Summary
The fission yeast Schizosaccharomyces pombe has a cylindrical rod-shape that is organized and maintained by interactions between the microtubule, cell membrane, and actin cytoskeleton; i.e., microtubules deliver factors to the cell tips that subsequently recruit the actin machinery to direct localized cell growth [1]. Mutations affecting any components in this pathway lead to bent, branched, or round cells [2]. In this context, the cytoskeleton controls cell polarity and thus dictates cell shape. Here, we use soft-lithography techniques to construct microfluidic channels in order to control the shape of cells. We show that by physically forcing wildtype rod-shaped cells to grow bent, they will re-organize their cytoskeleton and re-direct cell polarity to make new ectopic cell tips. In addition, by physically forcing bent or round mutant cells to conform to the wildtype rod-shape, cells will reverse their mutational phenotypes by re-organizing their cytoskeleton to maintain proper wildtype-like microtubule, cell membrane proteins, and actin localizations. Our study provides direct evidence that the cytoskeleton controls cell polarity and cell shape and demonstrates that cell shape also controls the organization of the cytoskeleton, in a feedback loop. We present a model of the feedback loop which explains how fission yeast cells maintain a rod-shape, and how perturbation of specific parameters of the loop can lead to different cell shapes.
Results and Discussion
Core mechanisms controlling cell polarity and cell shape are evolutionarily conserved [3, 4]. In general, localized dynamic interactions between the microtubule and actin cytoskeletons and the cell membrane dictate sites of polarized cell growth, giving rise to cell polarity and cell shape. The fission yeast Schizosaccharomyces pombe has proven to be an excellent model organism to study cytoskeletal organization, cell polarity, and cell shape [1]. Wildtype fission yeast cells are rod-shaped, grow in a bipolar fashion by cell tip extension, and divide by medial fission. Microscopy-based studies have revealed that microtubules are organized as several bundles along the long-axis of the cell, with minus ends bundled in antiparallel fashion at the cell center and dynamic plus ends interacting distally with the cell tips [5, 6]. Actin is organized into patches and cables which are localized to the growing cell tips [7, 8].
The current favored model suggests that microtubule plus ends deliver a group of proteins known as the +TIP complex (comprised of the conserved proteins tea1p (a kelch-repeat protein), tea2p (a kinesin-7 protein), tip1p (CLIP-170 protein) and mal3p (EB1 protein)) to the cell tip, where tea1p is docked to the membrane-bound receptor mod5p. Tea1p subsequently recruits the so-called polarisome protein complex (comprised of bud6p (a polarity protein) and for3p (a formin protein)), which nucleates the actin filaments that serve as tracks directing the growth machinery toward cell tips [1, 9]. Mutations affecting microtubule number or dynamics cause cells to grow bent or branched [10–13]. Mutations affecting actin localization at cell tips cause cells to grow round, while the use of actin-depolymerizing drugs inhibits cell growth [14–17]. This model implies that the actin cytoskeleton is responsible for maintaining cell polarity and cell growth per se while the microtubule cytoskeleton is responsible for fine-tuning the axis or direction of cell growth [1, 9]. However, newly divided fission yeast cells already have a rod-shape and defined cell tips, and at steady-state the microtubule-membrane-actin pathway exists in a closed loop, making it difficult to determine causality. In addition, fission yeast has a rigid cell wall which is remodeled by the cytoskeleton and imparts shape. How this cell wall-defined shape influences the underlying cytoskeleton is unknown. Here, we use fabricated μm-scale channels to control the shape of living yeast cells in order to investigate causal relationships between the cytoskeleton, cell polarity, and cell shape. We tested the current model, and our findings indicate that microtubules can initiate new sites of polarized cell growth while actin maintains sites of growth, and that externally-applied cell shape can reorganize the underlying cytoskeleton, partially reversing the mutational phenotype.
Soft-lithography and patterned adhesive surfaces had been successfully used to control the shape of bacteria and mammalian cells [18–21]. We combined soft-lithography and microfluidics technologies to create light microscopy-enabled, polydimethylsiloxane (PDMS) elastomer-based chambers containing μm-scale channels of controlled shapes and appropriate dimensions for fission yeast (Fig. 1A). The cells can be syringe-pumped into these channels, in which they grow normally while conforming to specific shapes (Fig. 1B), e.g., rod-shaped wildtype cells can be made to grow in a curved manner and bent or round mutant cells can be made to grow in a straight manner. In addition, inlet and outlet holes allow for continuous flow and exchange of liquid media or drugs (Fig. 1A). Cells expressing functional fluorescent fusion proteins were used in order to visualize structures involved in the pathway leading to directed cell growth – microtubles (atb2p [22]), +TIPs (tea1p [10]), the membrane receptor mod5p [23], the polarisome complex (bud6p [24]), and actin (crn1p [8]).
