Abstract
Background
Chronic kidney disease (CKD) is an increasingly common condition, especially among older adults. CKD manifests differently in older versus younger patients, with a risk of death that far outweighs the risk of CKD progressing to the point that dialysis is required. Current CKD guidelines recommend a blood pressure target of <130/80 mmHg for all CKD patients, but it is unknown how lower versus higher baseline blood pressures may affect older adults with CKD.
Study Design
Retrospective cohort study
Setting and Participants
Older patients (age 75+ years) with CKD (eGFR <60 ml/min/1.73 m2) in a community-based health maintenance organization.
Predictor
Baseline systolic blood pressure (SBP) <130, 130-160 (reference group), and >160 mm Hg.
Outcomes
Subjects followed for 5 years to examine rates of mortality (primary outcome) and cardiovascular disease hospitalizations (secondary outcome).
Results
At baseline, 3099 subjects (38.5%) had SBP <130 mm Hg, 3772 (46.9%) had SBP 131-160 mm Hg, and 1171 (14.6%) had SBP >160 mm Hg. A total of 3734 (46.4%) died and 2881 (35.8%) were hospitalized. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) for mortality in SBP <130 and >160 mm Hg group were 1.22 (1.11-1.34) and 0.99 (0.89-1.10), respectively. Adjusted HR (CI) for cardiovascular hospitalization in these groups were 1.10 (1.09-1.45) and 1.26 (1.09-1.45), respectively.
Limitations
While causality should not be implied from this retrospective analysis, the results from this study can generate hypotheses for future randomized controlled trials to investigate the relationship between blood pressure and outcomes in older CKD patients.
Conclusions
Our study suggests that lower baseline SBP (≤130 mmHg) may predict poorer outcomes in terms of both mortality and cardiovascular hospitalizations among older adults with CKD. Conversely, higher baseline SBP (>160 mmHg) may predict increased risk of cardiovascular hospitalizations but does not predict mortality. Clinical trials are required to test this hypothesis.
Keywords: aging, elderly, chronic kidney disease, blood pressure
Chronic kidney disease (CKD) is a significant medical problem affecting 16% of the US population. CKD is especially common among older adults; 37% of individuals over age 65 and 50% over age 85 are estimated to have CKD.1,2 Older adults manifest CKD differently, with high rates of mortality and hospitalization that outweigh their rate of progressing to require dialysis or transplant.2-4 Current CKD guidelines are age-neutral and are thus limited in their ability to account for possible age-related differences among CKD patients.5
Blood pressure control is known to slow CKD progression and is a cornerstone of CKD management.5 Current CKD guidelines recommend a blood pressure target of <130/80 mmHg for all patients.5 The trials upon which these recommendations are based, however, did not uniformly lower blood pressure to <130/80 mmHg and included predominantly patients 70 years old and younger.6-8 Recent research in elderly patients without CKD has demonstrated that hypertension control reduces stroke and mortality even among the oldest old; these studies lowered systolic blood pressure to 140-150 mmHg on average (well above those levels recommended for CKD patients).9-11 The potential benefits or harms of lowering blood pressure to <130mmHg systolic in older CKD patients remain unclear.
We performed a retrospective cohort analysis on community-dwelling older adults with CKD to examine the relationship between systolic blood pressure (SBP) and important outcomes in this population. The specific SBP thresholds examined are modeled upon current CKD guidelines and SBP targets identified as relevant for older adults without CKD, in order to examine how current hypertension management standards may affect older CKD patients. We hypothesized that older CKD patients might experience increased harm, in the form of increased mortality and hospitalizations, in association with lower baseline SBP values.
Methods
Sources of Data
We conducted a retrospective cohort study of older adults with stage 3-5 CKD, analyzed by SBP, to examine long term outcomes for this population including mortality. Patients in this cohort were identified from within the Kaiser Permanente Northwest health maintenance organization, which provides care for 450,000 individuals in the Portland, Oregon and Vancouver, Washington area. The demographic characteristics of these individuals mirror those of the surrounding community.12 Kaiser Permanente Northwest has had an electronic medical record in place as the sole medical record system since 1997. This study was reviewed and approved by the Kaiser Permanente Northwest and Oregon Health and Science University institutional review boards.
