Abstract
Bluetongue (BT) is still present in Europe and the introduction of new serotypes from endemic areas in the African continent is a possible threat. Culicoides imicola remains one of the most relevant BT vectors in Spain and research on the environmental determinants driving its life cycle is key to preventing and controlling BT. Our aim was to improve our understanding of the biotic and abiotic determinants of C. imicola by modelling its present abundance, studying the spatial pattern of predicted abundance in relation to BT outbreaks, and investigating how the predicted current distribution and abundance patterns might change under future (2011–2040) scenarios of climate change according to the Intergovernmental Panel on Climate Change. C. imicola abundance data from the bluetongue national surveillance programme were modelled with spatial, topoclimatic, host and soil factors. The influence of these factors was further assessed by variation partitioning procedures. The predicted abundance of C. imicola was also projected to a future period. Variation partitioning demonstrated that the pure effect of host and topoclimate factors explained a high percentage (>80%) of the variation. The pure effect of soil followed in importance in explaining the abundance of C. imicola. A close link was confirmed between C. imicola abundance and BT outbreaks. To the best of our knowledge, this study is the first to consider wild and domestic hosts in predictive modelling for an arthropod vector. The main findings regarding the near future show that there is no evidence to suggest that there will be an important increase in the distribution range of C. imicola; this contrasts with an expected increase in abundance in the areas where it is already present in mainland Spain. What may be expected regarding the future scenario for orbiviruses in mainland Spain, is that higher predicted C. imicola abundance may significantly change the rate of transmission of orbiviruses.
Introduction
Understanding the patterns and mechanisms of species occurrence and abundance is a central issue in ecology and epidemiology. Biogeography is an increasingly important discipline and is highly useful for addressing determinants of animal, plant or pathogen species distribution, since the broad-scale factors influencing species spatial patterns can be accurately identified from biogeographical modelling [1]. The increased application of spatial models to conservation biology and wildlife management has been driven, at least in part, by the advent of extensive computerised spatial databases — for example the Worldclim project (www.worldclim.org) — and powerful analytical tools [2].
This discipline has rarely focused on pathogen ecology (but see [3]), i.e. epidemiology [4], as was demonstrated at the 4th meeting of the International Biogeography Society in 2009, where a symposium was designed to introduce to biogeographers the many ways that this discipline can contribute to the study of disease ecology [5]. The scarcity of epidemiological studies conducted from a biogeographical perspective is probably due to the fact that epidemiological processes have key differences compared to other biological phenomena [6]. Thus, abiotic conditions may have less influence on pathogen distribution than on animal or plant species distribution. This is due to the dependence of pathogens on other species, for example hosts, which is even more complex in the case of vector-borne pathogens [7]. Hence, biotic factors in these systems are the main determinants of pathogen distribution, and abiotic factors may indirectly affect their distribution by interfering with host and vector distribution and abundance [8].
In this sense, some studies have been conducted to explain and predict vector distribution and abundance from an epidemiological perspective. Most of them were motivated by disease outbreaks [9], such as bluetongue, malaria or Crimean-Congo hemorrhagic fever [10]–[12]. Nevertheless, a better approach would be to determine the distribution and abundance of vectors in advance of disease outbreaks to establish sound disease risk management policies.
Bluetongue (BT) is one of the vector-borne diseases that has raised increasing interest in vector ecology among epidemiologists. Currently, BT outbreaks are still ongoing in Europe and new introductions remain a risk. An important route of the BT virus (BTV) being introduced into Europe is via wind-borne infected midges arriving from northern African countries. This potential threat should be addressed by research-based protection and prevention policies; in fact, ecological studies on BTV vectors are one of the main targets of scientific preventive research [13]. In this respect, Culicoides imicola (Diptera: Ceratopogonidae) is the major vector of BTV in the African continent and Mediterranean Europe [14] where it shares vector ability with other Culicoides spp. [15], [16].
C. imicola biogeography has already been studied from an epidemiological perspective. These models, based on climate and satellite imagery, identified some of the key parameters determining C. imicola distribution in the Mediterranean area. C. imicola spatial distribution in Europe is very patchy, showing a high dependence on local habitat conditions, such as soil type, soil moisture and topography [10], [17], [18]. Different Culicoides spp. have specialized in using different conditions [19], and breed in a range of moist microhabitats. Nonetheless, soil type strongly determines the ability of C. imicola to become established in any given zone, presumably by interfering with the availability of breeding sites [18]–[20]. In South Africa, C. imicola has been found to be absent from sandy areas [17], [21], whereas distance from moisture-retentive soil was the most important factor determining C. imicola presence in Italy [18]. However, to date, no study has been designed to explore the pure and combined effects of a wide variety of factors (both abiotic and biotic) that determine the distribution and abundance of this arthropod vector species, including the influence of hosts on C. imicola population dynamics.
Ever since the initial C. imicola distribution models were developed, in which a close link between species distribution and climatic variables was demonstrated, researchers began to assess the effects of climate change on this species [22]. The conventional wisdom was that global climate change would result in an expansion of tropical pathogens, particularly those transmitted by vectors, throughout temperate areas [23], [24]. Despite its high potential relevance, the first approaches conducted on C. imicola were quite simplistic — authors considered a putative increase of 2°C in mean temperature and then recalculated the potential species distribution using transformed climatic variables. Nonetheless, projected future scenarios of climate change suggested that global warming would be the main factor modulating the northward expansion of C. imicola into Europe [22]. However, the predictions of the climatic models did not perfectly fit the observed abundance data since other factors regulate species distribution (as mentioned) and therefore its expansion [25], [26]. C. imicola is able to spread northwards but probably only into those areas where non-climate factors are suitable for the species, as suggested by [10].
In this respect, few statistical models include potentially important non-climate variables. The accuracy of these predictions could be increased by including other ecogeographical variables in the models [27], such as soil characteristics [20], the presence of wild or domestic potential hosts [28] or geographical factors, e.g. showing species population dynamics [29].
In this context, we attempted to improve our understanding of the biotic and abiotic determinants of C. imicola as follows: i) by modelling its present abundance with topoclimatic, host, spatial and soil conditions using variation partitioning procedures; ii) by studying the spatial pattern of the predicted abundance of C. imicola relative to BT outbreaks to assess the spatial association between vector abundance and BT occurrence; and iii) by investigating how the predicted current distribution and abundance patterns of C. imicola might change under future (2011–2040) scenarios of climate change according to the Intergovernmental Panel on Climate Change.