Figure 1. Microfluidic channels can control the shape of fission yeast cells.
A. Microfluidic channels to constrain the shape of fission yeast cells. Shown is a typical cell chamber made by bonding a polydimethylsiloxane (PDMS) replica (p) onto a glass-bottom dish (g). The PDMS replica contains either curved or straight microfluidic channels (c) connected to inlet (i) and outlet (o) tubings for liquid exchange.
B. A curved microfluidic channel containing fission yeast cells. Cells growing outside the channel are free to take on their natural shape, while cells growing inside the channel conform to the shape of the channel.
C. Timelapse image of a wildtype cell expressing GFP-atb2p (tubulin) (PT.72) inside a curved channel. While growing inside the channel, the cell can progress normally through the cell cycle, forming a mitotic spindle (yellow arrow) during mitosis. Time, hr:min.
D. Wildtype cells expressing mCherry-atb2p (tubulin) and the conserved microtubule bundler ase1p-GFP (PT.802). Microtubule bundles inside the bent cell reflect global changes in their location, not changes in the number and inherent antiparallel organization of individual microtubule bundles by ase1p (n=8; control cells n=14). Bar, 10 μm.
E. Plot of cell arc Radius vs. cell Length. We calculate the arc radius as: (see Appendix). We defined three regions of interest: red zone – cells are short (<14 μm), and therefore their arc radii are large (>8 μm), and no reorganization of microtubules is apparent; yellow zone – cells are between 14.4 ± 1.7 μm to 17.7 ± 0.4 μm (n=16) with arc radii between 7.4 ± 0.1 μm to 7.7 ± 0.3 μm (n=16), and unambiguous microtubule reorganization to the convex side of the cell occurs; green zone – cells >18 μm with average arc radii of 7.5 μm (n=10), and frequent and sustained contacts of microtubule tips with ectopic virgin cell cortex occurs. Cells in the green zone are capable of forming ectopic cell tips – 70% of cells in this zone showed unambiguous ectopic tip protrusion.
First, we compared the behavior of the microtubule cytoskeleton in growing rod-shaped wildtype cells confined inside curved channels to control cells growing outside the channels using live-cell imaging (Fig. 1B). We observed that as wildtype cells grow longer inside the curved channels, their curvature increases, and their arc radii become smaller (Fig. 1C and Fig. 1E and Mov. 1A). Shorter cells have larger arc radii and therefore show no reorganization of the microtubules (Fig. 1E, red zone). At cell lengths between 14–18 μm, and arc radii of ~7.7 μm, microtubules unambiguously reorganize to the convex side of the cell (Fig. 1E, yellow zone). This distribution is a consequence of microtubule mechanical buckling or bending due to continued elongation upon contact with the curved cell wall at or adjacent to the cell tips (Fig. S1A and Mov. 1D). At cell lengths >18 μm and arc radii of ~7.5 μm, sustained interactions between microtubules and ectopic cell cortex occur (Fig. 1E, green zone; and Fig. S1B and Mov. 1E). In contrast, microtubules in control cells grown outside the channels remain distributed symmetrically with respect to the long-axis of the cell, irrespective of cell length (Fig. 1D and Mov. 1B and 1C). Microtubule re-organization reflects changes in the relative position of microtubule bundles within bent cells, but each microtubule bundle retains its basic structure of minus-end antiparallel bundling by the protein ase1p [25, 26] (Fig. 1D). These observations indicate that when straight fission yeast cells are forced to grow bent, they will reorganize their dynamic microtubule cytoskeleton in response to their new shape.
We next examined the consequences of microtubule reorganization and contact at ectopic cell cortex in bent wildtype cells by imaging, in combinations, proteins of the microtubule cytoskeleton, cell membrane, and/or polarisome and actin cytoskeleton. We chose cells >14 μm for analysis of cytoskeletal reorganization. In contrast to control cells, in which the actin marker crn1p preferentially localized to the growing cell tips, cells in bent channels showed diffuse localization of crn1p (Fig. 2A). One-hour time-integration of microtubule and crn1p dynamics revealed that the ectopic site of microtubule-cortex interaction had begun to accumulate crn1p, indicating new actin accumulation (Fig. 2A and Fig. S2A). In fact, this new site had also accumulated other representative proteins of the +TIP complex, cell membrane, and polarisome cytoskeleton (Fig. 2B and Fig. S2A). Using high temporal resolution imaging, we observed individual microtubules depositing tea1p at the ectopic cell cortex in the bent cells (Fig. S2B). Over time, this site initiated polarized cell growth and developed a new cell tip (Fig. S2C and Mov. 2A–D). We observed ~70% of cells creating unambiguous ectopic cell tip protrusion (n>40). No ectopic accumulation of tea1p or bud6p and no ectopic cell tips were observed inside the curved channels when cells were treated with the microtubule-depolymerization drug MBC (n>40) (Fig. S2D and S2E). Interestingly, the old cell tip that no longer received microtubule contact continued polarized cell growth (Fig. 2A and Fig. S2C and Mov. 2A–D). These findings are a direct demonstration of the current model [9, 27]: microtubules are required to initiate new sites of cell polarity via frequent contact with the cell membrane; however, once polarity sites have been established, polarized cell growth is accomplished and maintained by the actin cytoskeleton independent of microtubules.
