Abstract
Recent development in soft lithography and microfluidics enables biologists to create tools to control the cellular microenvironment. One such control is the ability to quickly change the temperature of the cells. Genetic model organism such as fission yeast has been useful for studies of the cell cytoskeleton. In particular, the dynamic microtubule cytoskeleton responds to changes in temperature. In addition, there are temperature-sensitive mutations of cytoskeletal proteins. We describe here the fabrication and use of a microfluidic device to quickly and reversibly change cellular temperature between 2°C and 50°C. We demonstrate the use of this device while imaging at high-resolution microtubule dynamics in fission yeast.
I. Introduction
The microtubule cytoskeleton is essential for cellular processes such as cell polarity or mitosis. Microtubules are dynamic biopolymers composed of α β-tubulin heterodimers. The fission yeast Schizosaccharomyces pombe has been effectively used to study the microtubule cytoskeleton. Historically, drugs have been used to modulate microtubule dynamics in fission yeast. For example, carbendazim (methyl benzimidazol-2-yl carbamate) is commonly used to depolymerize microtubules. Repo−lymerization is achieved upon drug washout. Microtubules also respond to temperature. Cells incubated in ice bath of below 6°C will completely depolymerize their microtubules. Repolymerization is achieved by heating up the cells. Microtubule dynamics in fission yeast are relatively fast—a typical microtubule has approximately 2 μm/min growth rate, 8 μm/min shrinkage rate, 0.02 min−1 catastrophe frequency, and little or no rescue. Thus, it is useful to be able to change drugs or temperature faster than the 1 min timescale to precisely observe microtubule dynamic responses.
The thermal time constant of a system decreases with decreasing size. Thus, miniaturized devices can achieve very fast temperature changes. Microfluidic systems, which enable fluid manipulation at the micron scale, are good candidates for fast temperature changes. Moreover, with recent development of technology based on the molding of PDMS (Polydimethylsiloxane), which is relatively inexpensive and easy to handle, microfluidics show a strong potential for fabrication of tools dedicated to cell biological experiments (Belanger et al., 2001, Charati and Stern, 1998, Duffy et al., 1998).
We present here a detailed protocol to fabricate and use a microfluidic temperature control device that enables temperature changes in the range of 2–50°C in less than 10 s. This device can be coupled to an oil immersion objective lens for high-resolution imaging. The device has been optimized for fission yeast studies, but can easily be adapted for other types of cells or organisms. Further, this device can be coupled with other microfluidic functionalities such as mechanical deformation or chemical perfusion. This device has been used to depolymerize microtubules at low temperature, and it has been also used to deactivate proteins at high temperature in the temperature-sensitive mutants widely available in fission yeast.
II. Device and Setup Presentation
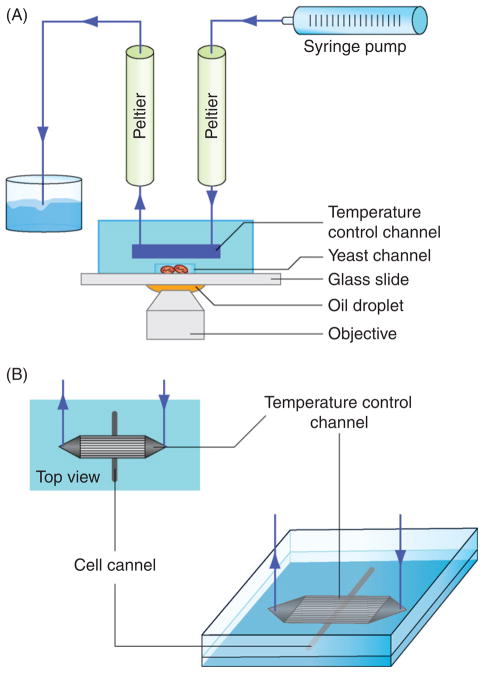
The temperature control device presented here is a bilayer PDMS device bonded onto a 150 μm thick glass coverslip. The bottom channel containing cells are in contact with the coverslip glass surface for imaging and are topped by a larger channel dedicated to temperature control (Fig. 1A). A thin 15 μm PDMS membrane separates the top and bottom channels to avoid direct fluid contact and serves to conduct temperature. Flowing water at controlled temperature through the top channel will, by heat diffusion that occurs through the thin PDMS membrane, change the temperature of the bottom channel containing cells. The top temperature control channel is simply a parallel network of 200 × 100 μm cross-section channel (1 cm long) for water circulation. In contrast, channels containing cells can be fabricated with different shapes and thicknesses depending on the type of cells involved (yeast, bacteria, etc.) and the required applications (cell deformation, drug screening, etc.). The temperature control setup is composed of two Peltier modules, a syringe pump, and a microscope (Fig. 1B). We use the Peltier module to control water temperature before pumping it into the microfluidic device. A typical Peltier can switch from 0°C to 50°C in less than 1 min. For our purpose, one Peltier module is plugged upstream (inlet) of the device and the second downstream (outlet). Once Peltier modules are at the desired temperatures, the temperature in the cells channel can be quickly changed from one to the other. This is possible because changing the direction of the water flow changes the Peltier in which the water goes through before entering the microfluidic device. The temperature change of the device is then limited by the time required to reverse the flow and not by the Peltier time constant.
