Morning medication (mg; l-dopa equivalent*) |
Total daily medication (mg; l-dopa equivalent*) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 66 | 8 | AR | 31 | 16 | 15 | L | 350 | 1150 |
| 2 | F | 48 | 8 | AR&T | 32 | 11 | 9 | R | 350 | 650 |
| 3 | F | 71 | 22 | T&AR | 63 | 23 | 23 | R | 100 | 600 |
| 4 | F | 73 | 18 | T&AR | 50 | 33 | 33 | L | 100 | 700 |
| 5 | M | 48 | 16 | T&AR | 60 | 12 | 26 | R | 300 | 900 |
| 6 | F | 61 | 17 | AR&T | 49 | 22 | 20 | L | 100 | 600 |
| 7 | M | 64 | 16 | AR | 40 | 24 | 10 | R | 250 | 1400 |
| 8 | M | 71 | 11 | AR | 37 | 11 | 24 | L | 325 | 850 |
| 9 | M | 69 | 14 | AR | 42 | 19 | 28 | L | 200 | 950 |
M – male, F – female, AR – akinetic-rigid, T – tremor, offmed – off medication, onmed – on medication, R – right, L – left.
At baseline evaluation.
Anti PD medication dosage is expressed as levodopa equivalent following published formulas: 1 mg pergolide = 1.05 mg pramipexole = 6 mg ropinirole = 1.5 mg cabergoline = 100 mg levodopa (Grosset et al., 2004).
Transcranial magnetic stimulation (TMS)
All transcranial magnetic stimulations, either single or repetitive, were performed with Magstim Rapid Transcranial Magnetic Stimulator (Magstim Company, Dyfed, UK). For “real” TMS a standard Magstim’s 70 mm figure-of-eight coil was used. The “sham” rTMS was carried out with Placebo Coil (Magstim Company) that looked the same and gave similar skin sensation and noise as a “real” coil but no effective magnetic field was generated. The hemisphere contralateral to the more severely affected side was target in all cases.
Neurophysiological assessment of cortical excitability
Cortical excitability was evaluated using single pulse TMS. The target muscle was the first dorsal interosseous muscle (FDI). At the beginning of each experiment the optimal scalp site (“hot-spot”) and the resting motor threshold (rMT) for FDI were determined following a standard procedure (Rossini et al., 1994). The determined coil position was marked on the head and its coordinates on midsagital (nasion–inion line) and biauricular (line connecting external auditory meati) axes in relationship to the vertex were recorded. In order to ensure consistent positioning of the coil throughout the experiment the same coordinates were used in further sessions. The hot-spot and MT finding procedures were replicated at each first treatment session (Mondays) and each evaluation session (Fridays) to check for consistency of coli positioning and MT changes following investigated procedures, respectively. No differences in hot-spot position were found at any of these occasions.
Neurophysiological testing was carried out first with FDI in complete rest. Ten stimuli were delivered at TMS intensities of 110%, 120%, 130%, and 150%rMT, each. Next, participants were asked to maintain voluntary contraction during TMS delivery. Series of ten stimuli of 120%rMT intensity were delivered during each of three different levels of background contraction – ‘mild’, ‘moderate’, and ‘maximal’. With the help of a custom made visual feedback device, participants were asked to maintain contraction of target FDI muscle either at 20 – 30% of the maximal voluntary contraction (MVC) strength (‘mild’ condition), 50 – 60%MCV (‘moderate’), or 90 – 100%MCV (‘maximal’). The MVC was determined beforehand and subjects trained to maintain required levels of contraction.
The peak-to-peak amplitude of each MEP was measured and the mean MEP amplitude was calculated separately for each condition. Also, in the conditions with voluntary contraction, the “silent-period” (SP) offset latency was measured from single traces and then averaged for each condition. The SP offset latency was determined as either the latency of the onset of a burst of EMG activity reaching at least 75% of the pre-stimulus background activity and lasting at least 20ms, or the latency of the onset of continuous EMG activity.
Low-frequency rTMS
During LF-rTMS three series of 600 stimuli of 1Hz rate, with one-minute breaks in between, were applied during each session (1800 stimuli in total, duration 32 minutes). Stimulation variables were in accordance with published safety recommendations (Wassermann 1998). The intensity was set individually to be just below active motor threshold (aMT). The aMT was determined with the target muscle maintaining 20% of the maximal voluntary contraction (MCV) strength and was defined as the minimum stimulator intensity capable to evoke a MEP of 200–300μV in amplitude at least in 50% of 10 consecutive trials (Rothwell et al., 1999). The aMT values were typically equal or below the 90% of resting motor threshold (rMT).
Data Analysis
From recoded data several outcome measures were derived: rest and active motor thresholds (rMT and aMT), rest MEP (measured with 120%rMT TMS intensity), MEP input-output (I-O) curve (MEPs measured with TMS intensities of 110, 120, 130, and 150%rMT, with FDI muscle at rest), MEP facilitation with voluntary contraction, and silent period (SP). For the later two, voluntary MEP facilitation and SP, the results were analysed in relationship to the level of the background muscle contraction (measured as mean of rectified EMG for 50ms interval before TMS pulse) regardless of the exact instruction set during which the measurement was made. The levels of the background muscle contraction were grouped into three levels: mild (10-33% of the maximal voluntary contraction [MCV]), moderate (34-66% MCV) and strong (67-100% MCV).
Data were analysed two-fold in a pair-wise fashion. Results obtained after one of the rTMS interventions (i.e. either real or sham) were first compared with results at baseline, and then with each other. For statistical assessment two-way and three-way repeated-measures analyses of variance (ANOVA) were used with factors rTMS type (real vs. sham) and dopaminergic treatment status (off vs. on medication, OFFMED and ONMED, respectively), as well as TMS intensity or level of background contraction, where appropriate. Results were considered as significant if P<0.05. Given that comparisons with baseline were planned hypothesis-driven, i.e. we wanted to check whether 1Hz rTMS could promote inhibitory mechanism manifested by shortening of SP and/or diminution of MEP, one-tailed directional probability was used. In contrast, since there was a possibility for a placebo effect of sham stimulation mimicking the expected effect of real stimulation, no reliable hypothesis could be formed whether TMS variables would be in any way different following real and sham rTMS, and thus for real versus sham comparisons two-tailed non-directional probability was used.
