Abstract
Objective
Angelman syndrome (AS) is a neurodevelopmental disorder caused by a deletion on chromosome 15, uniparental disomy (UPD), imprinting defect, or UBE3A mutation. It is characterized by intellectual disability with minimal speech and certain behavioral characteristics. We used standardized measures to characterize the developmental profile and to analyze genotype-phenotype correlations in AS.
Method
The study population consisted of 92 children, between 5 months and 5 years of age, enrolled in a Natural History Study. Each participant was evaluated using the Bayley Scales of Infant and Toddler Development (Third Edition) (BSID-III), the Vineland Adaptive Behavior Scales (Second Edition) (VABS-II), and the Aberrant Behavior Checklist.
Results
74% had a deletion and 26% had UPD, an imprinting defect or a UBE3A mutation (“non-deletion”). The mean±standard deviation (SD) BSID-III cognitive scale developmental quotient (DQ) was 40.5±15.5. Participants with deletions were more developmentally delayed than the non-deletion participants in all BSID-III domains except in expressive language skills. The cognitive DQ was higher than the DQ in each of the other domains, and the receptive language DQ was higher than the expressive language DQ. In the VABS-II, deletion participants had weaker motor and language skills than the non-deletion participants.
Conclusion
Children with AS have a distinct developmental and behavioral profile; their cognitive skills are stronger than their language and motor skills, and their receptive language skills are stronger than expressive language skills. Developmental outcomes are associated with genotype, with deletion patients having worse outcomes than non-deletion patients.
Key Terms: Angelman syndrome, Development, Behavior, Phenotype
INTRODUCTION
Angelman Syndrome (AS) is a neurodevelopmental disorder characterized by intellectual disability, a lack of speech, characteristic behavioral features, seizures, and a happy demeanor.1 It has a prevalence of between 1/10,000 and 1/20,000 individuals.2–4 In normal individuals, certain genes are expressed only when they are inherited from a specific parent (i.e., the genes are imprinted); one of these imprinted genes is UBE3A, which is expressed only when it is inherited from the mother. AS is caused by deficient expression of the maternal copy of the UBE3A gene due to one of four molecular etiologies: deletion of the AS critical region on maternal chromosome 15q11-q13, paternal uniparental disomy (UPD) for chromosome 15, an imprinting defect causing lack of expression of the maternal copy of UBE3A, and mutations in the maternally-inherited copy of UBE3A.1 Among individuals with a deletion, some have a common 5.9 Mb (class I) deletion, while others have a smaller 5.0 Mb (class II) deletion on chromosome 15q11-q13, differing only by the location of the proximal (centromeric) breakpoints of the deletion.5,6
The neurocognitive features of AS include severe developmental delay, usually noted within the first year of life. Most patients plateau at a developmental level between 24–30 months.7 Language development in this population is significantly impaired with most individuals lacking any speech and a minority of individuals having single word vocabularies.8 Although all skills are delayed, there is variability in adaptive behavior functioning, with relative strength in socialization and relative weakness in motor skills.7 Despite the characteristically happy demeanor of these subjects, many difficult behaviors have been identified, including brief attention span, hyperactivity, aggression, tantrums and behavioral noncompliance.9,10 Previous studies have suggested that children with AS due to deletions have lower developmental levels than those due to other molecular etiologies, and children with class I deletions appear to have weaker cognitive abilities than those with class II deletions. Children with imprinting defects and UPD demonstrate the most advanced developmental and language abilities.11–15 However, many of these studies used non-standardized measures such as counting the number of words,11 or asking questions about assistance with feeding, toileting, and dressing.16 Moreover, some of these studies were performed on relatively small numbers of patients.12,17
As part of a Natural History study on AS, we sought to delineate the developmental profile of a large sample of children aged five months to five years with AS due to different molecular etiologies using validated standardized instruments. In our exploratory analysis, we also present our findings of the developmental outcomes in participants of different ages. Changes in developmental progress with age in the AS population have not been previously reported. We believe that these exploratory data may shed light on whether their neurodevelopment stagnates beyond a given chronological age. Moreover, many parents as well as clinicians assessing younger AS children have often commented on how these younger children appear to perform much closer to the expected level of cognitive functioning for their chronological age in their daily lives, although there have not been any formal data supporting these anecdotal observations. We hope that our data will assist general and developmental pediatricians, child psychologists, and clinical geneticists provide better prognostic information and develop more targeted interventions.
Although mouse models of AS are now widely available,18,19 the few published studies on the development and behavioral features of these mice have been conducted at specific time points;20–22 hence the longitudinal developmental outcomes in AS mice cannot be gleaned from these previous studies. Our findings may serve to inform the design and conduct of such experiments in AS mice, which in turn may provide a model for assessing the efficacy of various potential therapeutic interventions. Moreover, by studying the neurodevelopment in AS using formal developmental instruments and performing genotype-phenotype correlations, we hope that this study can serve as an example for the investigation of developmental outcomes in other more common genetic conditions.
METHODS
Participants
All the participants in this report were enrolled in the AS Natural History study (ClinicalTrials.gov Identifier: NCT00296764) conducted by the Angelman, Rett, and Prader-Willi Syndromes Consortium of the NIH Rare Diseases Clinical Research Network (RDCRN) at one of four study sites – Rady Children’s Hospital San Diego, Texas Children’s Hospital, Greenwood Genetic Center, and Children’s Hospital Boston – between January 2006 and March 2008. The inclusion criteria were: (i) a molecular diagnosis of AS, and (ii) age between 1 day old and 60 years old. The exclusion criteria were: (i) the presence of a co-morbid disorder that is not a known feature of AS and (ii) born at less than 28 weeks gestation. Subjects were recruited through parent support groups such as the Angelman Syndrome Foundation and the AS listserv, and referrals from professional colleagues. In addition, the study was registered on the public web site, http://ClinicalTrials.gov/through which parents and healthcare professionals were able to contact the investigators. This study was approved by the Institutional Review Boards at each study site, as well as by the Data and Safety Monitoring Board of the RDCRN. Written consent was obtained from the legal guardian of each participant.