Cohort definition
Patients age 75 or older with stages 3-5 CKD who were enrolled in Kaiser Permanente Northwest from January 1, 1999 to Dec 31, 2003 were eligible for inclusion. CKD was defined as two estimated glomerular filtration rate (eGFR) values <60 ml/min/1.73m2 measured at least three, and fewer than 24, months apart (without intervening eGFR values of >60 ml/min/1.73m2). The index date was the date of the second eGFR of <60 ml/min/1.73m2. Patients were required to have one year of enrollment and one year of prescription benefits in the health plan prior to the index date. Patients were also required to have at least one outpatient blood pressure measurement in the year prior to index date; the SBP value closest and prior to the index date served as the baseline systolic blood pressure for that patient. Patients with a prior history of renal replacement therapy were excluded. (See Figure 1)
Figure 1.
Study design and derivation of the cohort.
SBP cohorts were assigned based on the baseline SBP value. Blood pressure in the outpatient setting at Kaiser Permanente Northwest is measured per routine clinic protocol. The SBP cohorts were: 1) SBP ≤ 130 mmHg, 2) SBP 131-160 mmHg, or 3) SBP >160 mmHg. Additional outpatient SBP readings during the 5 year follow-up period were not required for inclusion, but if available, follow-up SBP values were averaged by year for each patient.
Patient data were reviewed for up to three years prior to the index date to evaluate baseline characteristics. The majority of these characteristics were identified via ambulatory ICD-9 diagnostic codes. The sources of comorbid illness history and other co-variables are listed in Table 1. Height, weight, and blood pressure were obtained from the outpatient. Age, gender, time at-risk, and death information were obtained from the enrollment database.13
Table 1.
Sources of baseline characteristics
| Characteristic | Data source |
|---|---|
| Demographic characteristics | |
| Age | Membership file |
| Sex | Membership file |
| Clinical characteristics | |
| Hypertension | ICD9 codes 401, 402, 403, 404, 405; or treatment with antihypertensive medication |
| Current Smoking | Separate field in electronic medical record (smoking list); problem list (ICD9 code 305.1); reason for visit (smoking related visit) |
| Diabetes | Diabetes Registry |
| Coronary Artery Disease | ICD9 codes 410, 411, 412, 413, 414 and not including 414.10, 414.11, 414.19 |
| Congestive Heart Failure | ICD9 codes 428.0, 428.9, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 429.4A, 429.9B, 429.9A, 428.1 |
| Peripheral Vascular Disease | ICD9 codes 250.7x, 440.xx, 443.81, 443.9 |
| Acute Kidney Injury | ICD9 codes 584.xx (Acute Renal Failure code) |
| Hypertension | ICD9 codes 401.xx – 405.xx, 440.1; blood pressure values recorded as separate fields in the electronic medical record (using latest values in up to last three years prior to index date) |
| Fracture | ICD9 codes 806-829 and 733.10-733.19 for pathologic fractures |
| Anemiaa | Laboratory data within the electronic medical record |
| Use of ACEi, ARB | Pharmacy data within the electronic medical record |
| Use of statin | Pharmacy data within the electronic medical record |
| Use of diuretic | Pharmacy data within the electronic medical record |
| Death | Enrollment database |
| Renal replacement therapy | Transplant and dialysis registry |
| eGFRb | Electronic medical record |
| proteinuriac | Laboratory data within the electronic medical record |
ACEi: angiotensin converting enzyme inhibitor
ARB: angiotensin receptor blocker
eGFR: estimated glomerular filtration rate
ICD9: International Classification of Diseases, Ninth Revision
defined as hemoglobin < 12 g/dL
calculated using serum creatinine and the 4 variable Modification of Diet in Renal Disease (MDRD) Study equation
Urine protein-creatinine ratio > 300, urine dipstick 1+ or greater, or microalbuminuria
Outcome variables
Patients were followed for 5 years after their index date for death and hospitalizations. Death was the primary outcome; confirmation of death was obtained from the enrollment database. An internal review found this database to be more than 90% accurate when compared to statewide death records. Two secondary outcomes included cardiovascular and fracture-related hospitalizations. The reason for hospitalization was identified via the primary discharge ICD-9 code. We also examined three subgroups within this cohort: patients who specifically carried a diagnosis of hypertension, patients without a prior diagnosis of heart failure, and patients with incident CKD. A patient was defined as having incident CKD if their eGFR closest and prior to their two qualifying eGFRs was >60 ml/min/1.73m2. All patients who did not meet this definition for incident CKD were presumed to have prevalent disease.