Due to non-parametric nature of clinical measures used, correlations between clinical and neurophysiological data were carried out using non-parametric Spearman’s rank order correlation method with significance set at P<0.05 level. Given that this was an exploratory study, no adjustments for multiple measurements were applied.
Results
Questioned at the end of their participation in the study, none of the patients were able to identify which type of rTMS (i.e. whether real or sham) was delivered in each of the treatment sessions.
Neurophysiology data
Motor thresholds
There was no difference in rMT across all conditions regardless whether recorded on or off medication and at baseline or after real or sham rTMS (Table 2). Equally, there was no difference in aMT across all conditions (Table 2).
Table 2.
Motor thresholds at rest (rMT) and during activation (aMT), and MEP size at rest (values are presented as mean±SD), in off and on state
| Baseline | real rTMS | sham rTMS | ||||
|---|---|---|---|---|---|---|
| OFF | ON | OFF | ON | OFF | ON | |
| rMT 1 | 48.7±9.8% | 50.6±10.7% | 49.0±8.7% | 49.1±8.6% | 49.2±8.5% | 49.3±8.5% |
| aMT 1 | 43.7±7.8% | 43.6±7.7% | 43.2±8.0% | 43.0±7.9% | 43.4±7.7% | 43.6±7.7% |
| MEP 2 | 422.3±307.0 | 349.7±244.9 | 283.2±111.2 3 | 300.5±155.0 | 410.6±244.7 | 377.9±245.6 |
Motor threshold values are percentages of the stimulator output.
MEP amplitudes are expressed in μV.
Significant (P<0.05) difference vs. baseline/OFF (post-hoc least-square difference (LSD) pair-wise test).
MEP at rest and during voluntary activation
MEP at rest in OFFMED condition was slightly lower after real rTMS than it was at baseline and after sham rTMS, while in ONMED condition there was no obvious difference (Figure 1, Table 2). The data were analysed by two-way repeated measures ANOVA with factors rTMS type (i.e. baseline vs. real or sham, and real vs. sham), and medication status (i.e. OFFMED vs. ONMED). No significant effect of any of the two factors and their interactions was found for any of the comparisons (F(1,8)<1.00, P>0.1, in all cases). Only for baseline vs. real rTMS comparison the effect of rTMS type (F(1,8)=2.63) was slightly stronger (P=0.072), while post-hoc least-square difference (LSD) pair-wise test showed as significant (P=0.03) baseline vs. real rTMS difference in MEP in OFFMED phase.
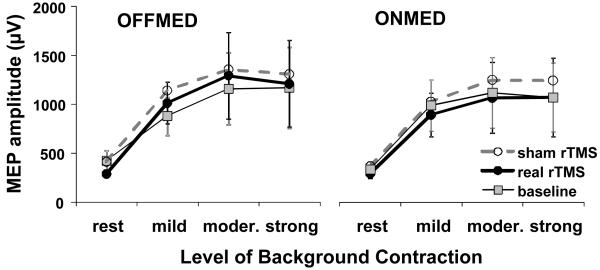
Figure 1.
MEP facilitation during various levels of voluntary contraction. Presented are group means with standard errors. Slightly lower MEP size can be seen at rest following real rTMS in ONMED condition. No major difference and large overlaps between responses at baseline and following both real and sham rTMS can be seen in MEP sizes recorded with different levels of background contraction.
MEP facilitation during various levels of voluntary contraction was analysed by three-way repeated measures ANOVA with factors rTMS type, medication status, and contraction level (i.e. rest vs. mild vs. moderate vs. strong). MEP recruitment curves showed no major differences regardless of the medication status and whether recorded at baseline or after either real or sham rTMS type (Figure 1, Table 3). Only contraction level and interactions between all three factors had significant effect in all pair-wise comparisons due to significant differences between MEP size at rest and MEP sizes at all three levels of contraction and between MEP size at mild contraction and MEP sizes at moderate and strong contractions; there was no significant difference between MEP sizes at moderate and strong contractions.
Table 3.