Neurodevelopmental Assessment Measures
Developmental assessment was performed by a child psychologist (JG, LH, RB-W, AB-C, SW, SP) at each study site using two standardized instruments – the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III)23 and the Vineland Adaptive Behavior Scales, Second Edition (VABS-II)24. In addition, problem behaviors were assessed using the Aberrant Behavior Checklist, Community version (ABC – Community).25
The BSID-III is a developmental tool that assesses cognitive, language, and motor functioning based on a set of standardized tasks administered by a psychologist to the child. The ability of the child to complete a set of developmentally appropriate tasks in each domain is recorded as the raw score, which is then converted to a developmental age. An earlier version of the BSID has been used in an AS population aged between 5 months and 10 years (mean: 3 years 8 months, SD: 2 years 5 months) in a research setting.7 Although the BSID-III has been standardized for children with a chronological age between 1 and 42 months, it is also used for children beyond that normative age range since children with developmental disabilities should be assessed using instruments that are appropriate for their developmental age rather than chronological age.26 When children score near the floor of a test (as often happens when an instrument is employed based on chronologic rather than developmental age), there is a lack of variability in test scores, and the standard scores are more likely to overestimate true functional abilities. A recent study in individuals with Fragile X syndrome highlighted that most patients scored at the floor of the intelligence test that was used.27 The authors demonstrated that meaningful variation in intellectual ability in children with Fragile X syndrome was obscured by the usual translation of raw scores into standardized scores.27
It has been noted that developmental quotients (DQ) instead of standard scores can be used to track the developmental progress of intellectually disabled children against their chronological ages and to compare their levels of functioning in different developmental domains.28 Using an earlier edition of the BSID, the DQ in children with intellectual disabilities correlated well with intelligence quotient (IQ) scores later in life.29 In the present study, we therefore calculated the DQ for each domain by dividing the developmental age by the chronological age and multiplying by 100. This controlled for chronological age as well as differences in the ages of participants with different molecular subtypes.
The VABS-II24 evaluates a child’s ability to perform specific tasks in 4 domains as a measure of overall adaptive skills (Communication, Daily Living Skills, Socialization, and Motor Skills) based on a set of questions administered to the parents/caregivers by a psychologist in a semi-structured interview. A developmental age for each subtest in each domain (i.e., receptive, expressive, and written communication skills; personal, domestic, and community daily living skills; interpersonal relationships, play and leisure time, and coping socialization skills; and gross and fine motor skills) is calculated based on the raw scores. The VABS-II has been validated in children and adults with intellectual disabilities and a prior edition has been used in studies with AS patients.7,30,31
The ABC is a questionnaire that evaluates the presence of specific maladaptive behaviors in five categories (Irritability, Lethargy/Withdrawal, Inappropriate Speech, Hyperactivity, and Stereotypic Behavior) and the extent to which these behaviors affect the child’s daily life. The ABC has recently been validated in a group of children between the ages of 14 and 43 months32 and has previously been used in the AS population.10,33 ABC raw scores for each of the five categories were analyzed, except for the “Inappropriate Speech” category because children with AS generally do not have speech.
Statistical analysis
To analyze the cognitive and behavioral outcomes, participants were grouped by molecular subtypes. The Student t-test was used to analyze the differences in the DQ and standard scores between the deletion and the non-deletion (i.e., combined UPD, Imprinting defects, and UBE3A mutations) subgroups on the BSID-III and VABS-II. One-way analysis of variance (ANOVA) was used to detect the presence of differences in BSID-III and VABS-II scores across the three major molecular sub-classes (i.e., Deletions, UPD/Imprinting defects combined, and UBE3A mutations). As the ABC raw scores were not normally distributed, the Mann-Whitney test and the Kruskal-Wallis test were used for equivalent comparisons. Means and standard deviations (SD) were calculated. Paired t-tests were used to analyze strengths and weaknesses (profiles) within the BSID-III and VABS-II. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Data on 92 participants are included in this report. The mean age was 34.4 months (SD: 14.0 months; Range: 5 months – 60 months). Age distribution and frequency of molecular sub-classes are shown in Table 1. Due to the small number of participants with either UPD (n=5) or an imprinting defect (n=7), these subjects were grouped together. Thus, the analyses of differences between molecular classes consisted of comparing children with deletions, those with UBE3A mutations, and those with UPD/imprinting defects. We also conducted analyses comparing all deletion versus non-deletion participants. Participants with deletions were significantly younger (mean age: 32.6 months, SD: 13.9) than the non-deletion participants (mean age: 39.4 months, SD: 13.4) (p=0.040); there was no significant difference in the mean age of those with class I and those with class II deletions (p=0.82), nor was there any difference between the mean age of males (mean: 36.8 months, SD: 13.5) and females (mean: 31.6 months, SD: 14.2) (p=0.075).
Table 1.
Age, Gender, and Molecular sub-class distribution among participants
| Total | Deletion class I | Deletion class II | Deletion (other)* | All Deletions | UPD/Imprinting defects | UBE3A mutation | ||
|---|---|---|---|---|---|---|---|---|
| Age(months) | 0 – 24 | 27 | 12 | 9 | 4 | 25 | 0 | 2 |
| 25 – 36 | 26 | 5 | 13 | 0 | 18 | 5 | 3 | |
| 37 – 60 | 39 | 12 | 13 | 0 | 25 | 8 | 6 | |
| Total | 92 | 29 (32%) | 35 (38%) | 4 (4%) | 68 (74%) | 13 (14%) | 11 (12%) | |
| Gender | M | 50 | 13 | 22 | 0 | 35 | 8 | 7 |
| F | 42 | 16 | 13 | 4 | 33 | 5 | 4 | |
These participants had a deletion that was not a typical class I or class II deletion
Cognitive Development
Objective measures of the participants’ intellectual functioning were assessed using the BSID-III scale. All subjects had severe global developmental delay; the cognitive age-equivalent ranged from 3 months to 27 months. The mean BSID-III cognitive scale DQ was 40.5 (SD: 15.5) for the entire cohort. Our ANOVA analyses revealed that there were statistically significant differences among molecular sub-classes in the BSID-III cognitive, gross motor, and fine motor DQs (p=0.001 for each domain); participants with UBE3A mutations had the highest mean DQ while those with deletions had the lowest mean DQ in each domain. In the cognitive domain, UBE3A participants had a mean DQ of 56.3 (SD: 17.3) while those with deletions had a mean DQ of 37.8 (SD: 15.1); in the gross motor domain, UBE3A participants had a mean DQ of 47.9 (SD: 19.6) and deletion participants had a mean DQ of 33.6 (SD: 10.4); in the fine motor domain, UBE3A participants had a mean DQ of 50.3 (SD: 23.6) and deletion participants had a mean DQ of 32.5 (SD: 13.6).
