Abstract
Methods for culturing mammalian cells ex vivo are increasingly needed to study cell and tissue physiology and to grow replacement tissue for regenerative medicine. Two-dimensional culture has been the paradigm for typical in vitro cell culture; however, it has been demonstrated that cells behave more natively when cultured in three-dimensional environments. Permissive, synthetic hydrogels and promoting, natural hydrogels have become popular as three-dimensional cell culture platforms; yet, both of these systems possess limitations. In this perspective, we discuss the use of both synthetic and natural hydrogels as scaffolds for three-dimensional cell culture as well as synthetic hydrogels that incorporate sophisticated biochemical and mechanical cues as mimics of the native extracellular matrix. Ultimately, advances in synthetic–biologic hydrogel hybrids are needed to provide robust platforms for investigating cell physiology and fabricating tissue outside of the organism.
Keywords: hydrogels, tissue engineering, 3D cell culture, biomaterials
Introduction
The culture of mammalian cells in vitro provides a defined platform for investigating cell and tissue physiology and pathophysiology outside of the organism. Traditionally, this has been done by culturing single cell populations on two-dimensional (2D) substrates such as tissue culture polystyrene (TCPS) or the surface of tissue analogs. Experiments with these 2D cell constructs have provided the base for our nascent interpretation of complex biological phenomena, including molecular biology, stem cell differentiation (Jaiswal et al., 1997), and tissue morphogenesis (Schnaper et al., 1993). Furthermore, 2D experiments have given rise to seminal findings in the dynamic relationship between cell function and interactions with the cellular microenvironment. Discher and coworkers demonstrated that the differentiation of human mesenchymal stem cells (hMSCs) is dependent on the mechanical stiffness of the 2D culture platform (Engler et al., 2007, 2006). Further, Ingber and coworkers have shown that the degree to which a cell is mechanically distended on a 2D scaffold dictates relative growth and apoptotic rates (Chen et al., 1997; Singhvi et al., 1994). Thus, in vitro cell constructs can be used to examine how epigenetic factors affect physiological phenomena; however, recent work has shown that cells often exhibit unnatural behavior when they are excised from native three-dimensional (3D) tissues and confined to a monolayer.
In their groundbreaking work, Bissell and coworkers demonstrated that human breast epithelial cells develop like tumor cells when cultured in two dimensions, but revert to normal growth behavior when cultured in 3D analogs of their native microenvironment (Petersen et al., 1992). Also, enhanced chondrogenesis of embryonic stem cells has been observed when cells are cultured in 3D embryoid bodies as compared to monolayer culture (Tanaka et al., 2004). These findings in oncogenesis and stem cell differentiation elucidate acute disparities in cell function between 2D and 3D culture and suggest that examining hierarchical biology in just two dimensions is insufficient.
As a result, biologists and bioengineers alike have investigated many three-dimensional scaffolds that recapitulate aspects of the native cellular microenvironment for in vitro cell culture. Among these, hydrogels—crosslinked networks that possess high water contents—demonstrate a distinct efficacy as matrices for 3D cell culture. Currently, the gamut of hydrogels used for mammalian cell culture ranges from purely natural to purely synthetic materials with each hydrogel system possessing its own advantages and limitations. As the field moves forward, the need for matrices that combine the benefits of natural and synthetic hydrogels is becoming more apparent, along with identifying the essential biophysical and biochemical signals to incorporate in these synthetic extracellular matrix (ECM) analogs. In this perspective, the necessity of 3D cell constructs is justified, the present state of hydrogels used as scaffolds in cell culture is addressed, and a look towards emerging synthetic–biologic hydrogels is presented.
The Context Matters
The paradigm that cellular scaffolds serve solely as passive vehicles with which to study the relationship between gene expression and cell function has become outdated. It is now evident that the cellular microenvironment contributes to the spatially and temporally complex signaling domain that directs cell phenotype. In fact, Bissell’s work establishes that phenotype can supersede genotype simply due to interactions with the ECM. Thus, a cell can no longer be thought of as a solitary entity defined by its genome, but must be evaluated in the context of the ECM, soluble growth factors, hormones, and other small molecules that regulate organ, and ultimately organism, formation and function. This dynamic, extracellular environment orchestrates an intracellular signaling cascade that influences phenotypic fate by altering gene and ultimately protein expression (Birgersdotter et al., 2005). The differences in cell behavior observed between 2D and 3D cultures come from perturbations in gene expression that stem from how the cell experiences its microenvironment differently in two dimensions as compared to the natural 3D surroundings (Fig. 1).
