Abstract
Hypoxic pulmonary hypertension is a disease of the lung vasculature that is usually quantified by pulmonary vascular resistance (PVR). However, a more complete description of lung vascular function and right ventricular afterload is provided by pulmonary vascular impedance (PVZ) from spectral analysis of pulsatile pressure-flow relationships. We studied pulsatile pressure-flow relationships in isolated, perfused lungs of mice in normoxia, after induction of hypoxic pulmonary hypertension by 10 days of hypoxic exposure, and after the administration of the vasoactive agents sodium nitroprusside and serotonin in order to gain insight into the effects of disease and vasoactive agents on afterload. Chronic hypoxia exposure increased 0 Hz impedance (Z0) from 2.0 ± 0.2 to 3.3 ± 0.2 mmHg min/ml but decreased characteristic impedance (ZC) from 0.21 ± 0.02 to 0.18 ± 0.01 mmHg min/ml (both P<0.05). Sodium nitroprusside only slightly decreased Z0 but increased ZC in normal lungs (P<0.05) and did not affect ZC and decreased Z0 in hypertensive lungs (P<0.05). Serotonin increased ZC in normal and hypertensive lungs but decreased Z0 in hypertensive lungs (P<0.05). There was an inverse correlation between mean pulmonary artery pressure and ZC in all circumstances. These findings demonstrate that vasoactive interventions can have different sites of action (i.e., proximal vs. distal segments) in the normal and chronically hypoxic pulmonary vasculature, and the pressure-dependency of ZC and RW. The measurement of PVZ in isolated lungs allows for an improved understanding of the modes of action of drugs and hypoxia on the pulmonary circulation.
Keywords: pulmonary circulation, serotonin, sodium nitroprusside, chronic hypoxia
Introduction
The functional state of the pulmonary circulation in vivo as well as in isolated perfused lungs is usually evaluated on the basis of the mean pulmonary artery pressure (PAP) and mean flow rate (Q) [1]. An improved estimation of pulmonary vascular resistance (PVR) can be obtained by measuring PAP for a range of Q values, thus generating a multipoint PAP-Q curve [1–3]. While single- and multi-point PVR measurements provide information about changes in the resistive properties of small, peripheral pulmonary arterioles that result from disease processes and pharmacological interventions, they neglect associated proximal compliance changes. Thus, PVR alone is an insufficient hemodynamic measure of the effects of disease and interventions on either the entire pulmonary vasculature or the right ventricle. Indeed, drugs efficacious for the treatment of severe pulmonary hypertension do not decrease PAP or PVR as much as expected given the resulting increases in patient exercise capacity and decreased mortality [4, 5]. It has been speculated that these drugs function by direct action on the right ventricle (i.e., by increasing contractility) and/or by decreasing the pulsatile hydraulic load [6], which depends on proximal compliance [7, 8].
A more complete description of the functional state of the pulmonary circulation including all forces that oppose right ventricular ejection can be obtained from the analysis of pulsatile PAP-Q relationships in the frequency domain [9, 10]. Using this technique, pulmonary arterial pressure and flow waves are decomposed into their harmonic constituents by a spectral analysis, and their information content is displayed as an impedance spectrum (PVZ) [9]. The zero Hz impedance, or Z0, is equivalent to the total pulmonary vascular resistance (PVR), or the slope of the mean PAP-Q curve. At high frequencies relative to the native heart rate, the impedance approaches a constant value, ZC, which corresponds to the square root of the inertance (per unit length) divided by the compliance (per unit length) of the proximal segments of the vasculature [9]. Alternatively, ZC can be calculated as the ratio of the fluid density times the pulse wave velocity divided by the luminal cross-sectional area of the proximal segments of the vasculature [11]. Essentially, ZC is the impedance of the proximal vasculature in the absence of wave reflections and neglecting viscous losses either at the luminal surface or in the walls of large arteries themselves. Given its direct dependence on proximal artery diameter and compliance, ZC is indispensible to the measurement of proximal artery changes associated with disease and vasoactivity [1, 9]. Also, the degree of mismatch between the Z0 and ZC can be used to estimate changes in wave reflection (RW) with disease processes and pharmacological interventions, which also affect right ventricular function [9]. The clinical relevance of ZC, RW, and the entire impedance spectrum has been increasingly recognized in diseases of the pulmonary vasculature such as pulmonary hypertension [10, 12].
We previously reported on the feasibility of measuring PVZ in isolated, perfused lungs of normal mice, those with embolic pulmonary hypertension and those with chronic hypoxia-induced pulmonary hypertension (hypoxia) [13, 14]. In the present study, we sought to demonstrate the utility of isolated lung impedance in differentiating between sites of action of vasoactive agents (i.e., proximal vs. distal) in the pulmonary circulation. We used serotonin and sodium nitroprusside, which were previously reported to increase and decrease PVR, respectively, and have variable effects on ZC in experimental animals [15–17]. In these studies, the observed effects on ZC could have been obscured by concomitant change in sympathetic nervous system tone [18] and anesthesia [19] which cannot be avoided in intact animal preparations. In addition, in isolated lungs, flow rate, airway pressure and left atrial pressure can be independently manipulated. The present results demonstrate that the effects of interventions on ZC and Z0 may be dissociated but that ZC is highly pressure dependent and is in fact inversely related to mean pulmonary artery pressure.
