Summary
Peroxisomes are indispensable for proper functioning of human cells. They efficiently compartmentalize enzymes responsible for a number of metabolic processes, including the absolutely essential β-oxidation of specific fatty acid chains. These and other oxidative reactions produce hydrogen peroxide, which is, in most instances, immediately processed in situ to water and oxygen. The responsible peroxidase is the heme-containing tetrameric enzyme, catalase.
What has emerged in recent years is that there are circumstances in which the tightly regulated balance of hydrogen peroxide producing and degrading activities in peroxisomes is upset - leading to the net production and accumulation of hydrogen peroxide and downstream reactive oxygen species. The factor most essentially involved is catalase, which is missorted in aging, missing or present at reduced levels in certain disease states, and inactivated in response to exposure to specific xenobiotics. The overall goal of this review is to summarize the molecular events associated with the development and advancement of peroxisomal hypocatalasemia and to describe its effects on cells. In addition, results of recent efforts to increase levels of peroxisomal catalase and restore oxidative balance in cells will be discussed.
Keywords: aging, catalase, peroxisome, protein import, reactive oxygen species, senescence
Introduction
How peroxisomes are formed has been a topic of great interest to many investigators for several years now. Proteins involved in various aspects of membrane assembly, protein import, and organelle maintenance and inheritance have been identified and how these molecules function mechanistically elucidated in many cases. Although important questions remain, it is clear that overall, peroxisome biogenesis is relatively well understood.
There is also a rich understanding of peroxisome biochemistry and function as well as of how the organelle is turned over during the processes known collectively as “pexophagy.” Furthermore, the molecular basis of many devastating peroxisomal diseases has been delineated. Of no less importance, but far less studied, are the questions of how the peroxisome functions in cells and organisms of advancing age and what contribution the organelle makes to the course of cellular aging. It is these latter questions that are the focus of this review.
Human cellular aging is multifactorial (see (1–3) and references therein); telomeres shorten, proteins are aberrantly glycated, DNA is damaged and inefficiently or improperly repaired, epigenetic events occur, protein expression is altered and reactive oxygen species accumulate and damage cellular constituents. No one process dominates under all circumstances - rather they appear to conspire in some, perhaps varying, combination. An important open question is what initiates the aging cascade. One possibility is reactive oxygen species; molecules with the capacity to damage, inactivate, and even destroy cellular DNA, proteins, and lipids including biological membranes.
Falling under the heading of reactive oxygen species and related compounds are singlet oxygen, superoxide anion, hydrogen peroxide, and the hydroxyl radical. These cellular reactive oxygen species are produced primarily by mitochondria and peroxisomes (4). Although peroxisomes probably do not generate near the amount of reactive oxygen species that mitochondria do (1,5), they may very well be making them earlier - an important point to be considered further below.
Peroxisome aging was initially investigated using a rat liver model. These important studies documented, for the first time, age-related differences in peroxisomal enzyme activities and overall organelle function (see detailed summary (6)). Important follow-up work by Badr and colleagues confirmed the reduced activities of peroxisomal β-oxidation enzymes and catalase in aging rat liver, and suggested that with respect to the former, diminished levels of the peroxisome proliferator-activated receptor alpha (PPARα)-binding partner, retinoid X receptor alpha (RXRα), may be responsible (7).
More recently, human fibroblasts have been employed to study the specific relationship of peroxisomes and cellular aging. These somatic cells may be cultured to various population doubling levels (PDL), with advancing PDLs being viewed as akin to aging. At a certain point, these cells experience an irreversible cell cycle arrest and are termed “senescent”. Characteristic biochemical and morphological changes accompany cells' entry into the end of their replicative lifespan. Perhaps the best validation of the model system comes from recent studies performed in primates demonstrating the presence of senescent cells at high frequencies in aged tissue (8).
A final note regarding cellular senescence; it has been suggested and evidence obtained to support the idea that cells enter replicative senescence to avoid transformation and becoming cancerous (9). Interestingly, the very environment that promotes senescence - that is, the presence of reactive oxygen species both within cells and in the surrounding stroma, also potentiates initiation and progression of cancerous transformation.