Materials and Methods
Study area and C. imicola abundance data
The study area was peninsular Spain. This is situated in southwest Europe and covers 493,518 km2 (nearly 85% of the Iberian Peninsula). It is a heterogeneous territory in climatic terms, with a mainly eastward and southward decreasing precipitation gradient (range 200–2000 mm) and a mainly northward decreasing temperature gradient [30]. The northern and Mediterranean coasts are bordered by mountain ranges and there are some east-to-west mountain chains in the centre of the Peninsula.
C. imicola capture data from 2005 to 2008 were provided by the Spanish bluetongue national surveillance programme; for details see [31]. In line with previous studies [31], only catch data obtained between April and October — annual peak abundance for C. imicola in Spain [32] — were used for analytical purposes. The only localities included in the analyses were those where sampling was performed at least once a month between April and October. For each sampled locality we obtained the maximum number of C. imicola captured per night during that period (April–October) and during the 3 years considered in this study as this abundance index (our response variable) has been shown to be consistently related to the real Culicoides spp. annual abundance [33].
The geographical coordinates of the sampled sites were recorded using a hand-held GPS receiver and this information was transferred to UTM 10×10 km square (n = 263, see Figure 1) which was the territorial unit – locality – used in this study.
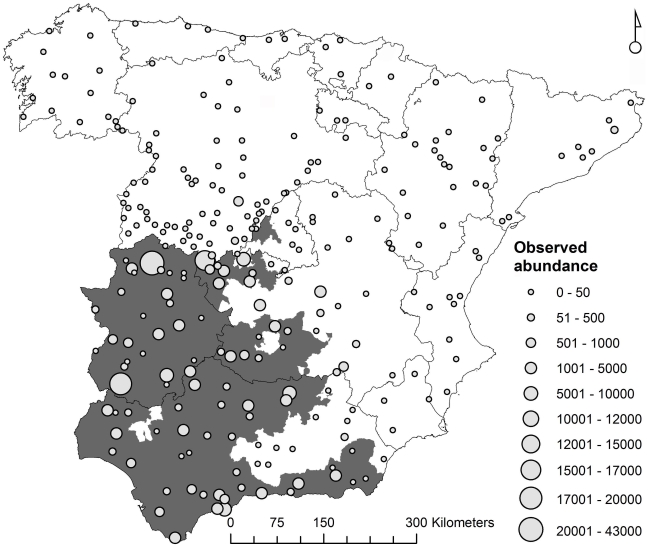
Figure 1. Location of the sampling localities.
Spatial distribution of the sites (UTM 10×10 km squares) in which the abundance of Culicoides imicola was recorded (n = 263) by the Spanish national bluetongue surveillance programme between 2005 and 2008 (symbol size is proportional to the maximum number of C. imicola captures per night). These were used as a training dataset to forecast the species abundance in peninsular Spain. Regional veterinary units in which bluetongue outbreaks were declared in livestock in 2007 are shown (grey areas).
Predictor variables
To identify the factors that affected C. imicola abundance in each square, we performed spatial modelling to compare the observed abundance of the species with 21 explanatory ecogeographical variables related to the following factors (see Table 1): spatial situation (2 variables), topoclimatic conditions (7 variables), hosts (5 variables), and soil (7 variables). These variables were chosen on the basis of their availability at our study scale and their potential predictive power according to previous knowledge on C. imicola ecology [16], [18], [19],[34],[35].
Table 1. Variables used to model the abundance of bluetongue vector Culicoides imicola.
| Code | Variable description | Factor | |
| LO | Longitude (m) | Spatial location | |
| LA | Latitude (m) | ||
| A | Mean altitude (masl) | Topography | Topoclimate |
| S | Slope (degrees; calculated from A) | ||
| T | Mean temperature in May–October (°C) | Climate | |
| Ts | Temperature seasonality | ||
| P | Precipitation in May–October (mm) | ||
| Ps | Precipitation seasonality | ||
| R | Annual radiation (Kwh m−2 day−1) | ||
| Fred | Favourability for red deer | Wild hosts | Hosts |
| Froe | Favourability for roe deer | ||
| Fwild | Favourability for wild boar | ||
| DL | Livestock density sheep/goat/cattle (ind/Ha) | Livestock | |
| DC | Cattle density (ind/Ha) | ||
| WL | Woodland (%) | Soil | |
| IL | Irrigated land (%) | ||
| UL | Sparsely vegetated areas (%) | ||
| SL | Scrublands (%) | ||
| NDVI | Normalized difference vegetation index (NDVI) | ||
| NDVIs | NDVI seasonality | ||
| SP | Soil permeability | ||
The spatial resolution of the predictors was not homogeneous between factors and thus all the ecogeographical information was finally translated into UTM 10×10 km squares using the Extract module of the Idrisi Andes software package.
Spatial factors
We included spatial variables (the longitude and latitude of each square's centroid point) in the models to reveal geographical trends in species distribution associated with historical events or species population dynamics [29], .
Topoclimatic factors: topography and climate
The importance of the topoclimatic factor in explaining species distribution and abundance at large spatial scales is well known [37], [38]. Thus, we compared the observed abundances with two predictor variables (altitude and slope) that provide orographical information. Altitude was available in digital format by the Land Processes Distributed Active Archive Center (http://LPDAAC.usgs.gov) at a resolution scale of 100×100 m, and slope was calculated based on altitude using the Idrisi SLOPE command [39].
Climate data (temperature and precipitation) were obtained from the Spanish ‘Agencia Estatal de Meteorología’ (AEMET; http://www.aemet.es). These data were created by the regionalization to Spain of the climate change models produced by the Intergovernmental Panel on Climate Change. This study used a general circulation model, CGM2, from the Canadian Climate Centre for Modeling and Analysis. CGM2 was run with the conditions forecast by the Special Report on Emissions Scenarios A2 and B2 [40] for the period 1961–1990 (later projected to future periods). Scenarios A2 and B2 represent an intermediate position of the range of projected temperature change scenarios for Spain, A2 being medium-high and B2 medium-low [41]. So, A2 is defined as a world of strengthening regional cultural identities, with an emphasis on family values and local traditions, high population growth, and less concern for rapid economic development. In turn, B2 is defined as a world in which the emphasis is on local solutions to economic, social, and environmental sustainability, with lower population growth than A2. The scenario A2 is predicted to change in temperature between periods at a higher rate than the B2 scenario, but in contrast, the precipitation is expected to change in a slightly higher rate in B2 than in A2 [41]. These scenarios are usually selected to study the effect of climate change on species distribution in the Iberian Peninsula [27], [42]. Further details on the peculiarities of each emission scenario and the expected climatic changes in the future can be revised, among others, in [27], [41].