Non-deletion participants also had better receptive language skills (mean DQ: 40.8, SD: 14.8) compared to deletion participants (mean DQ: 32.6, SD: 16.0) (p=0.028), but no differences were found in expressive language skills between the non-deletion (mean DQ: 31.0, SD: 17.5) and the deletion (mean DQ: 26.9, SD: 13.6) groups (p=0.302). The gross motor skills in the non-deletion participants (mean DQ: 42.8, SD: 15.1) were stronger than those in the deletion participants (mean DQ: 33.6, SD: 10.4) (p=0.0099), as were the fine motor skills (mean DQ: 42.1, SD: 18.8 vs. mean DQ: 32.5, SD: 13.6) (p=0.028). There were no significant differences in any of the BSID-III domains between participants with class I and class II deletions. The mean DQ for each skill assessed by the BSID-III is summarized in Figure 1. There were no statistically significant differences between the DQ of males and females in any of the BSID-III domains, regardless of molecular subtype.
Figure 1.
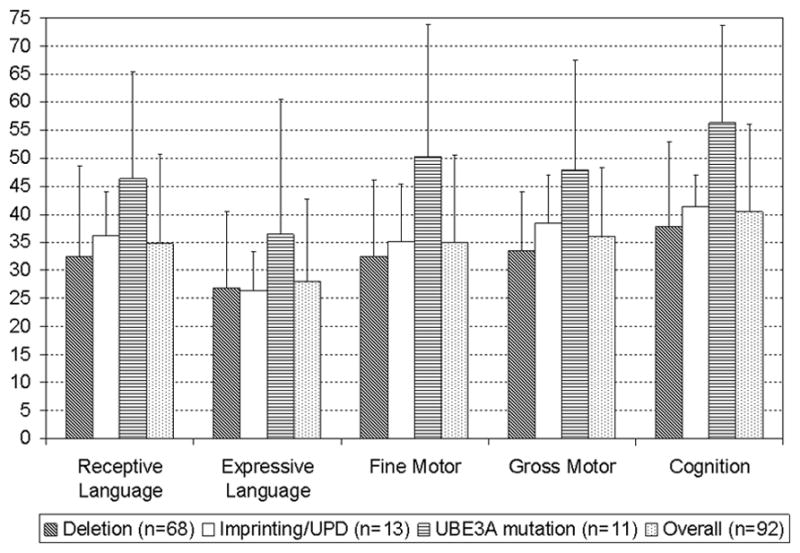
BSID-III Developmental quotients (Mean Age equivalents/Chronological age) of the cohort by molecular sub-types and developmental domains. Error bars indicate 1 standard deviation (SD).
Using the BSID-III to examine the strengths and weaknesses of participants, we found that receptive language skills were significantly better than expressive language skills (p<0.0001) in both the deletion and the non-deletion AS participants. For the entire cohort, there were no significant differences between gross motor and fine motor skills DQ on the BSID-III (p=0.39). The cognitive DQ was significantly higher than the receptive language DQ (p<0.001) and the expressive language DQ (p<0.001) for the entire cohort, and for both the deletion and non-deletion groups considered separately. The cognitive DQ was higher than the gross motor skills DQ (p=0.0001 for the entire cohort, p=0.0025 for the deletion group, p=0.011 for the non-deletion group) and the fine motor skills DQ in both the deletion and non-deletion participants (p<0.0001 for the entire cohort, p<0.0001 for the deletion group, p=0.016 for the non-deletion group).
Adaptive Behavior Functioning
The overall adaptive skills of the participants were evaluated using the VABS-II. As shown in Figure 2, among the different molecular sub-types, participants with UBE3A mutations were the least delayed in each of the four VABS-II domains (i.e., communication, daily living, socialization, and motor skills) while participants with deletions were the most delayed in all domains, except in the socialization domain in which participants with UPD/imprinting defects were marginally more delayed than those with deletions (mean ± SD standard score: 69.5 ± 4.3 vs. 70.3 ± 9.2 respectively). The language and motor domains standard scores showed a statistically significant difference across the three major sub-classes (p=0.006 and p<0.001 respectively). Similarly, the deletion participants had a significantly lower standard score than the non-deletion participants in the language domain (mean ± SD: 58.6 ± 10.9 vs. 65.0 ± 10.0, p=0.011) and in the motor domain (mean ± SD: 58.4 ± 7.7 vs. 66.5 ± 10.2, p=0.0012). There were no significant differences in the VABS-II standard scores between the deletion and non-deletion participants in the daily living skills (p=0.16) and socialization (p=0.32) domains. Of note, we were able to assess “Domestic Skills” in only 14 out of the 68 subjects with a deletion, 8 out of 13 subjects with imprinting defects/UPD, and 7 out of 11 subjects with UBE3A mutations, because the remaining children were not capable of performing even the most basic tasks in this domain. Similarly, “Community Skills” could only be assessed in 28 out of the 68 subjects with a deletion and 7 out of 11 subjects with UBE3A mutations. There were no statistically significant differences in the standard scores between males and females in any of the domains.
Figure 2.