Fig. 1.
Temperature control device schematic and setup. (A) Schematic of the complete setup. (B) Schematic of the bilayer PDMS device. The top layer contains microchannels for temperature changes. The bottom layer contains microchannels for holding cells.
III. Mold and Device Fabrication
Devices are fabricated using soft lithography of PDMS. This method enables fabricating hundreds of microfluidic devices using a single mold. PDMS has several advantages for the fabrication of microfluidic devices dedicated to cell biology. First, this elastomer is transparent, biocompatible (McDonald and Whitesides 2002), and permeable to gas (Unger et al., 2000). Those characteristics enable easy cell culture and microscope imaging. Second, from a technological point of view, PDMS material is cheap, easy to mold, has a low Young’s modulus, and can be easily covalently bonded to itself or glass using plasma ionization treatment. The small Young’s modulus of PDMS is particularly interesting for implementation of fluidic valves (Velve-Casquillas et al., 2010). Moreover, two or more PDMS replicas can be bonded together using plasma treatment that enables the fabrication of multilayer microfluidic devices.
A. Preliminary Step: Microfluidic Mold Fabrication
The first step required for microfluidic device fabrication is the mold fabrication. Microfluidic molds are fabricated using photolithography of SU8 onto silicon substrate. Briefly, a mold is made from transferring the channel designs from a mask onto a photoresist-coated silicon wafer substrate via UV photolithography. This wafer serves as the future mold. Since this method is extensively documented and well described in the Microchem SU8 datasheet, we will not describe it in detail here. Since our device is composed of two layers of channels, then two different molds are required. Before spin coating the proper photoresist onto the silicon substrate, a layer of omnicoat is used to promote subsequent adhesion between the photoresist and the silicon. For the temperature control channel mold, we used a 100 μm thick SU8-2050 (or 3050) photoresist. Similarly, for cell channel mold, we used a 5 μm thick SU8-2005 photoresist. Different types of SU8 enable different channel thickness.
Once the molds are made, it is then necessary to coat them with an anti-adhesive treatment to avoid removing the photoresist patterns during subsequent PDMS replication. For this purpose, we place the mold inside a closed Petri dish and add a 10 μl droplet of chlorotrimethylsilane for 3 min. Natural evaporation of the silane in the Petri dish will coat a thin layer of silane onto the mold surface. Since silane is harmful and volatile, this operation should be performed under a fume hood. Once molds are treated with silane, they can be used for repeated PDMS device fabrication (10–100 replications) before requiring a renewal of anti-adhesive treatment or a new mold.
Photolithography of features as small as 2 μm does not necessarily require clean room facilities such as found in physics, engineering, or material sciences labs. We find that for cellular dimensions, photolithography equipment can be installed in a classical biology fume hood. To fabricate our molds we used the OAI UV lamp (Fig. 2B), Laurell spin coater (Fig. 2C), and two Barnstead hotplates. With the exception of isopropanol, all mold making reagents are from Microchem.
Fig. 2.