Results of ANOVAs on silent period (SP), MEP facilitation with voluntary muscle contraction, and input-output (IO) MEP curve
| Factors | Interactions | ||||||
|---|---|---|---|---|---|---|---|
| rTMS type (1) |
on/off state (2) |
Muscle contraction (3) |
1×2 | 1×3 | 2×3 | 1×2×3 | |
| SP | |||||||
| Baseline vs. real rTMS |
F=3.728
P=0.047 |
F=9.631
P=0.009 |
F=26.248
P<0.001 |
F=0.339 P=0.289 |
F=4.624
P=0.014 |
F=1.281 P=0.154 |
F=2.922
P=0.043 |
| Baseline vs. sham rTMS |
F=1.448 P=0.134 |
F=19.162
P=0.002 |
F=21.802
P<0.001 |
F=0.381 P=0.278 |
F=0.210 P=0.406 |
F=4.662
P=0.014 |
F=1.477 P=0.131 |
| Real rTMS vs. sham rTMS |
F=7.318
P=0.030 |
F=10.286
P=0.015 |
F=32.246
P<0.001 |
F=0.010 P=0.924 |
F=1.102 P=0.360 |
F=4.271
P=0.036 |
F=1.850 P=0.194 |
| MEP facilitation | |||||||
| Baseline vs. real rTMS |
F=0.004 P=0.475 |
F=0.735 P=0.208 |
F=4.598
P=0.006 |
F=2.298 P=0.084 |
F=0.784 P=0.257 |
F=1.007 P=0.203 |
F=4.678
P=0.005 |
| Baseline vs. sham rTMS |
F=2.167 P=0.090 |
F=0.186 P=0.339 |
F=4.073
P=0.009 |
F=0.745 P=0.207 |
F=1.183 P=0.168 |
F=0.609 P=0.308 |
F=2.371
P=0.048 |
| Real rTMS vs. sham rTMS |
F=1.364 P=0.276 |
F=3.800 P=0.087 |
F=4.185
P=0.016 |
F=0.704 P=0.426 |
F=0.047 P=0.986 |
F=1.764 P=0.181 |
F=1.251 P=0.313 |
| IO MEP | (TMS intensity [3]) | ||||||
| Baseline vs. real rTMS |
F=3.375
P=0.052 |
F=0.721 P=0.210 |
F=25.614
P<0.001 |
F=0.064 P=0.403 |
F=0.728 P=0.273 |
F=1.248 P=0.157 |
F=2.278
P=0.053 |
| Baseline vs. sham rTMS |
F=0.178 P=0.342 |
F=0.497 P=0.250 |
F=46.203
P<0.001 |
F=0.084 P=0.389 |
F=1.981Z P=0.072 |
F=0.476 P=0.351 |
F=0.666 P=0.290 |
| Real rTMS vs. sham rTMS |
F=0.151 P=0.707 |
F=1.069 P=0.331 |
F=37.590
P<0.001 |
F=0.028 P=0.871 |
F=1.680 P=0.198 |
F=2.462 P=0.087 |
F=0.926 P=0.443 |
Significant (P<0.05) results are presented in bold.
I-O curve
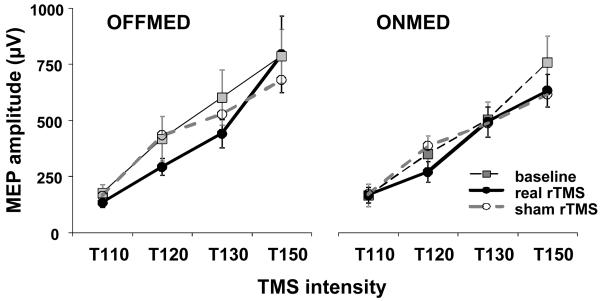
In off condition, I-O curve following real rTMS was slightly less steep than both at baseline and after sham rTMS (Figure 2). We did not formally calculate the slope but the I-O curves were analysed by three-way repeated measures ANOVAs (Table 3) with factors rTMS type, medication status, and test TMS intensity level (i.e. 110 vs. 120 vs. 130 vs. 150%rMT). When baseline and real rTMS were compared, the rTMS type and interaction of all three factors showed considerable effect (for both, P=0.05), and in particular MEP sizes at 120%rMT and 130%rMT intensities off medication, and at 150%rMT on medication were significantly smaller (Post-hoc LSD test, P=0.03, 0.007, and 0.03, respectively). No other comparison showed significant effects.
Figure 2.
Input-output (I-O) curves. Presented are group means with standard errors. Lower MEP sizes can be seen following real rTMS in responses recorded with single-pulse TMS intensities of 120% and 130% of rMT.
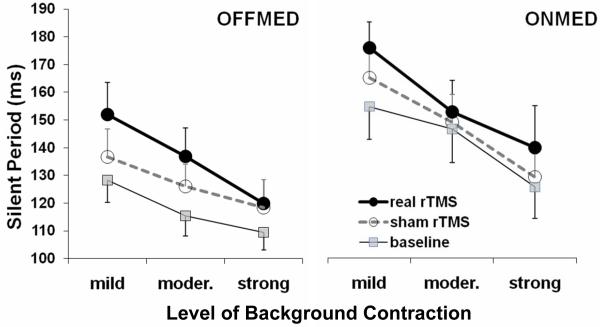
Silent Period
In one patient silent period (SP) could not be reliably determined due to excessive rest tremor in off condition. Therefore, analyses were carried out on data from eight patients (Figure 3, Table 3). The SP was analysed by three-way repeated measures ANOVA with factors rTMS type, medication status, and contraction level. As expected, SP was longer in ONMED than in OFFMED condition. In addition, level of background contraction had significant effect as well; the SP was progressively shorter with higher levels of background contraction. Interaction between two factors was also significant although not for baseline vs. real rTMS comparison.
Figure 3.
Silent period during various levels of voluntary contraction. Presented are group means with standard errors. Longer SP duration can be seen following real rTMS in responses recorded with different levels of background contraction in both OFFMED in ONMED conditions.
Following real rTMS the SP was significantly longer than both at baseline and following sham rTMS and the effect was independent from medication status while showing some interaction with contraction levels but only for baseline vs. real rTMS comparison (Table 3). Post hoc pair-wise LSD test (with all three factors) showed as significant baseline vs. real rTMS difference at mild, moderate, and strong levels of contraction in OFFMED condition (P=0.00002, 0.00005, and 0.01, respectively), and at mild and strong levels of contraction in ONMED condition (P=0.00005 and 0.0015, respectively). For real vs. sham rTMS comparison, the difference was significant at mild and moderate levels of contraction in OFFMED condition (P=0.004 and P=0.029, respectively), and at mild and strong levels of contraction in ONMED condition (P=0.029 and 0.034, respectively). No significant effect of order of rTMS series (i.e. whether real or sham was first) on SP prolongation following real rTMS in comparison to baseline was found. This was tested by the Mann-Whitney U Test for each of the medication states and contraction levels separately.
Clinical variables – neurophysiology interaction
Patients reported no side effects following rTMS. As already reported (Filipović et al., 2009), rTMS did not have any adverse effects on patients’ motor functions and other PD symptoms – total UPDRS score and Motor Section score (both in ON phase) did not differ after real and sham rTMS.
