Abstract
Protein tyrosine kinases (PTK) are key enzymes of mammalian signal transduction. For the fidelity of signal transduction, each PTK phosphorylates only one or a few proteins on specific Tyr residues. Substrate specificity is thought to be mediated by PTK–substrate docking interactions and recognition of the phosphorylation site sequence by the kinase active site. However, a substrate-docking site has not been determined on any PTK. C-terminal Src kinase (Csk) is a PTK that specifically phosphorylates Src family kinases on a C-terminal Tyr. In this study, by sequence alignment and site-specific mutagenesis, we located a substrate-docking site on Csk. Mutations in the docking site disabled Csk to phosphorylate, regulate, and complex with Src but only moderately affected its general kinase activity. A peptide mimicking the docking site potently inhibited (IC50 = 21 μM) Csk phosphorylation of Src but only moderately inhibited (IC50 = 422 μM) its general kinase activity. Determination of the substrate-docking site provides the structural basis of substrate specificity in Csk and a model for understanding substrate specificity in other PTKs.
Protein tyrosine kinases (PTK) are a large family of enzymes that transfer the γ-phosphate of ATP to Tyr hydroxyl group in proteins (1). This covalent modification is a fundamental mechanism in cellular regulation and signal transduction (2). Activation of specific PTKs is associated with various types of human cancer and other proliferative diseases, making many PTKs targets for drug discovery (3, 4). Although all PTKs share a highly conserved catalytic domain (5), each PTK phosphorylates only one or a few protein substrates on specific tyrosine residues, enabling them to transduce regulatory signals to specific targets. The tertiary structures of a dozen PTKs have been determined (5), but the mechanisms by which PTKs recognize their physiological substrates are still poorly understood.
One of the best understood PTK regulatory systems is the regulation of Src family PTKs (SFKs) (6). There are nine kinases in the Src family. They share an overall structural organization, containing a myristoylation motif, a unique region, an Src homology (SH)3 domain, an SH2 domain, a catalytic domain, and a regulatory C-terminal tail. SFKs are regulated by phosphorylation on two Tyr residues, one located on the activation loop (Yact) and the other on the C-terminal tail (Ytail). Yact is the site of autophosphorylation catalyzed by SFKs through an intermolecular mechanism, which activates SFKs (7). Ytail is phosphorylated by another family of PTKs containing two members, C-terminal Src kinase (Csk) (8) and Csk-homologous kinase (Chk) (9, 10). Phosphorylated Ytail binds to the SH2 domain intramolecularly (11, 12), which leads to inactivation of SFKs (13). Additionally, phosphorylation of Yact blocks the inactivation of SFKs by Ytail phosphorylation (14). Because the phosphorylation of Yact and Ytail has opposite effects on SFK function, it is critical that they are specifically phosphorylated by respective kinase activities. The phosphorylation of Ytail by Csk and Chk is indeed highly specific and exclusive (15). Only Csk and Chk phosphorylate the Ytail of SFKs, which are the only physiological substrates for Csk and Chk. It is not clear whether Csk and Chk have identical or different specificity among SFKs.
The C-terminal tails of SFKs have a consensus sequence of TATEXQYtailQXQ/G, where the X's are variable residues. Early efforts to understand Csk substrate specificity used peptides mimicking this phosphorylation site (8, 16). However, such peptides are ≈1,000 times less efficient than SFKs as substrates for Csk (8, 16, 17). By screening a random peptide library, Cole and coworkers (17) identified an optimal peptide substrate for Csk with the sequence of EEEIYFFF. The optimal peptide is 500 times better as a substrate than are peptides mimicking the Src C-terminal tail and bears little resemblance to the physiological phosphorylation site (17). These studies demonstrate that the C-terminal tail does not contain sufficient determinants for Csk recognition. Recent mutagenic studies of Src indicated that, although the local sequence surrounding the phosphorylation site played important roles, additional determinants residing outside the C-terminal tail are required for efficient Ytail phosphorylation by Csk (18). These observations suggest that Csk recognition of Ytail of SFKs involves two types of interactions: docking interactions between Csk and SFKs and local interactions between the active site of Csk and the tail peptide sequence. Such bivalent interactions would allow Csk to specifically recognize SFKs and position the Ytail into Csk active site for phosphorylation. The docking site on Csk and the docking determinants on SFKs have not been determined.
The tertiary structures of Csk (19, 20) and several SFKs (11, 12, 21) have been determined, but the structures do not provide clear clues to how Csk recognition of SFKs may be achieved. In this current study, using structure-guided site-specific mutagenesis, we determined a substrate-docking site on Csk that is critical for its ability to bind to, phosphorylate, and regulate SFKs but is not important for the general kinase activity. Furthermore, a peptide mimicking the substrate-docking site potently inhibited Csk phosphorylation of Src but only moderately inhibited the general kinase activity of Csk. To our knowledge, this is the first report of a substrate-docking site on any PTK.