Following the procedure to obtain the climatic variables described by [27], we calculated mean temperature and precipitation, and their seasonalities, for each emission scenario (A2 and B2) and study period (present and future 2011–2040). Mean temperature and precipitation were quantified from May to October only since this period includes the annual peak abundance for C. imicola [32], . Seasonality was measured as the variation coefficients of monthly means in a year [16]. Mean solar radiation was also considered as potential predictor of species abundance. Unfortunately, mean solar radiation was not included in the list of variables quantified for each emission scenario, and thus we used the same radiation data for both A2 and B2 emission scenarios.
Hosts: wild ungulates and livestock
In addition to livestock, several wild species were described as potential hosts for BTV in Spain [44], [45], [46] and therefore for BTV vectors. Thus, the relative abundances of both wild and domestic hosts were considered to explore their effect in explaining the abundance of C. imicola.
Unfortunately, data on wild species abundance at a geographical scale are very scarce. We therefore used the favourability function [47] to obtain the environmental favourability for wildlife, i.e. red deer (Cervus elaphus), roe deer (Capreolus capreolus) and wild boar (Sus scrofa), from presence/absence data, as an index of species abundance. The favourability for a species was significantly related to species abundance as described in a previous study [48]. Briefly, the favourability function is basically a logistic regression that assesses the local variations in presence probability relative to the overall species prevalence (ratio of the number of presences to absences). Using the favourability function, the values for all models are levelled according to the species prevalence in each area [47].
The environmental predictors shown in Table 2 were used to model the environmental favourability for potential wild hosts for C. imicola. Wild ungulate distribution data were extracted from the study by [49] and were offered for 10×10 km UTM cells. For each species, we performed a forward-backward stepwise logistic regression procedure to select a subset of significant predictors of the species distribution. Probabilities yielded by logistic regression (P) may be used to calculate favourability values (F), where n1 is the number of presences and n0 the number of absences [47].
Table 2. Variables used to model the environmental favourability for potential wild hosts of Culicoides imicola: red deer, roe deer and wild boar.
| Variable description | Red deer | Roe deer | Wild boar |
| Mean annual precipitation –P– (mm)(1) | + | + | |
| Maximum precipitation in 24 h –MP24– (mm)(1) | + | + | |
| Relative maximum precipitation ( = MP24/P) | |||
| Mean annual number of days with precipitation ≥0.1 mm(1) | − | ||
| Mean annual number of hail days(1) | |||
| Mean annual number of foggy days(1) | + | + | + |
| Mean annual potential evapotranspiration –PET– (mm)(1) | − | − | |
| Mean annual actual evapotranspiration (mm) ( = min [P,PET]) | + | + | |
| Mean relative air humidity in January at 07:00 h –HJN– (%)(1) | + | + | − |
| Mean relative air humidity in July at 07:00 h –HJL– (%)(1) | − | + | |
| Annual air humidity range (%) ( = HJL-HJN)(1) | |||
| Mean temperature in January –TJN– (°C)(1) | − | − | |
| Mean temperature in July –TJL– (°C)(1) | + | + | |
| Annual temperature range (°C) ( = TJL-TJN)(1) | + | ||
| Mean annual temperature (°C)(1) | + | ||
| Mean annual number of frost days (minimum temperature ≤0°C)(1) | − | ||
| Continentality index(1) | + | ||
| Humidity index(1) | + | + | + |
| Mean annual insolation (hours year−1)(1) | − | ||
| Distance to the nearest town with more than 100,000 inhabitants (km)(2) | + | + | |
| Distance to the nearest town with more than 500,000 inhabitants (km)(2) | + | ||
| Distance to the nearest highway (km)(2) | + | − | + |
Variables included in each model and the sign of their coefficients (positive or negative) are shown. All variables were retained at p<0.01.
Sources:
[30];
[53]; data on the number of inhabitants of urban centres taken from the ‘Instituto Nacional de Estadística’ (http://www.ine.es).
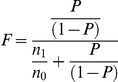 |
Data on livestock were provided by the Spanish ‘Ministerio de Medio Ambiente y Medio Rural y Marino’ (http://www.marm.es) at a regional veterinary unit level (n = 490 in continental Spain) for 2008. Census data on cattle and small ruminants (sheep and goats) were used to estimate two predictors, livestock density (sheep, goat and cattle) and cattle density. We considered that all domestic ruminants are susceptible to host BTV — and therefore to C. imicola — and thus they were together considered in a livestock abundance index which was used in the models. Cattle density was independently considered in the models because of the greater amount of dung production compared to other domestic ruminants. The higher amounts of organic material contributed by cattle may promote breeding sites favourable to C. imicola despite the fact that this midge species does not directly breed in cattle dung unlike other Culicoides spp. [19]. These variables were transferred from counties to UTM 10×10 km squares, assuming that the mentioned densities were constant through the regional veterinary unit. Therefore, all UTM squares included in a unit — the major part of its surface area — had the same density values.
Soil: land cover and pedological variables
Six predictors were included in our model which were related to land cover (including two NDVI-related predictors) and one related to soil permeability (Table 1). NDVI, and its seasonality, were included within this factor, and not within climate [50]; even when it is indirectly related to precipitation, NDVI is a measure of the amount and vigour of vegetation on the land surface directly related to soil moisture [51].
For each UTM square, the frequency of occurrence was calculated of four land cover variables (woodland, irrigated land, sparsely vegetated areas and scrubland) — which were obtained from the CORINE Land Use/Land Cover database [52] — at a spatial resolution of 250×250 m (Table 1). The annual mean value and seasonality (see above for details on the calculations) of the NDVI were derived from a monthly imagery dataset over an 18-year period (from 1982 to 2000) downloaded from the NASA Goddard DAAC website (ftp://daac.gsfc.nasa.gov/data/avhrr/) at a resolution scale of 1000 m. Finally, soil permeability was obtained from a map of synthesis of ground-water aquifers with three different permeability classes [53]. We determined soil permeability for each UTM 10×10 km square by calculating the average of the values assigned to the pixels within the square.
Statistical analyses
Abundance model
Firstly, we avoided correlations between predictor variables related to a specific factor since multicollinearity within a factor unnecessarily affects the automatic stepwise variable selection procedures in regression analysis. Two variables are correlated when the absolute value of Spearman's coefficient is higher than or equal to 0.8 [54]. When two or more explanatory variables were correlated we selected the variable that was most significantly related to the response variable [55] for inclusion in the models.