Methods
Animal Handling
Twelve male 10–12 week old C57BL6/J mice, 24.1 ± 1.6 grams weight were used (Jackson Laboratory, Bar Harbor, ME). The mice were subjected to hypoxia in the University of Wisconsin-Madison Biotron facility as previously described [14]. The mice were exposed to zero days (CTL) or 10 days (HPH) of hypobaric hypoxia (n=6 at each condition), such that the partial pressure of O2 was reduced by half. Mice were euthanized with an intraperitoneal injection of 150 mg/kg pentobarbital solution. All protocols and procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Isolated Lung Preparation
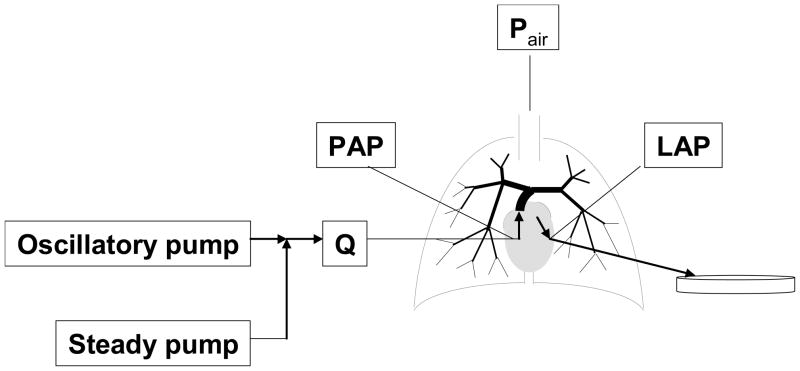
The isolated, ventilated, perfused mouse lung preparation was used as previously developed and validated for measurement of steady and pulsatile pressure-flow rate relationships [13, 14] with some changes to tubing type and tubing length to improve the frequency response. In brief, following euthanasia, the trachea, pulmonary artery and left atrium were cannulated for ventilation, perfusate inflow and perfusate outflow, respectively (Figure 1). The lungs were ventilated with room air and perfused with heated RPMI 1640 cell culture medium with 3.5% Ficoll (an oncotic agent). A syringe pump was used to create steady pulmonary vascular flow of perfusate whereas a high frequency oscillatory pump was used in parallel with the syringe pump to superimpose a pulsatile component on the pulmonary vascular flow. Pressure transducers (P75, Harvard Apparatus) measured the pulmonary artery pressure (PAP) and left atrial pressure (LAP). Flow rate (Q) was measured with an in-line flow meter (Transonic Systems, Inc., Ithaca, NY). Pressures and flows were monitored by continuous display on a laptop computer and recorded at 200Hz.
Figure 1.
Schematic of isolated lung set-up showing sites for measurement of instantaneous pulmonary artery flow rate (Q), pulmonary artery pressure (PAP), and left atrial pressure (LAP).
Pulsatile flow protocol
The pulsatile flow rate measurements were performed according to established methods [13, 14] after initial steady pressure-flow measurements of PAP, LAP and Q at 1 ml/min. Pulsatile pressure-flow data were recorded for flow rates of the form Q = 3 + 2 sin (2πf t) ml/min at frequencies f = 1, 2, 5, 10, 15 and 20 Hz. Multiple cycles of oscillation were performed at each frequency and three sequential trials were performed; each trial from 1 to 20 Hz was completed within 22 seconds. Pressure and flow data from the last cycle at each frequency were used, averaged over the three trials. No consistent differences in behavior from the first to the third trial were noted for any state in control or hypoxic lungs. Note, flow oscillations of this magnitude (1–5 ml/min) could not be consistently generated with our system at frequencies above 20 Hz (~2 HR for mice). All data were recorded with the lungs inflated at the end inspiratory pressure of 10 cm H2O. Between protocols, the lungs were allowed to rest for 1 min with normal ventilation (2 to 10 cm H2O at 90 breaths/min) and a steady flow rate of Q = 0.5 ml/min.
Serotonin and sodium nitroprusside
Following measurements in a normal state, serotonin (5-hydroxytryptamine, 5-HT; Sigma Chemical Company, St. Louis, MO) was added to the perfusate to a final concentration of 10−4 M to induce a constricted state. This concentration was selected based on previous experiments in isolated mouse lungs in which this dose was found to significantly increase PVR [20]. Initial, low steady flow rate measurements were taken 2–3 min after serotonin administration, followed by the pulsatile flow rate measurements. This time point was chosen from our own preliminary studies and from others’ work in which the response to serotonin was shown to peak at 5 min and gradually decrease thereafter [20]. Then, sodium nitroprusside (SNP; Sigma Chemical Company, St. Louis, MO) was added to fresh perfusate to a final concentration of 10−2 M in order to induce a dilated state. Low steady measurements were obtained beginning after 15 min of administration.