PTS1 Protein Import in Aging
The overwhelming majority of peroxisomal enzymes, including most oxidases which produce hydrogen peroxide, contain a carboxy-terminal tripeptide closely related or identical to serine-lysine-leucine (SKL) (10). Called peroxisomal targeting signal 1 (PTS1), this sequence and residues just upstream determine the enzymes' capacities to be recognized by the import receptor, Pex5p (10–12). Conventional wisdom in the field is that the strength of Pex5p's binding to a given substrate determines its import efficiency. “PTS1 predictor” (see http://mendel.imp.ac.at/mendeljsp/sat/pts1/PTS1predictor.jsp), a powerful tool for the in silico analysis of relative PTS1 strength, indicates that human peroxisomal oxidases consistently score in the 10–15 range - a point to be returned to shortly.
Using biochemically-defined in vitro assays, peroxisomal import of a PTS1 (SKL)-containing reporter protein was found to drop in cells of advancing age (13). Interestingly, this import defect appears first in cells of middle passage and is then exacerbated in late passage and senescent cells. Extensive follow-up studies indicate that irrespective of the human cell line examined or the import assay employed, the results were similar; aging compromises peroxisomal protein import.
As pointed out above, catalase levels are found to be reduced in peroxisomes of aged rat livers (6,7,14). Coupled with early reports that catalase was a rather poor substrate of the peroxisomal protein import apparatus (15), a more detailed examination of catalase's import properties as a function of age was prompted. Perhaps not surprisingly, catalase's import capacity too declined significantly with age (Terlecky, S.R., Koepke, J.I., and Walton, P.A., unpublished results). Catalase is a PTS1-containing enzyme whose import is mediated by Pex5p (16). However, its PTS1 is a tetrapeptide, lysine-alanine-asparagine-leucine (KANL) (16), largely unrelated to the consensus motif described above. This sequence is an extremely weak PTS1, only poorly directing catalase or other reporter proteins to which it is appended, to the organelle (13,17). Combined with its upstream amino acids (in catalase), its “PTS1 predictor” score is a paltry 1.5. A weak import substrate even in early passage cells, catalase's import is severely compromised in middle and late passage cells. Thus it appears that although aging acts on all PTS1 enzymes with respect to their import capacities, it is catalase with its weak KANL signal that is the most dramatically affected.
Pex5p
Binding
One straightforward explanation for the distinct import capacities of SKL-containing enzymes as compared to catalase is differential binding by Pex5p. Several experimental approaches were brought to bear on the issue including fluorescence anisotropy, ligand blots, solid-phase (ELISA-type) assays, two-hybrid analysis, and surface plasmon resonance, among others (12,13,18). The results were clear and consistent - proteins containing an SKL PTS1 were far better recognized and bound by Pex5p than the KANL-containing catalase molecule. Importantly, appending an SKL sequence in lieu of KANL increased catalase's recognition by Pex5p to the level seen with other SKL-containing enzymes (13). Catalase-SKL was not only recognized more avidly by Pex5p, but its import into peroxisomes was also enhanced (Terlecky, S.R., Koepke, J.I., and Walton, P.A., unpublished results).
What happens to Pex5p with age? Preliminary results suggest its affinity for PTS1 sequences is reduced (Terlecky, S.R., Koepke, J.I., and Walton, P.A., unpublished results). As will be discussed below, aging cells accumulate hydrogen peroxide and other reactive oxygen species. Perhaps Pex5p is oxidatively damaged in that environment. The observation that hydrogen peroxide-treated Pex5p binds PTS1 less well supports this notion. Investigation in this area continues.
Most of the binding studies described above involve measurement of direct protein-protein interactions between receptor and ligand. Such interactions may very well be facilitated or modulated in the cell by molecular chaperones or other factors. Hsp70 is one such factor - shown to regulate Pex5p's binding to PTS1 (19) and be a necessary component of (PTS1) protein import (20). Since its expression is reduced in aging (21), hsp70, or more accurately, its absence, may contribute to the processes associated with peroxisome senescence.