Methods
Chemicals and Reagents. All reagents used for bacterial culture and protein expression were purchased from Fisher. Chromatographic resins, glutathione-agarose, iminodiacetic acid-agarose, and Sephadex G25 were purchased from Sigma. DNA primers were synthesized by Integrated DNA Technologies (Coralville, IA). [γ-32P]ATP (6,000 Ci mol–1; 1 Ci = 37 GBq) was purchased from Perkin-Elmer. Peptides were synthesized by solid phase synthesis, purified by HPLC, and confirmed by electrospray mass spectrometry.
Recombinant Kinase and Substrate Expression and Site-Specific Mutagenesis. Wild type human Csk was expressed in E. coli (DH5α) by using pGEX-Csk-st plasmid (22, 23). Site-specific mutants were generated by using QuikChange (Stratagene). The entire coding regions of the mutant plasmids were sequenced to confirm that the correct mutations were incorporated. The GST–Csk fusion proteins were purified by glutathione affinity chromatography and stored in 50 mM Tris-Cl, pH 8.0, at –20°C in 30% glycerol. The chicken Src mutant devoid of kinase activity (kdSrc) was coexpressed with GroEL and GroES chaperone in BL21(DE3) as described (18). kdSrc was purified by a Ni2+-iminodiacetic acid agarose column as described (24). Lys295Met mutation abolishes the Src kinase activity but does not affect its ability to serve as a substrate for Csk (18, 25). Protein concentrations were determined by the Bradford method and A280 in the 6 M urea. Purity of the purified enzymes was examined by SDS/PAGE and Coomassie blue staining.
Kinase Activity Assays. Kinase activity of Csk and mutants was determined by using polyE4Y or kdSrc and [γ-32P]ATP (600 dpm pmol–1) as the substrates as described (26). Briefly, phosphorylation reactions were performed in 50-μl volumes at 30°Cinthe protein kinase assay buffer: 50 mM N-(2-hydroxyethyl)piperazine-N′-3-propanesulfonic acid (pH 8.0) containing 5% glycerol, 0.005% Triton X-100, and 0.05% 2-mercaptoethanol. The standard assay used 3 nM WT Csk, 12 mM MgCl2, 0.2 mM ATP, and 1 mg ml–1 polyE4Y or 10 μM kdSrc. After a 10-min reaction time, 35 μl of the reaction mixture was spotted onto Whatman filter paper squares (2 × 1 cm), which were washed in 5% trichloroacetic acid at 65°C three times for 20 min each. The radioactivity incorporated into polyE4Y or kdSrc was determined by liquid scintillation counting. Assays were performed in duplicate, and each assay was repeated at least twice with reproducible results.
To determine the catalytic parameters of Csk by using kdSrc as a substrate, 0.71–7.1 μM kdSrc was used as the variable substrate. The assays were performed as described above. The reaction minus Csk was used as background controls. The background was <2,000 cpm, and the signals were in the range of 10,000–100,000 cpm. No autophosphorylation or phosphorylation of polyE4Y by kdSrc was detected under these conditions. The Km and kcat values were determined by using double reciprocal plot.
To determine the inhibition of Csk activity by peptides, Csk activity in the presence of various concentrations of the peptide was determined. The kinase activity as a function of the peptide concentration was fitted into a curve-fitting program (labfit)to determine the IC50.
Csk Inactivation of Src. The ability of Csk and mutants to inactivate Src was determined as described (14). Appropriate level of Src activity was incubated with Csk or Csk mutants in the presence of 0.1 mM ATP and 12 mM MgCl2 at 30°C for 10 min. At the end of the incubation, 0.4 mg/ml RCM-lysozyme and [γ-32P]ATP was added. Because RCM-lysozyme was preferentially phosphorylated by Src, the residual Src activity after the initial incubation could be accurately determined without removing Csk from the incubation.
Pull-Down Assay. To determine the interaction between Csk and kdSrc, pull-down assays were performed. Purified GST, GST-Csk, or GST-mutant Csk fusion proteins (100 pmol) were incubated with 200 pmol of kdSrc in 50 μl of kinase assay buffer without ATP and MgCl2 at 30°C for 15 min with gentle agitation. Glutathione-agarose bead suspension (20 μl) was added into each incubation and incubated for another 15 min. The incubation mixtures were passed through a small column to collect the beads, which were then washed three times with 100 μl of kinase assay buffer each. The proteins associated with the beads were analyzed by SDS/PAGE.