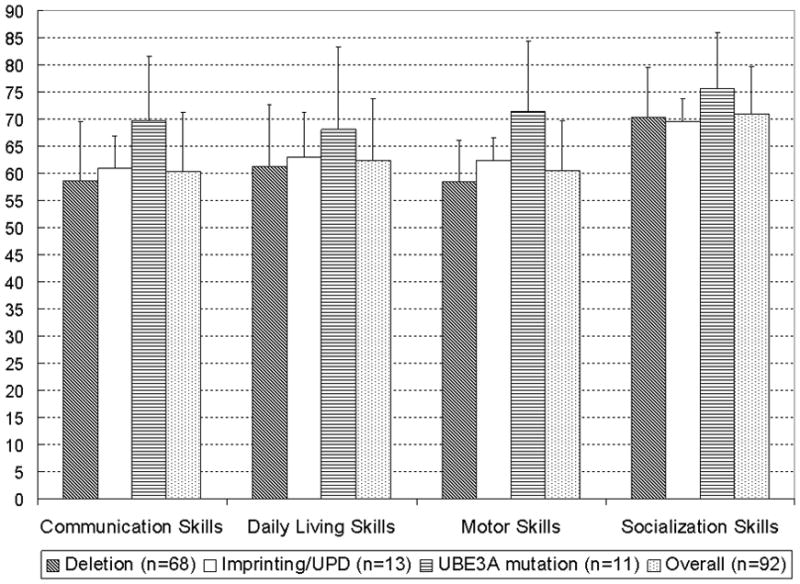
VABS-II (Adaptive Behavior Skills) Mean Domain Standard Scores of the cohort by molecular sub-types and developmental domains. Error bars indicate 1 standard deviation (SD).
When we analyzed profiles of strengths and weaknesses using standard scores of each VABS-II domain, we found that the standard scores in the socialization domain were much higher than those in the communication, motor, and daily living skills domains among participants with deletions (p<0.0001 for comparison of each domain against the socialization domain); the socialization domain standard scores were also higher than the scores in the other domains among the non-deletion participants (socialization vs. communication skills: p<0.0001, socialization vs. daily living skills: p=0.0003, socialization vs. motor skills: p=0.0002).
Maladaptive Behaviors
Raw scores on the ABC hyperactivity subscale were consistent with ratings of toddlers in clinical settings (Figure 3). The non-deletion participants had slightly higher ABC irritability scores (a marker of aggression) compared to those with deletions (p=0.004). There were no other statistically significant differences in the ABC raw scores between the deletion and non-deletion participants or among molecular classes.
Figure 3.
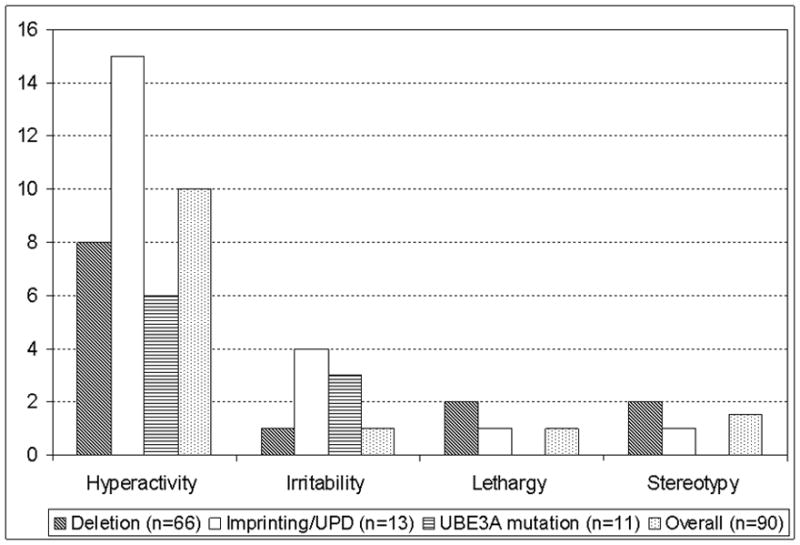
Aberrant Behavior Checklist (Median Raw Scores) for each category
Exploring changes in Developmental Profile with Age
We performed regression analyses to explore the relationships between the different developmental domains and chronological age. We found that our data best fit a cubic regression for the analysis of the BSID-III age-equivalent scores against chronological age, and a quadratic regression for the analysis of the VABS-II standard scores against chronological age.
The rate of overall cognitive development of participants with a deletion appeared to decrease and stagnate at a developmental age of approximately 13 months when the chronological age was approximately 45 months (Figure 4); in contrast, in non-deletion participants, cognitive development appeared to continue to progress. Nonetheless, there was a decline in the VABS-II adaptive behavior composite standard score (which may be used as a surrogate measure of overall development) with increasing age even in the non-deletion participants, although among the older participants, the rate of decline appeared to be slower in the non-deletion group compared to the deletion group (Figure 5). Similarly, in both the language and social skills domains of the VABS-II, the older participants had lower standard scores than the younger ones, and the rate of decline in the standard scores appeared to be greater in those with a deletion compared to those without a deletion (Figures 6 and 7).
Figure 4.
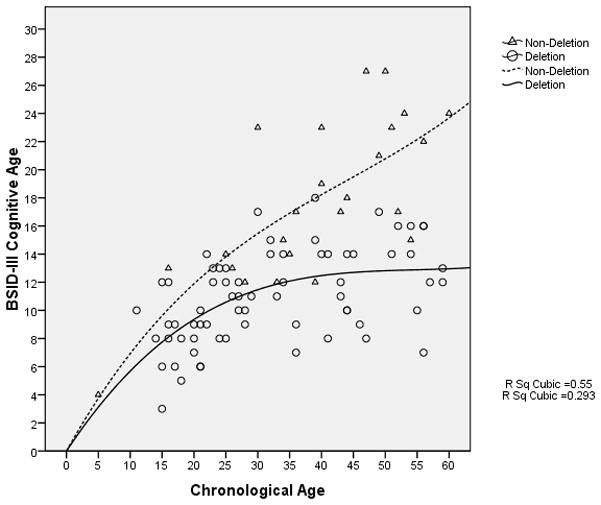
BSID-III Cognitive Age-equivalent scores vs. Chronological age (in months)
Figure 5.