Trafficking
Pex5p is a cycling receptor - binding PTS1 cargo in the cytosol and ferrying it to the peroxisome membrane. The receptor docks with the peroxisomal membrane protein Pex14p (22), and through a rather poorly understood series of steps, releases its cargo, is freed from Pex14p and then either enters the organelle (in whole or in part), or engages other membrane peroxins and recycles back to the cytosol. Pex5p cycling is critical - under certain conditions (e.g. ATP depletion or reduced temperatures) Pex5p accumulates on the peroxisome membrane and PTS1 protein import is slowed (23). Increased membrane-associated Pex5p is also seen in certain mutant backgrounds - for example, when the peroxisomal membrane proteins Pex2p, Pex10p, or Pex12p are defective or absent (23), or when activity of the AAA proteins Pex1p and Pex6p are reduced or eliminated (24,25). Of relevance here is the fact that Pex5p's cycling is reduced and accumulation on the peroxisome membrane enhanced in middle and late passage cells (13). Again, such slowing of Pex5p cycling is most certainly associated with reduced import rates. Pointing to the oxidative environment of aging cells as potentially once more eliciting these effects are the observations that treating (early passage) cells with hydrogen peroxide not only causes Pex5p to amass on the organelle membrane, but also significantly reduces PTS1 protein import (13).
Peroxisomes of Aging Cells
Proliferation
Detailed immunocytochemical analysis of middle and late passage human cells revealed progressively less intense staining of SKL-containing proteins (13). Rather, these molecules appeared to accumulate in ever-increasing amounts in the cytosol. Catalase was also mislocalized in aging cells, although to a far greater extent. Indeed, some late passage cells appeared completely devoid of peroxisomal catalase. Biochemical (latency) analysis confirmed the immunofluorescence work - catalase was clearly accumulating in the cytosol of aging cells (13).
As if the aging cell senses a reduced concentration of peroxisomal enzymes (or the accumulation of unmetabolized substrates), it responds by increasing peroxisome number - that is, the organelle proliferates (13). As compared to early passage cells, middle and late passage cells contain approximately twice as many punctate structures that may be immunodecorated with antibodies to the peroxisomal membrane protein of 70 kilodaltons (PMP70) or Pex14p (appropriate membrane markers).
Similarly, peroxisomes proliferate in human retinal epithelia of aged individuals or those suffering from (age-related) macular degeneration (26).
Peroxisome biogenesis is thought to be a delicate balance of organelle/membrane formation and assembly, protein import, and growth and division of the nascent structure. In aging, this balance is upset and organelle formation or division occurs even when protein import is corrupted. It is not clear what elicits this response (Pex11p involved?) and whether or not it represents some type of rescue mechanism.
Hydrogen Peroxide Production
In early passage cells, peroxisomes exist in oxidative balance - the organelle's oxidases consume molecular oxygen and convert it to hydrogen peroxide, while catalase decomposes the potentially toxic metabolite back to oxygen and water. A balance of peroxisomal pro- and antioxidants exists. With advancing age and the accompanying effects on peroxisomal protein import described above, this homeostatic balance is upset. Peroxisomal catalase in particular is reduced or lost. The result of this uncoupling is the net production of hydrogen peroxide and related reactive oxygen species. Specific oxidation-sensitive fluorescent dyes and enzymatic assays have been used to document dramatic increases in hydrogen peroxide and other reactive oxygen species in late passage cells (13). That these molecules amass in aged cells was well established in the literature (1); that the peroxisome contributes to this oxidative burden has only recently come to light.
Metal ion-catalyzed conversion of hydrogen peroxide to the hydroxyl radical via the Fenton reaction serves to increase the destructive capacity of the peroxisomal metabolic by-products formed. These molecules are quite clearly capable of damaging DNA, proteins, and lipids including biological membranes. Recent evidence suggests peroxisome-derived hydrogen peroxide and downstream reactive oxygen species may also damage mitochondria causing them to depolarize and produce damaging oxidants of their own (Terlecky, S.R., Koepke, J.I., and Walton, P.A., unpublished results).