As it was reported (Filipović et al., 2009), following real rTMS, scores on dyskinesia scale (CDRS) changed significantly in comparison to the baseline while following sham rTMS the change was not significant. However, on direct comparison, difference in CDRS scores following real and sham rTMS although obvious was not significant. Following real rTMS mean relative reduction in CDRS scores in comparison to baseline in the subpopulation of patients whose SP data were reported in this study (N=8) was 8.0% (SD 7.9), which was in keeping with the reduction reported for the whole group (N=10, 8.3%, SD 8.3; Filipović et al., 2009). In comparison to baseline, seven patients had CDRS scores reduced following real rTMS and one had score unchanged.
In order to check whether there are any clinical factors influencing SP measurements in this study, correlations were analysed between selected SP variables and relevant clinical variables. The SP variables were SP offset latencies at baseline and the SP offset latency change following real rTMS (calculated as a difference between measurements after real rTMS and at baseline), for all three levels of contraction. The clinical variables were age, duration of disease, UPDRS scores off and on medication at baseline, CDRS scores at baseline, and medication level (total daily levodopa equivalents). Only consistent correlation found was between UPDRS scores on medication and SP offset latencies at baseline (Table 4). The higher UPDRS scores, the longer SP offset latencies were recorded. The correlations were significant for mild and moderate levels of background contraction for SP offset latencies measured both in off and on medication states. There were two further isolated correlations as well. Duration of the disease correlated with SP offset latency at baseline in off medication state when the background contraction was moderate. The SP offset latency change following real rTMS correlated negatively with the amount of total daily medication. Furthermore, correlations were analysed between mentioned SP variables, on one side, and CDRS scores following real rTMS and the change in CDRS scores between baseline and post-real rTMS, on the other side (Table 4). Only one isolated correlation was found to be significant. The SP offset latency change following real rTMS, in on medication state when the background contraction was mild, correlated with the change in CDRS scores between baseline and post-real rTMS.
Table 4.
Results of Spearman Rank Order Correlation test for correlations between SP offset latency and clinical variables. The values of SP offset latencies recorded at baseline evaluation and the difference between values recorded following real rTMS and at baseline were analysed. The R coefficients are presented and significant results are marked in bold. Results are presented separately for medication conditions and levels of background contraction
| Baseline | Real rTMS - Baseline difference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Medication status | OFFMED | ONMED | OFFMED | ONMED | ||||||||
| Background contraction | Mild | Mod. | Max. | Mild | Mod. | Max. | Mild | Mod. | Max. | Mild | Mod. | Max. |
|
| ||||||||||||
| Age | 0.26 | 0.24 | −0.04 | 0.52 | 0.61 | 0.31 | 0.29 | 0.48 | 0.53 | 0.17 | 0.24 | 0.65 |
| UPDRS off @ Baseline | 0.38 | 0.59 | 0.59 | 0.40 | 0.52 | 0.59 | −0.05 | 0.05 | 0.33 | 0.12 | 0.26 | 0.52 |
| UPDRS on @ Baseline | 0.83 | 0.83 | 0.69 | 0.74 | 0.71 | 0.48 | −0.24 | −0.24 | 0.36 | −0.48 | −0.29 | 0.05 |
| PD duration | 0.55 | 0.75 | 0.67 | 0.52 | 0.65 | 0.65 | −0.10 | −0.07 | 0.48 | 0.05 | 0.19 | 0.47 |
| Daily medication | 0.43 | 0.26 | 0.26 | 0.19 | 0.00 | 0.07 | −0.33 | −0.43 | 0.05 | − 0.74 | −0.50 | −0.59 |
| CDRS @ Baseline | 0.19 | 0.19 | 0.05 | 0.33 | 0.31 | 0.14 | 0.38 | 0.59 | 0.48 | −0.14 | 0.29 | 0.62 |
| CDRS diff. Real - Base. | −0.45 | −0.27 | −0.31 | −0.34 | −0.06 | 0.16 | 0.52 | 0.39 | 0.36 | 0.87 | 0.63 | 0.63 |
Mod. – Moderate; Max. – Maximal. UPDRS – Unified Parkinson’s Disease Rating Scale; off – off medication; on – on medication; @ Baseline – recorded at baseline; CDRS – Clinical Dyskinesia Rating Scale; diff. Real – Base. – difference between scores obtained at evaluation session following real rTMS and at baseline evaluation session.
Discussion
The key finding of this study is a proof that, in PD patients, low-frequency rTMS delivered for four consecutive days is able to induce an increase of motor corticospinal inhibition as indexed by significant SP prolongation and a trend towards diminished slope of the MEP I-O recruitment curve, which are detectable 24 hours after the last rTMS delivery. As far as we are aware, this is first demonstration of such extended effect of rTMS on cortical excitability, not only in PD patients but also in human subjects in general.
Traditionally, cortical excitability is measured as either the resting motor threshold (rMT) or motor evoked potential (MEP) size (reviews in Pascual-Leone et al., 1998; and Fitzgerald et al., 2002a). Effect of 1Hz rTMS on rMT level in healthy subjects was assessed in several studies (reviewed in Fitzgerald et al., 2006). Most of them reported no change which is in keeping with the results of this study.
Most of the studies investigating post-train effects of low frequency stimulation on MEP size (amplitude, area or recruitment curve) showed a reduction in MEP size (reviewed in Fitzgerald et al., 2006). However, the effect appears to be intensity related; almost all studies providing stimulation at relatively low stimulation intensities (85–90% of rMT or 90% of active MT), comparable with intensities used in this study, reported no effects (Gerschlager et al., 2001; Modugno et al., 2003; Brighina et al., 2005; Houdayer et al., 2008). However, they were all single-session studies; the results of this study suggest that by repeated application of low frequency rTMS over several days it is possible to elicit a modest but sustainable effect on cortical excitability even when using sub-threshold TMS intensities.