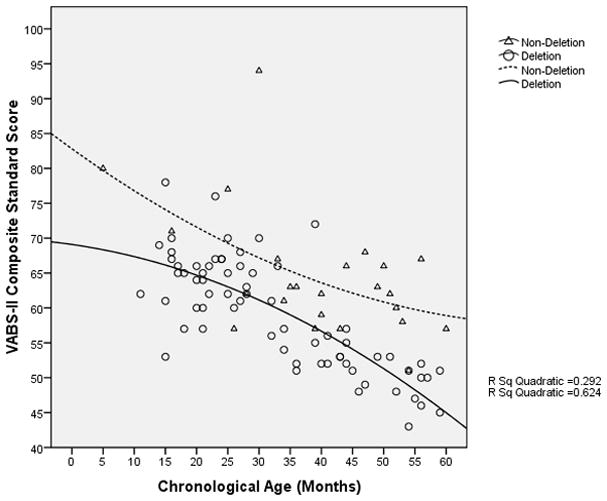
VABS-II Adaptive Behavior Composite Standard Score vs. Chronological age of participants (in months)
Figure 6.
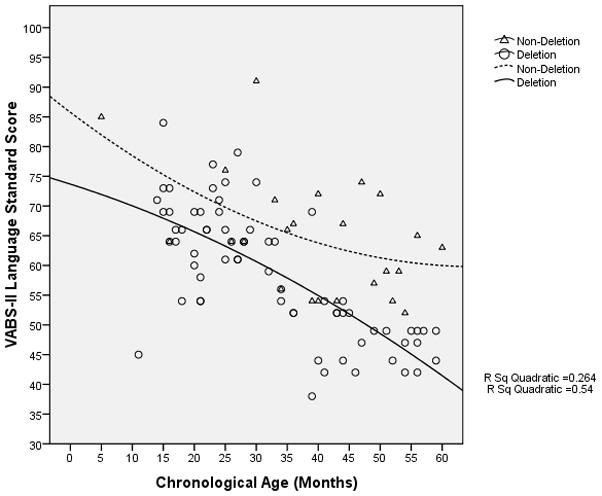
VABS-II Language Domain Standard Score vs. Chronological age of participants (in months)
Figure 7.
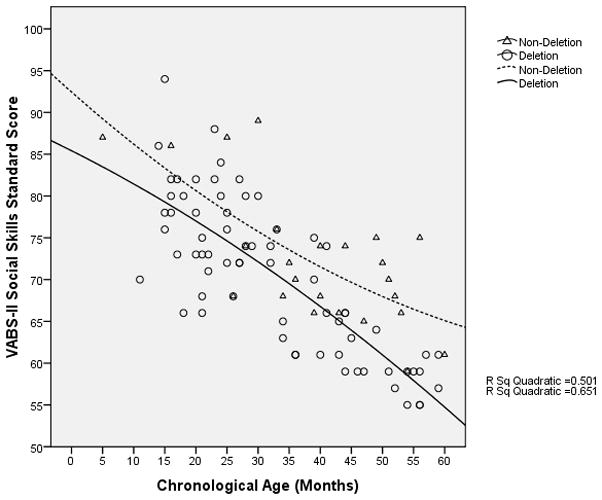
VABS-II Social Skills Domain Standard Score vs. Chronological age of participants (in months)
DISCUSSION
We have presented the baseline neurodevelopmental data from the AS Natural History study. Our analysis of the BSID-III developmental quotients (DQs) demonstrated that the non-deletion participants were more advanced than the deletion participants in all aspects of development except expressive language skills, which is consistent with the findings from previous studies.11–15 We also noted significant differences in the VABS-II language and motor standard scores between molecular subtypes where again, non-deletion patients scored higher as compared to deletion patients. It is notable that overall, the most consistent finding in our study is the uniformly poor expressive language skill in all participants regardless of their molecular sub-class. Since the single common molecular mechanism in all participants is the lack of UBE3A protein in parts of the brain where the UBE3A gene is imprinted, we hypothesize that UBE3A may be essential in the development of expressive language skills. Although expressive language delay is common in syndromes associated with intellectual disabilities, AS is unusual in that all individuals have extremely poor expressive language skills that are out of proportion to their overall level of intellectual disability and their receptive language skills (as shown by the BSID-III analyses herein), and even the highest-functioning adult individuals are not expected to develop more than a few short phrases. In our collective experience, the vast majority of AS individuals have little use of spoken words or phrases.
As found in previous studies, and consistent with the previously reported behavioral phenotype in AS,7 our study shows that AS children have a relative strength in their social skills (as indicated by the socialization domain in VABS-II).
A previous study on the developmental profile of 20 AS children between ages 5 months and 10 years using the BSID-II found that these children had a developmental age of no greater than 17 months, based on the Mental Development Index.7 In contrast, approximately 10% of our subjects had a developmental age of greater than 18.9 months (up to a maximum of 27 months), based on the Cognitive Scale of the BSID-III. One of the differences in these scores may be that the BSID-II Mental Development Index also included language-based items, whereas the BSID-III has separate cognitive and language domains. Thus, the cognitive scale of the BSID-III may be a more sensitive measure of cognition (as opposed to the BSID-II Mental Development Index that is more heavily weighted with language-based items).
There is little published data on changes in the developmental profile of AS individuals over time. It is also unclear whether the neurodevelopment in AS individuals continues throughout life or whether it inevitably stagnates at a pre-determined chronological or developmental age. We anticipate that longitudinal data from our AS natural history study will eventually help to answer these questions. Meanwhile, we performed exploratory regression analyses using our current baseline data to find out if there might be differences in the developmental profile between the younger and the older participants in our cohort. Our data suggest that the cognitive skills in children with deletions do not appear to develop much beyond 13 months while those in other molecular sub-classes continue to develop over time, albeit at a slower rate compared with a normally developing child. This is reflected both in the overall decline in the VABS adaptive behavior composite standard scores, which indicates that these children fall increasingly behind their age-matched peers in their neurodevelopment, and the widening difference in the VABS adaptive behavior composite standard scores between the deletion and non-deletion participants.
