Abstract
Elevated amounts of glutamate, which acts as a neurotransmitter but is also a neurotoxin, are a hallmark of the autoimmune neurological disease multiple sclerosis and may contribute to its pathology. The discovery that a receptor for glutamate can inhibit the development of autoimmunity and protect from neuroinflammation in a mouse model of multiple sclerosis suggests that glutamate may also have a protective role and that its receptor may represent a therapeutic target (pages 897–902).
Although we are inclined to view the nervous and immune systems of the body as separate entities, the reality is far more complex. At the molecular level, crosstalk between the nervous and immune systems has been well documented. Many molecules and mediators are common to both, and substances produced by the immune system act on the nervous system and vice versa. As an example, the cytokine interleukin-1 (IL-1), which is fundamental to the process of inflammation, was discovered largely because of its role in immunoreactive innervation of the hypothalamus to elicit fever1. Conversely, the neurotransmitter nitric oxide is also produced by cells of the immune system and has a role in inflammation and immunomodulation2,3.
In this issue of Nature Medicine, Fallarino et al.4 add the neurotransmitter glutamate to the list of substances that participate in crosstalk between the nervous and immune systems. High amounts of glutamate are found in the brain of individuals with multiple sclerosis, and whereas this is widely thought to contribute to neurotoxicity, the authors now present an alternative possibility—a role for glutamate in driving a regulatory T cell response that ameliorates neuroinflammation in a mouse model of this paralyzing disease. They show that this increase in regulatory T cells is mediated by the metabotropic glutamate receptor-4 (mGluR4) expressed on dendritic cells (DCs), a cell type crucial for supporting an immune response. Their data suggest that glutamate may have a protective role in inflammatory neurodegenerative diseases.
Glutamate is a highly abundant excitatory neurotransmitter and is essential for many aspects of normal brain function such as learning and memory5. Its actions are mediated by ionotropic (ion channel–forming) and by metabotropic (G protein–activating) glutamate receptors5. In excess, however, glutamate is harmful and causes neuronal death through a massive calcium influx via ionotropic glutamate receptor channels, with consequent damage to mitochondria and activation of proapoptotic genes. Glutamate toxicity occurs as part of the ischemic cascade associated with spinal cord injury, stroke, traumatic brain injury and in various diseases of the central nervous system, including Alzheimer’s disease, amyotrophic lateral sclerosis, Parkinson’s disease and multiple sclerosis5.
The adaptive immune response is orchestrated by antigen-specific T cells, which differentiate after activation into one of several functionally distinct T ‘helper’ lineages known as TH1, TH2 and TH17 (ref. 6). Each produces a distinct assortment of cytokines and chemokines under control of their respective lineage-specific transcription factors T-bet, GATA3 and ROR-γt. Whereas their role is to protect us from various types of pathogens, they can also be involved in tissue pathology6. For example, TH2 cells are involved in allergy and asthma, and TH1 cells, and even more so TH17 cells, are involved in autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis induced in mice by immunization with an antigenic peptide derived from brain myelin.
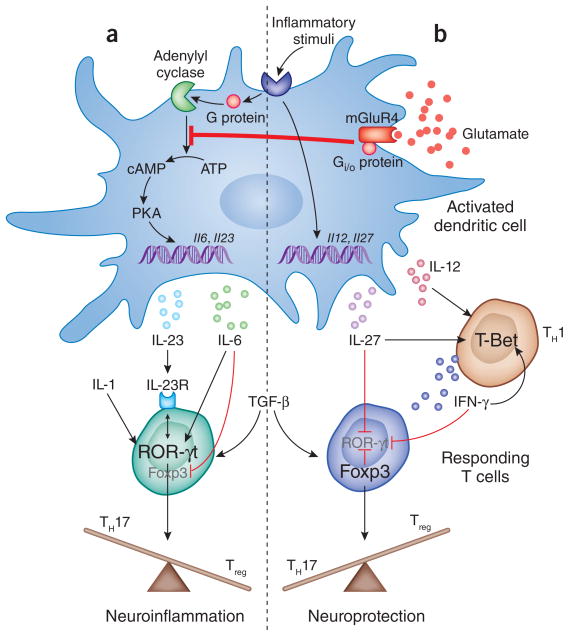
T cells respond to their antigen displayed on the surface of DCs, which also express co-stimulatory molecules and polarizing cytokines, and direct the responding T cells to adopt a particular lineage (Fig. 1). For example, IL-12 and IL-27 produced by DCs will specifically drive T-bet expression in the T cell, promoting a TH1 response dominated by the cytokine interferon-γ. Alternatively, release of transforming growth factor-β; (TGF-β), IL-1, IL-6 and IL-23 leads to ROR-γt expression by the T cells and differentiation toward the TH17 lineage. Expression of TGF-β in the absence of IL-6 also directs the induction of another T cell transcription factor known as FoxP3. FoxP3 can directly suppress expression of ROR-γt and causes the T cells to adopt a regulatory phenotype, yielding T regulatory cells (Treg cells) that inhibit inflammatory effector T cells.
Figure 1.