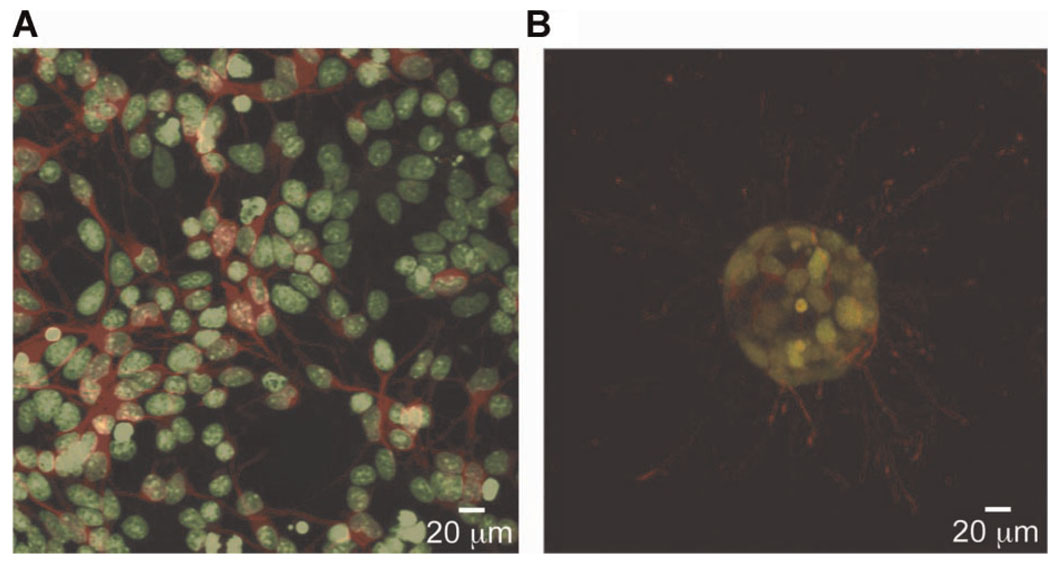
Figure 1.
Cells experience a drastically different environment between 2D and 3D culture. For instance, neural cells cultured in monolayer (A) are constrained to extend processes in the plane. Cell bodies are stained green and β-tubulin in axonal extensions is stained red. When cultured within hydrolytically degradable poly(ethylene glycol) based hydrogels (B) the same cells form neurospheres and extend processes isotropically in three dimensions. Images taken by M.J. Mahoney.
For instance, 2D culture polarizes cells such that only a segment of the cell’s membrane can interact with the ECM and neighboring cells, while the rest of the cell is exposed to the bulk culture media (Zhang et al., 2005). This leads to unnatural, polarized integrin binding and mechanotransduction, which both affect intracellular signaling and phenotypic fate (Gieni and Hendzel, 2008). The inherent polarity also leads to unnatural interactions with soluble factors. In 2D culture, cells experience the homogenous concentration of nutrients, growth factors, and cytokines present in the bulk media with the section of the membrane that contacts the surrounding media. In contrast, the concentrations of soluble factors that influence cell migration, cell–cell communication, and differentiation possess dynamic spatial gradients in vivo (Ashe and Briscoe, 2006).
Morphology alone has been shown to influence subtle cellular processes such as global histone acetylation (Le Beyec et al., 2007) as well as proliferation, apoptosis (Chen et al., 1997), differentiation, and gene expression (Birgersdotter et al., 2005). 2D culture confines cells to a planar environment and restricts the more complex morphologies observed in vivo.
Furthermore, differences in migration exist between a 2D surface and a 3D environment. Not only is a cell confined to a plane in 2D, but also encounters little to no resistance to migration from a surrounding ECM. This applies to other phenomena that occur over longer length scales, such as cancer metastasis and tissue organization, where the behavior is regulated by mechanical interactions with the surrounding cellular microenvironment. Thus, to properly study cell physiology, mechanotransduction, and tissue morphogenesis in vitro, cells should be cultured in 3D model microenvironments that recapitulate critical mechanical and biochemical cues present in the native ECM while facilitating hierarchical processes such as migration and tissue organization.
3D Culture Platforms
Over the past few decades, tissue engineers and cell biologists have begun to develop material systems to culture mammalian cells within 3D ECM mimics to circumvent the limitations posed by traditional 2D cell culture. To this end, cells have been encapsulated within microporous (Levenberg et al., 2003; Shea et al., 1999; Yim and Leong, 2005), nanofibrous (Semino et al., 2003; Silva et al., 2004), and hydrogel scaffolds (Peppas et al., 2006). Microporous scaffolds allow for convenient encapsulation of cells but they contain porosities (~100 µm) greater than the average cell diameter (~10 µm); thus, they effectively serve as 2D scaffolds with curvature. Nanofibrous scaffolds provide a 3D topology that better mimics the architecture formed by fibrillar ECM proteins; however, many are too weak to handle the stress needed for proper mechanotransduction. These limitations are not found in hydrogels, which capture numerous characteristics of the architecture and mechanics of the native cellular microenvironment (Saha et al., 2007).
Due to their ability to simulate the nature of most soft tissues, hydrogels are a highly attractive material for developing synthetic ECM analogs. These reticulated structures of crosslinked polymer chains possess high water contents, facile transport of oxygen, nutrients and waste, as well as realistic transport of soluble factors (Nguyen and West, 2002). Furthermore, many hydrogels can be formed under mild, cytocompatible conditions and are easily modified to possess cell adhesion ligands, desired viscoelasticity, and degradability (Lutolf and Hubbell, 2005).
Hydrogels used for cell culture can be formed from a vast array of natural and synthetic materials, offering a broad spectrum of mechanical and chemical properties. For an excellent review of the materials and methods used for hydrogel synthesis see Lee and Mooney (2001). At the simplest deconstruction, hydrogels are promoting of cell function when formed from natural materials and permissive to cell function when formed from synthetic materials (Fig. 2).