Calculations
Pulmonary vascular impedance magnitude (Z) and phase (Θ) were calculated from one full sinusoidal cycle of ΔP = PAP− LAP and Q at each pulsatile flow rate frequency. Input resistance Z0 was calculated from the average impedance at the 0th harmonic (f = 0 Hz) for all frequencies. Characteristic impedance ZC was calculated as the average of Z values between the first minimum (5 Hz) and the highest frequency imposed (20 Hz) and RW was calculated as (Z0−ZC)/(Z0+ZC) [21].
Statistics
Absolute changes in study endpoints with regard to state (normal tone, SNP or 5HT) and hypoxia exposure (CTL vs. HPH) were analyzed using a generalized least squares (gls) for repeated measures with an unstructured variance-covariance structure. Following a comparison of factors, we found in the linear model that the main effect of exposure was significant with significant interaction with frequency. The main effect of state was also significant, with significant interaction with frequency. We then built contrast matrices to investigate changes in Z as a function of state and frequency and, separately, changes in Z as a function of exposure and frequency. We could not conclude that the interaction between all three factors was significant in this sample size (n=6). No violations of the normality assumption were found and P-values less than 0.05 were considered significant. A Bonferroni adjustment was performed when comparing SNP or 5HT to the normal tone state. All P-values were two-sided and all statistical analyses were performed using R software (R-project.org, version 2.5.1). All data are presented in terms of means ± standard deviation.
Results
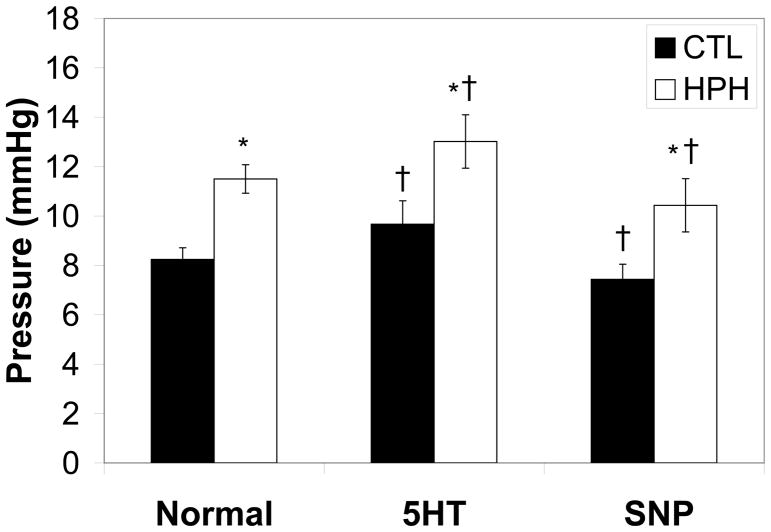
As expected, hypoxia increased PAP regardless of the presence or absence of vasoactive agent. In control and hypoxic lungs perfused with steady flow, serotonin increased PAP while sodium nitroprusside decreased it (Figure 2).