Although AS children are often regarded as being highly sociable, over time, their socialization skills lag increasingly behind age-matched peers, as seen in the decline in the VABS-II socialization domain standard scores as the chronological age of the participants increases. It is important to recognize that the assessment of socialization skills in the VABS-II is partly dependent on the linguistic capacity of the participant, suggesting that the decline in the socialization domain standard scores in the older participants may be a result of their inability to communicate well. Therefore, interventions to actively encourage communication using all modalities including gestures, sign language, and assisted communicative devices may help to promote the development of social skills in this population. We recognize the limitations of these regression analyses, including the use of a cross-sectional sample, the limited age range (up to only 60 months of age), and the relatively wide scatter in our data points. Nevertheless, we believe that these preliminary data suggest that the developmental profile of these individuals may change as they age, and hence the importance of obtaining longitudinal developmental data on AS individuals across a wide age range for at least 5 – 10 years. Although we acknowledge that most individuals with developmental delays will probably show a decline in their DQ and standard scores on standardized assessments with increasing age, it will be useful to know the rate and pattern of decline in these scores for any given condition because such information may have some prognostic value. In a longitudinal study on 55 boys with Fragile X syndrome aged between 8 and 68 months, Roberts et al. found that although the rate of increase in the developmental age, as measured using the Mullen Scales of Early Learning, lagged behind that of the chronological age, the rate of developmental progress was constant over that age range with no evidence of any decline or stagnation in development.34 An earlier study on the adaptive behavior skills of 80 children with Down syndrome between the ages of 1.08 to 11.5 years found that the rate of increase in their “adaptive age” was constant until about 7 years of age when there appeared to be a “plateau” in the development of their adaptive skills; however, the data points for participants older than 6 years of age were widely scattered suggesting that some individuals continued to progress while others stagnated or even regressed.28 In contrast, the rate of development in our cohort of AS children appeared to be decreasing or stagnating before the age of 4 years. Future studies examining the developmental trajectories of children with other genetic syndromes associated with intellectual disabilities may help elucidate the extent to which our preliminary observations are unique to AS. Moreover, such information provides useful and important baseline data for the development of future therapeutic trials that aim to reverse or slow this relative decline.
Analyses of the ABC scores confirmed that most of the participants were very hyperactive, which is consistent with frequent anecdotal parental reports of hyperactivity in these children. This finding is important because excessive activity may impede a child’s ability to concentrate and to learn new skills; hence, hyperactivity in these children may need to be actively managed. As has been reported by parents and observed by other clinicians, AS individuals with deletions had lower irritability (aggression) compared to those without deletions. This finding has also been noted in Prader-Willi syndrome in which those without deletions are more likely to have psychiatric difficulties and problem behaviors.35 In contrast, lethargy (withdrawal) was not a common problem in our population, which is consistent with a previous study in which participants with AS were found to be less lethargic when compared to individuals with developmental disabilities due to other etiologies using the ABC.33 Our participants did not demonstrate as many “problematic behaviors” based on the ABC as that observed in the aforementioned study, possibly because our participants are younger (mean age: 2 years 10 months vs. 9 years old) and may not have developed sufficiently to demonstrate many of the problem behaviors observed in the older AS population. Another possible hypothesis is that caregivers may find it easier to control maladaptive behaviors in younger children and hence do not perceive these behaviors as being “problematic”.
Strengths and Weaknesses of Study
Identifying an overall neurodevelopmental profile of young children with AS has been challenging due to the dearth of standardized instruments that can be used to evaluate subjects in the lowest ranges of cognitive functioning. One of the strengths of this study is the use of standardized and structured developmental instruments such as the BSID-III and VABS-II that allowed for the assessment of children with profound developmental delays. These instruments allowed us to objectively compare the developmental profile of children with AS to age-matched peers of all developmental levels. However, the VABS-II and the ABC rely on reports and opinions by parents or caregivers to assess the capabilities of the child. The subjective nature of this information may be inadvertently biased by the way in which parents and caregivers report behaviors or skills.
One of the very few standardized instruments that allows for discrimination among subjects with very low cognitive abilities is the BSID-III, which can discriminate among subjects performing at a developmental age up to 42 months old. Like most instruments that allow for discrimination in the low-functioning population, the BSID-III is not designed to be used in older children and adults. Nevertheless, since none of the subjects in our study were found to have a developmental age beyond 34 months in any of the developmental domains assessed by the BSID-III, we believe that this is a valid measure of developmental skills in this population. Moreover, the majority of our participants under 42 months of age scored at or near the floor of the test, yielding little to no variability in standard scores; hence DQs were calculated for all of our participants. Although it is recognized that developmental assessments in infancy and early childhood are not highly predictive of intelligence in later life due to the wide variability in that population,26,36 some authors have found that the predictability of developmental assessments increases with increasing severity of intellectual disabilities (i.e., they are more predictive of long-term intelligence among children who are more severely delayed).37 The use of DQ when the norms of a developmental instrument are not applicable to the population being assessed is a well-accepted practice, as DQ allows for the comparison of children at different ages and at different times28,38, and it also provides for a broader range of possible scores.31 Moreover, BSID-III DQ scores have been found to correlate well with subsequent scores on the Stanford-Binet Intelligence Scale in a group of children with intellectual disabilities.29
Although this is one of the largest studies on the neurodevelopmental profile of individuals with AS, there are relatively few subjects with molecular etiologies other than deletions, which limits our ability to perform comparisons and genotype-phenotype correlations among the non-deletion sub-populations. We also recognize that the younger mean age of our deletion participants compared to the non-deletion participants may have confounded our analysis of the gross and fine motor abilities in these two sub-groups, because abnormal muscle tone can lead to delays in the acquisition of motor skills, and 73% of our participants up to the age of 24 months had abnormalities in muscle tone compared to 43% of those who were at least 42 months old.