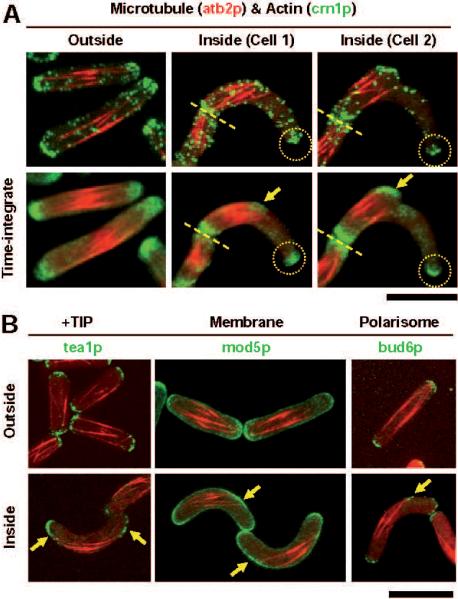
Figure 2. Cell shape-induced microtubule reorganization leads to new cell polarization sites.
A. Wildtype fission yeast cells expressing mCherry-atb2p (tubulin) and crn1p-GFP (a marker for actin patches) (PT.832). Top row shows single time point 2-color merged images of cells. Second row shows 2-color time-integrated images where 60 time points, representing 1 hour of cell growth, have been summed to show cumulative intensities over time. Cells inside the channel (cell 1) contain rigid microtubule bundles which can no longer extend to both cell tips. Instead, the microtubules reach one tip of the cell and make frequent and sustained contact with the cell cortex (yellow arrows) some distance away from the second tip (yellow dashed circle). Time-integration shows that actin patches accumulates at the ectopic site of microtubule and membrane contact. With longer growth times inside channels (cell 2) this site shows clearer localization of actin (yellow arrow) and the initiation of a new ectopic cell tip, making a branched wildtype cell. The old cell tip continues to be enriched in actin and grow in the absence of microtubule contact (yellow dashed circle) (n=12; control cells n>30).
B. Wildtype cells expressing mCherry-atb2p (tubulin) and proteins of the +TIP (tea1p-GFP) (PT.826), cell membrane (mod5p-GFP) (PT.907), or polarisome (bud6p-GFP) cytoskeleton (PT.853). Outside channels, cell tip proteins show clear symmetry in localization at the bipodal tips of the rod-shaped cells (n>30). Inside curved channels, tip protein symmetry is broken. Consistent with the reorganization of microtubules to the convex cell surface and microtubule contact at new ectopic membrane sites, all tip proteins show relocalization to the ectopic site (yellow arrows) while maintaining localization at the old cell tips (n=19). Bar, 10 μm.
Bent shape-manipulated wildtype cells which develop a new cell tip due to the reorganization of their cytoskeleton are reminiscent of the branched or T-shaped phenotypes exhibited by cells with genetic mutations in the microtubule cytoskeleton [10]. We therefore asked if mutant cells with abnormal shapes forced to grow inside straight channels would reorganize their microtubule and actin cytoskeletons and adopt a more wildtype-like phenotype. We first tested the round mutant orb6ts [16, 17]. The fission yeast protein orb6p is a kinase of the conserved NDR kinase family which controls cell morphology [16, 17]. Orb6p localizes to growing cell tips; a temperature-sensitive mutation of orb6p leads to mislocalized actin patches and a consequent spherical shape [16, 17]. We pumped orb6ts cells into straight channels at the permissive temperature (23°C) at which cells are rod-shaped, and then incubated the chamber for several hours at the restrictive temperature (36°C) in order to deactivate orb6p. Consistent with previous reports, at the restrictive temperature orb6ts cells outside the channels became round, microtubules were disorganized with no preferred axis of alignment, and tea1p, mod5p, bud6p, and crn1p were mislocalized with no preference for cell tips (Fig. 3A and Fig. S3A). Remarkably, inside straight channels, orb6ts cells contained microtubules which were aligned with the long-axis of the cell, and tea1p and proteins of the membrane, polarisome, and actin cytoskeleton were localized to the cell tips in a wildtype-like fashion (Fig. 3A and Fig. S3A and S3B). We concluded that the disorganized cytoskeletal phenotype of round mutant orb6ts cells can be partially rescued when cells are externally forced into a wildtype-like shape. Our results thus far suggest that there exists a positive feedback loop between cell shape and the cytoskeleton; i.e., cell shape leads to microtubule reorganization, which leads to repositioning of polarizome complexes, which defines actin-dependent sites of polarized cell growth, which leads to new cell shape, etc. (Fig. S4).