The fabrication process. (A) Detailed fabrication procedure of the PDMS microfluidic temperature control device. (B) Photograph of the UV light source used to expose the patterns from the mask onto the wafer coated with photoresist (the future mold). (C) Photograph of the spin coater used to coat materials evenly onto surfaces. (D) Photograph of the plasma cleaner used to oxidize surfaces for subsequent bonding. (E) Photograph of a homemade coverslip holder.
Mask design for the temperature control channels is an array of 12 parallel 1 cm long × 200 μm wide × 100 μm thick channels, spaced 100 μm apart. Mask design for the cell channels varies depending on the desired biological sample and experiment.
The temperature control device fabrication procedure is schematically described in Fig. 2A. The two layers of channels are fabricated independently and are then covalently bonded using plasma treatment. The full fabrication process is described below.
B. Step 1: PDMS Preparation
This step produces a homogenized mixture of PDMS and curing agent without air bubbles.
Pour into a cup liquid PDMS and then add curing agent to a ratio 9:1.
Stir vigorously for about 3 min (with a plastic fork or spoon) to homogenize the mixture. The stirring will generate small air bubbles, turning the clear mixture white.
Put the cup in a vacuum chamber for 30 min to degas to remove the air bubbles. The mixture should be transparent at the end.
1. Remarks!
The ratio of PDMS to curing agent affects the stiffness of the final PDMS product. More curing agent leads to stiffer PDMS products. We find that 9:1 ratio is optimal for our work, but small changes (7:1 to 12:1) will not be critical for our application.
C. Step 2a: Fabrication of the Temperature Control Channels
This step describes how to make PDMS replica of the microchannel pattern from the mold.
Once the PDMS mixture is degassed and there are no bubbles, pour it gently onto the temperature control mold (which is glued to the bottom of a Petri dish) to a height of approximately 3 mm. Wait a few minutes to allow any new air bubbles to reach the surface, then gently blow them away. Once no air bubbles remain on the PDMS surface, put the dish into an oven at 65°C for 2–4 h to cure, and harden the PDMS mixture.
Once the PDMS is cured or hardened, cut using a surgical scalpel a region of interest around the microchannel pattern and lift it up from the mold surface. This piece should look like a block of clear and flexible material.
Drill inlet and outlet holes onto the PDMS block using a 20-gauge needle.
You can store the PDMS block in a closed Petri dish with the channel side up.
1. Remarks!
The recommended 2–4 h curing time is not critical for our application. However, too long a curing time could lead to PDMS aging and brittleness.
During the cut out step, one should take a minimum of 2–3 mm margin around the microchannels. This will yield a large surface for better bonding and water tightness for the subsequent device assembly.
Avoid touching the PDMS microchannels with your fingers, since the surface properties of the material are critical for plasma treatment and bonding.
During inlet and outlet drilling, when the needle goes through the PDMS block a little PDMS cylinder remains at the end of the needle and should be taken off before needle removal. Needles used for drilling should be smaller than the steel tube adaptor to allow a tight seal between the PDMS block and the steel tube adaptor used during the subsequent injection procedure. Moreover, to avoid PDMS cracking during drilling, the needle edge should be previously smoothed using sandpaper.
D. Step 2b: Fabrication of Cell Channel Layer
To fabricate a bilayer PDMS device, in which the top temperature control channels are separated from the bottom cell channels by just a few microns, the bottom layer has to be a very thin PDMS layer. This step describes how to fabricate a 15 μm thin PDMS membrane on the mold containing the cell microchannels.
Place the cell microchannel mold onto a spin coater (Fig. 2C), and then pour degassed PDMS mixture on top to cover about 30% of the surface.
Launch the spin coater at 500 rpm for 10 s (acceleration 100 rot2/min), followed by 6000 rpm for 30 s (acceleration 500 rot2/min). The spinning will spread the PDMS mixture onto the mold as an even layer approximately 15 μm thin.
Put the mold onto a hotplate at 95°C for 30 min to cure the PDMS mixture.
You can store the mold with the PDMS membrane on top in a closed Petri dish with the PDMS side up.