Future studies should focus on the development of psychometric instruments that can discriminate among subjects who function at very low levels in each of the commonly-tested developmental domains (e.g. communication, motor skills, social skills, and activities of daily living), and the validation of such instruments in a wide age group from early infancy to adulthood. It is only with the availability and acceptance of such tools that accurate assessment of the developmental profile of children and adults with AS and other developmental disorders will be possible. There is also a need for large-scale longitudinal studies with subjects in a broad age group over an extended period of time, such as the ongoing AS Natural History study from which these baseline data were obtained. Such longitudinal evaluations will provide insights into the changes in the developmental profile that occur over time, and facilitate the creation of developmental growth curves for the cognitive, language, and motor functioning in AS individuals. This will enable providers and caretakers to understand the developmental trajectories of AS individuals and to anticipate level of functioning in adulthood.
Acknowledgments
The project described was supported by Grant Number NIH U54 RR019478 (awarded to ALB) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the Angelman Syndrome Foundation – Western Area Chapter. Funding through NCRR was through the cooperative agreement mechanism. The protocol was reviewed and approved by the NCRR Protocol Review Committee and subsequent study progress was monitored by their Data Safety Monitoring Board (DSMB). Data were imported into the Data Management Coordinating Center (DMCC). Data analysis and interpretation was conducted cooperatively with the DMCC statistician (H-SL). Manuscript preparation, review, and approval were solely that of the authors. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR, NIH, or the Angelman Syndrome Foundation. WHT is partly supported by a Children’s Hospital Boston Career Development Fellowship and the Clinical Investigator Training Program: Harvard/MIT Health Sciences and Technology – Beth Israel Deaconess Medical Center, in collaboration with Pfizer Inc. and Merck & Co. The sponsors had no role in this study nor in the preparation of this manuscript.
This study would not have been possible without the AS patients and their parents’ and guardians’ interest and devotion to this long-term study. We also express our appreciation to the General Clinical Research Centers (GCRC) at Children’s Hospital Boston and Texas Children’s Hospital for their support. The authors specifically thank: the study coordinators/nurses for their invaluable assistance–Beverly M. Feldman (Baylor College of Medicine), Vera Anastasoaie, Sharyn Lincoln, and Janette Z. Lawrence (Children’s Hospital Boston), Fran Annese and Joy Graham (Greenwood Genetic Center), Marla Hashiguchi (Rady Children’s Hospital San Diego); the clinical research staff at the Data Management Coordinating Center (DMCC) (Jennifer Pilger, Rachel Richesson, June T. Tran) for their technical support; and Alan K. Percy (University of Alabama at Birmingham) for his leadership and support. WHT would like to thank Virginia E. Kimonis (now at University of California, Irvine) for her assistance in initiating the study at Children’s Hospital Boston, and Alison Clapp, Medical Librarian at Children’s Hospital Boston, for her assistance in obtaining the many references.
References
- 1.Clayton-Smith J, Laan L. Angelman syndrome: a review of the clinical and genetic aspects. J Med Genet. 2003;40:87–95. doi: 10.1136/jmg.40.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen MB, Brondum-Nielsen K, Hansen LK, et al. Clinical, cytogenetic, and molecular diagnosis of Angelman syndrome: estimated prevalence rate in a Danish county. Am J Med Genet. 1995;60:261–262. doi: 10.1002/ajmg.1320600317. [DOI] [PubMed] [Google Scholar]
- 3.Kyllerman M. On the prevalence of Angelman syndrome. Am J Med Genet. 1995;59:405. doi: 10.1002/ajmg.1320590331. author reply 403–404. [DOI] [PubMed] [Google Scholar]
- 4.Buckley RH, Dinno N, Weber P. Angelman syndrome: are the estimates too low? Am J Med Genet. 1998;80:385–390. doi: 10.1002/(sici)1096-8628(19981204)80:4<385::aid-ajmg15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Knoll JH, Nicholls RD, Magenis RE, et al. Angelman syndrome: three molecular classes identified with chromosome 15q11q13-specific DNA markers. Am J Hum Genet. 1990;47:149–155. [PMC free article] [PubMed] [Google Scholar]
- 6.Christian SL, Robinson WP, Huang B, et al. Molecular characterization of two proximal deletion breakpoint regions in both Prader-Willi and Angelman syndrome patients. Am J Hum Genet. 1995;57:40–48. [PMC free article] [PubMed] [Google Scholar]
- 7.Peters SU, Goddard-Finegold J, Beaudet AL, et al. Cognitive and adaptive behavior profiles of children with Angelman syndrome. Am J Med Genet A. 2004;128:110–113. doi: 10.1002/ajmg.a.30065. [DOI] [PubMed] [Google Scholar]
- 8.Andersen WH, Rasmussen RK, Stromme P. Levels of cognitive and linguistic development in Angelman syndrome: a study of 20 children. Logoped Phoniatr Vocol. 2001;26:2–9. [PubMed] [Google Scholar]
- 9.Summers JA, Allison DB, Lynch PS, et al. Behaviour problems in Angelman syndrome. J Intellect Disabil Res. 1995;39 (Pt 2):97–106. doi: 10.1111/j.1365-2788.1995.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 10.Clarke DJ, Marston G. Problem behaviors associated with 15q- Angelman syndrome. Am J Ment Retard. 2000;105:25–31. doi: 10.1352/0895-8017(2000)105<0025:PBAWQA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Lossie AC, Whitney MM, Amidon D, et al. Distinct phenotypes distinguish the molecular classes of Angelman syndrome. J Med Genet. 2001;38:834–845. doi: 10.1136/jmg.38.12.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moncla A, Malzac P, Voelckel MA, et al. Phenotype-genotype correlation in 20 deletion and 20 non-deletion Angelman syndrome patients. Eur J Hum Genet. 1999;7:131–139. doi: 10.1038/sj.ejhg.5200258. [DOI] [PubMed] [Google Scholar]