Figure 3. Round- and bent-shaped mutant cells maintain wildtype-like cell polarity when constrained inside straight channels – a feedback loop.
A. Top, A straight microfluidic channel containing orb6ts mutant cells growing at the restrictive 36°C temperature. Bottom, orb6ts cells expressing mCherry-atb2p (tubulin) and tea1p-GFP (+TIP) (PT.893). Outside the straight channel, orb6ts cells have lost their bipodal polarity and are round with microtubules oriented in all directions and with +TIP protein tea1p delocalized (observed in ~87% of control cells; n=30). Inside the channel, orb6ts cells are forced to maintain a rod-shape with microtubules maintaining their alignment along the cell long-axis and +TIP protein tea1p specifically localized to the growing cell tips (observed in 60% of cells; n=15). Bar, 10 μm.
B. Top, a straight microfluidic channel containing mto1Δ mutant cells. Bottom, mto1Δ cells expressing mCherry-atb2p (tubulin) and tea1p-GFP (+TIP) (PT.910). Outside the channel, the single microtubule bundle is located at the convex surface of the bent mto1Δ cell. The +TIP protein tea1p shows asymmetry in its localization at the old cell tips and also begins to localize ectopically at a new cortical site (yellow arrow). Inside the channel, the microtubule bundle of mto1Δ cells shows no preference for a particular cell surface, but instead interacts with the bipodal cell tips. As a consequence, cell tip proteins show symmetrical localization at the cell tips and not at other ectopic sites (observed in ~88% of cells, n=17; control cells n=17). Bar, 10 μm.
C. Top, mto1Δ cells from (B). We defined the Length Ratio as the ratio of the width of the tea1-GFP (or bud6-GFP) signal divided by the width of the cell tip. Bottom, comparison plots of Length Ratios of tea1-GFP and bud6-GFP in mto1Δ cells growing outside versus inside the straight channels.
We tested our hypothesis that a feedback loop exists between the cytoskeleton and cell shape. We reasoned that even a minor shape-induced focusing of microtubules would enhance subsequent focusing of +TIP and polarisome complexes in a feedback loop. We therefore examined the distribution of tea1p and bud6p proteins in mto1Δ cells. Fission yeast mto1p is a conserved protein involved in microtubule organization [11]. Cells deleted of mto1p grow in a bent fashion and usually contain only one bundle of microtubules, located at the convex surface of the bent cell, as opposed to the multiple microtubule bundles of wildtype cells [11]. In straight channels, mto1Δ cells appear rod-shaped but still contain a single microtubule bundle (Fig. 3B). However, the distribution of microtubules, as well as tea1p and bud6p appear more symmetrical and focused at the cell tips than in control mto1Δ cells outside the channels (Fig. 3B and 3C and Fig. S3C). In control mto1Δ cells, tea1p and bud6p covers 81 ± 6% (n=10) and 82 ± 8% (n=14) of the cell tip, respectively. In contrast, inside the straight channels, tea1p and bud6p covers only 54 ± 14% (n=16) and 61 ± 9% (n=6) (p<0.001) of the cell tip, respectively (Fig. 3B and 3C). These results are consistent with the existence of feedback loop which focuses tea1p and bud6p at the tip of the cells in a microtubule-dependent manner.
A feedback loop implies that rod-shaped cells will continue to grow straight and round cells will continue to grow round. How then can cells transition from a rod to round cell shape, as in the orb6ts mutant? We reasoned that proper retention of either the +TIP proteins or the polarisome proteins is an important parameter of the feedback loop. We therefore measured the retention of tea1p and bud6p at the cell tips in wildtype and orb6ts cells constrained in straight channels in the presence or absence of microtubules. Twenty minutes after MBC treatment, both tea1p and bud6p significantly delocalize from the cell tips of wildtype and orb6ts cells. Tea1p was retained at similar levels of 53 ± 16% (n=12) in wildtype cells and 55 ± 18% (n=14) in orb6ts cells (p=0.722) (Fig. 4A and 4B). In contrast, bud6p was retained at 78 ± 16% (n=14) in wildtype cells but only at 46 ± 16% (n=13) in the orb6ts cells (p<0.001) (Fig. 4A and 4B). This indicates that microtubule-dependent tea1p delivery and retention at cell tips is not affected in orb6ts cells, while bud6p retention at the cell tips is orb6p-dependent. We found that orb6ts cells transititon from straight to round by slowly decreasing their lengths and increasing their widths through successive cell cycles, so that the aspect ratio of the cell tends toward unity after 2–4 generations (Fig. 4C and 4D). These data are also consistent with our feedback loop model, where different mutant cell shapes can evolve from an initial rod-shaped cell (Fig. 4E and Fig. S4).