Figure 2.
Pulmonary artery pressure (PAP) at a flow rate of 1 ml/min for control (CTL) and chronically hypoxic (HPH) mouse lungs in the normal tone state, with serotonin and sodium nitroprusside. *P<0.05 vs. 0-day; †P<0.05 for change from normal to serotonin or sodium nitroprusside.
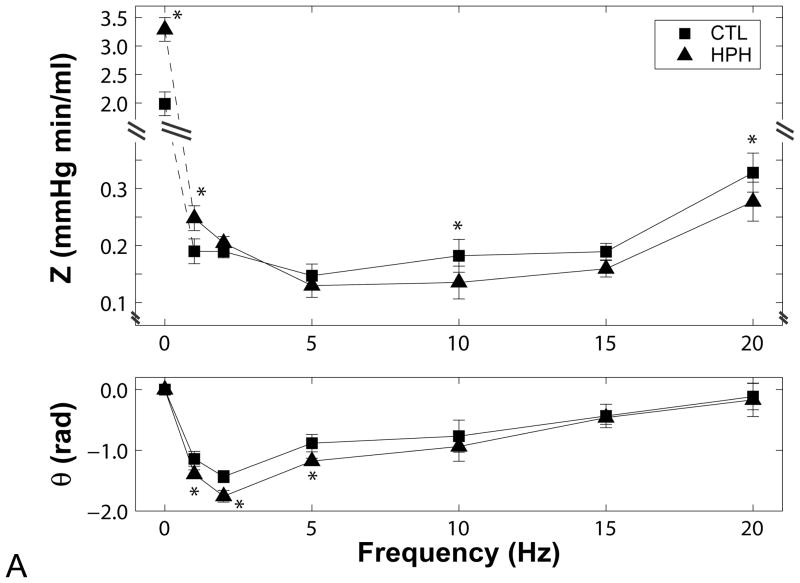
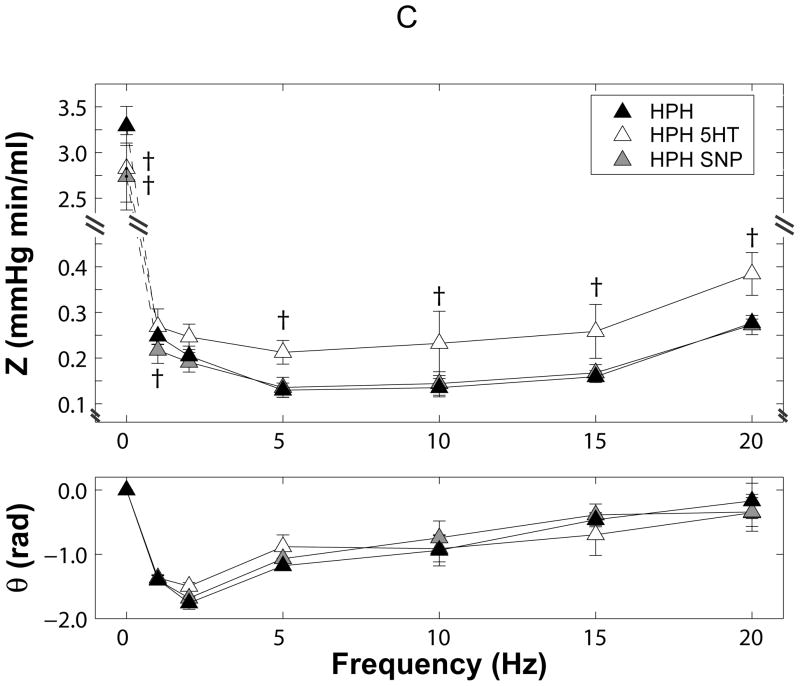
All impedance spectra had the expected “L”-shape with a high amplitudes at zero frequency and a rapid decline to low amplitudes at higher frequencies with moderate oscillations (Figure 3). The phase angles were negative at low frequencies and approached zero at higher frequencies.
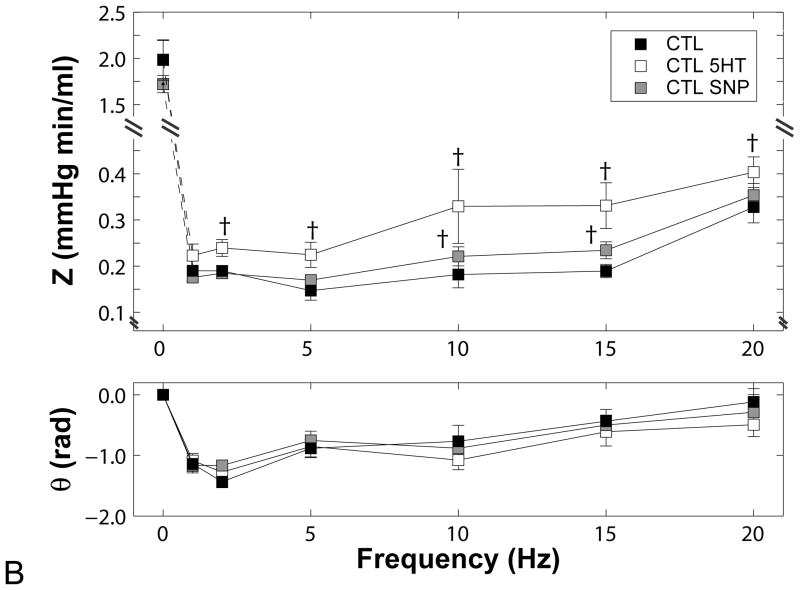
Figure 3.
Pulmonary vascular impedance magnitude (Z) and phase (θ) spectra in isolated perfused lungs from (A) control (CTL) and chronically hypoxic (HPH) mouse lungs under normal tone conditions, (B) CTL lungs exposed to serotonin (5HT, white) and sodium nitroprusside (SNP, grey) and (C) HPH lungs exposed to serotonin (5HT, white) and sodium nitroprusside (SNP, grey). Note the changes in scale on the y-axis for each panel. *P<0.05 vs. 0-day; †P<0.05 for change from normal tone to serotonin or sodium nitroprusside.
Hypoxia increased the magnitude at 0 Hz (Z0) and decreased the magnitude at higher frequencies, resulting in a cross-over between the two spectra. Hypoxia also decreased phase angle at low frequencies (Figure 3A).
In control lungs (Figure 3B), serotonin had no effect on Z0 but otherwise shifted the ratio of pressure to flow moduli to higher pressures at all frequencies. As a consequence, serotonin increased ZC; the increase in ZC relative to Z0 resulted in decreased RW (Table 1). Phase angle was unaffected. Sodium nitroprusside tended to decrease Z0 and increase impedance magnitudes at higher frequencies such that ZC increased and RW decreased. Again, phase angle was unaffected.
Table 1.