Results
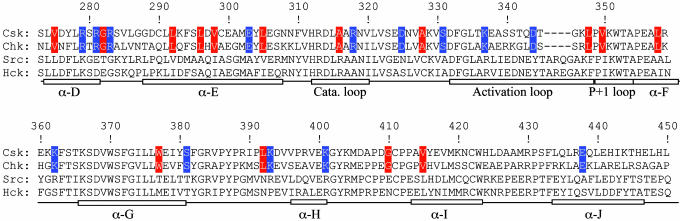
Homology Alignment and Ala Scanning Identify a Potential Substrate-Docking Site in Csk. Csk contains an SH3 and an SH2 domain (27, 28). Previous studies suggest that the SH3 and SH2 domains do not directly contribute to substrate recognition by Csk (29, 30). The Csk catalytic domain is composed of an ATP-binding lobe and a peptide-binding lobe (19, 20). We focused our search for the substrate-docking site on the peptide-binding lobe, which contains 180 residues (271–450). Because Csk and Chk are the only two PTKs capable of phosphorylating SFKs on Ytail, the docking site must be conserved in Csk and Chk but not in other PTKs. To locate the structural features uniquely conserved in Csk and Chk, primary sequences of Csk (28), Chk (31), Src (32), and Hck (33) in the peptide-binding lobe were aligned. This alignment identified 29 residues that are uniquely conserved in the Csk family (Fig. 1). Because Csk phosphorylation of kdSrc is highly sensitive to ionic inhibition (data not shown), we first focused on 14 polar and charged residues (Arg-279, Arg-281, Arg-283, Glu-303, Arg-318, Asp-325, Ser-331, Lys-337, Asp-344, Lys-362, Ser-381, Lys-393, Lys-401, and Glu-438). Among the 14, Ser-331 is buried in the interior of Csk, and Arg-318, Lys-337, and Asp-344 have been previously excluded as possible residues to interact with Src (24, 34, 35), leaving 10 residues as potentially part of the substrate-docking site. No obvious spatial pattern was observed when the uniquely conserved residues were mapped onto the tertiary structure of Csk.
Fig. 1.
Alignment of the amino acid sequences of Csk, Chk, Src, and Hck in the peptide-binding lobe. Residues that are uniquely conserved in the Csk family are highlighted blue (polar or charged) or red (hydrophobic or Gly).
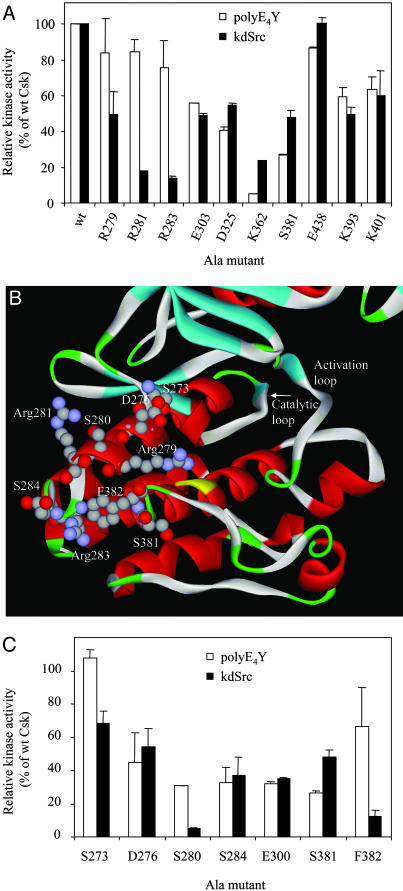
To determine which of these residues may be part of a substrate-docking site, they were individually mutated to Ala and the mutant enzymes were purified to apparent or near homogeneity. For some reason, one of the mutants, Arg359Ala, was not produced. By functional nature of the hypothetical substrate-docking site, we reasoned that this site would be critical for Csk activity toward its physiological substrate but not important for phosphorylation of an artificial substrate. Thus, as the initial screening, the kinase activities of the mutants toward an artificial and a physiological substrate were determined (Fig. 2A). The artificial substrate used was polyE4Y, a random co-polymer of Glu and Tyr in the ratio of 4:1. PolyE4Y lacks a defined phosphorylation site or higher orders of structure and is commonly used as a generic PTK substrate. The activity toward polyE4Y, therefore, is considered as general kinase activity. The physiological substrate used is a recombinantly expressed kinase-defective Src (kdSrc) that contains a Lys295Met mutation. The mutation inactivates Src but does not affect its ability to serve as a specific substrate for Csk or Chk (10, 18). The use of kdSrc instead of active Src eliminates interference to the assay by Src autophosphorylation.
Fig. 2.
Ala scanning mutagenesis to identify the substrate-docking site on Csk. (A) Ala scanning of polar or charged residues that are uniquely conserved in the Csk family. The activity of the Ala mutants toward an artificial and a physiological substrate are determined. (B) Structure of the Csk peptide-binding lobe. Residues that are potentially part of the substrate-docking site, the catalytic loop, and the activation loop are indicated. (C) Ala scanning of residues in or near the identified substrate-docking site.