There are no studies on LF-rTMS effect on MEP facilitation during voluntary contraction. In studied group of PD patients no significant changes were seen following either real or sham rTMS suggesting lack of LF-rTMS effects on facilitatory corticospinal mechanisms in PD. Relatively weak changes in MEP facilitation with various levels of voluntary contractions found in this study were in keeping with previously reported results in PD patients (Valls-Solé et al., 1994).
Studies of 1Hz rTMS effects on SP in healthy subjects provided conflicting results. Two studies reported a shortening (Fierro et al., 2001; Fitzgerald et al., 2004), three no change (Fitzgerald et al., 2002b; Gilio et al., 2003; Modugno et al., 2003), one some increase (Daskalakis et al., 2006), and only one clear increase (Stinear and Byblow, 2004) of SP duration following a single session of 1Hz rTMS.
Data for PD are scarce. In a group of twelve PD patients off medication, about half-an-hour after single 20min-long session of 0.5Hz rTMS applied at 80% rMT intensity (600 stimuli in total) over the left primary motor cortical area, Lefaucheur et al. (2004) found significant prolongation of the SP and increase in short-latency intra-cortical inhibition (SICI) in comparison to the pre-rTMS values; sham rTMS did not elicit such changes. These results would be very much complimentary with the ones from our study providing a proof that even a single LF-rTMS session is able to induce SP prolongation in PD patients.
Apparently contradictory are the results from the Wagle-Shukla et al. (2007) study. Using a comprehensive battery of TMS measures of cortical excitability, which included MEP at rest and MEP IO curve as well as SP, in six PD patients, they did not find any significant change 24h after 10 successive daily 15min-long sessions of 1Hz rTMS applied at 90%rMT (900 stimuli) in comparison to the pre-rTMS values. Interestingly, they found significant reduction of dyskinesia following rTMS treatment which was in keeping with the clinical results of this study (Filipović et al., 2009).
The difference in results regarding SP between Wagle-Shukla et al. (2007) and this study may be due to slight differences in methodology. In our study twice as much stimuli were applied. Longer duration of rTMS trains may be necessary for physiological effects to be detectable after 24h even if clinical effects are clearly present. In addition, Lafoucher et al. (2004), who also found SP prolongation following slow rTMS, tested their patients in off phase. We tested our participants in clearly defined off phase and subsequently in on phase following their usual morning medication. Wagle-Shulka et al. (2007) carried out neurophysiology testing after the levodopa challenge test using 125% of patients’ usual morning levodopa equivalent dose. Levodopa and dopaminergic drugs prolong SP in healthy subjects (Ziemann et al., 1997) and PD patients (Priori et al., 1994; Ridding et al., 1995; Diószeghy et al., 1999; Strafella et al., 2000; Pierantozzi et al., 2001). We also found a significant effect of levodopa and dopaminergic medication on SP prolongation which was independent from and statistically stronger than rTMS effect. It may be that the magnitude of physiological response to higher doses of levodopa used for levodopa challenge in Wagle-Shulka et al. (2007) study blurred the effect of rTMS.
Siebner et al. (2000) reported a significant prolongation of the SP following a single session of subthreshold 5Hz rTMS in non-medicated PD patients but not in healthy subjects. This may suggest an increased susceptibility towards inhibitory effects of rTMS in PD, which extends beyond the range of rTMS frequencies known to promote inhibitory mechanisms in healthy subjects. Increased susceptibility to rTMS inhibitory effects has been already demonstrated in another basal ganglia disorder, focal hand dystonia (Siebner et al., 2003). This is an issue that requires further investigation.
Although following real rTMS CDRS scores diminished and SP offset latencies became longer in comparison to baseline, there was no real correlation between two. This was surprising, since both changes happened following the same intervention. Small sample size might have precluded detection of statistically significant link. Alternatively, the finding may indicate that SP duration and dyskinesia are not causally related but rather are manifestation of close but not identical physiological processes.
In patients with PD several indices of motor cortex inhibition show impairments. Apart from the most consistently reproduced finding of shortened duration of the SP after TMS in the patients when off medication or when taking low levodopa doses (Cantello et al. 1991; Priori et al. 1994; Nakashima et al. 1995; Valzania et al. 1997; Diószeghy et al. 1999), reduction in short-latency intracortical inhibition (SICI) (Ridding et al. 1995; Hanajima et al. 1996; Strafella et al., 2000) and long-latency afferent inhibition (LAI) (Sailer et al., 2003; Tamburin et al., 2003) were also found. Low frequency rTMS in PD most likely does not affect only mechanisms responsible for SP generation but a range of different inhibitory mechanisms, such as one responsible for SICI (Lefaucheur et al., 2004). It may well be that dyskinesias are mostly mediated through impairment in these other mechanisms and thus the lack of correlation between SP prolongation and clinical dyskinesia improvement in this study. As a further proof for this, increase of SICI was consistently reported after subthalamic nucleus (STN) deep-brain stimulation (DBS) (Cunic et al., 2002; Däuper et al., 2002; Pierantozzi et al., 2002) and similar findings were reported for internal globus pallidus DBS (Pierantozzi et al., 2002). In addition, increase of LAI was reported after STN DBS (Sailer et al., 2007). Both DBS procedures are well known to be exceptionally effective in calming dyskinesia in PD (Benabid, 2003; Anderson et al., 2005).
In healthy subjects, dopaminergic stimulation was found to enhance LF rTMS induced motor cortex inhibition, measured by changes in MEP amplitude (Lang et al., 2008). However, in this study, medication status had no effect of rTMS variables and in particular rTMS induced SP prolongation was independent from the medication effect, which also caused SP prolongation. Our findings were in keeping with previous findings of Morgante et al. (2006) with another non-invasive method for motor cortex excitability modulation, paired-associated stimulation (PAS). While PAS method they used caused significant SP prolongation in healthy subjects, it failed to do the same in PD patients; levodopa caused SP prolongation, but did not improve response to PAS. The lack of levodopa modulatory effect on deficient inhibitory system plasticity may play a role in the pathophysiology of dyskinesia in PD.