Metrics of impedance (Z0, ZC, RW) for control (CTL) and hypoxic (HPH) mouse lungs in the normal tone state or treated with serotonin (5HT) or sodium nitroprusside (SNP).
| Z0 (mmHg min/ml) | ZC (mmHg min/ml) | RW (dimensionless) | ||||
|---|---|---|---|---|---|---|
| CTL | HPH | CTL | HPH | CTL | HPH | |
| Normal | 2.0 ± 0.2 | 3.3 ± 0.2* | 0.21 ± 0.02 | 0.18 ± 0.01* | 0.81 ± 0.03 | 0.90 ± 0.01* |
| 5HT | 2.0 ± 0.2 | 2.8 ± 0.4*† | 0.32 ± 0.04† | 0.27 ± 0.04† | 0.72 ± 0.03† | 0.82 ± 0.02*† |
| SNP | 1.7 ± 0.1 | 2.7 ± 0.4*† | 0.24 ±0.1† | 0.18 ± 0.01* | 0.75 ± 0.02† | 0.87 ± 0.02*† |
P<0.05 vs. 0-day;
P<0.05 for change from normal tone to serotonin or sodium nitroprusside
In hypoxic lungs (Figure 3C), serotonin paradoxically decreased Z0 and shifted the ratio of pressure to flow moduli to higher pressures at all frequencies, thereby increasing ZC. Here, the combined effects of decreased Z0 and increased ZC resulted in decreased RW. Sodium nitroprusside decreased Z0 and had no effects at higher frequencies such that ZC remained constant. The drop in Z0 alone was sufficient to decrease RW. Phase angle was unaffected by either serotonin or sodium nitroprusside.
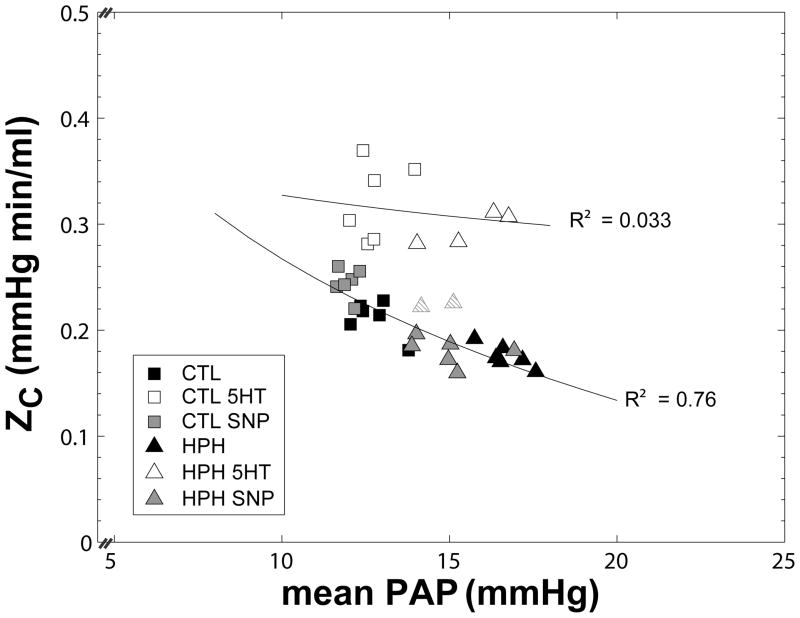
To examine how changes in ZC and RW due to hypoxia and vasoactive agents were dependent on pressure, we plotted each versus mean PAP (estimated from mean Q × Z0 + mean LAP) (Figures 4 and 5). While hypoxia decreased ZC, it also increased mean PAP such that the ZC-PAP relationship for control and hypoxic lungs in the normal state was inversely curvilinear. Sodium nitroprusside had the opposite effect of hypoxia, decreasing mean PAP and increasing ZC (Figure 4). As a result, ZC for control and hypoxic lungs in the normal state and as treated with sodium nitroprusside could be plotted along one curve, demonstrating an inverse correlation (R2 = 0.76, P<0.001). In contrast, serotonin shifted the ZC-PAP relationship upwards. An inverse relationship was still evident but administration of serotonin increased scatter (R2= 0.033), examples of which are the two hypoxia lungs (striped triangles) that are close to the dilated state values. The proximity of these points to the normal and sodium nitroprusside ZC-PAP curve suggest that serotonin did not act on the arteries in these lungs to the same degree or in the same way as in the other lungs.
Figure 4.
Characteristic impedance (ZC) as a function of mean pulmonary artery pressure (mean PAP) for control (CTL, squares) and hypoxic (HPH, triangles) mouse lungs in a normal tone state and treated with serotonin (5HT, white) or sodium nitroprusside (SNP, gray). Note that serotonin shifts the ZC-PAP relationship upward. (Striped triangles represent where serotonin did not generate the same effect).
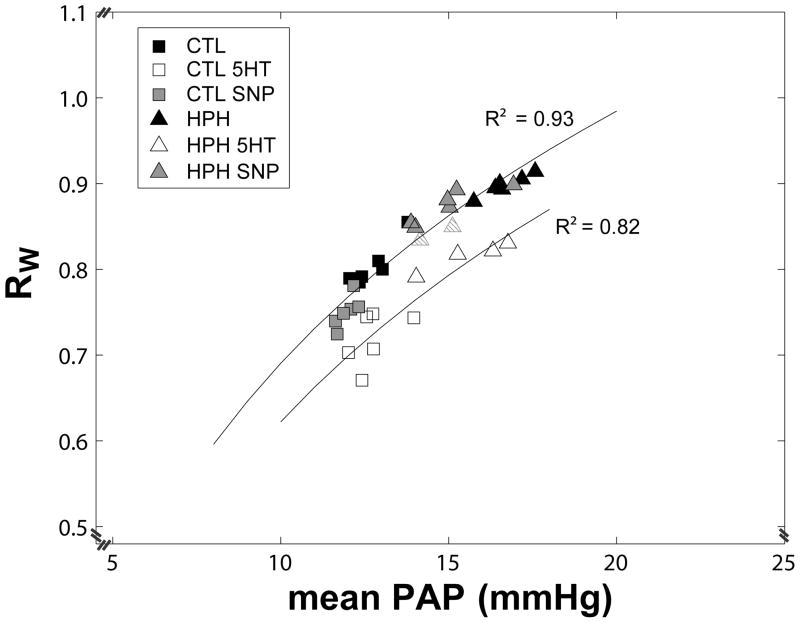
Figure 5.