Statistical analysis
We utilized Cox proportional hazards regression to examine outcomes by SBP cohort; the SBP 131-160 mmHg group served as the reference group. Patients were censored for loss of benefits and for initiation of renal replacement therapy for the mortality outcome, and for loss of benefits, initiation of renal replacement therapy, and death in non-mortality outcomes. We used a completed cases analysis, including only variables with less than 15% missing values in our adjusted models. The one exception was proteinuria, with an average of 36% missing data, which was included based on likelihood of high clinical impact.
Results
At total of 8,042 patients met inclusion criteria; baseline characteristics are described in Table 2. Of note, 71.5% of patients in the SBP ≤130 mmHg cohort were receiving at least 1 antihypertensive medication, compared to 76.8% of the SBP >160 mmHg cohort. The average number of days between the baseline SBP and index date was similar across cohorts (SBP ≤130 mmHg, 76.2 days; SBP 131-160 mmHg, 81.3 days; SBP >160 mmHg 70.4). We examined subsequent outpatient SBP values (when available) to determine the percent of patients whose SBP remained consistent with their cohort assignment (90% after year 1 for SBP cohorts 131-160 and >160 mmHg versus 83% after year 1 for SBP cohort <130 mmHg).
Table 2.
Baseline characteristic by blood pressure groups
| Variable | SBP group | ||
|---|---|---|---|
| ≤130 mmHg (n = 3099) |
131-160 mm Hg (n = 3772) |
>160 mmHg (n = 1171) |
|
| Age | |||
| 75-85 | 74.1 | 77.2 | 75.7 |
| >85 | 25.9 | 22.8 | 24.3 |
| Female | 40.5 | 33.0 | 27.8 |
| *Tobacco use | |||
| current | 26.4 | 26.9 | 25.3 |
| ever | 59.9 | 56.3 | 52.8 |
| CKD Stagea | |||
| 3 | 90.5 | 91.6 | 90.4 |
| 4 | 8.9 | 7.7 | 9.3 |
| 5 | 0.6 | 0.7 | <1 |
| Hypertension diagnosis | 67.5 | 78.5 | 88.6 |
| Acute kidney injury | 2.8 | 1.8 | <5 |
| Diabetes | 22.1 | 22.3 | 24.0 |
| Coronary Artery Disease | 44.3 | 33.7 | 29.5 |
| Congestive Heart Failure | 41.0 | 25.3 | 21.2 |
| Peripheral Vascular Disease | 15.3 | 11.6 | 13.2 |
| ±Use of antihypertensive medications | |||
| ACEi/ARB | 34.5 | 32.0 | 40.5 |
| spironolactone | 4.1 | 2.6 | 1.9 |
| CCBs | 10.6 | 11.6 | 13.8 |
| alpha blockers, clonidine, and vasodilators |
4.3 | 6.2 | 10.6 |
| beta blockers | 36.1 | 33.6 | 39.8 |
| ¥ Receiving no antihypertensive medications |
28.5 | 30.8 | 23.2 |
| ¥ Receiving ≥3 antihypertensive medications |
13.5 | 12.6 | 17.4 |
| Use of statin medications | 25.1 | 22.2 | 22.0 |
| Anemia (Hb <12 g/dL) | 69.7 [6.6] | 70.8 [8.9] | 69.8 [10.3] |
| ‡Proteinuria | 6.6 [33.6] | 7.3 [36.1] | 10.5 [39.1] |
| †Cancer | 7.2 | 6.8 | 5.7 |
| BMI <30 kg/m2 | 61.2 [18.8] | 58.2 [15.8] | 59.0 16.0] |
| Albumin level <2 | <1 [74.0] | <1 [76.1] | <1 [77.5] |
N for total cohort is 8042. All values shown are percentages; however, “<5” signifies fewer than 5 patients per cohort with that trait (in such cases, percentages were not calculated). Numbers in brackets, where shown, denote percent missing.
Tobacco use current: use within 3 years of index date
Cancer includes any diagnosis code for cancer in the three years prior to inception date into the cohort
Proteinuria as listed below is a composite of urine protein/creatinine ratio >300, urine dipstick 1+ or greater, or microalbuminuria.
Antihypertensive medication use by cohort was determined by examining prescriptions for patients in the 90 days prior to index date.
Medication categories included in the determination of zero or 3 more antihypertensive medications by cohort included central and dihydropyridine CCBs, alpha blockers, clonidine, vasodilators, ACEi, ARBs, spironolactone, loop diuretics, Thiazide-type diuretics, and beta blockers.