It has been show that duration of SP is sensitive to GABA-B modulation (Werhahn et al., 1999). It is thought that dopaminergic medication exercise its influence on SP duration through enhancement of post-synaptic sensitivity to GABA (Beauregard and Ferron, 1991). The mechanism through LF rTMS exercise its effect on cortical excitability is not certain yet (Fitzgerald et al., 2006). Most likely, they are similar to the mechanisms involved in the phenomenon of long term depression (LTD) seen in animal experiments (Ziemann, 2004). The lack of statistical interactions between rTMS and medication effects in this study suggests that two interventions cause change in SP duration through different mechanisms. This finding provides further proof for the feasibility of therapeutical use of LF rTMS.
An interesting finding of this study was that severity of PD motor symptoms affected SP duration. Higher UPDRS scores on medication were significantly associated with longer SP duration both off and on medication. The physiological and clinical significance of this relationship is not clear. Nevertheless, severity of PD motor symptoms did not have any obvious effect on the principal finding of this study, the SP prolongation following real rTMS in comparison to baseline. Other significant correlations between SP duration and clinical variables were not consistent and most likely were detected by chance due to multitude of comparisons.
Another interesting finding of this study is that the duration of the SP in studied PD patients has been significantly influenced by the degree of contraction. It is commonly considered that in healthy subjects degree of background contraction has no particular influence on the SP duration (e.g. Chen et al., 2008). This is certainly true for higher intensities of stimulation, however, the available evidence seems to rather reliably suggest that at the low stimulus intensities, at or below 120% MT, and with the explicit instruction given to the participants to keep the contraction level constant, the SP gets progressively shorter with increase of background contraction (Cantello et al. 1992; Wilson et al. 1993; Mathis et al. 1998; Filipović et al., 2008). Results of this study suggest that the relationship might be even more pronounced in PD or at least in a subset of PD patients displaying prominent dyskinesia as further manifestation of impaired inhibitory mechanisms. This is an issue that may be of interest for further studies.
Dopaminergic medication was shown to induce changes in cortical interactions and plasticity (Mir et al., 2005; Morgante et al., 2006). The rTMS in this study was delivered with patients on medication. It is yet unknown whether the effects may be different and even stronger if rTMS is delivered off medication. This issue requires to be evaluated further.
Before concluding, it should be noted that in this study the strength of rTMS pulses was relatively low. It was set for each patient separately to be just below active motor threshold (aMT) to avoid the difference between “active” and “sham” sessions to be perceived by patients. However, weaker rTMS pulses induce weaker physiological effects (Fitzgerald et al., 2000b) and it might well be that with the same experimental setting as in this study stronger rTMS would be able to induce more pronounced effects on cortical excitability in general and inhibitory mechanisms in particular. This is an issue that would require further studies.
The encouraging results of this study regarding both physiological and clinical effects, suggest a need for to further studies that would systematically evaluate relevant methodological features able to establish more prominent and longer lasting effects. That is, clinical trials that would involve not only stronger TMS stimuli, but also more days of rTMS and bilateral stimulation.
Acknowledgements
This work was supported by the Parkinson’s Disease Society of the United Kingdom (grant number 4034). SRF was partially supported by the grant (project number 145083) from the Serbian Ministry for Science and Technology. Authors have nothing else to disclose. There has been no conflict of interest.
REFERENCES
- Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs. subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554–560. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Ferron A. Dopamine modulates the inhibition induced by GABA in rat cerebral cortex: an iontophoretic study. Eur J Pharmacol. 1991;205:225–231. doi: 10.1016/0014-2999(91)90902-3. [DOI] [PubMed] [Google Scholar]
- Benabid AL. Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol. 2003;13:696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Prager A, Muellbacher W, Cohen LG. Reduction of human visual cortex excitability using 1-Hz transcranial magnetic stimulation. Neurology. 2000;54:1529–1531. doi: 10.1212/wnl.54.7.1529. [DOI] [PubMed] [Google Scholar]
- Brighina F, Giglia G, Scalia S, Francolini M, Palermo A, Fierro B. Facilitatory effects of 1 Hz rTMS in motor cortex of patients affected by migraine with aura. Exp Brain Res. 2005;161:34–38. doi: 10.1007/s00221-004-2042-7. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Bettucci D, Civardi C, De Angelis MS, Mutani R. Parkinson’s disease rigidity: magnetic motor evoked potentials in a small hand muscle. Neurology. 1991;41:1449–1456. doi: 10.1212/wnl.41.9.1449. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Civardi C, Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology. 1992;42:1951–1959. doi: 10.1212/wnl.42.10.1951. [DOI] [PubMed] [Google Scholar]