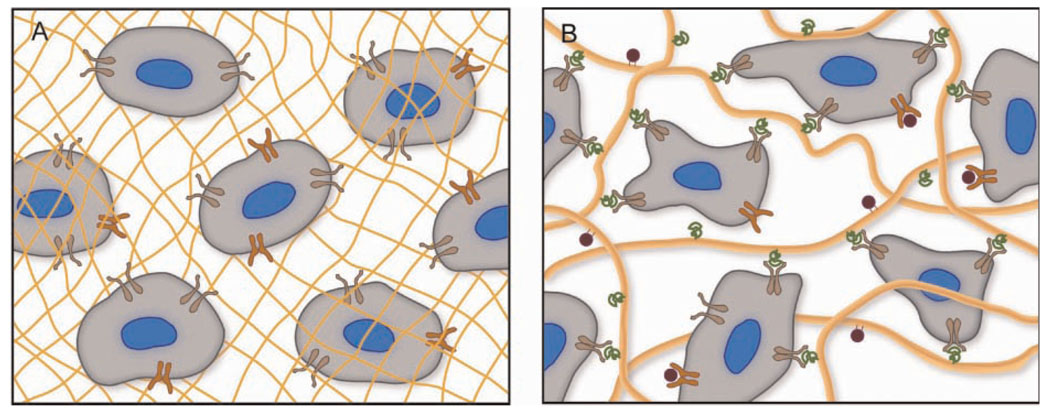
Figure 2.
Permissive hydrogels (A) composed of synthetic polymers (yellow mesh) provide a 3D environment for culturing cells; however, they fail to activate integrins (brown) and other surface receptors (orange). The synthetic environment simply permits viability as cells remodel their surrounding microenvironment. On the other hand, promoting hydrogels (B) formed from naturally derived polymers present a myriad of integrin-binding sites (green) and growth factors (red) coordinated to the ECM (yellow fibers), which direct cell behavior through signaling cascades that are initiated by binding events with cell surface receptors.
Permissive and Promoting Hydrogels
Natural gels for cell culture are typically formed of proteins and ECM components such as collagen (Butcher and Nerem, 2004), fibrin (Eyrich et al., 2007), hyaluronic acid (Masters et al., 2004), or Matrigel, as well as materials derived from other biological sources such as chitosan (Azab et al., 2006), alginate (Barralet et al., 2005), or silk fibrils. Since they are derived from natural sources, these gels are inherently biocompatible and bioactive (Dawson et al., 2008). They also promote many cellular functions due to the myriad of endogenous factors present, which can be advantageous for the viability, proliferation, and development of many cell types. However, such scaffolds are complex and often ill-defined, making it difficult to determine exactly which signals are promoting cellular function (Cushing and Anseth, 2007). Furthermore, tuning their material properties such as mechanics and biochemical presentation can be difficult, there is risk of contamination, they can be degraded or contracted too quickly, and possess an inherent batch-to-batch variability that confounds the effect of the scaffold on cell proliferation, differentiation, and migration.
On the other hand, hydrogels can be formed of purely non-natural molecules such as poly(ethylene glycol) (PEG; Sawhney et al., 1993), poly(vinyl alcohol) (Martens and Anseth, 2000), and poly(2-hydroxy ethyl methacrylate) (Chirila et al., 1993). PEG hydrogels have been shown to maintain the viability of encapsulated cells and allow for ECM deposition as they degrade (Bryant and Anseth, 2002), demonstrating that synthetic gels can function as 3D cell culture platforms even without integrin-binding ligands. Such inert gels are highly reproducible, allow for facile tuning of mechanical properties, and are simply processed and manufactured. However, they lack the endogenous factors that promote cell behavior and act mainly as a template to permit cell function (Cushing and Anseth, 2007).
These synthetic scaffolds offer a minimalist approach to the culture of mammalian cells outside of the body and have been used for clinical applications as well as for fundamental studies of cell physiology (Shu et al., 2006). However, in order to properly mimic the native ECM, some of its complexity must be integrated into these permissive hydrogels.
Native Extracellular Matrix
In vivo, cells grow within a complex and bioactive hydrogel scaffold that provides mechanical support while directing cell adhesion, proliferation, differentiation, morphology, and gene expression—the ECM. Functional scaffolds for three-dimensional cell culture that permit cell growth while promoting function and tissue organization, should emulate this prototypical hydrogel (West, 2005) on multiple length scales (Fig. 3).
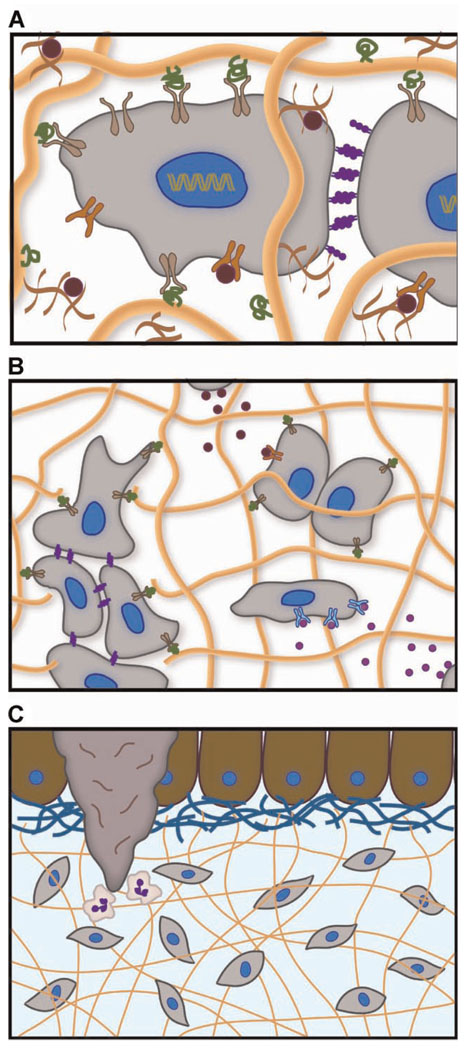
Figure 3.