Stage 3 CKD corresponds to eGFR 31-60 ml/min/1.73m2; Stage 4, eGFR 15-30 ml/min/1.73m2; Stage 5, eGFR <15 ml/min/1.73m2.
SBP systolic blood pressure; ACEi: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; CCB, calcium channel blocker; CKD, chronic kidney disease; Hb, hemoglobin; BMI, body mass index; eGFR, estimated glomerular filtration rate
The global likelihood ratio test of blood pressure found that blood pressure predicted mortality (p < 0.001). The unadjusted mortality rate during the five year follow-up was 55.3% in the SBP ≤ 130 mmHg group, and 44.2% and 44.3% for the SBP 131-160 and SBP >160 mmHg cohorts respectively. The SBP ≤ 130 mmHg had an increased unadjusted hazard for mortality (HR, 1.43; 95% CI, 1.33-1.52), whereas the unadjusted hazard for mortality in the SBP >160 mmHg cohort was inconclusive (HR, 0.98; 95% CI, 0.88-1.08). (Table 3) The global likelihood ratio test confirmed the prognostic value of blood pressure for cardiovascular hospitalization (p = 0.003), but not for fracture-related hospitalization (P = 0.7). We continued to include and discuss fracture-related hospitalization in our analyses because, while a clear relationship between fracture-related hospitalization and SBP cannot be defined by this study, the information derived may be of use to future investigators and trials which may further examine this outcome. The unadjusted hazards for cardiovascular hospitalization were 1.20 (95% CI 1.11-1.31) and 1.18 (95% CI 1.06-1.31) for the SBP ≤ 130 and >160 mmHg cohorts respectively. For the same cohorts, the hazards for fracture-related hospitalizations were 1.11 (95% CI, 0.95-1.31) and 1.03 (95% CI 0.83, 1.28).
Table 3.
Cox proportion hazard model results
| Mortality |
Cardiovascular hospitalizations |
Fracture-related hospitalizations |
|
|---|---|---|---|
| Unadjusted model (n = 7496) | |||
| SBP ≤130 mmHg | 1.43 (1.33, 1.53) | 1.20 (1.11, 1.31) | 1.13 (0.96, 1.34) |
| SBP = 131-160 mmHg | 1.00 | 1.00 | 1.00 |
| SBP >160 mmHg | 0.98 (0.88, 1.08) | 1.18 (1.06, 1.31) | 0.99 (0.79, 1.24) |
| Adjusted model (n = 4561) | |||
| SBP ≤130 mmHg | 1.22 (1.11; 1.34) | 1.10 (0.99; 1.23) | 1.06 (0.86; 1.32) |
| SBP = 131-160 mmHg | 1.00 | 1.00 | 1.00 |
| SBP >160 mmHg | 1.06 (0.93; 1.22) | 1.26 (1.09; 1.45) | 0.96 (0.71; 1.30) |
c The adjusted model controls for age, gender, CKD stage (3, 4, or 5), coronary artery disease, diabetes, heart failure, peripheral vascular disease, anemia, prior diagnosis of hypertension, angiotensin converting enzyme inhibitor or angiotensin receptor blocker use, diuretic use, spironolactone use, and dipstick positive proteinuria. Patients with diagnosis of cancer in the three years prior to the index date were excluded.
The adjusted hazard for mortality for the SBP ≤ 130 mmHg cohort was 1.22 (95% CI, 1.11-1.34), while the adjusted hazard for mortality in the SBP >160 mmHg cohort remained inconclusive (HR 1.06, 95% CI 0.93, 1.22). The Kaplan-Meier survival graph for this analysis (Figure 2) shows clear and consistent separation for the absolute risk of mortality between the lowest SBP cohort and the two higher SBP cohorts. The adjusted hazard for cardiovascular hospitalizations for the SBP >160 mmHg cohort was 1.26 (95% CI 1.09, 1.45), whereas the hazard ratio was 1.10 (95% CI 0.99, 1.23) for the SBP ≤ 130 mmHg. The hazard for fracture-related hospitalization was 1.06 (95% CI 0.86, 1.32) for the SBP ≤ 130 mmHg cohort and 0.96 (95% CI 0.71, 1.30) for the SBP >160 mmHg cohort. (Table 3)
Figure 2.