If a residue is part of the substrate-docking site of Csk, its mutation to Ala will likely more dramatically decrease Csk activity toward kdSrc than toward polyE4Y. Three of the 10 mutants, Arg279Ala, Arg281Ala, and Arg283Ala, displayed this property, with the latter two exhibiting >80% of WT activity toward polyE4Y but <20% of WT activity toward kdSrc. Lys362Ala displayed the opposite effect, having a more dramatic effect on polyE4Y phosphorylation than kdSrc phosphorylation. This is representative of a group of Csk mutants that preferentially affect phosphorylation of polyE4Y over kdSrc, which were separately characterized (unpublished data). Mutation of the other residues had similar effects on polyE4Y and kdSrc phosphorylation. Overall, Ala scanning mutagenesis implicated Arg-281, Arg-283, and Arg-279 as part of the substrate-docking site.
Additional Residues Are Identified to Be Critical for Src Phosphorylation. Arg-279, Arg-281, and Arg-283 are located on α-helix D, a short helix located near the active site of Csk. In the tertiary structure, these three residues form a triangle (Fig. 2B). Each side of this triangle measures 15–16 Å. Two other residues, Ser-280 and Phe-382, although not uniquely conserved in Csk family, also fall within or near the area defined by the Arg triangle and could be part of the substrate-docking site. They were individually mutated to Ala, and the ability of the mutants to phosphorylate kdSrc and polyE4Y was determined. Ser280Ala and Phe382Ala displayed significantly less relative activity toward kdSrc than toward polyE4Y (Fig. 2C), indicating that these two residues are also part of the substrate-docking site. Ser-284 and Ser-381 are located just outside of the Arg triangle (Fig. 2B). The mutation of either one to Ala affected polyE4Y and kdSrc phosphorylation equally (Fig. 2C), indicating that these two residues were not specifically important for Src recognition.
Two residues, Ser-273 and Asp-276, are located on the α-helix D, and between the Arg triangle and the active site (Fig. 2B). If the Arg triangle and the active site of Csk form a continuous binding surface for Src interaction, then these residues are also likely to be important for Src phosphorylation by Csk. To test this possibility, they were mutated to Ala and the mutants were purified and analyzed (Fig. 2C). Ser273Ala displayed WT level activity toward polyE4Y and ≈70% WT activity toward kdSrc. Asp276Ala retained ≈40% of WT activity toward either polyE4Y or kdSrc. This result suggests that Ser-273 is also part of the substrate-docking site.
The above Ala scanning study identified six residues (Ser-273, Arg-279, Ser-280, Arg-281, Arg-283, and Phe-382) as specifically important for kdSrc phosphorylation. The first five are located on α-helix D, and the last one is located next to the helix in the tertiary structure. Several residues immediately outside this region were not specifically important for kdSrc phosphorylation. Therefore, these six residues represent the major determinants of the substrate-docking site.
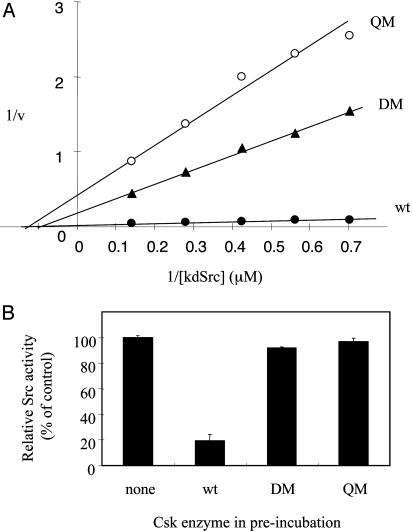
Mutations of the Putative Substrate-Docking Site Convert Csk into a Generically Active Kinase Unable to Phosphorylate, Regulate, or Bind to Src. To further verify the substrate-docking site, two mutants, one containing double mutations (DM) of Arg281Ala, and Arg283Ala and the other containing quadruple mutations (QM) of Ser280Ala, Arg281Ala, Arg283Ala, and Phe382Ala, were generated. Ser-273 and Arg-279 were not included in these mutants, because mutation of each had relatively minor effects on kdSrc phosphorylation (Fig. 2). Fig. 3A compares the Lineweaver-Burk plots of WT and mutant enzymes using kdSrc as the variable substrate. Both mutants had dramatically decreased kcat and mildly higher Km values. Table 1 summarizes the catalytic parameters of the two mutants using both kdSrc and polyE4Y as the variable substrates. Toward kdSrc, the Km increased ≈1.5-fold for DM and 3-fold for QM. Both mutants had kcat values <15% of that of WT, resulting in a 20-fold or more reduction in kcat/Km ratio. In contrast, the mutants had similar kcat and moderately increased Km toward polyE4Y, resulting in a reduction of kcat/Km ratio by <3-fold. This result demonstrates that the mutated residues are specifically important for kdSrc phosphorylation.