Index of wave reflection (RW) as a function of mean pulmonary artery pressure (mean PAP) for control (CTL, squares) and hypoxic (HPH, triangles) mouse lungs in a normal tone state and treated with serotonin (5HT, white) or sodium nitroprusside (SNP, gray). Note that serotonin shifts the RW-PAP relationship downward. (Striped triangles represent where serotonin did not generate the same effect).
RW also exhibited a marked dependency on mean PAP (Figure 5). While hypoxia increased mean PAP and RW, sodium nitroprusside decreased mean PAP and RW, creating a highly correlated and significant RW-PAP relationship (R2=0.93; P<0.001). Serotonin shifted the RW-PAP relationship curve downward by decreasing RW with no change to mean PAP. As with ZC, serotonin appears to not have had the same effect on two lungs (striped triangles), which appear similar to those treated with sodium nitroprusside.
Discussion
The novel experimental findings in the present study are the relationships between ZC and mean PAP and between RW and mean PAP, which allow us to identify potentially independent effects that vasoactive agents and hypoxia can have on proximal and distal sites in the pulmonary circulation, which we discuss below.
ZC can be calculated as the square root of inertance divided by compliance or the ratio of the fluid density (ρ) times the pulse wave velocity divided by the luminal cross-sectional area of the proximal segments of the vasculature [11]. For a single, straight tube with elastic modulus E, radius r and wall thickness h, then, ZC is proportional to . As determined by E, proximal arterial radius r increases with mean PAP and by conservation of mass, h decreases. In addition, in arteries, which are strain-stiffening [22], E also may be a function of mean PAP. The consequence of these theoretical relationships is that distal vascular changes that increase PVR and thus mean PAP increase r, which decreases ZC in an inversely curvilinear way (Figure 4; CTL, CTL SNP, HPH SNP data). Proximal decreases in r that are not caused by a decrease in mean PAP increase ZC by shifting this curve upward (Figure 4; CTL 5HT and HPH 5HT data).
In these ways, the ZC-mean PAP relationship can provide important insights into the independent effects of vasoactive agents and hypoxia on the proximal and distal vasculature, with the caveat that changes in diameter and stiffness cannot be independently ascertained. In particular, our data suggest that hypoxia acts distally to increase PVR, which in turn causes proximal artery dilation. Hypoxia may also act proximally to cause dilation and proximal artery stiffening. Sodium nitroprusside acts distally to decreases PVR, which decreases proximal artery diameter but does not appear to independently dilate the proximal arteries. In contrast, under pulsatile flow conditions, serotonin had little effect distally but significant impact proximally, decreasing diameter and possibly also stiffness.
These experiments were performed using isolated, perfused mouse lungs to avoid the effects of systemic hemodynamic changes associated with physiological and pharmacological interventions. It has been shown that anesthesia [19], volume status [23] and level of sympathetic nervous system activation [18] can markedly affect pulmonary vascular impedance and function. Using the isolated lung system, we can investigate the impact of vasoactive agents and disease on pulmonary vascular function independent of these variables. In addition, this system allows independent control of flow rate, flow pulsation frequency, airway pressure and left atrial pressure. Finally, by utilizing mouse lungs, future comparisons to pulmonary vascular function defects in genetically engineered strains are possible.
Previously, hypoxia has been shown to increase Z0 but otherwise act on pulmonary vascular impedance in variable ways depending on the animal model used. In isolated mouse lungs, our group previously found that chronic hypoxia increased Z0 but decreased ZC [14] as confirmed here. A few differences from this previous report are worth mentioning. First, variability in the impedance was lower in the current study (with the same sample size), which we attribute in part to the use of only male mice. Second, impedance magnitude at 20 Hz was greater for control and hypoxic mice in the current study (although within the standard deviations of the prior study) because of small modifications to the testing system such as the use of stiffer tubing and shorter lengths of tubing, which increased the frequency response. These last changes in particular likely affected the measured impedance phase, which was largely frequency-independent in prior work [13] or had such large variability that no pattern could be discerned [14]. In the current study, impedance phase increased with increasing frequency above 5 Hz and was significantly different between control and hypoxic lungs at 1, 2 and 5 Hz (Figure 3A).