Figure 4. A mechanism for the cell shape-cytoskeleton feedback loop – bud6p retention at cell tips is important for shape changes.
A. Straight microfluidic channels containing wildtype and orb6ts mutant cells growing at the restrictive 36°C temperature (channels are indicated by dashed yellow lines). Inside the channel, orb6ts cells are constrained and maintain a somewhat elongated rod-shape. Wildtype and orb6ts cells are expressing mCherry-atb2p (tubulin) and bud6p-GFP (polarisome) (PT.924 and PT.894, respectively). During imaging, 25 μg/ml of MBC (microtubule-depolymerizing drug) was perfused through the channels. Approximately 20 minutes after MBC-treatment, no microtubules were present, and a significant fraction of bud6p-GFP no longer localized to the cell tips (yellow arrows). This experiment was also performed for wildtype and orb6ts mutant cells expressing mCherry-atb2p (tubulin) and tea1p-GFP (+TIP) (PT.922 and PT.893, respectively).
B. Normalized intensity plots comparing the retention of tea1p-GFP and bud6p-GFP at the cell tips before (pre-MBC) and 20 minutes after (post-MBC) microtubule depolymerization with 25 μg/ml of MBC.
C. Brightfield time-lapse images of orb6ts cells growing at the restrictive 36°C temperature. Cells transition from straight to round shape by decreasing their lengths and increasing their widths during successive cell cycles (septa are indicated by red arrows).
D. Aspect ratio of orb6ts cells through successive cell cycles. Tracing of cells from (C). Aspect ratio is defined as Length/Width.
E. A model for cell shape – a positive feedback loop. At steady-state, cells maintain a Rate of Delivery (RD) of proteins such as tea1p to the cell tips, and a Rate of Retention (RR) of proteins such as bud6p at the cell tips. When RD > RR, cells maintain a straight shape. When RD < RR, cells evolve into a round shape after successive cell cycles.
Our current work shows that S. pombe morphogenesis is governed by a feedback loop between the cytoskeleton and cell shape. We have identified two key steps in this loop that allow cells to maintain a rod shape: 1) focusing of microtubules by cell shape induces focusing of polarisome deposition at cell tips; and 2) retention of a focused polarisome is necessary to maintain straight cell growth at cell tips. It will be interesting to test the robustness, evolutionary conservation, and/or disease reversal implications of this proposed cytoskeleton and cell shape feedback loop. For example, will cells maintain their newly acquired cytoskeleton and shape phenotypes when removed from the confinement of microfluidic channels either immediately or after a certain number of generations? There has been an indication from studies in bacteria that shape can be maintained once freed from external constraints [20]. Second, which aspects of the feedback loop are conserved? One key parameter of the feedback loop determined by this study is the proper retention of the polarisome protein bud6p at the cell tips. This may be analogous to the polar cap formation mechanism shown in budding yeast [28]. And finally, can externally imposed shape such as that found in multi-cellular organisms compensate for or reverse the cytoskeletal phenotypes of a mutant cell located inside a tissue composed mainly of wildtype cells? There are indications that cancer cells can be made to form morphologically normal structures and are more resistant to apoptosis when grown in certain three dimensional contexts [29, 30].
Supplementary Material
2-color merged timelapse movie of several wildtype fission yeast cells expressing mCherry-atb2p (tubulin) (red) and the membrane receptor GFP-mod5p (green) (PT.908) growing inside a curved channel. The cell on the far left attempts to form a new cell tip where its microtubules make frequent contact with virgin membrane cortex. This is evident by an increase in the GFP-mod5p signal, indicative of new polarized cell growth, and attempted protrusion of a new cell tip. However, this new cell tip remains confined within the channel. Serendipitously, due to a small piece of dirt (green amorphous spot) which slightly lifts the PDMS away from the glass surface and creates a small opening, the cell on the far right can successfully make a new protruding cell tip at the site of microtubule and virgin membrane contact.
Timelapse movie of a wildtype fission yeast cell expressing GFP-atb2p (tubulin) (PT.72) growing inside a curved channel. As the cell grows longer inside the channel, it conforms to the shape of the curved channel and appears progressively bent. This shape change causes the microtubule bundles to redistribute to the convex cortex of the cell. This cell can progress normally through the cell cycle, as evident by the breakdown of interphase microtubules and the formation of a mitotic spindle at the end of the movie.
3D rotation of control wildtype fission yeast cells expressing mCherry-atb2p (tubulin) (PT.832) growing outside a curved channel. These cells are rod-shaped with a linear growth-axis. Their interphase microtubule bundles are aligned parallel with the cell growth-axis and are distributed more-or-less evenly around this axis.