Glutamate activation of mGluR4 signaling in dendritic cells prevents the production of IL-6 and IL-23, favoring the development of Treg cells over TH17 cells in EAE. In the absence of mGluR4, DCs respond to an inflammatory signal such as LPS by upregulating both TH1-specific (IL-12 and IL-27) and TH17-specific (IL-6 and IL-23) cytokines through different pathways. Transcription of IL-6 and IL-23 is induced via the production of cAMP, which is catalyzed from ATP by the enzyme adenylyl cyclase, and, in turn, activates downstream mediators such as protein kinase A (PKA), which leads to gene expression. These cytokines, along with TGF-β and IL-1, induce the transcription factor ROR-γt in the responding T cell, upregulating TH17-specific cytokines and the IL-23 receptor, which is required later for maintenance of the TH17 phenotype. IL-6 directly suppresses Foxp3, leading to the promotion of TH17 at the expense of Treg cells and the development of neuroinflammation. Fallarino et al.4 show that activation of mGluR4 by glutamate inhibits adenylyl cyclase and the production of cAMP, preventing expression of IL-6 and IL-23. In their absence, Foxp3 is upregulated by the activating T cell in the presence of TGF-β and blocks TH17 differentiation by direct inhibition of ROR-γt, tipping the balance toward a neuroprotective response. The TH1 response is unaffected by this process, and IL-27 and interferon-γ (IFN-γ ) also contribute to the promotion of Treg cell differentiation through inhibition of ROR-γ t.
Through the expression of these different cytokines, DCs act as master regulators of the immune response, controlling not only whether an inflammatory immune response will occur, but also its nature, depending on the T cell lineage that is induced6. What environmental cues control the function and decision making of these master regulators? Fallarino et al.4 show that one of these cues is glutamate, which acts through metabotropic glutamate receptors that are expressed by DCs.
Previous studies have examined the effect of various neurotransmitters on the immune response, but they preceded the identification of TH17 cells as pathogenic effectors and largely ignored a role for DCs. Armed with the information now available regarding the TH17 response, the authors used a combination of mice deficient in mGluR4 and specific allosteric compounds that enhance mGluR4 receptor signaling in wild-type mice to examine the role of this receptor in EAE4. They found that mice deficient in mGluR4 developed exacerbated disease with a higher mortality, whereas enhanced glutamate signaling through mGluR4 ameliorated disease and favored the induction of Treg cells at the expense of TH17 development4. Interestingly, although high receptor expression was found on T cells and on DCs, the protective effect of mGluR4 signaling was mediated entirely by DCs and occurred via mGluR4’s inhibitory effect on intracellular activation of cAMP. Inhibition of cAMP activation prevents IL-6 and IL-23 production and skews the DC cytokine response to a profile favoring induction of Treg cells, which turn out to be responsible for the resulting protection from EAE in mice—as shown by the ability of T cells obtained from mice protected by mGluR4 stimulation to transfer protection to untreated recipients, which was reversed by removal of Treg cells.
Notably, human DCs also expressed mGluR4, and activation of this receptor with the same allosteric stimulator used in mice had comparable effects in human DCs. Currently approved therapies for autoimmune neurological diseases have variable efficacy, major side effects and can only slow, and not stop, the neurodegenerative process7. Although the data of Fallarino et al.4 are promising, more studies are needed to clarify issues relevant to the clinical setting. Protection, for instance, does not seem to be maintained after treatment cessation, despite the development of Treg cells—a disappointing result. Further treatment, however, is not required to maintain Treg cell–mediated protection upon transfer of these cells to new recipients4. Whereas mGluR4 stimulation is effective in disease prevention, it is unclear whether it would be effective after the onset of clinical symptoms.
This study also raises questions relevant to an ongoing clinical trial for treatment of Parkinson’s disease in which a mGluR4 enhancer is used to reduce glutamate release in the brain8. If this therapy proves effective, could hitherto unknown immunological effects be involved? Could suppression of TH17 responses, important in antimicrobial defense, be an unwanted side effect of this treatment? Answers to these questions could have implications not only for treatment of conditions associated with glutamate toxicity, but also for a broad range of debilitating autoimmune disorders that have been linked to aberrant TH17 responses, including rheumatoid arthritis, psoriasis, inflammatory bowel disease and uveitis9,10.
The study by Fallarino et al.4, and others to come, will continue to blur the distinctions between the signals driving the nervous, immune and endocrine systems, revealing unknown therapeutic potential and also highlighting the consequences that perturbing one of them may have on the others.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Breder CD, Dinarello CA, Saper CB. Science. 1988;240:321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan C. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 3.Coleman JW. Int Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 4.Fallarino F, et al. Nat Med. 2010;16:897–902. doi: 10.1038/nm.2183. [DOI] [PubMed] [Google Scholar]
- 5.Platt SR. Vet J. 2007;173:278–286. doi: 10.1016/j.tvjl.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, Yamane H, Paul WE. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compston A, Coles A. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 8.East SP, Gerlach K. Expert Opin Ther Pat. 2010;20:441–445. doi: 10.1517/13543770903551295. [DOI] [PubMed] [Google Scholar]
- 9.Crome SQ, Wang AY, Levings MK. Clin Exp Immunol. 2010;159:109–119. doi: 10.1111/j.1365-2249.2009.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damsker JM, Hansen AM, Caspi RR. Ann NY Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]