Hydrogen peroxide and related reactive oxygen species initiate a negative spiral of molecular events resulting in oxidative damage to cellular constituents. Cell growth rates are reduced, perhaps due to effects on the mitogenic cell-signaling extracellular signal-regulated protein kinase 1/2 (“Erk 1/2”) pathway, known to be sensitive to hydrogen peroxide (27).
Ames and colleagues demonstrated a direct connection between accumulation of reactive oxygen species and aging by showing non-toxic concentrations of hydrogen peroxide drove (early passage) cells to a senescent-like state (27). Cell morphology, growth, and function were all affected. Similar approaches employed recently revealed that hydrogen peroxide actually damages the PTS1-protein import machinery suggesting the notion of a self-sustaining “peroxisome deterioration spiral” as illustrated in Figure 1. In this model, age-related peroxisomal hypocatalasemia results in increased cellular concentrations of hydrogen peroxide. Other more damaging reactive oxygen species may form and harm cellular components and functions. Peroxisomes themselves are a target of oxidant-induced damage - further exacerbating the problem by potentially perpetuating the spiral.
Figure 1. Peroxisome deterioration spiral.
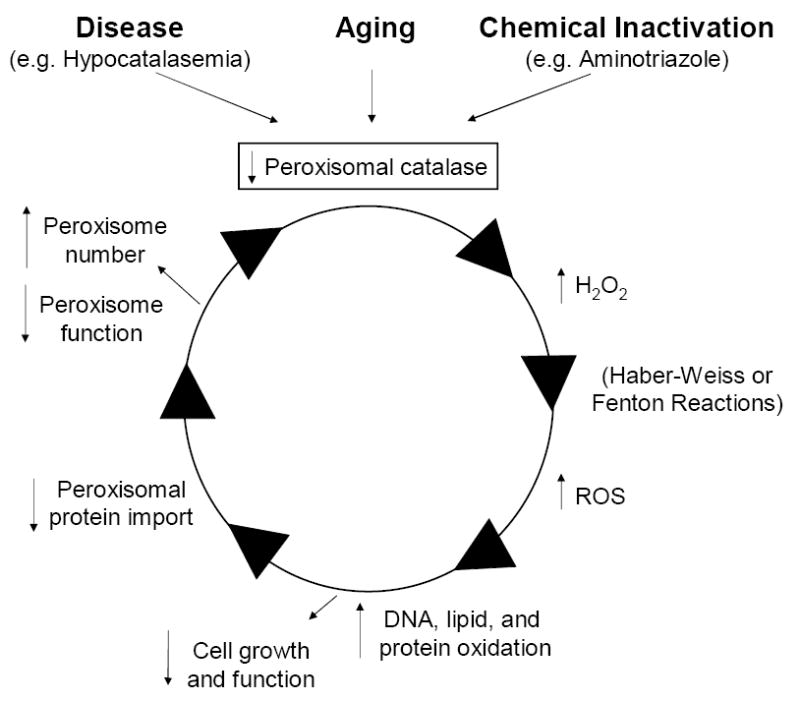
See text for further explanation.
Hypocatalasemia and Disease
Many individuals worldwide are hypocatalasemic, not due to aging's effects, but rather to a reduction in cellular catalase expression (29,30) or stability (29,31). Detailed epidemiological studies suggest these individuals experience the premature onset of age-related disease, including anemia, atherosclerosis, tumors, and type 2 diabetes as well as the eye disorders, cataracts and macular degeneration (32–35). Cells from a hypocatalasemic patient (expressing approximately 25% of normal catalase levels) were found to have accumulated hydrogen peroxide and harbored age-associated pathologies (17). These cells were oxidatively damaged - proteins were excessively carbonylated, DNA contained oxidative lesions, and growth rates were reduced. Peroxisome function was compromised as was PTS1 protein import. Presumably one or both of the latter phenomena explain the reduced activity measured of dihydroxyacetone-phosphate acyl-transferase, the rate-limiting enzyme in plasmalogen biosynthesis. This is an important point as plasmalogens are a critical class of cellular lipids found in virtually all membranes within the cell. They serve to protect the cell against various damaging agents including reactive oxygen species (36). Their relative absence would render the cell and its constituent membranes that much more susceptible to toxic insult. What other peroxisomal enzyme activities are reduced or lost potentially further jeopardizing cell physiology remain to be determined.