Fig. 3.
Effect of DM and QM on Csk kinase function. The details and rationale of the mutations are described in the text. (A) Effect of docking site mutations on Csk phosphorylation of kdSrc. (B) Effect of docking site mutations Csk's ability to inactivate Src.
Table 1. Catalytic parameters of Csk mutants.
| Activity with kdSrc
|
Activity with polyE4Y
|
|||||
|---|---|---|---|---|---|---|
| kcat, min-1 | Km, μM | Relative kcat/Km | kcat, min-1 | Km, μg/ml | Relative kcat/Km | |
| WT | 53.8 ± 17.2 | 5.7 ± 2.2 | 1 | 89.6 ± 5.6 | 95.4 ± 16.6 | 1 |
| DM | 6.5 ± 1.2 | 13.9 ± 3.6 | 0.05 | 124.1 ± 29.8 | 274.1 ± 6.7 | 0.48 |
| QM | 7.6 ± 4.2 | 22.9 ± 9.4 | 0.03 | 111.1 ± 27.8 | 322.2 ± 32.7 | 0.37 |
Relative kcat/Km = actual kcat/Km/actual kcat/Km of WT.
Because the Csk mutants were defective in phosphorylating kdSrc, it was expected that they would be defective in regulating Src by phosphorylation. This possibility was tested by a Src inactivation assay (14) (Fig. 3B). Src expressed in and purified from insect cells (36, 37) was incubated with WT or mutant Csk (equal polyE4Y kinase activity were used for WT or mutant Csk) in the presence of ATP and MgCl2 for 10 min, and then the Src activity in the incubation was determined. If Csk or the mutants were able to inactivate Src, the preincubation would result in a decrease in Src activity. As expected, WT Csk inactivated Src, but the two mutants did not. This result confirmed that the mutations converted Csk into a generically active kinase without the ability to recognize and phosphorylate Src.
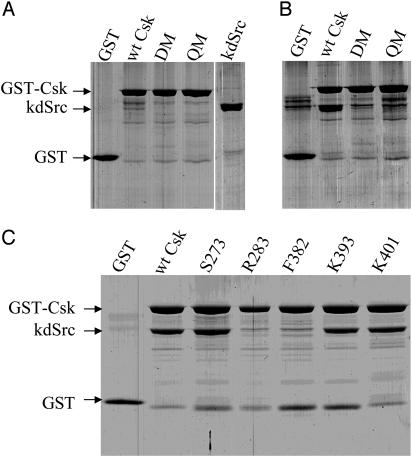
If the mutated residues in DM and QM are indeed the substrate-docking site, the mutants should have a much weaker interaction with kdSrc than WT Csk. Pull-down assays were performed, as described in Methods, to evaluate the interaction of Csk to kdSrc (Fig. 4). GST-wtCsk, but not GST, was able to pull down kdSrc (close to a 1:1 ratio), indicating that kdSrc was binding specifically to Csk. Similarly, Csk mutants S273, K393, and K401 were also able to pull down kdSrc, correlating to their significant residual kdSrc kinase activity. In contrast, DM, QM, R284, and F382 were not able to pull down kdSrc, in good agreement with their inactivity to phosphorylate kdSrc. This result further confirmed that the identified docking residues were specifically responsible for binding to Src. Because these residues are responsible for binding to Src and rendering Csk effective in phosphorylating Src, we conclude that these residues constitute the major determinants of the substrate-docking site.
Fig. 4.
Pull-down assay to determine the interaction of Csk and mutants with kdSrc. (A) Purified GST, GST-Csk, GST-DM, and GST-QM. (B and C) kdSrc pull-down assay with various Csk variants. Each of the purified GST or fusion proteins (100 pmol) was incubated with purified kdSrc (200 pmol) and precipitated with glutathione-agarose. The proteins retained by the beads were analyzed by SDS/PAGE and Coomassie blue staining.