1. Remarks!
The relative thinness of the membrane is important since it plays a critical role in heat transfer between the temperature and the cell channels. The thinness of the membrane depends mainly of two parameters: the viscosity of the PDMS and the rotation speed during spin coating. The PDMS mixture should be freshly made and degassed (<1 h) before spin coating, because the PDMS mixture will slowly cure even at ambient room temperature and change its viscosity.
The PDMS surface containing the microchannels should not be touched when handling, since the surface properties of PDMS are crucial for proper plasma treatment and bonding.
E. Step 3: Plasma Treatment and Bonding of Both PDMS Layers (Temperature Control and Cell Microchannels)
To fabricate the PDMS bilayer it is necessary to covalently stick the top temperature control PDMS block (Step 2a) onto the bottom cell PDMS mold (Step 2b). For this purpose, the most common technique is plasma ionization treatment. We use a Plasma Cleaner (Fig. 2D), with a plasma flow module to control the pressure inside the plasma chamber.
Place both top PDMS block (microchannel side up) and bottom PDMS mold into the plasma chamber. Start the vacuum.
Once the air pressure inside the plasma chamber is stabilized between 500 mTorr and 1000 mTorr, turn the radio frequency RF power on high for 30 s. You should see a purple glow inside the chamber. This glow indicates that the surfaces of the PDMS are being ionized.
Release the vacuum and immediately take out the PDMS blocks (take care not to touch the surfaces). Then place the top temperature block (microchannel side down) directly on top of the bottom PDMS mold. The two PDMS surfaces should start to bond covalently.
Put the bilayer assembly on a hotplate at 95°C for 30 min. You now have a bilayer PDMS block on top of the cell microchannel mold.
Use a surgical scalpel to cut out bottom PDMS layer (which is now bonded to the top PDMS layer) and peel up the complete bilayer PDMS block.
Drill inlet and outlet holes onto the cell microchannels of the PDMS bilayer block using a 20-gauge needle.
You can store this bilayer PDMS device in a closed Petri dish with the microchannel side up.
1. Remarks!
To eliminate possible dust settling on the surface of PDMS block prior to plasma treatment and bonding, one can use an air gun to blow on the PDMS. An alternative to the air gun is to quickly stick on and then remove Scotch tape (3M) on the block surfaces just before plasma treatment.
Plasma treatment is critical for microfluidic fabrication. Mistake in the exposure time, the pressure, or the presence of impurities in the vacuum chamber can lead to inefficient plasma treatment. In good normal condition, the plasma should have a purple color. White or pink plasma indicates too high a pressure, and evanescent or clear plasma indicates too low a pressure.
The presence of refluxing oil from the vacuum pump into the plasma vacuum chamber also leads to inefficient plasma treatment and subsequent bonding. If the plasma treatment time is too long (>1 min) subsequent bonding between PDMS surfaces will be inefficient.
Contact between the two PDMS blocks has to be done within 1 min after plasma treatment. Longer waiting time will lead to less efficient bonding. Moreover, once the two blocks are in contact, do not try to reposition or readjust them.
The bottom cell channels should be positioned under the center of the top temperature control channels to reach optimal temperature uniformity.
After the bilayer PDMS block is cut from the mold, one can clean the mold of residual PDMS coating by using a tweezer or rolling on the surface with a gloved finger.
F. Step 4: Plasma Bonding of the Bilayer PDMS Assembly onto a Glass Coverslip
This step will bond the bilayer PDMS block onto the glass coverslip to create the final enclosed device.
Place both the glass coverslip and the bilayer PDMS block (microchannels facing up) into the plasma chamber. Start the vacuum.
Once the air pressure inside the plasma chamber is stabilized between 500 mTorr and 1000 mTorr, turn the radio frequency RF power on high for 30 s. You should see a purple glow inside the chamber. This glow indicates that the surfaces of the PDMS are being ionized.
Release the vacuum and immediately take out the glass coverslip and PDMS block (take care not to touch the surfaces). Then place the PDMS block (microchannel side down) directly on top of the glass coverslip. The two surfaces should start to bond covalently immediately.
Put the device on a hotplate at 95°C for 30 min. You now have a bilayer PDMS block on top of the glass coverslip.