Absent from these analyses was any evidence that these cells were genuinely senescent. It appears cells from hypocatalasemic patients enter the “peroxisome deterioration spiral” not due to mislocalized catalase, but rather because of its relative absence. Oxidative damage is the price paid - which once again only serves to further ratchet down all peroxisomal protein, but especially of catalase.
Cells may also be rendered hypocatalasemic by inhibiting the enzyme with 3-amino-1,2,4-triazole (aminotriazole). In experiments where aminotriazole is added to cells continuously for several passages at a concentration that reduces catalase activity to approximately 35% of normal, the cells behave rather predictably (Terlecky, S.R., Koepke, J.I., and Walton, P.A., unpublished results). They amass hydrogen peroxide and other reactive oxygen species, contain oxidatively damaged protein and DNA, and exhibit slowed growth rates. Their peroxisomes proliferate and are less import competent; senescence markers appear. In short, the cells have entered the peroxisome deterioration spiral. Quite clearly, catalase inactivation accelerates aging in human cells.
Peroxisomal Catalase
Other Model Systems
To this point, the review has focused on hypocatalasemia as it relates to the human condition. However, the effects of altered peroxisomal catalase levels have been examined in a number of other model systems. For example, in the nematode Caenorhabditis elegans, loss of peroxisomal catalase results in the organism manifesting a progeric phenotype (37). (Note that these animals contain distinct cytosolic and peroxisomal forms of catalase; loss of the cytosolic enzyme has no effect on aging parameters.) Generation and processing of reactive oxygen species appears altered, peroxisome morphology is changed, and the organism's lifespan is shortened. Similarly, lifespan of the yeast Saccharomyces cerevisiae is significantly reduced when its (peroxisomal) catalase is knocked out (37).
In rats and mice, a general trend emerges. Cellular catalase levels drop with age, accompanied by an increase in reactive oxygen species and resultant oxidative stress (14,38,39). Calorically restricted animals reverse this trend - they express elevated levels of catalase and are more long-lived (40).
Restoration
To directly evaluate the role of catalase in organismal longevity, Schriner and coauthors (41) created a series of transgenic mice overexpressing catalase in various subcellular organelles. They directed the catalase to mitochondria, to the nucleus, and to peroxisomes by engineering the enzyme with appropriate organellar targeting signals. (With respect to the peroxisomally-targeted enzyme, the naturally occurring KANL PTS1 was employed.) Their results were dramatic - overexpression of mitochondrial catalase increased lifespan by nearly 20%. An anti-aging strategy based on “antioxidant prophylaxis” was a viable one. Nuclear-targeted catalase showed only insignificant increases while peroxisomally-directed catalase increased lifespan only some 10%. Perhaps these results reflect the importance both of catalase as a critical cellular anti-oxidant and mitochondria as the major source of damaging reactive oxygen species. However, based on what is now known regarding peroxisomal trafficking of catalase with age, it would be very interesting to know what happens to animals overexpressing catalase with an SKL-targeting signal. Such animals have been created and longitudinal aging studies begun (Terlecky, S.R., Koepke, J.I., and Walton, P.A., unpublished results).
Restoration of peroxisomal catalase has been achieved at the cellular level (16 and Terlecky, S.R., Koepke, J.I., and Walton, P.A., unpublished results). Human fibroblasts from a hypocatalasemic individual transduced with catalase-SKL had their levels of hydrogen peroxide and related oxidants reduced nearly 80%. In fact, their oxidant level after treatment was only slightly higher than that seen in normal (early passage) fibroblasts. Catalase-KANL similarly introduced into hypocatalasemic cells quenched less than 50% of the hydrogen peroxide and related reactive oxygen species. If hypocatalasemic cells are in fact generating hydrogen peroxide and other reactive oxygen species in peroxisomes, then catalase directed to the organelle (via an SKL-PTS1) might be expected to be the most effective strategy. Supporting this idea is the observation that in aging human cells nuclear microinjected with plasmids encoding catalase-SKL or catalase-KANL derivatives, it is the catalase-SKL molecule that is far more effective at eliminating hydrogen peroxide and related reactive oxygen species.