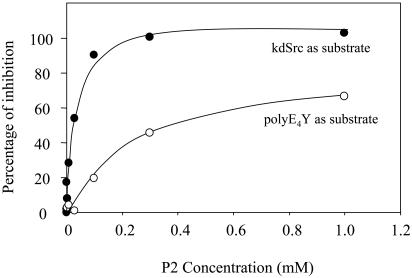
Peptidic Mimic of the Substrate-Docking Site Specifically Inhibits the Phosphorylation of kdSrc by Csk. As another independent test for the identified substrate-docking site, the ability of peptides mimicking the docking site as inhibitors for Csk was determined. Peptides mimicking the substrate-docking site structure would be expected to compete against Csk in binding to Src. Thus, they should inhibit Csk phosphorylation of kdSrc but not that of polyE4Y. To test this idea, three peptides were synthesized: VDYLRS (P1), RSRGRS (P2), and RSVLGG (P3). They cover residues V275 through Ser-280 (P1), Arg-279 through Ser-284 (P2), and Arg-283 through Gly-288 (P3). Note that P2 contained four residues, Arg-279, Ser-280, Arg-281, and Arg-283, that are key residues of the substrate-docking site of Csk. In contrast, P1 or P3 contained only at most two residues important for SFK binding. P1 and P3 (up to 1 mM) did not inhibit Csk phosphorylation of polyE4Y or kdSrc (data not shown). In contrast, P2 potently inhibited Csk phosphorylation of kdSrc (IC50 = 21 ± 3.8 μM) but only moderately inhibited polyE4Y phosphorylation (IC50 = 422 ± 46 μM) (Fig. 5). The differential inhibition of kdSrc and polyE4Y phosphorylation indicated that P2 specifically interfered with the interaction between Csk and kdSrc. This result further indicates that the residue cluster located on α-helix D and mimicked by P2 contained key determinants of the substrate-docking site.
Fig. 5.
Inhibition of Csk activity by peptide P2 that mimics the substrate-docking site. The Ki of P2 inhibition of Csk is 21 μM toward kdSrc but 422 μM toward polyE4Y.
Discussion
PTKs of the Csk family specifically phosphorylate SFKs on a C-terminal tail Tyr residue and regulate their activity. This system is chosen to investigate PTK substrate specificity because the exclusive PTK–substrate relationship is well established in vivo and in vitro, and extensive structural and biological data are available on Csk and SFKs to assist such studies. One unique feature of this system that was central to our strategy was that Csk and Chk shared functional identity but relatively low sequence identity (54%). This feature allowed us to locate some key residues in the docking site by evaluating uniquely conserved residues in Csk family.
The identified substrate-docking site of Csk is composed of six residues, Ser-273, Arg-279, Ser-280, Arg-281, Arg-283, and Phe-382. This identification of the substrate-docking site is supported by several lines of evidence. First, several of the key residues of the docking site are uniquely conserved in Csk and Chk. The unique conservation is consistent with Csk and Chk being the only two kinases able to phosphorylate SFKs on Ytail. Second, mutations of the residues within the docking site abolished Csk activity toward Src without significantly affecting its general kinase activity. Mutation of many residues outside this region did not preferentially affect Csk's ability to phosphorylate the physiological substrate. Third, Csk mutants containing multiple point mutations in the docking site resulted in a >95% loss of Csk activity toward kdSrc but only a modest decrease in activity toward artificial substrate, effectively converting Csk into a generic PTK unable to phosphorylate Src. Correspondingly, the Csk mutants were unable to regulate Src activity. Fourth, the loss of activity toward kdSrc correlates to the loss of ability to physically bind to kdSrc, indicating that the mutated site is indeed critical for Csk–Src interaction. Fifth, a peptide mimicking part of the docking site potently inhibited Csk phosphorylation of kdSrc but only moderately inhibited Csk activity toward an artificial substrate. These results demonstrate that the substructure consisting of the α-helix D and Phe-382 is indeed the docking site that specifically interacts with SFKs for efficient phosphorylation.
At present, it is difficult to assign quantitative contributions to the individual residues. The identified residues (Fig. 2B), containing three positively charged residues, two polar residues, and one hydrophobic residue, appear well suited to provide a complex surface for highly specific and unique interaction with the substrate. The docking site is located near the active site (Fig. 6) and appears well positioned for Src docking. Crystallized IRK–peptide substrate complexes (38) and mutagenic studies of other PTKs (39) indicate that the P + 1 loop provides the main platform for peptide substrate binding to PTKs. It is likely that the P + 1 loop in Csk performs the same function in binding to SFK C-terminal tail and presents Ytail to the active site. The docking site is located on the same side of the kinase molecule as the P + 1 loop. It can be envisioned that substrate-docking site would interact with the docking determinants on SFKs, which would bring the C-terminal tail peptide to Csk active site. Because Src binding and phosphorylation are both abolished by docking site mutations, it appears that the docking interaction may be primarily responsible for SFKs recognition by Csk.