For each emission scenario we related the observed C. imicola abundance (response variable) to the distribution of the climatic variables and the other predictors previously described (Table 1). Given the over-dispersion of our data, for modelling purposes we used generalized linear models (GLM) with a negative binomial distribution and a logarithmic link function [56]. Count models, such as Poisson and negative binomial, were successfully used for studying factors affecting population abundance and for conducting population estimates [57], [58]. We estimated the scale parameter (K) for the dependent variable [59], with x and s2 as the mean and variance of the data:
To select a subset of significant predictors we used a forward-backward stepwise model-selection procedure. All steps were assessed to decrease the Akaike Information Criterion, AIC [60].
Finally, we compared the predicted C. imicola abundance with the number of BT outbreaks detected in livestock at the regional veterinary unit level (outbreak data were taken from: http://rasve.mapa.es) to assess the spatial association between vector abundance and the BT occurrence rate [50], [61]. For this purpose, five thresholds were fitted to define the highest abundance of C. imicola (500, 5000, 12000, 20000 and 50000). We quantified the percentage of localities under each threshold in which at least one BT outbreak was detected in 2007 as an index of spatial overlap between C. imicola abundance and the number of outbreaks. Data on BT outbreaks were considered for 2007 only because of the emergence of the highly pathogenic BTV s1 in the Iberian Peninsula and the absence of preventive vaccination of livestock at that time. Thus, the number of BTV outbreaks in 2007 resembled the natural expansion of BTV within the Spanish mainland. This allowed us to associate an epidemiologic meaning to C. imicola abundance predictions under scenarios of future climate change. All statistical calculations were made using the SPSS 17 software.
Variation partitioning procedure
Variation partitioning is a quantitative method in which the variation in a dependent variable can be separated into independent components reflecting the relative importance of different groups of explanatory variables (factors) and their joint effects. This allows us to specify how much of the variation of the final model is explained by the pure effect of each factor, i.e. not affected by collinearity with other factors in the model, and which proportion is attributable to their shared effect [36], [62], [63].
To do this, independent models for each factor were developed using the statistically significant variables of each factor included in the final model. These partial models are a measure of all the variability explained by each factor (the circles in the Venn diagram). Similarly, we developed partial models for each pair and trio of factors. Then, variation partitioning procedures were applied to the final model output (FMO), i.e. C. imicola abundance predicted with all factors. For the partition of 4 factors (a, b, c and d), the FMO was correlated against the partial predicted abundance calculated with the retained variables pertaining to 3 of the factors (a, b and c, for example). The residuals of this correlation represent the pure effects of the 4th factor (d in this example), i.e. the part of the FMO not explained by the other 3 factors. The amount of variation explained by the pure effect of d (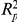 ) was obtained with the Pearson's coefficient (squared to obtain the amount of variation explained) obtained correlating the FMO with the partial predicted abundance with the 3 included factors as follows:
) was obtained with the Pearson's coefficient (squared to obtain the amount of variation explained) obtained correlating the FMO with the partial predicted abundance with the 3 included factors as follows: 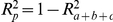 . This process was repeated to obtain the variation explained by the pure effect of each factor. The variation explained simultaneously by two factors (
. This process was repeated to obtain the variation explained by the pure effect of each factor. The variation explained simultaneously by two factors (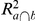 ; combined effects) was obtained using the partial predicted abundance calculated with the other two factors, specifically with the R2 obtained by correlating FMO and this partial predicted abundance, and the R2 of the pure effects of the two factors involved in the intersection as follows:
; combined effects) was obtained using the partial predicted abundance calculated with the other two factors, specifically with the R2 obtained by correlating FMO and this partial predicted abundance, and the R2 of the pure effects of the two factors involved in the intersection as follows: 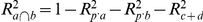 . The variation attributable to intersections among trios can be analogously subtracted as follows:
. The variation attributable to intersections among trios can be analogously subtracted as follows: 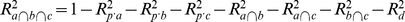 [63]. For applications and further details see [8], [29], [64], [65].
[63]. For applications and further details see [8], [29], [64], [65].
Future projections: comparing present and future C. imicola abundances
The predicted abundance of C. imicola for each emission scenario was projected to the future by replacing the current temperature and precipitation variables in the models with those expected according to each climate change scenario for the future period. Thus, two predictions of C. imicola abundance were forecast, one per emission scenario. To do this, the values of the other variables included in the final models (spatial, topography, host and soil factors) were not modified between periods [27].
Multicollinearity among predictors can be a real problem when a model is projected in other spatial or temporal situations outside the range where it was calibrated [66]. So, we used each predictors' variance inflation factor (VIF) to quantify collinearity among predictors in the models for the present (A2 and B2) because they were projected to a future situation. VIFs were calculated for each predictor as the inverse of the coefficient of non-determination for a regression of that predictor on all others (see [67]). VIF is a positive value representing the overall correlation of each predictor with all others in a model. Previous authors used a value of VIF>10 as the threshold over which multicollinearity can be considered a problem [68], but a more stringent approach is to use values as low as 3 [67].
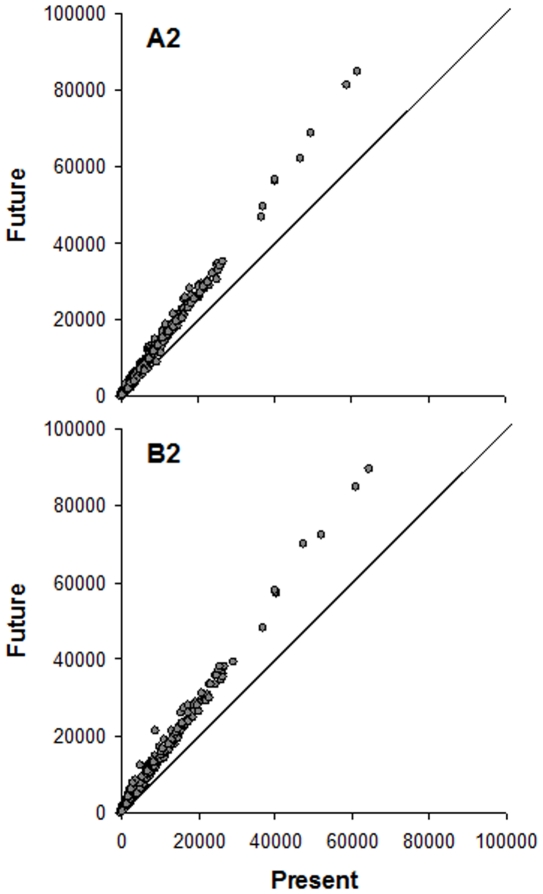
We used a dual approach to compare present and future C. imicola abundances. On the one hand, we quantitatively assessed the relationship between C. imicola abundances predicted for the present and abundances forecast for the future. This was performed to assess if the predicted abundances would be higher (or lower) in the future than those predicted for the present at a locality level. Thus, we simply represented in a scatterplot the abundance forecast for the future (y-axis) relative to the abundance predicted for the present (x-axis), and visually evaluated if values for the future were over (or under) the diagonal, which represents the situation where the abundance forecast for the future equals that predicted for the present. On the other hand, we assessed the differences between periods (present and future) in terms of the number of localities with ‘high’ predicted C. imicola abundance. To this end we used the same procedure based on C. imicola abundance thresholds previously described. We quantified (as percentages) the localities with a predicted abundance over each threshold for the present that were also forecast over this threshold for the future as an index of the localities maintaining high C. imicola abundance. We also quantified localities that were forecast to be over the threshold for the future and that were predicted to be under the threshold in the model for the present, which is indicative of the localities where C. imicola was forecast to substantially increase its abundance in the future.