1. Remarks!
If the plasma treatment works well the contact area between PDMS block and glass coverslip should spread and bond within seconds. If this is not the case, and some noncontact regions remained (white area), one can push the PDMS block gently down onto the glass coverslip with a pair of tweezers. Do not apply too much force or you risk collapsing the microchannels.
To facilitate device handling and microscope imaging, we use a homemade glass coverslip holder as shown in Fig. 2E.
IV. Setup Installation
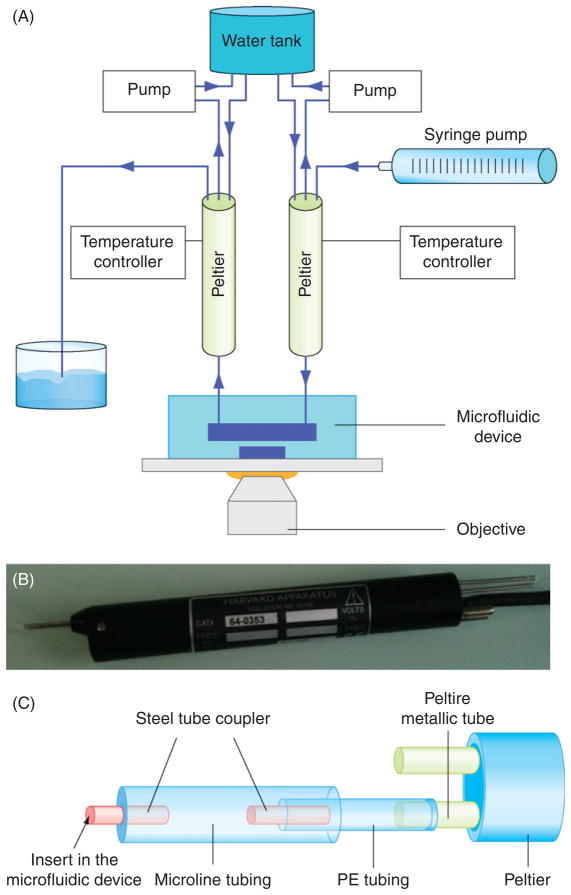
The experimental setup is composed of an inverted microscope (Nikon TE2000e, 100×/1.4NA oil immersion objective), two Peltier modules, a water tank, and a syringe pump. Beginning from this basic setup it is necessary to add two Peltier temperature controllers and two peristaltic pumps to control and maintain the Peltier temperature (Fig. 3A and B).
Fig. 3.
Peltier setup. (A) Schematic of the complete setup for the Peltier modules. (B) Photograph of the Peltier module used for fast temperature changes. (C) Schematic of the tubing assembly between the Peltier outlet and the PDMS microfluidic device.
A. Step 1: Peltier Module Microfluidic Connection
In this step we will prepare the Peltier modules to fit with the PDMS microfluidic device. Each Peltier has an inlet–outlet metal tube. Water is pumped through the inlet, is heated up or cooled down as it travels through the Peltier, and then exit through the outlet into the PDMS device. The Peltier itself requires cooling.
Connect the Peltier outlet to the PDMS device. To ensure good fitting, we connect the Peltier metallic outlet to a 2 cm (1.14 mm ID) polyethylene (PE) tubing and then connect this to a 4 cm “Microline” tubing (0.5 mm ID). This is terminated by a stainless steel tube adaptor that will fit directly into the inlet hole of the PDMS device. This 6 cm long assembly is long enough to easily handle and plug into the device, but short enough to limit heat dissipation prior to reaching the cells.
Repeat for the second Peltier. With the two configurations, the first Peltier is used for heating and the second for cooling.
Connect the Peltier inlet to the water source. The tubing assembly is the same as above; however, the length of tubing is not important here.
Repeat for the second Peltier. Note that the first Peltier will be connected to a syringe pump, while the second will be submerged into a water bath. The syringe pump acts to push water through the first Peltier for heating and to pull water through the second Peltier for cooling.
1. Remarks!
To avoid accidental lifting of tubings (and therefore water leakage), one should tape the tubings at different strategic points on or around the microscope stage.
At low temperature settings, condensation can appear on the Peltier module. To avoid water leakage onto the microscope, one can tie a sponge or paper towel around the Peltier module.