Concluding Comments/Future Considerations
One of the questions that arises regarding peroxisomal hypocatalasemia and cellular and organismal aging is why does human catalase have a “weak” targeting signal? A difficult question to answer - however, it does not appear to be an anomaly specific to humans. That is, examination of PTS1 sequences of at least 10 other animals including dogs, pigs, cows, and orangutans, reveals low PTS1-predictor scores (range of 1.5 – 4.4) for all. It is probably not likely that cells missort catalase by design - consider the patient with Zellweger-like neurological impairment described by Singh and colleagues (42). This individual's cells simply do not traffic catalase, in contrast to other peroxisomal enzymes that appear correctly targeted. Coupled with the evidence summarized here regarding the association of catalase mislocalization and age-related cellular pathologies, there must be another explanation. The more likely reason that catalase contains a weak targeting signal is that there was no evolutionary pressure to select against it. In humans, where due to environmental conditions, food supplies, disease, and other factors, life expectancy was not far beyond reproductive age, the naturally occurring catalase signal was sufficient. Only recently as humans have started to live longer has the issue of peroxisomal trafficking efficiency and accumulation of cellular reactive oxygen species become a concern. Mice die in the wild largely of hypothermia, not from the accumulated effects of cellular aging.
Going forward, basic and translational scientists will continue to try to understand the cellular factors and molecular mechanisms contributing to aging and related degenerative disease. Such understanding will foster the development of pharmaceutical therapeutics designed not only to treat aging, but also to slow it down or indeed, prevent it. Oxidant-induced damage accumulates over years - facilitating the development and progression of age-related disease. The work summarized herein identifies a new source of reactive oxygen species in cells and a potential new target for intervention. Reducing the oxidative load contributed to the cells by peroxisomal hypocatalasemia may prove to be very beneficial in treating aging pathologies and, importantly, be an attainable goal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Aging: Phenomena and theories. Ann NY Acad Sci. 1998;854:1–7. doi: 10.1111/j.1749-6632.1998.tb09886.x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson FB, Sinclair DA, Guarente L. Molecular biology of aging. Cell. 1999;96:291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- 4.Moldovan L, Moldovan NI. Oxygen free radicals and redox biology of organelles. Histochem Cell Biol. 2004;122:395–412. doi: 10.1007/s00418-004-0676-y. [DOI] [PubMed] [Google Scholar]
- 5.Lee HC, Wei YH. Mitochondrial alterations, cellular response to oxidative stress and defective degradation of proteins in aging. Biogerontology. 2001;2:231–244. doi: 10.1023/a:1013270512172. [DOI] [PubMed] [Google Scholar]
- 6.Perichon R, Bourre JM, Kelly JF, Roth GS. The role of peroxisomes in aging. Cell Mol Life Sci. 1998;54:641–652. doi: 10.1007/s000180050192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao C, Youssef J, Rezaiekhaleigh M, Birnbaum L, Badr M. Senescence-associated decline in hepatic peroxisomal activities corresponds with diminished levels of retinoid X receptor alpha, but not peroxisome proliferator-activated receptor alpha. Mech Ageing Dev. 2002;123:1469–1476. doi: 10.1016/s0047-6374(02)00086-6. [DOI] [PubMed] [Google Scholar]
- 8.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 9.Campisi J. Suppressing cancer: the importance of being senescent. Science. 2005;309:886–887. doi: 10.1126/science.1116801. [DOI] [PubMed] [Google Scholar]