Results
Wildlife abundance indices
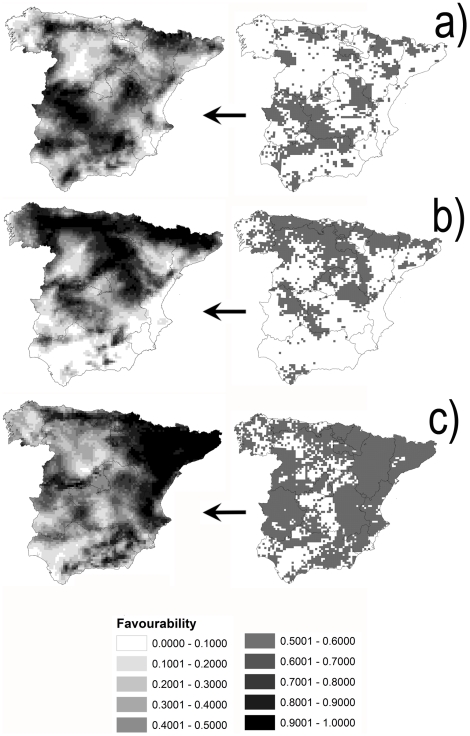
The final functions for wild ungulate favourability models are shown in Table 2. Favourability values for red deer were high across the southwestern and northern areas of peninsular Spain (Figure 2). Higher favourability values were found in the northern half of peninsular Spain for roe deer and in eastern and northeastern areas for wild boar.
Figure 2. Maps of potential wild hosts abundance.
Favourability, where 0 represents minimum favourability and 1 represents maximum favourability, in UTM 10×10 km squares for potential wild hosts of Culicoides imicola: red deer (a), roe deer (b) and wild boar (c). Current distributions of these species, referring to 10×10 km UTM grid cells, are depicted in the maps of the right; adapted from [49].
Environmental conditionants of C. imicola abundance
The mean observed abundance of C. imicola, quantified as the maximum number captured per night between April and October, was (mean ± SE, minimum–maximum): 679.19±200.39, (0–43000) in the period 2005–2008.
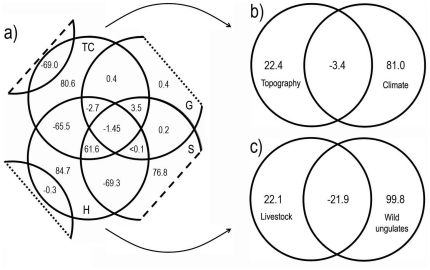
Only 3 out of the 21 considered predictors (latitude, temperature seasonality and annual radiation) were not included in the final A2 and B2 models due to collinearity. The final models for A2 and B2 scenarios explained 59.1% and 59.2% of the total deviance, respectively, and retained variables of the four factors (see Table 3). Table 3 shows the variables which were found to drive C. imicola abundance in peninsular Spain. The models obtained for the studied emission scenarios were very similar both in relation to the variables retained and the predicted abundance (Figure 3) regarding the deviation explained. Given the high similarity detected between both final models, we performed the variation partitioning procedure on one of them only. The one selected was B2 since it explained a higher deviance than A2. Variation partitioning demonstrated that the pure effect of host and topoclimate factors explained a high percent (>80%) of the variation (see Figure 4a). The pure effect of soil was the next in importance in explaining the abundance of C. imicola. As expected, a high amount of variation was explained by the combined effect of hosts-soil, hosts-topoclimate and soil-topoclimate, since there is a close relationship between each pair of factors, such as the NDVI linking topoclimate and soil. Partitioning complex factors (topoclimate and hosts) into their components (topography/climate and wild/domestic hosts, respectively) demonstrated that within topoclimate the highest amount of variation was explained exclusively by climate (Figure 4b), whereas wild ungulates explained a higher variation than livestock within host factors (Figure 4c).
Table 3. Variables included in the Culicoides imicola abundance model (GLM binomial negative distribution with logarithmic link function) according to the CGM2 circulation model and the A2 and B2 emission scenarios.
| Variable | A2 | B2 | ||
| Estimate | Wald | Estimate | Wald | |
| LO | 2.11*10−6 | 57.624 | 1.80*10−6 | 43.931 |
| A | −0.004 | 1435.267 | −0.004 | 1540.023 |
| S | 0.366 | 824.084 | 0.369 | 846.350 |
| T | 0.134 | 42.766 | 0.147 | 51.287 |
| P | −0.010 | 248.338 | −0.011 | 276.241 |
| Ps | −0.010 | 9.811 | −0.017 | 29.835 |
| Fred | 7.551 | 2273.938 | 7.619 | 2314.680 |
| Froe | −2.465 | 298.214 | −2.530 | 310.008 |
| Fwild | −3.911 | 871.300 | −3.932 | 882.893 |
| DL | 0.258 | 33.594 | 0.255 | 32.732 |
| DC | −1.925 | 123.697 | −1.929 | 124.670 |
| WL | −0.038 | 270.870 | −0.038 | 270.036 |
| IL | 0.007 | 23.012 | 0.007 | 23.607 |
| UL | 0.016 | 44.732 | 0.017 | 52.074 |
| SL | 0.010 | 30.608 | 0.010 | 30.282 |
| NDVI | 0.033 | 213.084 | 0.036 | 248.979 |
| NDVIs | 0.068 | 232.390 | 0.069 | 243.169 |
| SP | 1.196 | 1666.474 | 1.201 | 1687.036 |
| Intercept | −2.945 | 48.666 | −2.882 | 46.331 |
All variables were retained at p<0.01. Variables coded as in Table 1.