To position the Peltier modules directly near the PDMS microfluidic device inlet, we used a common stand.
This microfluidic temperature control system can be used with all types of inverted microscope.
B. Step 2: Connection of Peristaltic Pump to Peltier Module
The Peltier modules need internal cooling to function properly. We directly cool the two Peltier modules using a closed-loop 2 l water reservoir bottle connected to Tygon R-1000 silicon tubing driven by peristaltic pumps.
1. Remarks!
When using Peltier as low as 1°C, the Peltier module may not be able to maintain this temperature. To solve this problem one can increase the peristaltic pump flow rate or decrease the reservoir water temperature by putting the water bottle on ice.
For low temperature experiments lasting several hours, the water reservoir should be at least 2 l, immersed in ice, and with a peristaltic pump rate of 6 l/h.
Other solutions for cooling the Peltier module are to use the thermal cooling module TCM1 from Warner Instrument or use a chiller.
Because of the high water flow rate involved for Peltier cooling, one should take care that tubings are connected tightly and securely to avoid leakage. Moreover, one should take care to examine frequently the ageing of the silicon tubing in contact with the rotary part of the peristaltic pump.
For more security against leakage, peristaltic pumps should be placed inside plastic bowls.
V. Biological Experiments
A. Step1: Device Preparation and Cell Injection
Use a small 2–3 ml syringe and a 24-gauge needle connected to a “Microline” tube with a stainless steel tubing adaptor for cell handling.
Fill the syringe with cells. One should avoid the presence of air bubbles in the syringe or tubing.
Plug the steel tubing end into the PDMS device cell inlet and inject gently the cells into the cell channels. Be sure that no air bubbles remain in the device at the end of the operation.
1. Remarks!
If the cell channels are not designed to allow media renewal and if the experiment will run for more than 2–3 h, then the cells may begin to miss fresh nutriment and the media in the channels may begin to dry up due to evaporation through PDMS. To limit this, one should plug a 1 cm long “Microline” tube at the outlet and inject media from the inlet to fill the outlet tubing without bubble. Once the outlet tube is full of media then inject cells from the inlet and cut the inlet tube at the same length as the outlet one. This extra tubings and media will help renew the cell media and enable experiments up to 9 h depending on the cell concentration.
While injecting cells, one could put the PDMS device onto a black background piece of paper to facilitate visualization of air bubbles. In contrast to water (which has little refractive index difference compared to PDMS), air has a high refractive index difference compared to PDMS. Thus, air bubbles appear white inside the PDMS device against the black background. Water would be transparent, like PDMS.
B. Step 2: Installing the PDMS Device on the Peltier Setup
Install a large 200-ml syringe filled with water onto the syringe pump.
Connect both Peltier modules to the PDMS device, the syringe pump, and the peristaltic pump cooling system (refer to Section IV Setup Installation).
Start the syringe pump until all tubings connected to the temperature channels are filled with water. Be sure that the entire tubing capillary is filled, which is essential for fast temperature change. During this step, the Peltier modules should be at ambient temperature to avoid temperature changes in the device during capillary filling. Once the capillary is filled, the syringe pump can be stopped.
Start the peristaltic pumps at 100 ml/min rate.
Set the Peltier modules at the desired temperatures.
1. Remarks!
At this stage, if no leakage occurs, you are ready to put the PDMS device onto the microscope to search for cells.
C. Step 3: Performing Temperature Changes
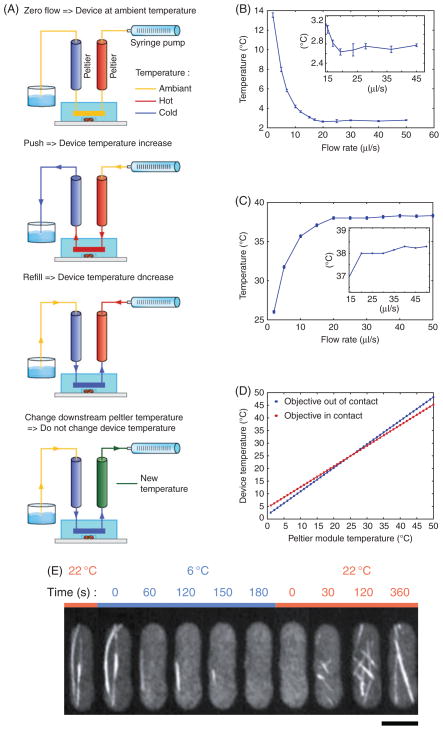
To change the temperature in the capillary, it is necessary to push or pull water through the Peltier modules and the PDMS device by the syringe pump (Fig. 4A). The upstream Peltier (connected to the syringe pump) should be hot and the downstream Peltier should be cold. They are set within the range of temperature changes desired.