3D rotation of a wildtype fission yeast cell expressing mCherry-atb2p (tubulin) (PT.832) growing inside a curved channel. The cell conforms to the curved shape of the channel and appears bent, with a curved long-axis. The interphase microtubule bundles appear buckled and bent, in alignment with the curved growth-axis and are distributed mostly at the convex cell cortex.
Timelapse movie of the re-organization of interphase microtubule bundles in a wildtype fission yeast cell expressing GFP-atb2p (tubulin) (PT.72) inside a curved channel. Due to the bent shape of the cell, most of the microtubule bundles are already present at the convex cell cortex. A new microtubule bundle is seen elongating at the convex side of the cell. This microtubule grows long, hits the cell wall, and buckles toward the convex cell cortex. Microtubule buckling may be a mechanism responsible for microtubule re-organization at the convex cell cortex.
Timelapse movie of a wildtype fission yeast cell expressing GFP-atb2p (tubulin) (PT.72) growing inside a curved channel. As the cell grows bent, the microtubules buckle and re-organize to the convex cell cortex. Overtime, the length and curvature of the cell dictates that the microtubules make contact at virgin cell cortex, away from the old cell tips. This new site of microtubule-cell membrane contact will organize a new site of cell polarity.
Timelapse movie of control wildtype fission yeast cells expressing mCherry-atb2p (tubulin) (left panel) and crn1p-GFP (a marker for actin patches) (right panel) (PT.832). At any single time point, microtubules are aligned along the linear growth-axis and actin patches are preferentially polarized at the growing cell tips.
Timelapse movie of a wildtype fission yeast cell expressing mCherry-atb2p (tubulin) (left panel) and crn1p-GFP (a marker for actin patches) (right panel) (PT.832) growing inside a curved channel. Microtubules are making frequent contact with one old cell tip and a new region of virgin membrane at the cell cortex; the second old cell tip has no interaction with microtubules. At early time points, actin patches appear delocalized from the cell tips. At later time points, actin begins to show preferential localization to the new site of microtubule contact, as well as the old cell tips. This new site of microtubule-virgin membrane contact will become a new cell polarity site.
Timelapse movie of a wildtype fission yeast cell expressing mCherry-atb2p (tubulin) (left panel) and crn1p-GFP (a marker for actin patches) (right panel) (PT.832) growing inside a channel. Once a new site of cell polarity has been established, polarized cell growth will occur at this site, leading to the protrusion of a new cell tip. The old cell tip continues to grow, independent of microtubule contact. This suggests that microtubules initiate new cell polarity sites while actin maintains polarized cell growth.
Acknowledgements
We thank the laboratories of Fred Chang, Sophie Martin, Dan McCollum, Ken Sawin, Roger Tsien, and Fulvia Verde for generously providing reagents. We thank Guillaume Stirnemann and Joseph Wong for technical helps. The Piel Lab is supported by grants from the ANR and HFSP. The Tran Lab is supported by grants from the NIH, ACS, ANR, FRM, LaLigue, and HFSP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Experimental Procedures See supplemental section.
References
- 1.Martin SG, Chang F. Cell cycle. Vol. 4. Georgetown, Tex: 2005. New end take off: regulating cell polarity during the fission yeast cell cycle; pp. 1046–1049. [PubMed] [Google Scholar]
- 2.Hayles J, Nurse P. A journey into space. Nature reviews. 2001;2:647–656. doi: 10.1038/35089520. [DOI] [PubMed] [Google Scholar]
- 3.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes & development. 2007;21:483–496. doi: 10.1101/gad.1511207. [DOI] [PubMed] [Google Scholar]
- 5.Drummond DR, Cross RA. Dynamics of interphase microtubules in Schizosaccharomyces pombe. Curr Biol. 2000;10:766–775. doi: 10.1016/s0960-9822(00)00570-4. [DOI] [PubMed] [Google Scholar]
- 6.Tran PT, Marsh L, Doye V, Inoue S, Chang F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. The Journal of cell biology. 2001;153:397–411. doi: 10.1083/jcb.153.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alfa CE, Hyams JS. Distribution of tubulin and actin through the cell division cycle of the fission yeast Schizosaccharomyces japonicus var. versatilis: a comparison with Schizosaccharomyces pombe. Journal of cell science. 1990;96(Pt 1):71–77. doi: 10.1242/jcs.96.1.71. [DOI] [PubMed] [Google Scholar]
- 8.Pelham RJ, Jr., Chang F. Role of actin polymerization and actin cables in actin-patch movement in Schizosaccharomyces pombe. Nature cell biology. 2001;3:235–244. doi: 10.1038/35060020. [DOI] [PubMed] [Google Scholar]
- 9.Sawin KE, Snaith HA. Role of microtubules and tea1p in establishment and maintenance of fission yeast cell polarity. Journal of cell science. 2004;117:689–700. doi: 10.1242/jcs.00925. [DOI] [PubMed] [Google Scholar]