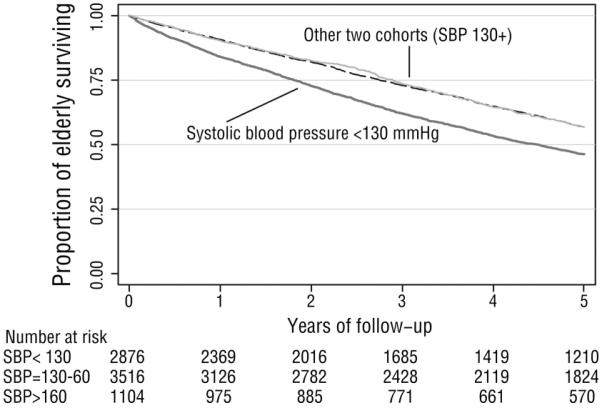
Kaplan-Meier curve for mortality in original cohort (death versus time). The thick dark line represents the lowest blood pressure cohort (SBP<130 mmHg); the other two blood pressure cohorts (SBP 131-160 and SBP >160 mmHg) are superimposed on the upper line.
For each subgroup analysis, we examined the p values for interaction between that subgroup characteristic, SBP, and mortality. The only significant interaction found was between systolic blood pressure and patients with a prior diagnosis of hypertension (P = 0.03) for the outcome of mortality. We also examined the p values for interaction between the subgroup characteristic, SBP, and cardiovascular hospitalizations; none of those p values reached significance.
In the subgroup analysis including only patients with a prior diagnosis of hypertension, the adjusted hazard for mortality for the SBP ≤130 mmHg cohort and SBP >160 mmHg cohort did not differ meaningfully from those identified within the entire study population (HR 1.32, 95% CI 1.19, 1.47 and HR 1.11, 95% CI 0.96, 1.29 respectively). (Table 4) Based upon the significant interaction between SBP and this subgroup (P = 0.03), we also examined the converse population – patients without a prior diagnosis of hypertension. In the adjusted model of this population, there was no increased risk of mortality in either SBP cohort (HR 0.99, 95% CI 0.82; 1.19 and HR 0.92, 95% CI 0.63; 1.34 for SBP cohorts ≤130 and >160 mmHg respectively). The p-value for interaction for the subgroup of patients with a prior diagnosis of hypertension was non-significant for cardiovascular hospitalizations, so we cannot reliably examine the relationship between SBP and this outcome for this subgroup. This analysis suggests that the risk for cardiovascular hospitalization may be increased for both the SBP ≤ 130 and >160 mmHg cohorts within this subgroup (HRs of 1.13 [95% CI 1.00-1.28] and 1.29 [95%CI, 1.11-1.50], respectively), but additional studies would be needed to truly clarify this relationship.
Table 4.
Cox Proportional Hazards model on CKD subgroups of patients with and without prior diagnosis of hypertension
| Mortality | Cardiovascular hospitalization | |
|---|---|---|
| CKD patients with prior diagnosis of HTN (n = 3432 ) | ||
| SBP ≤130 mmHg | 1.32 (1.19; 1.47) | 1.13 (1.00; 1.28) |
| SBP = 131-160 mmHg | 1.00 | 1.00 |
| SBP >160 mmHg | 1.11 (0.96; 1.29) | 1.29 (1.11; 1.50) |
| CKD patients without prior diagnosis of HTN (n = 1129) | ||
| SBP ≤130 mmHg | 0.99 (0.82; 1.99) | 0.99 (0.79; 1.24) |
| SBP = 131-160 mmHg | 1.00 | 1.00 |
| SBP >160 mmHg | 0.92 (0.63; 1.34) | 1.03 (0.67; 1.62) |
HR (95% CI) reported. These models are all adjusted and control for gfr, age, gender, cad, diabetes, anemia, ace/arb, diuretic, spironolactone, proteinuria, and peripheral vascular disease. Patients with prior diagnosis of cancer were excluded from all models.
The non-significant p-values for interaction for the incident subgroup and subgroup of patients without heart failure make it impossible to establish the relationships between these subgroups and mortality or cardiovascular hospitalizations within this study. We did examine these outcomes as these results might identify future research questions or areas of interest for future researchers. We found that patients with incident CKD may experience an increased hazard for mortality of 1.37 (95% CI 1.20, 1.56) for the SBP ≤ 130 mmHg cohort, but the hazard for mortality was inconclusive among the SBP >160 mmHg cohort (HR 0.99, 95% CI 0.80, 1.23). Risk of cardiovascular hospitalization among incident CKD patients was similar, with increased risk for the SBP ≤130 mmHg cohort (HR 1.18, 95% CI 1.02, 1.37) and inconclusive risk for the SBP >160 mmHg cohort (HR 1.19, 95% CI 0.96; 1.47). When the adjusted models were repeated with the exclusion of patients with a prior diagnosis of heart failure, results mirrored those of the original cohort analysis. The SBP ≤130 mmHg cohort continued to experience increased risk of mortality (HR 1.15, 95% CI 1.01; 1.31) but indeterminate risk of cardiovascular hospitalizations (HR 1.07, 95% CI 0.93; 1.24). The SBP >160 mmHg cohort experienced increased risk of cardiovascular hospitalization (HR 1.27, 95% CI 1.07; 1.50) but their mortality risk was inconclusive (HR 1.04, 95% CI 0.88; 1.23).