Serotonin has been shown to have variable effects on the pulmonary circulation in terms of site of action (proximal versus distal), efficacy and duration of efficacy. We perfused isolated lungs with serotonin under low, steady flow conditions and demonstrated an increase in PAP in control and hypoxic lungs. However, during pulsatile perfusion, no elevation of Z0 was evident. Indeed, in hypoxic lungs, serotonin decreased Z0. This confounding result is likely explained by the different pulmonary vascular responses to steady and pulsatile flow and the loss of efficacy of serotonin over time. With regard to the former, we have previously shown that exposing isolated lungs to pulsatile flow decreases PVR and have suggested that the mechanism is akin to mechanical pre-conditioning [14]. With regard to the latter, it is well known that the vascular response to serotonin decreases with time [20] and we could not measure the responses to steady and pulsatile flow simultaneously.
Here we also showed that serotonin increased characteristic impedance. Prior intact animal studies that have shown that serotonin either increases [24, 25] or does not change ZC [15, 16]. However, two key differences exist between these prior studies and ours. First, as noted above, our results are not confounded by the consequences of changes in blood volume, sympathetic nervous system activity and anaesthetic agent since they were obtained in isolated lungs. Second, and perhaps more importantly, in [15, 16, 24, 25], ZC was measured when pulmonary artery pressures were elevated, presumably due to the effects of serotonin on the distal pulmonary vasculature. In our study, PAP was not elevated when ZC was measured. Thus, we are able to separate the effect of increased pressure – which decreases ZC by increasing proximal artery radius – from serotonin-induced proximal constriction – which increases ZC. Here then we demonstrate the utility of the isolated lung system for differentiating between proximal and distal effects of serotonin, which have long been controversial in the literature. In particular, for the time point at which our pulsatile flow data were collected, our results demonstrate that the effects of serotonin were largely proximal in both the control and hypoxic lungs; the distal effects were either non-existent or paradoxical (Figure 4). This situation may occur because metabolic inactivation of serotonin is faster in the distal arteries than in the proximal arteries in mouse lungs.
Sodium nitroprusside is an effective pulmonary vasodilator but also well known activator of the sympathetic nervous system. Thus, in an intact animal, the direct effects of sodium nitroprusside on pulmonary arteries may be confounded by systemic effects. Previously, sodium nitroprusside has been found to shift PAP-Q relationships to lower pressures without changing ZC in intact dogs exposed to acute hypoxia [17]. However, since sodium nitroprusside did not eliminate the effects of acute hypoxia on PAP, these measurements were taken with the animal in a relatively hypertensive state [17]. Our current analysis suggests that ZC is highly dependent on mean PAP and should be interpreted with close attention to mean PAP. Here we found that sodium nitroprusside tended to decrease Z0 and increased ZC in control lungs; in contrast, in hypoxic lungs, Z0 decreased significantly and ZC did not change. To interpret these findings, we note that the inverse curvilinear relationship makes ZC more sensitive to small changes in mean PAP (and thus in Z0) at low pressures and less sensitive to small changes in mean PAP at high pressures. As discussed above, these ZC-mean PAP relationships suggest that sodium nitroprusside acts mostly distally, when it acts, and does not appear to have independent proximal effects in either control or hypoxic lungs.
Limitations
Limitations in this study must be acknowledged. We did not create dose response curves for control and hypoxic mouse lungs to sodium nitroprusside or serotonin. Instead, we selected doses that produced the expected effects on pulmonary artery pressure and used the same concentration of both drugs for control and hypoxia. It is likely that different concentrations of drugs would have different proximal and distal effects and durations of effect. In addition, we did not obtain PVZ results above 20 Hz, which is only twice the native heart rate of the mouse [26], because the system cannot generate higher frequency oscillations in flow of the same magnitude (1 to 5 ml/min). We cannot exclude that additional information could be obtained at higher frequencies and that our calculation of ZC would change if these were included. However, in vivo the lower harmonics contain the most power and thus the most information [9]. In addition, our calculations of impedance spectra are based on pulsatile waveforms with single frequencies that were imposed in a fixed order. Time-dependent effects, such as loss of drug efficacy, could therefore have affected our higher frequency data more than our lower frequency data. However, given the short duration of all pulsatile flow trials (66 seconds in total for three sequential trials from f=1 to 20 Hz), this effect is likely to be small.
In the isolated perfused lung system, due to the lower than physiological flow rate (by about 3-fold) and perfusate viscosity (by 3- to 4-fold), mean PAP is lower than commonly reported in vivo in control and hypoxic mice [27]. Since we found here that ZC and RW are highly pressure dependent, this difference likely dramatically affects the measured values of impedance metrics. Thus, at this time, our results cannot be compared to in vivo measurements of impedance.
Conclusion
Experimentally, measurement of pulsatile pressure-flow relationships in isolated lungs enables a more complete understanding of the effects of hypoxia and drugs on pulmonary vascular function. The feasibility shown here for isolated perfused mouse lungs is particularly relevant to future studies on the pulmonary vascular consequences of specific gene mutations and deletions in genetically engineered mouse models.
Acknowledgments
This research was supported by the Belgium-Luxembourg Fulbright Scholar Program (NCC), National Institutes of Health grants HL086939 (NCC) and 1UL1RR025011 (from the Clinical and Translational Science Award program of the National Center for Research Resources). The authors would also like to thank Jens Eickhoff for guidance on the statistical analyses.