The native ECM is the prototypical hydrogel that regulates cell function on many length scales. A: Integrin-binding with ECM proteins (green ligands and tan receptors), growth factor sequestration within proteoglycans (red), and cell–cell contact via cadherins (purple) occur on the scale of tens of nanometers to microns. B: Migration, which is critical in tissue regeneration, cancer metastasis, and wound healing, initiates on the scale of tens to hundreds of microns. Paracrine signaling that directs differentiation (pink growth factors) and proliferation (red growth factors) is also mediated on this length scale. C: Tissue homeostasis, development, and wound healing are regulated over hundreds of microns to centimeters. Here, we illustrate neutrophils being recruited to the site of a wound in the epithelium.
The ECM’s backbone—a complex architecture of structural, fibrous proteins such as fibronectin, collagen, and laminin—provides the matrix’s mechanical properties. Cells sense these mechanics through binding events between integrins on the cell surface and binding motifs of the ECM proteins. Hydrated proteoglycans fill the interstitial voids of this backbone, sequestering soluble biomolecules: growth factors, small integrin-binding glycoproteins (SIBLINGS), and matricellular proteins. Cells dynamically restructure the microenvironment to release signaling molecules, allow migration, or accommodate cell function via ECM-cleaving proteins, such as metalloproteinases (MMPs), and the deposition of ECM components, both of which are regulated by integrin-mediated signaling pathways (Daley et al., 2008). This remodeling is necessary for proper tissue homeostasis and becomes more pronounced in pathological and developing states. Although ECM composition varies significantly from tissue to tissue within the organism, understanding its general composition and how remodeling functions in development and wound healing points to important design criteria for 3D cell culture platforms. For a complete review of ECM remodeling see Daley et al. (2008).
Design Criteria
Developing bioactive hydrogels for 3D cell culture is an archetypal engineering problem, requiring control of physical and chemical properties on length scales from microns to centimeters and time scales from seconds to weeks. In order to develop a functional ECM mimic, the gel’s mechanical properties, adhesive ligand and growth factor presentation, transport and degradation kinetics must be tuned to the given culture’s needs a priori in a cytocompatible, reliable, and cost effective fashion (Griffith and Naughton, 2002; Saha et al., 2007). Furthermore, these gels’ chemistries and their degradation products cannot have a deleterious effect on encapsulated cells.
Traditionally, a scaffold’s mechanical and chemical properties are set during encapsulation with little user- or cell-defined control post-fabrication. In order to truly mimic the ECM, it is necessary to develop materials whose mechanical and chemical properties can be tuned on the time and length scales of cell development, exogenously by the user, or endogenously by the cells. Although scaffolds are designed to mimic the native ECM, the prime culture conditions are not precisely known a priori so the expression of one or more facets of the gel can be altered to approach the proper conditions for a desired cellular response. Ultimately, the goal is to rationally engineer biomaterial scaffolds that meld the benefits of synthetic and natural gels to satisfy the required needs of a given culture system in a robust scaffold.
Bridging the Gap
Recent work in scaffold engineering demonstrates that 3D synthetic microenvironments can be designed to promote cell viability and direct cell adhesion (Lee et al., 2008), differentiation (Salinas and Anseth, 2008b), proliferation (Mann and West, 2002), and migration (West and Hubbell, 1999) through the controlled presentation of mechanical and biochemical cues. Such instructive materials are bridging the gap between promoting and permissive gels by incorporating biomimetic signals into synthetic materials that elicit desired cell–gel interactions. These scaffolds can be tailored to the specific cell culture requirements and design criteria and are providing novel and well-defined ECM mimics for controlled hypothesis testing in cell biology and regenerative medicine (Cushing and Anseth, 2007).
In vivo, the ECM provides a milieu of binding ligands for cell adhesion that connect cells’ cytoskeletons to the cellular microenvironment (Wang et al., 1993). These ligands encourage integrin-binding events that communicate the mechanics of the ECM to the cell and direct cell fate through intracellular signaling pathways (Giancotti and Ruoslahti, 1999). Thus, integrin-binding events play a critical role not only in cell adhesion, but also in most cellular processes (Howe et al., 1998). In the simplest case, these binding ligands are recapitulated in synthetic hydrogels by physically entrapping ECM proteins, such as collagen, laminin, or fibronectin, into the network. These large proteins provide binding domains for integrin adhesion and have been shown to improve cell viability and function (Weber et al., 2008). However, entrapped proteins can denature, aggregate, introduce multiple binding motifs, and are often heterogeneously distributed throughout the gel, all of which confound their effects.