We also examined the average systolic and diastolic blood pressures and pulse pressures by year and SBP cohort. Systolic blood pressure largely reflects the SBP cohort assignment for SBP group <130 and 131-160 mmHg (SBP 129-130 and 136-140 mmHg respectively), but is lower than the expected 160 mmHg (range 141-151 mmHg) for the SBP >160 mmHg cohort. Pulse pressure increases across increasing SBP cohorts.
Discussion
Our study suggests a relationship between lower systolic blood pressure and mortality among the older adults with CKD. Individuals in our population in the SBP ≤130 mmHg cohort appeared to have a higher risk of death compared to their counterparts with SBP 131-160mmHg (HR 1.22, 95% CI 1.11, 1.34). The association between SBP and mortality was attenuated in the adjusted model (unadjusted HR 1.43 versus adjusted HR of 1.22).
We are unable to draw conclusions about the risk of mortality among individuals in the SBP >160 mmHg cohort (Hazard ratio 1.06, 95% CI 0.93, 1.22), but our analysis suggests an increased hazard for cardiovascular hospitalization for this higher SBP cohort (HR 1.26), compared to their counterparts with SBP 131-160mmHg. The adjusted hazard for cardiovascular hospitalization in the SBP >160 mmHg was actually higher than the unadjusted ratio (1.26 compared to 1.18 respectively). This difference may reflect the impact of potentially protective medication use (specifically renin angiotensin aldosterone system blockade) which was more common in the SBP >160 mmHg cohort and which was controlled for in the adjusted analysis. We did not find a significant relationship between systolic blood pressure and fracture-related hospitalizations for any SBP cohort.
The reason for the apparent increase in mortality risk in older CKD patients with SBP ≤130 mmHg within our cohort is likely multi-factorial. The clinical characteristics of older CKD patients may predict decreased potential benefit from lower blood pressures in terms of mortality and rate of decline in kidney function.8,14,15 The impact of hypertension management on outcomes for CKD patients may hinge upon the presence and degree of proteinuria.16 This may have particular relevance for older CKD patients, because kidney disease is less likely to be proteinuric in older compared to younger adults.17
We reviewed follow-up outpatient blood pressure values to assess the potential impact of pulse pressure on our findings, as higher pulse pressure has been associated with adverse outcomes. Vaccarino and colleagues found a 32% increase in risk of heart failure with each 10 mmHg rise in pulse pressure.18 Data from the Framingham Heart Study also identified an increased risk of heart disease among individuals with a widened pulse pressure, which has been associated with an increased risk of cardiovascular events.19 Within our study, however, it is unlikely that pulse pressure is playing a significant role in the increased hazard for mortality in the SBP ≤ 130 mmHg cohort as pulse pressure is lowest within that cohort.
Of note, the retrospective nature of our study may also cause concern for residual confounding as a potential contributor to our results; this is of greatest concern in the lowest SBP cohort, in that a low baseline blood pressure can be a marker of a more severe illness profile. This concern is reinforced by the differentially high rates of heart disease and heart failure in the lowest SBP cohort. (Table 2) We have attempted to limit the risk of residual confounding by controlling for multiple comorbid illnesses in our adjusted Cox model, and also by examining the subgroup analysis of only patients without a prior diagnosis of heart failure (which was reassuring as the point estimates for mortality do not change in this subgroup analysis).