- 10.Mata J, Nurse P. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell. 1997;89:939–949. doi: 10.1016/s0092-8674(00)80279-2. [DOI] [PubMed] [Google Scholar]
- 11.Sawin KE, Lourenco PC, Snaith HA. Microtubule nucleation at non-spindle pole body microtubule-organizing centers requires fission yeast centrosomin-related protein mod20p. Curr Biol. 2004;14:763–775. doi: 10.1016/j.cub.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Toda T, Umesono K, Hirata A, Yanagida M. Cold-sensitive nuclear division arrest mutants of the fission yeast Schizosaccharomyces pombe. Journal of molecular biology. 1983;168:251–270. doi: 10.1016/s0022-2836(83)80017-5. [DOI] [PubMed] [Google Scholar]
- 13.Umesono K, Toda T, Hayashi S, Yanagida M. Cell division cycle genes nda2 and nda3 of the fission yeast Schizosaccharomyces pombe control microtubular organization and sensitivity to anti-mitotic benzimidazole compounds. Journal of molecular biology. 1983;168:271–284. doi: 10.1016/s0022-2836(83)80018-7. [DOI] [PubMed] [Google Scholar]
- 14.Hirata D, Kishimoto N, Suda M, Sogabe Y, Nakagawa S, Yoshida Y, Sakai K, Mizunuma M, Miyakawa T, Ishiguro J, et al. Fission yeast Mor2/Cps12, a protein similar to Drosophila Furry, is essential for cell morphogenesis and its mutation induces Wee1-dependent G(2) delay. The EMBO journal. 2002;21:4863–4874. doi: 10.1093/emboj/cdf495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonhard K, Nurse P. Ste20/GCK kinase Nak1/Orb3 polarizes the actin cytoskeleton in fission yeast during the cell cycle. Journal of cell science. 2005;118:1033–1044. doi: 10.1242/jcs.01690. [DOI] [PubMed] [Google Scholar]
- 16.Verde F, Mata J, Nurse P. Fission yeast cell morphogenesis: identification of new genes and analysis of their role during the cell cycle. The Journal of cell biology. 1995;131:1529–1538. doi: 10.1083/jcb.131.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verde F, Wiley DJ, Nurse P. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7526–7531. doi: 10.1073/pnas.95.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brock A, Chang E, Ho CC, LeDuc P, Jiang X, Whitesides GM, Ingber DE. Geometric determinants of directional cell motility revealed using microcontact printing. Langmuir. 2003;19:1611–1617. doi: 10.1021/la026394k. [DOI] [PubMed] [Google Scholar]
- 19.Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. Faseb J. 2002;16:1195–1204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi S, DiLuzio WR, Weibel DB, Whitesides GM. Controlling the shape of filamentous cells of Escherichia coli. Nano letters. 2005;5:1819–1823. doi: 10.1021/nl0507360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thery M, Racine V, Piel M, Pepin A, Dimitrov A, Chen Y, Sibarita JB, Bornens M. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19771–19776. doi: 10.1073/pnas.0609267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding DQ, Chikashige Y, Haraguchi T, Hiraoka Y. Oscillatory nuclear movement in fission yeast meiotic prophase is driven by astral microtubules, as revealed by continuous observation of chromosomes and microtubules in living cells. Journal of cell science. 1998;111(Pt 6):701–712. doi: 10.1242/jcs.111.6.701. [DOI] [PubMed] [Google Scholar]
- 23.Snaith HA, Sawin KE. Fission yeast mod5p regulates polarized growth through anchoring of tea1p at cell tips. Nature. 2003;423:647–651. doi: 10.1038/nature01672. [DOI] [PubMed] [Google Scholar]
- 24.Glynn JM, Lustig RJ, Berlin A, Chang F. Role of bud6p and tea1p in the interaction between actin and microtubules for the establishment of cell polarity in fission yeast. Curr Biol. 2001;11:836–845. doi: 10.1016/s0960-9822(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 25.Loiodice I, Staub J, Setty TG, Nguyen NP, Paoletti A, Tran PT. Ase1p organizes antiparallel microtubule arrays during interphase and mitosis in fission yeast. Molecular biology of the cell. 2005;16:1756–1768. doi: 10.1091/mbc.E04-10-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashita A, Sato M, Fujita A, Yamamoto M, Toda T. The roles of fission yeast ase1 in mitotic cell division, meiotic nuclear oscillation, and cytokinesis checkpoint signaling. Molecular biology of the cell. 2005;16:1378–1395. doi: 10.1091/mbc.E04-10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawin KE, Nurse P. Regulation of cell polarity by microtubules in fission yeast. The Journal of cell biology. 1998;142:457–471. doi: 10.1083/jcb.142.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, Turley EA, Bissell MJ. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. Journal of the National Cancer Institute. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald JC, Whitesides GM. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Accounts of chemical research. 2002;35:491–499. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- 32.Russell P, Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986;45:145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- 33.Tran PT, Paoletti A, Chang F. Methods. Vol. 33. San Diego, Calif: 2004. Imaging green fluorescent protein fusions in living fission yeast cells; pp. 220–225. [DOI] [PubMed] [Google Scholar]