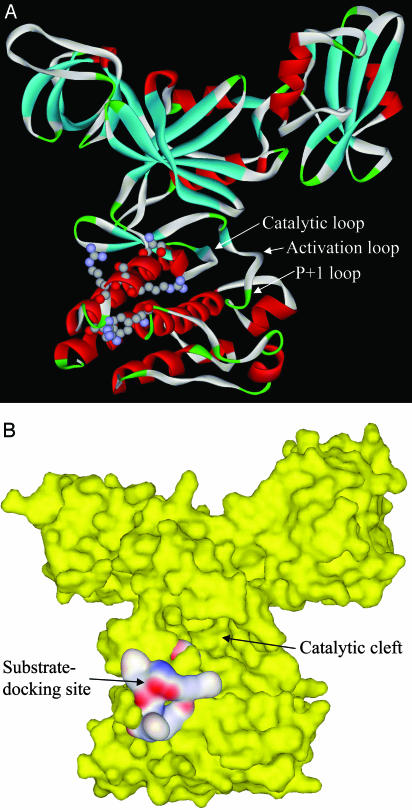
Fig. 6.
Structures of Csk catalytic domain and the substrate-docking site. (A) Ribbon structure of Csk with identified residues in the substrate-docking site shown in a ball-and-stick model. Several loop structures relevant to peptide substrate binding and catalysis are indicated by arrows. (B) Surface structure of Csk (yellow) and the substrate-docking site (colored by electrostatic potential). The active site cleft is indicated.
This work raises several important questions that remain to be answered. First, what are the specificity determinants on SFKs that allow them to be specifically recognized by Csk and Chk? We reason that such determinants would be a surface area complementary to the Csk substrate-docking site. The identification of the docking site on Csk will facilitate the identification of such determinants on SFKs. Second, it is unclear whether other PTKs will use a similar mechanism for recognizing their physiological substrates. Csk family PTKs have unusually strict substrate specificity in that they only phosphorylate SFKs. Other PTKs may phosphorylate multiple families of protein substrates. Alignments of primary and tertiary structures of PTKs indicate that the structures of α-helix D are highly variable between PTK families, consistent with the possibility of its being a key structure for substrate specificity determination. Third, it is interesting that mutations of the docking site only moderately affected the Km of Csk for kdSrc but more dramatically affected the kcat. We started this work with the expectation that the docking site would mainly affect the complementarity between the enzyme and the substrate, and thus perturbation to the docking site would more significantly affect the Km. The large decrease in kcat caused by the mutations suggest that the docking interaction is critical for Csk transition state complementarity with SFKs instead.
Acknowledgments
We thank J. F. Sperry and P. S. Cohen for critically reading the manuscript, P. A. Cole (The Johns Hopkins University, Baltimore) for providing pREP4groESL plasmid, and R. Jove (University of South Florida, Tampa) for providing pMcSrc Met-295 plasmid. DNA sequencing was performed at the University of Rhode Island Genomics and Sequencing Center. This work was supported by grants from the National Institutes of Health (1 P20 RR16457) and the University of Rhode Island Research Council.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Csk, C-terminal Src kinase; Chk, Csk-homologous kinase; DM, double mutant; kdSrc, kinase-defective Src that contains a Lys295Met mutation; PTK, protein tyrosine kinase; SFK, Src family PTK; QM, quadruple mutant; SH, Src homology; Yact, the Tyr residue in the activation loop; Ytail, the Tyr residue in the C-terminal tail of Src family kinases.
References
- 1.Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. (2002) Science 298, 1912–1934. [DOI] [PubMed] [Google Scholar]
- 2.Hunter, T. (1995) Cell 80, 225–236. [DOI] [PubMed] [Google Scholar]
- 3.Blume-Jensen, P. & Hunter, T. (2001) Nature 411, 355–365. [DOI] [PubMed] [Google Scholar]
- 4.Capdeville, R., Buchdunger, E., Zimmermann, J. & Matter, A. (2002) Nat. Rev. Drug Discov. 1, 493–502. [DOI] [PubMed] [Google Scholar]
- 5.Hubbard, S. R. & Till, J. H. (2000) Annu. Rev. Biochem. 69, 373–398. [DOI] [PubMed] [Google Scholar]
- 6.Thomas, S. M. & Brugge, J. S. (1997) Annu. Rev. Cell Dev. Biol. 13, 513–609. [DOI] [PubMed] [Google Scholar]
- 7.Brown, M. T. & Cooper, J. A. (1996) Biochim. Biophys. Acta 1287, 121–149. [DOI] [PubMed] [Google Scholar]
- 8.Okada, M., Nada, S., Yamanashi, Y., Yamamoto, T. & Nakagawa, H. (1991) J. Biol. Chem. 266, 24249–24252. [PubMed] [Google Scholar]