- 10.Subramani S. Components involved in peroxisome import, biogenesis, proliferation, turnover and movement. Physiol Rev. 1998;78:171–180. doi: 10.1152/physrev.1998.78.1.171. [DOI] [PubMed] [Google Scholar]
- 11.Subramani S, Koller A, Snyder WB. Import of peroxisomal matrix and membrane proteins. Ann Rev Biochem. 2000;69:399–418. doi: 10.1146/annurev.biochem.69.1.399. [DOI] [PubMed] [Google Scholar]
- 12.Lametschwandtner G, Brocard C, Fransen M, Van Veldhoven P, Berger J, Hartig A. The difference in recognition of terminal tripeptides as peroxisomal targeting signal 1 between yeast and human is due to different affinities of their receptor Pex5p to the cognate signal and to residues adjacent to it. J Biol Chem. 1998;273:33635–33643. doi: 10.1074/jbc.273.50.33635. [DOI] [PubMed] [Google Scholar]
- 13.Legakis JE, Koepke JI, Jedeszko C, Barlaskar F, Terlecky LJ, Edwards HJ, Walton PA, Terlecky SR. Peroxisome senescence in human fibroblasts. Mol Biol Cell. 2002;13:4243–4255. doi: 10.1091/mbc.E02-06-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beier K, Volkl A, Fahimi HD. The impact of aging on enzyme proteins of rat liver peroxisomes: quantitative analysis by immunoblotting and immunoelectron microscopy. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63:139–146. doi: 10.1007/BF02899254. [DOI] [PubMed] [Google Scholar]
- 15.Lazarow PB, Robbi M, Fujiki Y, Wong L. Biogenesis of peroxisomal proteins in vivo and in vitro. In: Kindl H, Lazarow PB, editors. Peroxisomes and Glyoxysomes. Vol. 386. Annals of the New York Academy of Sciences; New York, NY: 1982. pp. 285–300. [DOI] [PubMed] [Google Scholar]
- 16.Purdue PE, Lazarow PB. Targeting of human catalase to peroxisomes is dependent upon a novel COOH-targeting sequence. J Cell Biol. 1996;134:849–862. doi: 10.1083/jcb.134.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood CS, Koepke JI, Teng H, Boucher KK, Katz S, Chang P, Terlecky LJ, Papanayotou I, Walton PA, Terlecky SR. Hypocatalasemic fibroblasts accumulate hydrogen peroxide and display age-associated pathologies. Traffic. 2006;7:97–107. doi: 10.1111/j.1600-0854.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 18.Gatto GJ, Jr, Maynard EL, Guerrerio AL, Getsbrecht BV, Gould SJ, Berg JM. Correlating structure and affinity for Pex5:PTS2 complexes. Biochemistry. 2003;42:1660–1666. doi: 10.1021/bi027034z. [DOI] [PubMed] [Google Scholar]
- 19.Harano T, Nose S, Uezu R, Shimizu N, Fujiki Y. Hsp70 regulates the interaction between the peroxisome targeting signal type 1 (PTS1)-receptor Pex5p and PTS1. Biochem J. 2001;357:157–165. doi: 10.1042/0264-6021:3570157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walton PA, Wendland M, Subramani S, Rachubinski RA, Welch WJ. Involvement of 70 kDa heat-shock proteins in peroxisomal import. J Cell Biol. 1994;125:1037–1046. doi: 10.1083/jcb.125.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 22.Fransen M, Terlecky SR, Subramani S. Identification of a human PTS1 receptor docking protein directly required for peroxisomal protein import. Proc Natl Acad Sci USA. 1998;95:8087–8092. doi: 10.1073/pnas.95.14.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dodt G, Gould SJ. Multiple PEX genes are required for proper subcellular distribution and stability of Pex5p, the PTS1 import receptor: evidence that PTS1 protein import is mediated by a cycling receptor. J Cell Biol. 1996;135:1763–1774. doi: 10.1083/jcb.135.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platta HW, Grunau S, Rosenkranz K, Girzalsky W, Erdmann R. Functional role of the AAA peroxins in dislocation of the cycling PTS1 receptor back to the cytosol. Nature Cell Biology. 2005;7:817–822. doi: 10.1038/ncb1281. [DOI] [PubMed] [Google Scholar]