- 13.Varela MC, Kok F, Otto PA, et al. Phenotypic variability in Angelman syndrome: comparison among different deletion classes and between deletion and UPD subjects. Eur J Hum Genet. 2004;12:987–992. doi: 10.1038/sj.ejhg.5201264. [DOI] [PubMed] [Google Scholar]
- 14.Sahoo T, Peters SU, Madduri NS, et al. Microarray based comparative genomic hybridization testing in deletion bearing patients with Angelman syndrome: genotype-phenotype correlations. J Med Genet. 2006;43:512–516. doi: 10.1136/jmg.2005.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahoo T, Bacino CA, German JR, et al. Identification of novel deletions of 15q11q13 in Angelman syndrome by array-CGH: molecular characterization and genotype-phenotype correlations. Eur J Hum Genet. 2007;15:943–949. doi: 10.1038/sj.ejhg.5201859. [DOI] [PubMed] [Google Scholar]
- 16.Smith A, Marks R, Haan E, et al. Clinical features in four patients with Angelman syndrome resulting from paternal uniparental disomy. J Med Genet. 1997;34:426–429. doi: 10.1136/jmg.34.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith A, Wiles C, Haan E, et al. Clinical features in 27 patients with Angelman syndrome resulting from DNA deletion. J Med Genet. 1996;33:107–112. doi: 10.1136/jmg.33.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albrecht U, Sutcliffe JS, Cattanach BM, et al. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat Genet. 1997;17:75–78. doi: 10.1038/ng0997-75. [DOI] [PubMed] [Google Scholar]
- 19.Gabriel JM, Merchant M, Ohta T, et al. A transgene insertion creating a heritable chromosome deletion mouse model of Prader-Willi and angelman syndromes. Proc Natl Acad Sci U S A. 1999;96:9258–9263. doi: 10.1073/pnas.96.16.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura K, Kishino T, Li E, et al. Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiol Dis. 2002;9:149–159. doi: 10.1006/nbdi.2001.0463. [DOI] [PubMed] [Google Scholar]
- 21.Weeber EJ, Jiang YH, Elgersma Y, et al. Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J Neurosci. 2003;23:2634–2644. doi: 10.1523/JNEUROSCI.23-07-02634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Woerden GM, Harris KD, Hojjati MR, et al. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nat Neurosci. 2007;10:280–282. doi: 10.1038/nn1845. [DOI] [PubMed] [Google Scholar]
- 23.Bayley N. Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) San Antonio, TX: Harcourt Assessment, Inc; 2005. [Google Scholar]
- 24.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales, Second Edition (Vineland-II) Upper Saddle River, NJ: Pearson Education, Inc; 2005. [Google Scholar]
- 25.Aman MG, Singh NN. Aberrant Behavior Checklist, Residential/or Community Version. East Aurora, NY: Slosson Educational Publications, Inc; 1994. [Google Scholar]
- 26.Lichtenberger EO. General measures of cognition for the preschool child. Ment Retard Dev Disabil Res Rev. 2005;11:197–208. doi: 10.1002/mrdd.20076. [DOI] [PubMed] [Google Scholar]
- 27.Hessl D, Nguyen DV, Green C, et al. A solution to limitations of cognitive testing in children with intellectual disabilities: the case of fragile X syndrome. J Neurodev Disord. 2009;1:33–45. doi: 10.1007/s11689-008-9001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dykens EM, Hodapp RM, Evans DW. Profiles and development of adaptive behavior in children with Down syndrome. Am J Ment Retard. 1994;98:580–587. [PubMed] [Google Scholar]
- 29.Goldstein DJ, Sheaffer CI. Ratio developmental quotients from the Bayley are comparable to later IQs from the Stanford-Binet. Am J Ment Retard. 1988;92:379–380. [PubMed] [Google Scholar]
- 30.Duker PC, van Driel S, van de Bercken J. Communication profiles of individuals with Down’s syndrome, Angelman syndrome and pervasive developmental disorder. J Intellect Disabil Res. 2002;46:35–40. doi: 10.1046/j.1365-2788.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- 31.Perry A, Flanagan HE, Dunn Geier J, et al. Brief report: the Vineland Adaptive Behavior Scales in young children with autism spectrum disorders at different cognitive levels. J Autism Dev Disord. 2009;39:1066–1078. doi: 10.1007/s10803-009-0783-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karabekiroglu K, Aman MG. Validity of the aberrant behavior checklist in a clinical sample of toddlers. Child Psychiatry Hum Dev. 2009;40:99–110. doi: 10.1007/s10578-008-0108-7. [DOI] [PubMed] [Google Scholar]
- 33.Summers JA, Feldman MA. Distinctive pattern of behavioral functioning in Angelman syndrome. Am J Ment Retard. 1999;104:376–384. doi: 10.1352/0895-8017(1999)104<0376:DPOBFI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 34.Roberts JE, Mankowski JB, Sideris J, et al. Trajectories and predictors of the development of very young boys with fragile X syndrome. J Pediatr Psychol. 2009;34:827–836. doi: 10.1093/jpepsy/jsn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dykens E, Shah B. Psychiatric disorders in Prader-Willi syndrome: epidemiology and management. CNS Drugs. 2003;17:167–178. doi: 10.2165/00023210-200317030-00003. [DOI] [PubMed] [Google Scholar]
- 36.Rapin I. Value and limitations of preschool cognitive tests, with an emphasis on longitudinal study of children on the autistic spectrum. Brain Dev. 2003;25:546–548. doi: 10.1016/s0387-7604(03)00127-x. [DOI] [PubMed] [Google Scholar]
- 37.Largo RH, Graf S, Kundu S, et al. Predicting developmental outcome at school age from infant tests of normal, at-risk and retarded infants. Dev Med Child Neurol. 1990;32:30–45. doi: 10.1111/j.1469-8749.1990.tb08464.x. [DOI] [PubMed] [Google Scholar]
- 38.Jonsdottir SL, Saemundsen E, Asmundsdottir G, et al. Follow-up of children diagnosed with pervasive developmental disorders: stability and change during the preschool years. J Autism Dev Disord. 2007;37:1361–1374. doi: 10.1007/s10803-006-0282-z. [DOI] [PubMed] [Google Scholar]