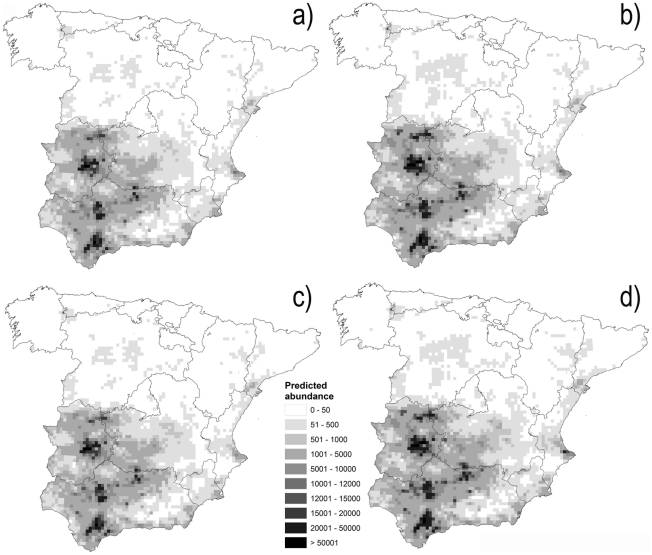
Figure 3. Predicted Culicoides imicola abundance.
Current predicted Culicoides imicola abundance (maximum number of captures per night) according to the CGM2 circulation model and the A2 (a) and B2 (c) emission scenarios (see text for details). Abundance was forecasted for the 2011–2040 period using CGM2 circulation model and the A2 (b) and B2 (d) emission scenarios.
Figure 4. Variation partitioning results.
Results of variation partitioning of the final model for the B2 emission scenario (a), and of the partial models obtained for the topoclimatic factor (b), and for the host — wild ungulates and livestock — factor (c). Values shown in the diagrams are the percentages of variation explained exclusively by topoclimate (TC), hosts (H), spatial location (G), and soil (S) and by the combined effect of these factors. See Table 2 for details of the variables included in each of the mentioned factors.
C. imicola abundance and risk of BT outbreaks
A close link was confirmed between C. imicola abundance and BT outbreaks, even when the vector abundance predicted for the present was low (Table 4), i.e. over 500 individuals maximum capture. In addition, only around 5% of localities where C. imicola abundance was predicted as absent (zero abundance) for the present had at least one BTV outbreak. We should mention that the predicted maximum C. imicola abundance of over 500 individuals in the present was almost exclusively restricted to those areas where BT outbreaks took place in 2007 (see Figure 1).
Table 4. Spatial overlap between predicted Culicoides imicola abundance for present and future periods according to the CGM2 circulation model.
| Thresholds/Model | P | FP | BT | |
| 500 | A2 | 100 | 2.72 | 89.62 |
| B2 | 100 | 4.36 | 88.93 | |
| 5000 | A2 | 100 | 1.67 | 98.71 |
| B2 | 100 | 2.34 | 99.13 | |
| 12000 | A2 | 100 | 0.79 | 100 |
| B2 | 100 | 0.98 | 100 | |
| 20000 | A2 | 100 | 0.42 | 100 |
| B2 | 100 | 0.63 | 100 | |
| 50000 | A2 | 100 | 0.08 | 100 |
| B2 | 100 | 0.08 | 100 | |
The A2 and B2 emission scenarios are shown. Different thresholds for the abundance of C. imicola were fitted to conduct the estimations. We estimated the percentage of localities with a predicted abundance over each threshold for the present that were also over the same threshold for future periods (P). Additionally, we estimated the percentage of localities predicted over each threshold for the future and under the same threshold in the present model (FP). Similarly, we estimated the percentage of localities with C. imicola abundance over the threshold — only with models for the present — with at least one bluetongue outbreak detected in 2007 (BT).
C. imicola abundance under future scenarios of climate change
The projection of the final C. imicola abundance models to future climatic scenarios showed an expected increasing total predicted abundance for each locality (Figures 3 and 5) although this increase was not marked. According to the obtained VIF values no relevant effects of multicollinearity are expected in the projections (mean VIF value and range: 2.778, 1.346–4.433; 2.632, 1.345–4.164; for A2 and B2 scenarios, respectively). Our predictions suggested that the distribution area of this vector species will remain quasi-constant in the future (Figure 3, Table 4). Nonetheless, the abundance of C. imicola will substantially increase in the localities already occupied. Finally, the higher rates of increases in distribution between periods were obtained for the lower threshold, that is, the increased distribution area is expected to present low abundance of C. imicola. The depicted situation was consistent for both emission scenarios; nevertheless, the obtained increment rates were higher for B2 than A2 emission scenarios (Figure 5, Table 4).
Figure 5. Present/future comparisons in Culicoides imicola abundance.
Relationships between the predicted Culicoides imicola abundance (maximum number of captures per night) for present and future periods according to the CGM2 circulation model and the A2 and B2 emission scenarios.
Discussion
Considerations regarding the methodological approach
We studied the relative contribution of several factors to determine the spatial pattern of abundance of a disease vector arthropod using variation partitioning techniques. This was done with the aim of deepening our understanding of the probable causalities and explanatory powers of predictors in multivariate models, but not with the aim of generating a predictive equation [69]. Thus, it is relevant to enhance the explanatory power of spatial predictive models. The variation partitioning procedure has been widely used to explain the distribution of biodiversity [29], but it has been less frequently applied to epidemiological studies [8], [70].
A large diversity of factors influencing C. imicola life-cycle were considered in the present study, and the application of variation partitioning allowed us to determine how much of the variation in the predicted C. imicola abundance was explained by the pure effect of each factor (topography and climate, host, soil and spatial factors), and what proportion could only be attributed to their shared effects. To date, only predictors related to climate, topography and soil have been considered for determining the distribution and abundance of C. imicola [10], [16], [18], [22], [71]. To the best of our knowledge, this is the first broad assessment of factors determining C. imicola — and any vector species — abundance at a large geographical scale. In view of the emergence of the concept of ‘environmental health’ (http://www.oneworldonehealth.org), variation partitioning may help to analyse data on emerging diseases as follows: i) by identifying the most relevant factors determining disease prevalence and spread; and ii) by delimitating epidemiological management units in relation to the factors involved in the transmission of the studied pathogen, e.g. by using freely available data from web-based epidemiological surveillance networks [72].
To date, the effect of climate change on C. imicola distribution and abundance only has been assessed in an oversimplified way using idealized scenarios, sensu [73], in which an increase in temperature is assumed to be constant for all territorial units in the study area [22]. In this study, changes in climate between periods were considered according to one circulation model and two emission scenarios following the guidelines of the Intergovernmental Panel on Climate Change. In addition, some authors have questioned the validity of models based only on climatic variables for forecasting future species distributions [74], [75], since many factors other than climate play an important role in determining species distributions and their dynamics over time. Consequently, and as conducted in this study, climate effects on species distributions should be considered together with other influential factors to be able to forecast modifications in species ranges due to climate change [27].