- Cantello R, Tarletti R, Civardi C. Transcranial magnetic stimulation and Parkinson’s disease. Brain Res Rev. 2002;38:309–327. doi: 10.1016/s0165-0173(01)00158-8. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rösler KM, Triggs WJ, Ugawa Y, Ziemann U. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Cunic D, Roshan L, Khan FI, Lozano AM, Lang AE, Chen R. Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson’s disease. Neurology. 2002;58:1665–1672. doi: 10.1212/wnl.58.11.1665. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Möller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174:403–412. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- Däuper J, Peschel T, Schrader C, Kohlmetz C, Joppich G, Nager W, Dengler R, Rollnik JD. Effects of subthalamic nucleus (STN) stimulation on motor cortex excitability. Neurology. 2002;59:700–706. doi: 10.1212/wnl.59.5.700. [DOI] [PubMed] [Google Scholar]
- Diószeghy P, Hidasi E, Mechler F. Study of central motor functions using magnetic stimulation in Parkinson’s disease. Electromyogr Clin Neurophysiol. 1999;39:101–105. [PubMed] [Google Scholar]
- Fierro B, Piazza A, Brighina F, La Bua V, Buffa D, Oliveri M. Modulation of intracortical inhibition induced by low- and high-frequency repetitive transcranial magnetic stimulation. Exp Brain Res. 2001;138:452–457. doi: 10.1007/s002210100728. [DOI] [PubMed] [Google Scholar]
- Filipovic SR, Papathanasiou I, Whurr R, Rothwell JC, Jahanshahi M. Differential effect of linguistic and non-linguistic pen-holding tasks on motor cortex excitability. Exp Brain Res. 2008;191:237–246. doi: 10.1007/s00221-008-1517-3. [DOI] [PubMed] [Google Scholar]
- Filipović SR, Rothwell JC, van de Warrenburg BP, Bhatia K. Repetitive transcranial magnetic stimulation for levodopa-induced dyskinesias in Parkinson’s disease. Mov Disord. 2009;24:246–253. doi: 10.1002/mds.22348. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ. The application of transcranial magnetic stimulation in psychiatry and neurosciences research. Acta Psychiatr Scand. 2002a;105:324–340. doi: 10.1034/j.1600-0447.2002.1r179.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, Chen R, Kulkarni J. Intensity dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin Neurophysiol. 2002b;113:1136–1141. doi: 10.1016/s1388-2457(02)00145-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Marston NA, Oxley T, De Castella A, Daskalakis ZJ, Kulkarni J. Reduced plastic brain responses in schizophrenia: a transcranial magnetic stimulation study. Schizophr Res. 2004;71:17–26. doi: 10.1016/j.schres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Fox P, Ingham R, George MS, Mayberg H, Ingham J, Roby J, Martin C, Jerabek P. Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport. 1997;8:2787–2791. doi: 10.1097/00001756-199708180-00027. [DOI] [PubMed] [Google Scholar]
- Fregni F, Otachi PTM, do Valle A, Boggio PS, Thut G, Rigonatti SP, Pascual-Leone A, Valente KD. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol. 2006;60:447–455. doi: 10.1002/ana.20950. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilio F, Rizzo V, Siebner HR, Rothwell JC. Effects on the right motor hand-area excitability produced by low-frequency rTMS over human contralateral homologous cortex. J Physiol. 2003;551:563–573. doi: 10.1113/jphysiol.2003.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosset K, Needleman F, Macphee G, Grosset D. Switching from ergot to nonergot dopamine agonists in Parkinson’s disease: a clinical series and five□drug dose conversion table. Mov Disord. 2004;19:1370–1374. doi: 10.1002/mds.20210. [DOI] [PubMed] [Google Scholar]
- Hagell P, Widner H. Clinical rating of dyskinesias in Parkinson’s disease: use and reliability of a new rating scale. Mov Disord. 1999;14:448–455. doi: 10.1002/1531-8257(199905)14:3<448::aid-mds1010>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Ogata K, Kanazawa I. Ipsilateral cortico-cortical inhibition of the motor cortex in various neurological disorders. J Neurol Sci. 1996;140:109–116. doi: 10.1016/0022-510x(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Houdayer E, Degardin A, Cassim F, Bocquillon P, Derambure P, Devanne H. The effects of low- and high-frequency repetitive TMS on the input/output properties of the human corticospinal pathway. Exp Brain Res. 2008;187:207–217. doi: 10.1007/s00221-008-1294-z. [DOI] [PubMed] [Google Scholar]
- Lang N, Speck S, Harms J, Rothkegel H, Paulus W, Sommer M. Dopaminergic potentiation of rTMS-induced motor cortex inhibition. Biol Psychiatry. 2008;63:231–233. doi: 10.1016/j.biopsych.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Von Raison F, Ménard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clin Neurophysiol. 2004;115:2530–2541. doi: 10.1016/j.clinph.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Motor cortex dysfunction revealed by cortical excitability studies in Parkinson’s disease: influence of antiparkinsonian treatment and cortical stimulation. Clin Neurophysiol. 2005;116:244–253. doi: 10.1016/j.clinph.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual–Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Mathis J, de Quervain D, Hess CW. Dependence of the transcranially induced silent period on the ‘instruction set’ and the individual reaction time. Electroencephalogr Clin Neurophysiol. 1998;109:426–435. doi: 10.1016/s0924-980x(98)00042-3. [DOI] [PubMed] [Google Scholar]
- Mir P, Matsunaga K, Gilio F, Quinn NP, Siebner HR, Rothwell JC. Dopaminergic drugs restore facilitatory premotormotor interactions in Parkinson disease. Neurology. 2005;64:1906–1912. doi: 10.1212/01.WNL.0000163772.56128.A8. [DOI] [PubMed] [Google Scholar]
- Modugno N, Curra A, Conte A, Inghilleri M, Fofi L, Agostino R, Manfredi M, Berardelli A. Depressed intracortical inhibition after long trains of subthreshold repetitive magnetic stimuli at low frequency. Clin Neurophysiol. 2003;114:2416–2422. doi: 10.1016/s1388-2457(03)00262-1. [DOI] [PubMed] [Google Scholar]
- Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain. 2006;129:1059–1069. doi: 10.1093/brain/awl031. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behaviour. Clin Neurophysiol. 2000;111:1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Wang Y, Shimoda M, Sakuma K, Takahashi K. Shortened silent period produced by magnetic cortical stimulation in patients with Parkinson’s disease. J Neurol Sci. 1995;130:209–214. doi: 10.1016/0022-510x(95)00029-2. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Pascual–Leone A, Valls–Sole J, Wassermann EM, Hallet M. Response to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pierantozzi M, Palmieri MG, Marciani MG, Bernardi G, Giacomini P, Stanzione P. Effect of apomorphine on cortical inhibition in Parkinson’s disease patients: a transcranial magnetic stimulation study. Exp Brain Res. 2001;141:52–62. doi: 10.1007/s002210100839. [DOI] [PubMed] [Google Scholar]