References
- 1.Naeije R. Pulmonary vascular function. In: AJ P, LJ R, editors. Pulmonary Circulation. London: Arnold; 2004. [Google Scholar]
- 2.Long L, et al. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res. 2006;98(6):818–27. doi: 10.1161/01.RES.0000215809.47923.fd. [DOI] [PubMed] [Google Scholar]
- 3.Chemla D, et al. Haemodynamic evaluation of pulmonary hypertension. Eur Respir J. 2002;20(5):1314–31. doi: 10.1183/09031936.02.00068002. [DOI] [PubMed] [Google Scholar]
- 4.Castelain V, et al. Pulmonary artery pressure-flow relations after prostacyclin in primary pulmonary hypertension. Am J Respir Crit Care Med. 2002;165(3):338–40. doi: 10.1164/ajrccm.165.3.2106033. [DOI] [PubMed] [Google Scholar]
- 5.Galie N, et al. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30(4):394–403. doi: 10.1093/eurheartj/ehp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voelkel N, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114(17):1883–91. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 7.Gan C, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest. 2007;132(6):1906–12. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 8.Mahapatra S, et al. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47(4):799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 9.Milnor WR. In: Hemodynamics. , editor. Baltimore: Williams & Wilkins; 1989. p. 419. [Google Scholar]
- 10.Hunter K, et al. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am Heart J. 2008;155(1):166–74. doi: 10.1016/j.ahj.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westerhof N, Stergiopulos N, Noble MIM. Snapshots of Hemodynamics An Aid for Clinical Research and Graduate Education. 1. Dordrecht/Boston/London: Springer; p. 192. [Google Scholar]
- 12.Champion H, Michelakis E, Hassoun P. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120(11):992–1007. doi: 10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- 13.Tuchscherer HA, Webster EB, Chesler NC. Pulmonary Vascular Resistance and Impedance in Isolated Mouse Lungs: Effects of Pulmonary Emboli. Annals of Biomedical Engineering. 2006;34(4):660–8. doi: 10.1007/s10439-005-9050-z. [DOI] [PubMed] [Google Scholar]
- 14.Tuchscherer HA, Vanderpool RR, Chesler NC. Pulmonary vascular remodeling in isolated mouse lungs: Effects on pulsatile pressure-flow relationships. Journal of Biomechanics. 2007;40(5):993–1001. doi: 10.1016/j.jbiomech.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Bergel D, Milnor W. Pulmonary vascular Impedance in the dog. Circulation Research. 1965;16:401–15. doi: 10.1161/01.res.16.5.401. [DOI] [PubMed] [Google Scholar]
- 16.Reuben S, et al. Impedance and transmission properties of the pulmonary arterial system. Cardiovasc Res. 1971;5(1):1–9. doi: 10.1093/cvr/5.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Fesler P, et al. Journal of Applied Physiology. Vol. 101. 2006. Effects of sildenafil on hypoxic pulmonary vascular function in dogs; pp. 1085–1090. [DOI] [PubMed] [Google Scholar]
- 18.Pace J. Sympathetic control of pulmonary vascular impedance in anesthetized dogs. Circ Res. 1971;29(5):555–68. doi: 10.1161/01.res.29.5.555. [DOI] [PubMed] [Google Scholar]
- 19.Ewalenko P, et al. Pulmonary vascular impedance vs. resistance in hypoxic and hyperoxic dogs: effects of propofol and isoflurane. J Appl Physiol. 1993;74(5):2188–93. doi: 10.1152/jappl.1993.74.5.2188. [DOI] [PubMed] [Google Scholar]
- 20.Held H, Martin C, Uhlig S. Characterization of airway and vascular responses in murine lungs. British Journal of Pharmacology. 1999;126(5):1191–1199. doi: 10.1038/sj.bjp.0702394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols WW, et al. McDonald’s blood flow in arteries : theoretical, experimental, and clinical principles. 5. London: Arnold; 2005. [Google Scholar]
- 22.Humphrey JD, Delange SL. An introduction to biomechanics : solids and fluids, analysis and design. xviii. New York: Springer; 2004. p. 631. [Google Scholar]
- 23.Dujardin J, et al. Effects of blood volume changes on characteristic impedance of the pulmonary artery. Am J Physiol. 1982;242(2):H197–202. doi: 10.1152/ajpheart.1982.242.2.H197. [DOI] [PubMed] [Google Scholar]
- 24.Bargainer J. Pulse wave velocity in the main pulmonary artery of the dog. Circ Res. 1967;20(6):630–7. doi: 10.1161/01.res.20.6.630. [DOI] [PubMed] [Google Scholar]
- 25.Hammon JJ, et al. Analysis of pulsatile pulmonary artery blood flow in the unanesthetized dog. J Appl Physiol. 1981;50(4):805–13. doi: 10.1152/jappl.1981.50.4.805. [DOI] [PubMed] [Google Scholar]
- 26.Schwenke D, et al. Long-term monitoring of pulmonary arterial pressure in conscious, unrestrained mice. J Pharmacol Toxicol Methods. 2006;53(3):277–83. doi: 10.1016/j.vascn.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Fagan K, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287(4):L656–64. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]