Factors determining C. imicola abundance
The models obtained for the two studied emission scenarios were very similar regarding both their explanatory and predictive power. This result agrees with previous studies in which low levels of uncertainty between emission scenarios were reported when modelling species distribution [42], [65], [76]. The climate scenario modelled for the present using the CGM2 circulation model obtained higher precipitations under the A2 emission scenario than under the B2 scenario, although they were quite similar. Differences between scenarios were even less in terms of monthly temperatures. For example, a 1 mm and 0.02°C difference was observed between the A2 and B2 scenarios for the present period relative to annual precipitation and annual mean temperature, respectively [27].
Hosts
Variation partitioning showed that the abundance of potential hosts, regardless of the other factors considered, attained the highest explanatory power among the factors considered to predict C. imicola abundance. To our knowledge, host abundance has never been considered in predictive modelling for an arthropod vector species, despite hosts being suggested as potential conditioning factors of vector distribution patterns [9], [22]. Additionally, host competence (referring to livestock only) in the epidemiology of BT was recently demonstrated when analyzing factors determining the occurrence of BT outbreaks in Spain [61]. The high explanatory power of wildlife abundance, which is substantially higher than even that attained by livestock density, may be mediated by different explanations as follows: i) wildlife — mainly red deer — may really be playing an important role in the ecology of C. imicola, since high densities – up to 69 deer/100 ha [77] – are present in southwestern mainland Spain; and ii) wildlife abundance was modelled with climatic variables, and thus the percentage explained by wildlife can probably be attributed in part to the topoclimate factor.
Unfortunately, the true role of wildlife in explaining C. imicola abundance cannot be inferred from our study design, and experimental studies may be necessary. Nonetheless, it should be mentioned that C. imicola may feed on wild ungulates, as BTV was detected in these animals [44] in areas where other competent BTV reservoir Culicoides spp. are not abundant [16]. Thus, local variations in host availability and composition may impair suitability for C. imicola and thus drive its local abundance and local C. imicola-borne disease epidemiology. In this respect, and even though our results should be taken with caution, a gap in our knowledge concerning the role of wildlife in C. imicola ecology and BTV epidemiology was found and this should be addressed in future studies.
Topography and climate
Topography and climate play a relevant role in spatial modelling since the geographic ranges of species at large-spatial scales are limited by abiotic conditions [38]. Thus, our results showed a high explanatory power of the pure effect of the topoclimate factor, mainly due to climatic conditions. Precipitation and its seasonality, and to a lesser extent temperature, were the climatic variables represented in the final models. Our findings contrast with previous studies in which temperature-related variables achieved the highest explanatory power for C. imicola distribution models [22], suggesting a degree of temperature-related limitation of vector persistence [35]. The high weight of precipitation found may agree with the requirement of C. imicola for humid organically enriched soil as breeding sites [17]. The importance of precipitation over temperature was also reported when modelling BT outbreaks in Spain [61]. Thus, different climatic requirements are probably modulating species distribution and species abundance at a biogeographical scale. Abundance models for C. imicola are ecologically (climatically) more similar to BTV distribution models than species distribution models; thus, the former are probably more suitable for consideration in BTV epidemiological studies.
Soil
The pure effect of soil (land cover and soil permeability) was the next in importance in explaining C. imicola abundance. In our model, the high explanatory power of NDVI, and its seasonality, is consistent with previous studies modelling both C. imicola distribution [16], [71], [78] and BT occurrence [50], [61]. Even when NDVI variables were retained in the final models, soil permeability — a variable closely related to the water-holding capacity of soil — was shown to be the most relevant of the soil-related variables [18]. These relationships between NDVI and soil permeability and C. imicola abundance can be interpreted from the perspective of the basic requirements of C. imicola larvae [79]. Whereas moisture is critical to their survival, nutrients are essential for their development and for the completion of their life-cycle, as mentioned. Thus, C. imicola prefers water-saturated, barely permeable, soil with high levels of organic matter, as shown by NDVI values. The land cover variables considered in our study were also retained in the final models, but were less significant than the remaining soil-related variables. This result is consistent with the findings of [20] who reported the limited ability of CORINE classification to accurately predict Culicoides breeding in Danish farmland.
Spatial components
C. imicola abundance was barely explained by the spatial component, demonstrating the absence (or weak presence) of a spatial structure in the abundance data [36]. This factor should be considered in all spatially explicit models in order to reveal geographical trends associated with historical events or species population dynamics [29]. The results obtained can be understood by the high dependence of C. imicola on suitable local conditions [10], [17], [18]. To the best of our knowledge, only [61] included the autologistic term in their models on BT epidemiology. Similarly, they found a low degree of spatial correlation, which was attributed to the BT data used in modelling that probably oversimplified the true spatial structure of BTV occurrence.
Combined effects
Our results show that a high amount of variation can only be explained by the combined effect of two (or more) factors. Specifically, topoclimate, hosts and soil, in pairs, attained higher amounts of variation. This is due to the interactions between factors and the effects being overlaid subsequently [70]. In this regard, the results obtained were expected since the factors mentioned above are interrelated, such as NDVI linking topoclimate and soil. Variation partitioning or similar tools [80] allow measuring the pure effect of each factor involved in a multi-factorial analysis.
Projections to future scenarios of climate change
Forecasted projections of C. imicola abundance for the near future are only based on changes in precipitation and temperature according to the Intergovernmental Panel on Climate Change [40]. However, indirect effects of climate on land use, host distribution or host population dynamics may modulate the life-cycle of C. imicola in the future and hence our predictions. Predicting climate change-associated indirect effects on these factors is difficult but they have to be borne in mind when interpreting our predictions on C. imicola abundance.
The projection of the final models to future climatic scenarios showed that the forecast C. imicola abundance is expected to increase in each locality (Figure 5), whereas its forecast distribution area will increase by a smaller amount (increase rate less than 4.5%). A stable trend in the C. imicola distribution range was recently reported using field data from surveillance programmes, such as those conducted in Portugal [81] and in Italy [26]. C. imicola has not appeared to increase its distribution range in Portugal since the mid-1990s, and the results from Italy demonstrated no detectable species range expansion between 2002 and 2007. However, it has been suggested that C. imicola is undergoing range expansion in the Mediterranean region, based on field data and on modelling [22], demonstrating a contrasting species response to changing climatic conditions. Other authors concluded that these regional differences are probably related to climatic characteristics [82]. Thus, this species has mainly expanded into warm areas (eastern Spain, northern Italy, southern France and northeastern parts of Greece), whereas areas where temperatures have remained largely unchanged, such as Portugal, have not experienced this type of expansion.