The results of our study highlight concerns surrounding hypertension management for older CKD patients. Importantly, data on safety and efficacy of very low blood pressure targets are lacking even among older adults without CKD. One prospective analysis identified a non-linear J-shaped relationship between blood pressure and outcomes for the elderly.20 In the SHEP (Systolic Hypertension in the Elderly Program) study, systolic blood pressure control was found to reduce the risk of fatal and non-fatal stroke in older adults; the systolic blood pressure achieved in the active treatment arm of this trial, however, was 143 mmHg.10 Similarly, HYVET (Hypertension in the Very Elderly Trial) found higher rates of mortality and cardiovascular events among untreated hypertensive older veterans.11 The average SBP in the treated arm of this trial was 140-150 mmHg. Both SHEP and HYVET demonstrate risk reduction with antihypertensive therapy, but neither study lowered blood pressure to the level currently recommended by CKD guidelines (<130/80 mmHg).5 Additionally, these trials excluded patients with abnormal kidney function. It remains unknown how applicable trials examining hypertension control in older adults without CKD are to an age-matched CKD population, but our study suggests that more aggressive antihypertensive management may not be without risk for older CKD patients.
Our analysis of patients with a prior diagnosis of hypertension demonstrated increased mortality risk for the SBP ≤ 130 mmHg cohort, which was greater than the risk of mortality for the same cohort in the entire population (HR 1.32 versus 1.22 respectively). Importantly, the converse was also true, and patients without a prior diagnosis of hypertension did not incur increased hazard for mortality or cardiovascular hospitalization based on baseline SBP. The reason for this difference is unknown, but could suggest that blood pressure changes (high or low) among patients without a history of hypertension may reflect acute changes in status as opposed to chronic illness and therefore play a lesser role in prognosticating longer term mortality and hospitalization outcomes. Within the incident subgroup, the lowest SBP cohort continued to have an increased risk of mortality (HR 1.37) compared to incident CKD patients with SBP 131-160 mmHg, indicating that duration of CKD may not be harmful in relation to important outcomes. The analysis of patients without a prior diagnosis of heart failure continued to show increased mortality risk for the lowest SBP cohort (HR 1.15).
The use of a single outpatient SBP value to define the baseline SBP presents a potential source of error. We chose to use a single SBP value as SBP baseline in order to maintain as broad a cohort as possible. Limiting our cohort to patients with two or more SBP values would potentially have excluded the sickest patients (who may have met CKD criteria but died prior to contributing their first SBP value). The use of a single SBP value may have resulted in some regression dilution bias, but in this scenario regression dilution bias would make it less likely to detect a significant difference between groups. As we were able to detect a difference between SBP cohorts, we suspect that correcting for regression dilution bias may have strengthened the harmful effect of a low systolic blood pressure at baseline.
We also could have potentially increased our cohort size by not including proteinuria in our adjusted analysis (given the high missing percent for this characteristic). We felt that the ability to examine the impact of the presence of proteinuria outweighed the harm of excluding those patients without a proteinuria measurement. In the future, prospective studies on this population could curb this limitation by improving the completeness of proteinuria data collection.
In examining our results, we considered how an alternate index date, such as using the first SBP value after the second qualifying eGFR <60 ml/min/1.73m2, might have impacted our results. A sensitivity analysis of three additional subgroups (only incident CKD patients with an SBP value within 90 days of the index date, only prevalent CKD patients, and only patients with at least one SBP value after their second low eGFR) yielded very similar hazard ratios to our original cohort analysis, with no change in directionality of point estimates.
Our decision to not pursue time varying analysis may be viewed as a limitation. We feel modeling BP level as a time-varying exposure could provide interesting information, but would essentially address a different research question. A time varying analysis, looking at blood pressures closest in time to outcomes, would examine the acute role of blood pressure and the outcome; our primary interest, however, was to examine the prognostic significance of blood pressure for these outcomes over time.
Our study specifically examined SBP values in alignment with current CKD guidelines goals (SBP <130 mmHg) and SBP values found to be associated with benefit (SBP 140-150 mmHg) and harm (SBP >160 mmHg) among older adults without CKD. We focused our analyses on these SBP cohorts in order to speak specifically to the relationship between significant outcomes and current hypertension management goals. Ongoing clinical trials, including SPRINT (the Systolic Blood Pressure Intervention Trial) will examine a target SBP of <140 versus <120 mmHg among CKD patients and will include older adults within the study population; results from this trial and other future research will augment our understanding of the relationship between different levels of blood pressure control on mortality, cardiovascular outcomes, and CKD progression among older CKD patients.