- 9.Klages, S., Adam, D., Class, K., Fargnoli, J., Bolen, J. B. & Penhallow, R. C. (1994) Proc. Natl. Acad. Sci. USA 91, 2597–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayrapetov, M. K., Lee, S. & Sun, G. (2003) Protein Expression Purif. 29, 148–155. [DOI] [PubMed] [Google Scholar]
- 11.Xu, W., Harrison, S. C. & Eck, M. J. (1997) Nature 385, 595–602. [DOI] [PubMed] [Google Scholar]
- 12.Sicheri, F., Moarefi, I. & Kuriyan, J. (1997) Nature 385, 602–629. [DOI] [PubMed] [Google Scholar]
- 13.Cooper, J. A. & Howell, B. (1993) Cell 73, 1051–1054. [DOI] [PubMed] [Google Scholar]
- 14.Sun, G., Sharma, A. K. & Budde, R. J. (1998) Oncogene 17, 1587–1595. [DOI] [PubMed] [Google Scholar]
- 15.Imamoto, A. & Soriano, P. (1993) Cell 73, 1117–1124. [DOI] [PubMed] [Google Scholar]
- 16.Ruzzene, M., Songyang, Z., Marin, O., Donella-Deana, A., Brunati, A. M., Guerra, B., Agostinis, P., Cantley, L. C. & Pinna, L. A. (1997) Eur. J. Biochem. 246, 433–439. [DOI] [PubMed] [Google Scholar]
- 17.Sondhi, D., Xu, W., Songyang, Z., Eck, M. J. & Cole, P. A. (1998) Biochemistry 37, 165–172. [DOI] [PubMed] [Google Scholar]
- 18.Wang, D., Huang, X. Y. & Cole, P. A. (2001) Biochemistry 40, 2004–2010. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa, A., Takayama, Y., Sakai, H., Chong, K. T., Takeuchi, S., Nakagawa, A., Nada, S., Okada, M. & Tsukihara, T. (2002) J. Biol. Chem. 277, 14351–14354. [DOI] [PubMed] [Google Scholar]
- 20.Lamers, M. B., Antson, A. A., Hubbard, R. E., Scott, R. K. & Williams, D. H. (1999) J. Mol. Biol. 285, 713–725. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi, H. & Hendrickson, W. A. (1996) Nature 384, 484–489. [DOI] [PubMed] [Google Scholar]
- 22.Smith, D. B. & Johnson, K. S. (1988) Gene 67, 31–40. [DOI] [PubMed] [Google Scholar]
- 23.Sun, G. & Budde, R. J. (1995) Anal. Biochem. 231, 458–460. [DOI] [PubMed] [Google Scholar]
- 24.Lin, X., Lee, S. & Sun, G. (2003) J. Biol. Chem. 278, 24072–24077. [DOI] [PubMed] [Google Scholar]
- 25.Jove, R., Kornbluth, S. & Hanafusa, H. (1987) Cell 50, 937–943. [DOI] [PubMed] [Google Scholar]
- 26.Sun, G. & Budde, R. J. (1997) Biochemistry 36, 2139–2146. [DOI] [PubMed] [Google Scholar]
- 27.Nada, S., Okada, M., MacAuley, A., Cooper, J. A. & Nakagawa, H. (1991) Nature 351, 69–72. [DOI] [PubMed] [Google Scholar]
- 28.Brauninger, A., Holtrich, U., Strebhardt, K. & Rubsamen-Waigmann, H. (1992) Gene 110, 205–211. [DOI] [PubMed] [Google Scholar]
- 29.Sun, G. & Budde, R. J. (1999) Arch. Biochem. Biophys. 367, 167–172. [DOI] [PubMed] [Google Scholar]
- 30.Sondhi, D. & Cole, P. A. (1999) Biochemistry 38, 11147–11155. [DOI] [PubMed] [Google Scholar]
- 31.Sakano, S., Iwama, A., Inazawa, J., Ariyama, T., Ohno, M. & Suda, T. (1994) Oncogene 9, 1155–1161. [PubMed] [Google Scholar]
- 32.Anderson, S. K., Gibbs, C. P., Tanaka, A., Kung, H. J. & Fujita, D. J. (1985) Mol. Cell. Biol. 5, 1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziegler, S. F., Marth, J. D., Lewis, D. B. & Perlmutter, R. M. (1987) Mol. Cell. Biol. 7, 2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams, D. M., Wang, D. & Cole, P. A. (2000) J. Biol. Chem. 275, 38127–38130. [DOI] [PubMed] [Google Scholar]
- 35.Lee, S., Lin, X., McMurray, J. & Sun, G. (2002) Biochemistry 41, 12107–12114. [DOI] [PubMed] [Google Scholar]
- 36.Budde, R. J., Ramdas, L. & Ke, S. (1993) Prep. Biochem. 23, 493–515. [DOI] [PubMed] [Google Scholar]
- 37.Budde, R. J., Ramdas, L. & Sun, G. (2001) J. Mol. Catal. 11, 805–809. [Google Scholar]
- 38.Hubbard, S. R. (1997) EMBO J. 16, 5572–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokoyama, N. & Miller, W. T. (1999) FEBS Lett. 456, 403–408. [DOI] [PubMed] [Google Scholar]