- 25.Miyata N, Fujiki Y. Shuttling Mechanism of Peroxisome Targeting Signal Type 1 Receptor Pex5: ATP-independent import and ATP-dependent export. Mol and Cell Biol. 2005;25:10822–10832. doi: 10.1128/MCB.25.24.10822-10832.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feher J, Kovacs I, Artrico M, Cavallotti C, Papale A, Balacco-Gabriel C. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiology of Aging. 2005;27:983–993. doi: 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Kim HS, Song MC, Kwak IH, Park TJ, Lim IK. Constitutive induction of p-Erk1/2 accompanied by reduced activities of protein phosphatases 1 and 2A and MKP3 due to reactive oxygen species during cellular senescence. J Biol Chem. 2003;278:37497–37510. doi: 10.1074/jbc.M211739200. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q, Ames BN. Senescent-like growth arrest induced by hydrogen peroxide in human diploid fibroblasts. Proc Natl Acad Sci USA. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eaton JW, Ma M. Acatalasemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 1995. pp. 2371–2383. [Google Scholar]
- 30.Wen JK, Osimi T, Hashimoto T, Ogata M. Diminished synthesis of catalase due to the decrease in catalase mRNA in Japanese-type acatalasemia. Physiol Chem Phy Med NMR. 1988;20:171–176. [PubMed] [Google Scholar]
- 31.Crawford DR, Mirault ME, Moret R, Zbinden I, Cerutti PA. Molecular defect in human acatalasia fibroblasts. Biochem Biophys Res Commun. 1988;153:59–66. doi: 10.1016/s0006-291x(88)81189-6. [DOI] [PubMed] [Google Scholar]
- 32.Goth L, Eaton JW. Hereditary catalase deficiencies and increased risk of diabetes. Lancet. 2000;356:1820–1821. doi: 10.1016/S0140-6736(00)03238-4. [DOI] [PubMed] [Google Scholar]
- 33.Goth L. Lipid and carbohydrate chemistry in acatalasemia. Clin Chem. 2000;46:564–566. [PubMed] [Google Scholar]
- 34.Goth L, Vitai M. Hypocatalesemia in hospital patients. Clin Chem. 1996;42:341–342. [PubMed] [Google Scholar]
- 35.Yildirim O, Ates NA, Tamer L, Musler N, Ercan B, Atik U, Kanik A. Changes in antioxidant enzyme activity and malondialdehyde level in patients with age-related macular degeneration. Ophthalmologica. 2004;218:202–206. doi: 10.1159/000076845. [DOI] [PubMed] [Google Scholar]
- 36.Morand OH, Zoeller RA, Raetz CRH. Disappearance of plasmalogens from membranes of animal cells subjected to photosensitized oxidation. J Biol Chem. 1988;263:11597–11606. [PubMed] [Google Scholar]
- 37.Petriv OI, Rachubinsky RA. Lack of peroxisomal catalase causes a progeric phenotype in Caenorhabditis elegans. J Biol Chem. 2004;279:19996–20001. doi: 10.1074/jbc.M400207200. [DOI] [PubMed] [Google Scholar]
- 38.Ishii K, Zhen LX, Wang DH, Funamori Y, Ogawa K, Taketa K. Prevention of mammary tumorigenesis in acatalasemic mice by vitamin E supplementation. Jpn J Cancer Res. 1996;87:680–684. doi: 10.1111/j.1349-7006.1996.tb00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito A, Watanabe H, Aoyama H, Nakagawa Y, Mori M. Effect of 1,2-dimethylhydrazine and hydrogen peroxide for the duodenal tumorigenesis in relation to blood catalase activity in mice. Hiroshima J Med Sci. 1986;35:197–200. [PubMed] [Google Scholar]
- 40.Rao G, Xia E, Nadakavukaren MJ, Richardson A. Effect of dietary restriction on the age-dependent changes in the expression of antioxidant enzymes in rat liver. J Nutr. 1990;120:602–609. doi: 10.1093/jn/120.6.602. [DOI] [PubMed] [Google Scholar]
- 41.Schriner S, Linfor NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpresson of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 42.Sheikh FG, Pahan K, Khan M, Barbosa E, Singh I. Abnormality in catalase import into peroxisomes leads to a severe neurological disorder. Proc Natl Acad Sci USA. 1998;95:2961–2966. doi: 10.1073/pnas.95.6.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]