Protein engineering has evolved such that we can identify active peptide sequences from desired proteins and incorporate them into synthetic hydrogels (Ruoslahti, 1996). This allows the controlled placement of specific binding domains onto an otherwise bioinert background (Lutolf and Hubbell, 2005) to study the interactions between adhesive peptide sequences, such as RGD and IKVAV, and cell function. In PEG scaffolds the incorporation of pendant RGD—the known binding domain of fibronectin—has been shown to increase viability and adhesion of encapsulated cells (Nuttelman et al., 2005; Park et al., 2005; Patel et al., 2005). Further studies with RGD tethered to synthetic gels have indicated ideal clustering and ligand density for cell spreading (Massia and Hubbell, 1991) and migration (Gobin and West, 2002). Novel polymerization mechanisms, such as photoinitiated thiol-acrylate and thiol-ene chemistries (Khire et al., 2006; Rydholm et al., 2008; Salinas and Anseth, 2008a), are allowing facile incorporation of peptides within routinely used synthetic gels.
Natively, the presentation of such binding domains is regulated spatially and temporally. For example, during chondrogenesis fibronectin is downregulated within 7–12 days of hMSCs differentiating to chondrocytes by upregulating the production of matrix metalloproteinase 13 (MMP-13), which cleaves fibronectin (Sekiya et al., 2002). To mimic this temporal control, an RGD peptide sequence that was susceptible to MMP-13 cleavage was built into a PEG gel so that hMSCs could remove RGD, the fibronectin analog, as they would naturally. This successfully upregulated chondrogenesis in the synthetic environment as the removal of fibronectin does in vivo (Salinas and Anseth, 2008b). This is not a singular example of temporal presentation and it is becoming increasingly important to design sophisticated gel niches that afford temporal regulation of such instructive cues.
Similar concepts can be extended to other functional peptide sequences. In the native ECM, the delivery of chemokines, such as growth factors, to specific locations at specific times is mediated by controlled storage and release (Ramirez and Rifkin, 2003). Like ECM proteins, growth factors can simply be entrapped within hydrogel scaffolds and released upon network degradation such that the release is dependent on diffusion and degradation rates (Chen and Mooney, 2003). To replicate the natural harboring of growth factors within the proteoglycans of the ECM, hydrogels have been synthesized that incorporate heparin to associate proteins with the network that can subsequently be released (Yamaguchi and Kiick, 2005). Improvements to this system have been made by covalently linking protein specific ligands to the gel’s backbone (Willerth et al., 2007). This work points to the need for gels that contain binding ligands that selectively associate desired growth factors and release the factors based on cellular uptake. Furthermore, controlling the local concentration of tethered peptide ligands creates spatial gradients in chemokine availability. In vivo, multiple soluble factors act synergistically or antagonistically to develop more sophisticated signaling regimes that direct tissue development and homeostasis (Ashe and Briscoe, 2006). To recapitulate these signaling domains, multiple functionalities need to be incorporated within the gel that sequester and present several, orthogonal cues at desired time points.
Cell demanded release of growth factors has been achieved by encapsulating them within gels that have MMP cleavable sequences in the network backbone (Zisch et al., 2003). Specifically, Michael addition has been used to construct a network of end-functionalized PEG and thiol-labeled MMP cleavable peptide sequences. Incorporating vascular endothelial growth factor (VEGF) into this network induced vascularization as cells exposed VEGF by cleaving the MMP susceptible peptide sequences (Zisch et al., 2003). This cell-mediated release is a better mimic of native growth factor sequestration and can be coupled with peptide binding systems for a more dynamic system.
As biochemical techniques progress and new small molecule targets, such as micro RNAs (mRNA), small interfering RNAs (siRNA), and RNA aptamers, are better understood, the approaches that are used to deliver large soluble factors will need to be extended to present small molecules that can assist in the precise regulation of gene expression within a 3D environment.
While these approaches mimic biochemical aspects of the ECM, synthetic hydrogels often fail to capture the biophysical structure of the cellular microenvironment (e.g., the fibrillar structure of collagen and the potential for cellular remodeling of the ECM). To recreate the native restructuring of the cellular microenvironment, it is often necessary to engineer degradation into synthetic ECM analogs so that viable cells can deposit their own ECM (Bryant and Anseth, 2002), migrate (Raeber et al., 2007), and undergo morphogenesis (Mahoney and Anseth, 2006). Synthetic hydrogels have been designed to hydrolytically degrade by incorporating poly(lactic acid) (Metters et al., 2000) or poly(caprolactone) (Nuttelman et al., 2006) units into the network backbone. In these scaffolds the initial number of hydrolytic bonds present dictates the rate of degradation, but in general, the rate is on a slower time scale than normal cellular processes. Michael addition and photoinitiated reactions of end-functionalized PEG and thiol-labeled MMP cleavable peptide sequences can also be used to create synthetic hydrogels whose degradation is cellularly driven (Lutolf et al., 2003) and on a much shorter time scale. Increased production of MMPs allows cells to remodel this synthetic environment, migrate, and deposit their own ECM much like they do in vivo. Such systems that possess dynamic feedback between the microenvironment’s structure and cell behavior will be extremely useful to study migration, tumor morphogenesis, and cancer metastasis.
Even with degradable synthetic hydrogels, the networks’ subcellular porosities can pose a barrier for cell migration, proliferation, and differentiation as well as for the proper distribution of soluble factors. These networks often do not possess the fibrillar network structure of the ECM protein backbone. To address this, scaffolds need to be devised that couple robust self-assembly (Hartgerink et al., 2001) or nanofabrication techniques with degradable synthetic hydrogels to better recreate critical aspects of the biomechanical structure of the native ECM.