Fig. 4.
Device characterization. (A) Schematic detailing the temperature control procedure. When the syringe pump is OFF, the device remains at ambient temperature, independent of Peltier temperature. When the syringe pump is pushing or pulling (refilling), the temperature change is dependent on the Peltier setting upstream of the water flow. Changing Peltier settings downstream of the water flow does not affect the device temperature. (B) Plot of device temperature versus syringe pump flow rate—cold. Peltier set at 1°C. The objective is not in contact with the coverslip. (C) Plot of device temperature versus syringe pump flow rate— hot. Peltier set at 39°C. The objective is not in contact with the coverslip. (D) Calculation of device temperature as a function of Peltier module temperature with 30 μl/s flow rate with ambient temperature of 25°C. Device temperature is given by the following equation: Tdevice = Tpeltier + A(Tambient − Tpeltier), A, 0.071 when the objective is in contact with the coverslip; A, 0.187 when objective is not in contact with the coverslip. (E) A time-lapse montage of microtubule dynamics in fission yeast responding to temperature changes. The cell is expressing GFP-atb2p (tubulin). Imaging is done with a 1003/1.4NA oil immersion objective. At 6°C the microtubules depolymerized to completion. At ambient room temperature of 23°C the microtubules repolymerized. Bar, 5 μm.
For heating, the syringe pump should push at flow rate of 2.5 ml/min to thermalize the PDMS microfluidic device with water passing through the upstream Peltier. There is a fluidic delay of 10–15 s between the action of the syringe pump and the beginning of the temperature change experienced by the cells. This delay should be taken into consideration for precisely timed experiments. Once the temperature begins to change, 10 more seconds is necessary to reach the desired temperature value (with a precision <1°C).
For cooling, the syringe pump should pull (reverse direction) at flow rate of 2.5 ml/ min to thermalize the PDMS microfluidic device with water passing through the downstream Peltier.
1. Remarks!
To prepare further temperature change, one can change the temperature of the downstream Peltier module without influencing the microfluidic device temperature. Peltier modules used here generally require 1–3 min to reach the desired temperature. Reaching Peltier temperature close to 0°C could take longer than 3 min depending on the peristaltic pump flow rate and temperature.
Water flow rate from the syringe pump is a critical parameter for temperature control. Figure 4B and C shows the dependence of temperature as a function of water flow rate with the Peltier modules set at 1°C and 39°C.
The temperature measured at the cells will be different from the Peltier module temperature setting. This is due to heat dissipation throughout the tubings and the PDMS device. For example, with the Peltier modules set at 1°C and ambient 24°C, the temperature experienced by the cells will be 2.7°C. Figure 4D gives the correlation between Peltier and device temperature.
The presence of the oil immersion objective acts as a strong heat sink and further increases the PDMS microfluidic device temperature. For example, with the cool Peltier module set at 1°C, the oil immersion objective will shift the cell temperature to 4.5°C. One can limit this problem by moving the position of the objective away from the cells being imaged during the interval between time points.
When using oil immersion objective lenses, temperature changes will generate materials dilation/contraction leading to focus drifting. The resulting drift is about 0.5 μm/°C. Although temperature changes inside the PDMS microfluidic device is very fast, a transient temperature gradient remains at the objective lens for 2–3 min before reaching steady state, leading to focus drift during this period. Nevertheless, because temperature changes obtained with this setup is reproducible, the extend of focus drift is thus predictable and therefore can be corrected during image acquisition.
During syringe pulling, bubbles may appear in the tubing and the syringe, leading to a higher fluidic time constant. Moreover, the presence of air in the tubing may lead to the microchannels filling up with air bubbles, leading to temperature nonuniformity.