The retrospective nature of our data limits the conclusions that can be derived from our results, in that this study cannot establish causation between SBP and outcomes such as mortality and hospitalizations. This study does describe an interesting relationship between SBP and these outcomes to generate future hypotheses for research. The increased risk of mortality described for older adults with CKD who have baseline SBP ≤ 130 mmHg in our study population remains unexplained by conventional knowledge and raises new questions regarding the appropriateness of current CKD hypertension guidelines among the elderly with CKD. Future trials among older CKD patients are needed to help tailor CKD guidelines and care for this unique patient group.
Acknowledgements
The authors would like to thank Dr. Sharon Anderson for her input and feedback on this manuscript.
Support: Funding for this work was provided through the Oregon Clinical and Translational Institute Career development pilot project grant (UL1 RR024140). Dr Weiss is also supported by an Agency for Healthcare Research and Quality T32 training grant (HS017582).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: the authors declare that they have no relevant financial interests.
Reference List
- 1.US Renal Data System . Chronic kidney disease. Vol. 1. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; Bethesda, MD: 2009. U.S. Renal Data System, USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease in the United States. Report No. [Google Scholar]
- 2.O’Hare AM, Bertenthal D, Covinsky KE, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol. 2006 March;17(3):846–53. doi: 10.1681/ASN.2005090986. [DOI] [PubMed] [Google Scholar]
- 3.O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007 October;18(10):2758–65. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 4.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006 January;69(2):375–82. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 5.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003 October;42(Supplement 3):1–201. [PubMed] [Google Scholar]
- 6.Shulman NB, Ford CE, Hall WD, et al. The Hypertension Detection and Follow-up Program Cooperative Group Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. Hypertension. 1989 May;13(5 Suppl):I80–I93. doi: 10.1161/01.hyp.13.5_suppl.i80. [DOI] [PubMed] [Google Scholar]
- 7.Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD, The MRFIT Research Group Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. JAMA. 1992 December 2;268(21):3085–91. [PubMed] [Google Scholar]
- 8.Wright JT, Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002 November 20;288(19):2421–31. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 9.Glynn RJ, Field TS, Rosner B, Hebert PR, Taylor JO, Hennekens CH. Evidence for a positive linear relation between blood pressure and mortality in elderly people. Lancet. 1995 April 1;345(8953):825–9. doi: 10.1016/s0140-6736(95)92964-9. [DOI] [PubMed] [Google Scholar]
- 10.SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991 June 26;265(24):3255–64. [PubMed] [Google Scholar]
- 11.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008 May 1;358(18):1887–98. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 12.Selby J, Smith D, Johnson ES, Rabel MA, Friedman GD, McFarland B. Kaiser Permanente Medical Care Program. In: Strom BL, editor. Pharmacoepidemiology. 4th ed John Wiley & Sons; New York: 2005. pp. 241–60. [Google Scholar]
- 13.Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek KD. Deaths: final data for 1999. Natl Vital Stat Rep. 2001 September 21;49(8):1–113. [PubMed] [Google Scholar]
- 14.Agarwal R. Blood pressure components and the risk for end-stage renal disease and death in chronic kidney disease. Clin J Am Soc Nephrol. 2009 April;4(4):830–7. doi: 10.2215/CJN.06201208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toto RD, Mitchell HC, Smith RD, Lee HC, McIntire D, Pettinger WA. “Strict” blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney Int. 1995 September;48(3):851–9. doi: 10.1038/ki.1995.361. [DOI] [PubMed] [Google Scholar]
- 16.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994 March 31;330(13):877–84. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 17.O’Hare AM, Kaufman JS, Covinsky KE, Landefeld CS, McFarland LV, Larson EB. Current guidelines for using angiotensin-converting enzyme inhibitors and angiotensin II-receptor antagonists in chronic kidney disease: is the evidence base relevant to older adults? Ann Intern Med. 2009 May 19;150(10):717–24. doi: 10.7326/0003-4819-150-10-200905190-00010. [DOI] [PubMed] [Google Scholar]
- 18.Vaccarino V, Berger AK, Abramson J, et al. Pulse pressure and risk of cardiovascular events in the systolic hypertension in the elderly program. Am J Cardiol. 2001 November 1;88(9):980–6. doi: 10.1016/s0002-9149(01)01974-9. [DOI] [PubMed] [Google Scholar]
- 19.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999 July 27;100(4):354–60. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 20.Oates DJ, Berlowitz DR, Glickman ME, Silliman RA, Borzecki AM. Blood pressure and survival in the oldest old. J Am Geriatr Soc. 2007 March;55(3):383–8. doi: 10.1111/j.1532-5415.2007.01069.x. [DOI] [PubMed] [Google Scholar]