Looking Forward
While advances in polymer chemistry are driving the evolution of sophisticated synthetic–biologic gels, 3D culture of mammalian cells in such microenvironments is not without challenges. First, oxygen availability requires special notice, since cells are less than 100 µm from a high-oxygen source in metabolically active tissue (Palsson and Bhatia, 2004). Second, moving to the third dimension exaggerates the heterogeneities present in the synthetic cellular microenvironment, compared to 2D surfaces. In 3D scaffolds, gradients and defects can occur in material properties, proteins can become diffusion limited leading to heterogeneous distributions, and oxygen and nutrient gradients arise as the culture medium diffuses through the gel. Third, regulating the distribution of soluble growth factors, which influence cellular differentiation and tissue homeostasis, becomes more complicated within 3D networks as the distribution depends on the bulk concentration in the media, diffusion within the gel, and cellular uptake. Finally, the standard techniques for imaging and analyzing cell function and protein distribution are more involved in the 3D environment. When working in a 3D network, cells have limited accessibility for immunostaining or DNA/RNA extraction and secreted proteins can be difficult to extract from the gels. Cell imaging is often complex as light scattering, refraction, and attenuation occur in a 3D composite, cell-laden gel.
These challenges point to the need for sophisticated techniques, as well as sophisticated hydrogel environments, to combine the ability for real-time biological analyses with real-time manipulation of the material environment. The native ECM is far from static; therefore, to facilitate complex cellular behavior ECM mimics must also be dynamic. To use these systems for hypothesis testing, it is important to possess user-defined control over the spatio-temporal presentation of integrin-binding ligands, growth factor release, and biomechanical properties (Fig. 4). This field has provided the basis for dynamic scaffold fabrication and emerging work is offering user-defined control. As an example, “click” chemistries are being exploited to encapsulate cells and attach adhesive ligands to the network post-fabrication while photolabile chemistries are being used to spatially and temporally regulate the gel’s mechanical and biochemical properties (Kloxin et al., 2009). Coupling these and other cytocompatible, orthogonal chemistries will provide a template for testing cell–ECM interactions and mechanotransduction in a defined, three-dimensional synthetic environment ex vivo. Finally, approaches to release cells, proteins, and other biological molecules from their 3D material environments need to be built into these platforms so that sophisticated biological assays can provide better insight into the role of matrix interactions on cell function.
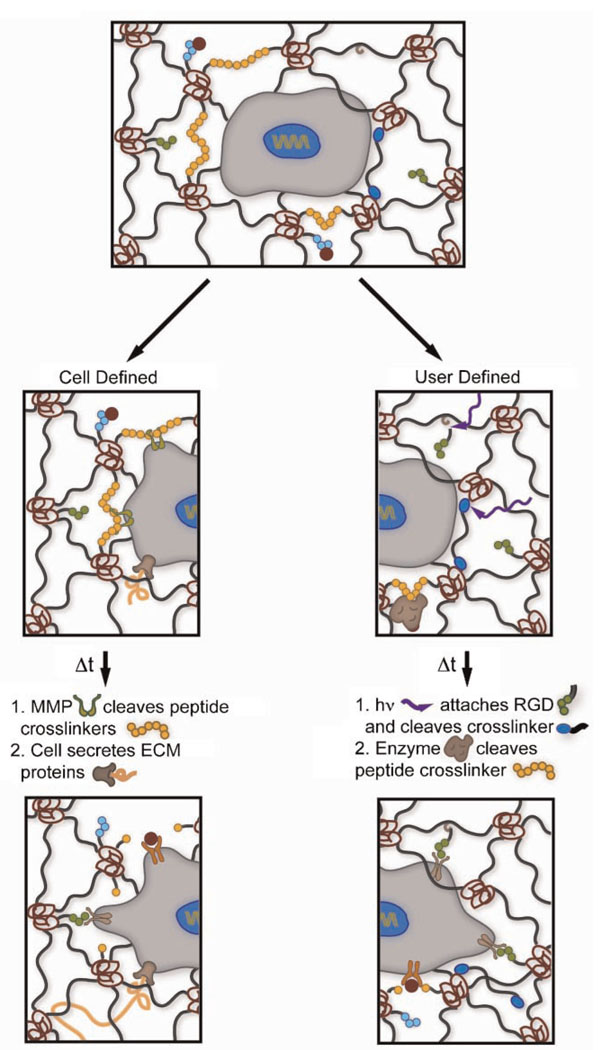
Figure 4.
Synthetic–biologic hydrogels that incorporate several well-defined and orthogonal chemistries serve as robust ECM mimics for 3D cell culture. Depending on the application, it may be advantageous to incorporate cell- or user-defined regulation of the material properties to emulate the native dynamic environment. However, in many cases, synthetic hydrogels that incorporate both cell- and user-defined chemistries will be necessary. Here, we illustrate a cell cleaving MMP degradable crosslinks (yellow circles) that allow it to access sequestered growth factors (red) and integrin-binding sites, such as RGD (green circles). Ultimately, this cleavage allows cell motility and the deposition of ECM proteins (orange fiber). User-defined chemistries, such as photodegradable crosslinks (blue ellipses) and post-gelation attachment of RGD to the network backbone, afford facile control of the dynamic biochemical and biophysical properties of the gel, thereby directing cell attachment and motility. Further, exogenous application of enzymes (brown) can allow user-defined release of sequestered growth factors.