Figure 4E shows an example of a cooling experiment. A fission yeast S. pombe cell expressing GFP-atb2p (tubulin) is cooled down to depolymerize the microtubules and then heated up to repolymerize the microtubules. The cooling Peltier was set at 1°C, and the effective temperature experienced by cells is 6°C. There is minor heat dissipation along the tubings, the PDMS device, and the oil contact between the glass coverslip and the microscopy objective.
VI. Conclusion
We described here a protocol which enables fabrication and use of a fast micro-fluidic temperature control device and setup. This kind of system allows fine control of microtubule polymerization dynamics. This system can also be used with temperature-sensitive mutant strains. The ability to couple this temperature control device with other microfluidic functionalities such as cell deformation and chemical perfusion will open new opportunities for cell biological experiments.
VII. Materials
A. Mold Fabrication
Spincoater, Laurell, CZ-650 series. (www.laurell.com)
Hotplate, Barnstead, model HP131720-33
UV lamp, OAI, model 30 with OAI intensity controller model 2105C2 (www.oainet.com)
Photoresist, Microchem, SU8 2005 (www.microchem.com)
Photoresist, Microchem, SU8 2050
Photoresist, Microchem, SU8 developer
Photoresist, Microchem, omnicoat
Isopropanol
B. Device Fabrication
Hotplate, Barnstead int, model HP131720-33
Oven, MEMMERT, 14L UNB100
Plasma cleaner, Harrick plasma, “Extended plasma” cleaner with “plasmaflow” pressure controller (www.harrickplasma.com)
Inlet and outlet drilling: 20-gauge needle smoothed with sandpaper
PDMS, Sylgard, 184
Coverglass, Dow Corning, 24 × 40 mm ref 2940-244
C. Temperature Control Setup
Peltier, Warner SC-20 Dual In-line Solution Heater/Cooler (www.warneronline.com)
Peltier controller, CL-100 Bipolar Temperature Controller (www.harvardapparatus.ciom)
Syringe Pump, Harvard Apparatus Remote Infuse/Withdraw PHD 4400 Hpsi Programmable
Peristaltic pump, Harvard Apparatus, Peristaltic Pump 66
Peristaltic pump tubing: Tygon, R-1000 1/8 in. ID * 1/4 in. OD
Syringe pump/Peltier tubing, Harvard Apparatus, “Microline” tubing 0.5 mm ID * 1.5 mm OD
Peltier metal tube/“Microline” tubing interface, Warner, Polyethylene tubing 1.57OD * 1.14ID (furnished with Peltier module)
Microfluidic device/“Microline” tubing interface, Harvard Apparatus, Stainless steel tubing coupler, 23-Gauge, 8 mm
Syringe for water injection, Monoject, 140cc Syringe with Luer Lock Tip
D. Cell Injection
2 ml syringe
24-gauge needle
“Microline” tubing 0.5 mm ID * 1.5 mm OD, Harvard Apparatus
Microfluidic device/“Microline” tubing interface, Harvard Apparatus, Stainless steel tubing coupler, 23-gauge, 8 mm
Acknowledgments
G.V-C. is supported by a postdoctoral fellowship from ARC; J.C. is supported by a predoctoral fellowship from FCT and ED Complexite du Vivant. This work is supported by grants from NIH, ACS, HFSP, FRM, ANR, LaLigue, and MarieParis.
References
- Belanger MC, Marois Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: a review. J Biomed Mater Res. 2001;58:467–477. doi: 10.1002/jbm.1043. [DOI] [PubMed] [Google Scholar]
- Charati SG, Stern SA. Diffusion of Gases in Silicone Polymers: Molecular Dynamics Simulations. Macromolecules. 1998;31:5529–5535. [Google Scholar]
- Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Rapid prototyping microfluidics systems in poly(dimetylsiloxane) Anal Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- McDonald JC, Whitesides GM. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc Chem Res. 2002;35:491–499. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 2000;288:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- Velve-Casquillas G, Le Berre M, Piel M, Tran PT. Microfluidic tools for cell biological research. Nano Today. 2010;5:28–47. doi: 10.1016/j.nantod.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]