- 34.Castagnetti S, Novak B, Nurse P. Microtubules offset growth site from the cell centre in fission yeast. Journal of cell science. 2007;120:2205–2213. doi: 10.1242/jcs.03464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2-color merged timelapse movie of several wildtype fission yeast cells expressing mCherry-atb2p (tubulin) (red) and the membrane receptor GFP-mod5p (green) (PT.908) growing inside a curved channel. The cell on the far left attempts to form a new cell tip where its microtubules make frequent contact with virgin membrane cortex. This is evident by an increase in the GFP-mod5p signal, indicative of new polarized cell growth, and attempted protrusion of a new cell tip. However, this new cell tip remains confined within the channel. Serendipitously, due to a small piece of dirt (green amorphous spot) which slightly lifts the PDMS away from the glass surface and creates a small opening, the cell on the far right can successfully make a new protruding cell tip at the site of microtubule and virgin membrane contact.
Timelapse movie of a wildtype fission yeast cell expressing GFP-atb2p (tubulin) (PT.72) growing inside a curved channel. As the cell grows longer inside the channel, it conforms to the shape of the curved channel and appears progressively bent. This shape change causes the microtubule bundles to redistribute to the convex cortex of the cell. This cell can progress normally through the cell cycle, as evident by the breakdown of interphase microtubules and the formation of a mitotic spindle at the end of the movie.
3D rotation of control wildtype fission yeast cells expressing mCherry-atb2p (tubulin) (PT.832) growing outside a curved channel. These cells are rod-shaped with a linear growth-axis. Their interphase microtubule bundles are aligned parallel with the cell growth-axis and are distributed more-or-less evenly around this axis.
3D rotation of a wildtype fission yeast cell expressing mCherry-atb2p (tubulin) (PT.832) growing inside a curved channel. The cell conforms to the curved shape of the channel and appears bent, with a curved long-axis. The interphase microtubule bundles appear buckled and bent, in alignment with the curved growth-axis and are distributed mostly at the convex cell cortex.
Timelapse movie of the re-organization of interphase microtubule bundles in a wildtype fission yeast cell expressing GFP-atb2p (tubulin) (PT.72) inside a curved channel. Due to the bent shape of the cell, most of the microtubule bundles are already present at the convex cell cortex. A new microtubule bundle is seen elongating at the convex side of the cell. This microtubule grows long, hits the cell wall, and buckles toward the convex cell cortex. Microtubule buckling may be a mechanism responsible for microtubule re-organization at the convex cell cortex.
Timelapse movie of a wildtype fission yeast cell expressing GFP-atb2p (tubulin) (PT.72) growing inside a curved channel. As the cell grows bent, the microtubules buckle and re-organize to the convex cell cortex. Overtime, the length and curvature of the cell dictates that the microtubules make contact at virgin cell cortex, away from the old cell tips. This new site of microtubule-cell membrane contact will organize a new site of cell polarity.
Timelapse movie of control wildtype fission yeast cells expressing mCherry-atb2p (tubulin) (left panel) and crn1p-GFP (a marker for actin patches) (right panel) (PT.832). At any single time point, microtubules are aligned along the linear growth-axis and actin patches are preferentially polarized at the growing cell tips.
Timelapse movie of a wildtype fission yeast cell expressing mCherry-atb2p (tubulin) (left panel) and crn1p-GFP (a marker for actin patches) (right panel) (PT.832) growing inside a curved channel. Microtubules are making frequent contact with one old cell tip and a new region of virgin membrane at the cell cortex; the second old cell tip has no interaction with microtubules. At early time points, actin patches appear delocalized from the cell tips. At later time points, actin begins to show preferential localization to the new site of microtubule contact, as well as the old cell tips. This new site of microtubule-virgin membrane contact will become a new cell polarity site.
Timelapse movie of a wildtype fission yeast cell expressing mCherry-atb2p (tubulin) (left panel) and crn1p-GFP (a marker for actin patches) (right panel) (PT.832) growing inside a channel. Once a new site of cell polarity has been established, polarized cell growth will occur at this site, leading to the protrusion of a new cell tip. The old cell tip continues to grow, independent of microtubule contact. This suggests that microtubules initiate new cell polarity sites while actin maintains polarized cell growth.