Conclusion
When employing synthetic hydrogels as ECM mimics, it is necessary to understand the cell’s native environment—how the cells interact with, remodel, and migrate through the ECM. To garner biologically relevant conclusions from in vitro cell culture, critical matrix factors must be recapitulated in a 3D environment. In cases of tissue development, it may be advantageous to allow cells to dictate changes in their own environment much like they do in vivo; however, user-defined control of the mechanical and biochemical properties can be advantageous to test complex hypotheses about the effect of specific cell–ECM interactions in 3D tissue models (Fig. 4). The path to designing the ideal ECM mimic is dependent on the culture at hand, but will likely require multiple, orthogonal chemistries. For instance, photolabile chemistries can be used to erode regions of a gel and the newly exposed surfaces can subsequently be painted with adhesive ligands to encourage cell adhesion and migration. More complex scaffolds, formed of interpenetrating networks of cell- and user-controlled chemistries, will arise. Ultimately, there is no single network that will mimic the complex ECM of every tissue type, but rationally incorporating bioinspired cues into synthetic gels can provide robust and diverse scaffolds for many cell culture systems.
References
- Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133(3):385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- Azab AK, Orkin B, Doviner V, Nissan A, Klein M, Srebnik M, Rubinstein A. Crosslinked chitosan implants as potential degradable devices for brachytherapy: In vitro and in vivo analysis. J Control Release. 2006;111(3):281–289. doi: 10.1016/j.jconrel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Barralet JE, Wang L, Lawson M, Triffitt JT, Cooper PR, Shelton RM. Comparison of bone marrow cell growth on 2D and 3D alginate hydrogels. J Mater Sci Mater Med. 2005;16:515–519. doi: 10.1007/s10856-005-0526-z. [DOI] [PubMed] [Google Scholar]
- Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro—A growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15(5):405–412. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59(1):63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- Butcher JT, Nerem RM. Porcine aortic valve interstitial cells in three-dimensional culture: Comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis. 2004;13:478–485. [PubMed] [Google Scholar]
- Chen RR, Mooney DJ. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20(8):1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chirila TV, Constable IJ, Crawford GJ, Vijayasekaran S, Thompson DE, Chen YC, Fletcher WA, Griffin BJ. Poly(2-hydroxyethel methacrylate) sponges as implant materials: In vivo and in vitro evaluation of cellular invasion. Biomaterials. 1993;14(1):26–38. doi: 10.1016/0142-9612(93)90072-a. [DOI] [PubMed] [Google Scholar]
- Cushing MC, Anseth KS. Hydrogel cell cultures. Science. 2007;316(5828):1133–1134. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121(3):255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- Dawson E, Mapili G, Erickson K, Taqvi S, Roy K. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60(2):215–228. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Rehfeldt F, Sen S, Discher DE. Microtissue elasticity: Measurements by atomic force microscopy and its influence on cell differentiation. In: Wang Y-L, Discher DE, editors. Methods in cell biology: Cell mechanics. Amsterdam: Elsevier; 2007. pp. 521–545. [DOI] [PubMed] [Google Scholar]
- Eyrich D, Brandl F, Appel B, Wiese H, Maier G, Wenzel M, Staudenmaier R, Goepferich A, Blunk T. Long-term stable fibrin gels for cartilage engineering. Biomaterials. 2007;28(1):55–65. doi: 10.1016/j.biomaterials.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: Are the pieces now in place? J Cell Biochem. 2008;104(6):1964–1987. doi: 10.1002/jcb.21364. [DOI] [PubMed] [Google Scholar]
- Gobin AS, West JL. Cell migration through defined, synthetic extracellular matrix analogues. FASEB J. 2002;16(3):751–760. doi: 10.1096/fj.01-0759fje. [DOI] [PubMed] [Google Scholar]
- Griffith LG, Naughton G. Tissue engineering—Current challenges and expanding opportunities. Science. 2002;295(5557):1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-ampiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10(2):220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64(2):295–312. [PubMed] [Google Scholar]
- Khire VS, Benoit DSW, Anseth KS, Bowman CN. Ultrathin gradient films using thiol-ene polymerizations. J Polym Sci Part A Polym Chem. 2006;44(24):7027–7039. [Google Scholar]
- Kloxin AM, Kasko AK, Salinas CN, Anseth KS. Photolabile hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Beyec J, Xu R, Lee SY, Nelson CM, Rizki A, Alcaraz J, Bissell MJ. Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp Cell Res. 2007;313(14):3066–3075. doi: 10.1016/j.yexcr.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1880. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Lee SH, Moon JJ, West JL. Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials. 2008;29(20):2962–2968. doi: 10.1016/j.biomaterials.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100(22):12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc Natl Acad Sci USA. 2003;100(9):5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MJ, Anseth KS. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials. 2006;27(10):2265–2274. doi: 10.1016/j.biomaterials.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Mann BK, West JL. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60(1):86–93. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- Martens P, Anseth KS. Characterization of hydrogels formed from acrylate modified poly(vinyl alcohol) macromers. Polymer. 2000;41(21):7715–7722. [Google Scholar]