- Pierantozzi M, Palmieri MG, Mazzone P, Marciani MG, Rossini PM, Stefani A, Giacomini P, Peppe A, Stanzione P. Deep brain stimulation of both subthalamic nucleus and internal globus pallidus restores intracortical inhibition in Parkinson’s disease paralleling apomorphine effects: a paired magnetic stimulation study. Clin Neurophysiol. 2002;113:108–113. doi: 10.1016/s1388-2457(01)00694-0. [DOI] [PubMed] [Google Scholar]
- Post RM, Kimbrell TA, McCann UD, Dunn RT, Osuch EA, Speer AM, Weiss SR. Repetitive transcranial magnetic stimulation as a neuropsychiatric tool: present status and future potential. J ECT. 1999;15:39–59. [PubMed] [Google Scholar]
- Priori A, Berardelli A, Inghilleri M, Accornero N, Manfredi M. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial brain stimulation in normal subjects, patients with Parkinson’s disease and drug-induced parkinsonism. Brain. 1994;117:317–323. doi: 10.1093/brain/117.2.317. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol. 1995;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Romeo S, Gilio F, Pedace F, Ozkaynak S, Inghilleri M, Manfredi M, Berardelli A. Changes in the cortical silent period after repetitive magnetic stimulation of cortical motor areas. Exp Brain Res. 2000;135:504–510. doi: 10.1007/s002210000541. [DOI] [PubMed] [Google Scholar]
- Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol. 2002;113:101–107. doi: 10.1016/s1388-2457(01)00693-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroenceph Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W, The International Federation of Clinical Neurophysiology Magnetic stimulation: motor evoked potentials. Electroenceph Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- Sailer A, Cunic DI, Paradiso GO, Gunraj CA, Wagle-Shukla A, Moro E, Lozano AM, Lang AE, Chen R. Subthalamic nucleus stimulation modulates afferent inhibition in Parkinson disease. Neurology. 2007;68:356–363. doi: 10.1212/01.wnl.0000252812.95774.aa. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson’s disease. Brain. 2003;126:1883–1894. doi: 10.1093/brain/awg183. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Filipovic SR, Rowe JB, Cordivari C, Gerschlager W, Rothwell JC, Frackowiak RSJ, Bhatia KP. Patients with focal arm dystonia have increased sensitivity to slow-frequency repetitive TMS of the dorsal premotor cortex. Brain. 2003;126:2710–2725. doi: 10.1093/brain/awg282. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Mentschel C, Auer C, Lehner C, Conrad B. Repetitive transcranial magnetic stimulation causes a short-term increase in the duration of the cortical silent period in patients with Parkinson’s disease. Neurosci Lett. 2000;284:147–150. doi: 10.1016/s0304-3940(00)00990-3. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Tormos JM, Ceballos–Baumann AO, Auer C, Catala MD, Conrad B, Pascual-Leone A. Low frequency repetitive transcranial magnetic stimulation of the motor cortex in writer’s cramp. Neurology. 1999;52:529–537. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Impaired modulation of corticospinal excitability following subthreshold rTMS in focal hand dystonia. Hum Mov Sci. 2004;23:527–538. doi: 10.1016/j.humov.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Valzania F, Nassetti SA, Tropeani A, Bisulli A, Santangelo M, Tassinari CA. Effects of chronic levodopa and pergolide treatment on cortical excitability in patients with Parkinson’s disease: a transcranial magnetic stimulation study. Clin Neurophysiol. 2000;111:1198–1202. doi: 10.1016/s1388-2457(00)00316-3. [DOI] [PubMed] [Google Scholar]
- Tamburin S, Fiaschi A, Idone D, Lochner P, Manganotti P, Zanette G. Abnormal sensorimotor integration is related to disease severity in Parkinson’s disease: a TMS study. Mov Disord. 2003;18:1316–1324. doi: 10.1002/mds.10515. [DOI] [PubMed] [Google Scholar]
- Tergau F, Naumann U, Paulus W, Steinhoff BJ. Low-frequency repetitive transcranial magnetic stimulation improves intractable epilepsy. Lancet. 1999;353:2209. doi: 10.1016/S0140-6736(99)01301-X. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Brasil-Neto JP, Cammarota A, McShane L, Hallett M. Abnormal facilitation of the response to transcranial magnetic stimulation in patients with Parkinson’s disease. Neurology. 1994;44:735–741. doi: 10.1212/wnl.44.4.735. [DOI] [PubMed] [Google Scholar]
- Valzania F, Strafella AP, Quatrale R, Santangelo M, Tropeani A, Lucchi D, Tassinari CA, De Grandis D. Motor evoked responses to paired cortical magnetic stimulation in Parkinson’s disease. Electroencephalogr Clin Neurophysiol. 1997;105:37–43. doi: 10.1016/s0924-980x(96)96517-0. [DOI] [PubMed] [Google Scholar]
- Wagle-Shukla A, Angel MJ, Zadikoff C, Enjati M, Gunraj C, Lang AE, Chen R. Low-frequency repetitive transcranial magnetic stimulation for treatment of levodopa-induced dyskinesias. Neurology. 2007;68:704–705. doi: 10.1212/01.wnl.0000256036.20927.a5. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroenceph Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol (Lond) 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SA, Lockwood RJ, Thickbroom GW, Mastaglia FL. The muscle silent period following transcranial magnetic cortical stimulation. J Neurol Sci. 1993;114:216–222. doi: 10.1016/0022-510x(93)90301-e. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W. Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electroenceph Clin Neurophysiol. 1997;105:430–437. doi: 10.1016/s0924-980x(97)00050-7. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS induced plasticity in human cortex. Rev Neurosci. 2004;15:253–266. doi: 10.1515/revneuro.2004.15.4.253. [DOI] [PubMed] [Google Scholar]