- Massia SP, Hubbell JA. An RGD spacing of 440 nm is sufficient for integrin avb3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J Cell Biol. 1991;114(5):1089–1100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters KS, Shah DN, Walker G, Leinwand LA, Anseth KS. Designing scaffolds for valvular interstitial cells: Cell adhesion and function on naturally derived materials. J Biomed Mater Res A. 2004;71(1):172–180. doi: 10.1002/jbm.a.30149. [DOI] [PubMed] [Google Scholar]
- Metters AT, Anseth KS, Bowman CN. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer. 2000;41(11):3993–4004. [Google Scholar]
- Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23(22):4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24(3):208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Nuttelman CR, Kloxin AM, Anseth KS. Temporal changes in PEG hydrogel structure influence human mesenchymal stem cell proliferation and matrix mineralization. In: Fisher JP, editor. Tissue engineering. Berlin: Springer-Verlag; 2006. pp. 135–149. [DOI] [PubMed] [Google Scholar]
- Palsson BO, Bhatia SN. Tissue engineering. Upper Saddle River, NJ: Pearson Prentice Hall; 2004. [Google Scholar]
- Park KH, Na K, Chung HM. Enhancement of the adhesion of fibroblasts by peptide containing an Arg-Gly-Asp sequence with poly(ethylene glycol) into a thermo-reversible hydrogel as a synthetic extracellular matrix. Biotechnol Lett. 2005;27(4):227–231. doi: 10.1007/s10529-004-8297-z. [DOI] [PubMed] [Google Scholar]
- Patel PN, Gobin AS, West JL, Patrick CW. Poly(ethylene glycol) hydrogel system supports preadipocyte viability, adhesion, and proliferation. Tissue Eng. 2005;11(9–10):1498–1505. doi: 10.1089/ten.2005.11.1498. [DOI] [PubMed] [Google Scholar]
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345–1360. [Google Scholar]
- Petersen OW, Ronnovjessen L, Howlett AR, Bissell MJ. Interaction with basement-membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89(19):9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeber GP, Lutolf MP, Hubbell JA. Mechanisms of 3-D migration and matrix remodeling of fibroblasts within artificial ECMs. Acta Biomater. 2007;3(5):615–629. doi: 10.1016/j.actbio.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Rifkin DB. Cell signaling events: A view from the matrix. Matrix Biol. 2003;22(2):101–107. doi: 10.1016/s0945-053x(03)00002-7. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev of Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- Rydholm AE, Held NL, Benoit DSW, Bowman CN, Anseth KS. Modifying network chemistry in thiol-acrylate photopolymers through postpolymerization functionalization to control cell-material interactions. J Biomed Mater Res A. 2008;86(1):23–30. doi: 10.1002/jbm.a.31526. [DOI] [PubMed] [Google Scholar]
- Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11(4):381–387. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas CN, Anseth KS. Mixed mode thiol-acrylate photopolymerizations for the synthesis of PEG-peptide hydrogels. Macromolecules. 2008a;41(16):6019–6026. [Google Scholar]
- Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008b;29(15):2370–2377. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney AS, Pathak CP, Hubbell JA. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(a-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26(4):581–587. [Google Scholar]
- Schnaper HW, Grant DS, Stetlerstevenson WG, Fridman R, Dorazi G, Murphy AN, Bird RE, Hoythya M, Fuerst TR, French DL, Quigley JP, Kleinman HK. Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J Cell Physiol. 1993;156(2):235–246. doi: 10.1002/jcp.1041560204. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA. 2002;99(7):4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semino CE, Merok JR, Crane GG, Panagiotakos G, Zhang SG. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation. 2003;71(4–5):262–270. doi: 10.1046/j.1432-0436.2003.7104503.x. [DOI] [PubMed] [Google Scholar]
- Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17(6):551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- Shu XZ, Ahmad S, Liu YC, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A. 2006;79(4):902–912. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DIC, Whitesides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264(5159):696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Murphy CL, Murphy C, Kimura M, Kawai S, Polak JM. Chondrogenic differentiation of murine embryonic stem cells: Effects of culture conditions and dexamethasone. J Cell Biochem. 2004;93(3):454–462. doi: 10.1002/jcb.20171. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve β-cell survival and insulin secretion in three-dimensional culture. Tissue Eng A. 2008;14(12):1959–1968. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JL. Scaffolding in tissue engineering. Boca Raton, FL: CRC Press; 2005. Bioactive hydrogels: Mimicking the ECM with synthetic materials; pp. 275–281. [Google Scholar]
- West JL, Hubbell JA. Ma PX, Elisseeff J, editors. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32(1):241–244. [Google Scholar]
- Willerth SM, Johnson PJ, Maxwell DJ, Parsons SR, Doukas ME, Sakiyama-Elbert SE. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J Biomed Mater Res A. 2007;80(1):13–23. doi: 10.1002/jbm.a.30844. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Kiick KL. Polysaccharide-poly(ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules. 2005;6(4):1921–1930. doi: 10.1021/bm050003+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim EKF, Leong KW. Proliferation and differentiation of human embryonic germ cell derivatives in bioactive polymeric fibrous scaffold. J Biomater Sci Polym Ed. 2005;16(10):1193–1217. doi: 10.1163/156856205774269485. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhao X, Spirio L. PuraMatrix: Self-assembling peptide nanofiber scaffolds. In: Ma PX, Elisseeff J, editors. Scaffolding in tissue engineering. Boca Raton, FL: CRC Press; 2005. pp. 217–238. [Google Scholar]
- Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17(13):2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]