Several hypotheses have been proposed to explain C. imicola range stability [26], some of which aid in interpreting the results obtained in this study. The first hypothesis is related to the fact that C. imicola may be expanding its distribution ranges at rates which were too low to be detected during our study periods. If the models obtained are projected to more future periods, then significant species expansions will probably be detected. However, the accuracy of the predictions will be reduced, and thus their applicability to disease risk management policy, due to the current uncertainty associated with circulation models and emission scenarios [42]. In addition, a clear increase in abundance was forecast, demonstrating an effective response of the species to climate change between the study periods. Thus, some evidence suggests that factors other than climatic ones may be involved [9], [83].
In this sense, an alternative hypothesis emerges. As previously stated, several factors play an important role in explaining vector and host dynamics over time [75]. Thus, they could determine species ranges in future scenarios [27], with subsequent implications for pathogen emergence and spread [84]. A broad assessment, as conducted in this study, suggests that C. imicola may spread, but probably only into those areas where other requirements are fulfilled, rather than moving along a wide front of increased temperature, as suggested by [22].
Relationships between vector abundance and BTV
Although our study focussed on the factors driving C. imicola abundance, our main aim was epidemiological and centred on the study of the determinants of orbiviruses threatening animal health in Europe. Our findings on C. imicola abundance suggest that the geographic distribution of orbiviruses expected in future scenarios would not increase if C. imicola was the only, or at least the most relevant, competent reservoir of orbiviruses. Nonetheless, recent evidence shows that the ecology of orbiviruses in Europe is more complex than previously thought due to other Culicoides spp. acting as new competent vectors [13], [15]. We suggest that a higher C. imicola abundance may significantly change the rate of transmission of orbiviruses and facilitate more severe epidemics. It is nonetheless essential to conduct specific studies on the epidemiologic factors driving orbivirus circulation rates, including the influence of competent vectors, before being able to accurately forecast future epidemics.
Acknowledgments
We are grateful to the “Subdirección General de Sanidad de la Producción Primaria” of the Spanish Ministry of the Environment and Rural and Marine Affairs for providing data from the Spanish Bluetongue National Surveillance Programme and from the national livestock census. We acknowledge the great contribution of an anonymous reviewer to the final version of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grant POII09-0141-8176 (Castilla-La Mancha) and by funds from Castilla-La Mancha and Principado de Asturias. P. Acevedo currently holds a Juan de la Cierva research contract awarded by the Spanish Ministry of Science and Innovation-European Social Fund and partially by the project CGL2009-11316/BOS - FEDER. F. Ruiz-Fons is currently supported by the Spanish Ministry of Science and Innovation under a contract awarded by the ‘Instituto de Salud Carlos III’. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Soberón J. Niche and area of distribution modeling: a population ecology perspective. Ecography. 2010;33:159–167. [Google Scholar]
- 2.Guisan A, Zimmermann NE. Predictive habitat distribution models in ecology. Ecol Model. 2000;135:147–186. [Google Scholar]
- 3.Peterson AT, Sánchez-Cordero V, Bear CB, Ramsey JM. Ecological niche modeling and potential reservoirs for Chagas diseases, Mexico. Emerg Infect Dis. 2002;7:662–667. doi: 10.3201/eid0807.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP. Oxford: Oxford University Press; 2002. The ecology of wildlife diseases.218 [Google Scholar]
- 5.Scheiner SM. The intersection of the sciences of biogeography and infectious diseases ecology. EcoHealth. 2010;6:483–488. doi: 10.1007/s10393-010-0298-x. [DOI] [PubMed] [Google Scholar]
- 6.Peterson AT. Biogeography of diseases: a framework for analysis. Naturwissenschaften. 2008;95:483–491. doi: 10.1007/s00114-008-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estrada-Peña A, Acevedo P, Ruiz-Fons F, Gortázar C, de la Fuente J. Evidence of the importance of host habitat use in predicting the dilution Effect of wild boar for deer exposure to Anaplasma spp. PLoS ONE. 2008;3(8):e2999. doi: 10.1371/journal.pone.0002999. doi: 10.1371/journal.pone.0002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alzaga V, Tizzani P, Acevedo P, Ruiz-Fons F, Vicente J, et al. Deviance partitioning of host factors affecting parasitisation in the European brown hare (Lepus europaeus). Naturwissenschaften. 2009;96:1157–1168. doi: 10.1007/s00114-009-0577-y. [DOI] [PubMed] [Google Scholar]
- 9.Baylis M, O'Connell L, Purse BV. Modelling the distribution of bluetongue vectors. Vet Ital. 2004;40:176–181. [PubMed] [Google Scholar]
- 10.Calistri P, Goffredo M, Caporale V, Meiswinkel R. The distribution of Culicoides imicola in Italy: application and evaluation of current Mediterranean models based on climate. J Vet Med B. 2003;50:132–138. doi: 10.1046/j.1439-0450.2003.00631.x. [DOI] [PubMed] [Google Scholar]
- 11.Gu W, Regens JL, Beier JC, Novak RJ. Source reduction of mosquito larval habitats has unexpected consequences on malaria transmission. P Natl Acad Sci USA. 2006;103:17560–17563. doi: 10.1073/pnas.0608452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estrada-Peña A, Venzal JM. Climate niches of ticks species in the Mediterranean region: modeling the occurrence data, distributional constraints, and impacts of climate change. J Med Entomol. 2007;44:1130–1138. doi: 10.1603/0022-2585(2007)44[1130:cnotsi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Anonymous. Bluetongue. Scientific opinion of the Panel on Animal Health and Welfare on a request from the European Comission (DG SANCO) on bluetongue. The EFSA Journal. 2008;735:1–69. doi: 10.2903/j.efsa.2008.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PPC, et al. Climate change and the recent emergence of bluetongue in Europe. Nat Rev Microbiol. 2005;3:171–181. doi: 10.1038/nrmicro1090. [DOI] [PubMed] [Google Scholar]
- 15.Caracappa S, Torina A, Guercio A, Vitale M, Calabro A, et al. Identification of a novel bluetongue virus vector species of Culicoides in Sicily. Vet Rec. 2003;153:71–74. doi: 10.1136/vr.153.3.71. [DOI] [PubMed] [Google Scholar]
- 16.Calvete C, Estrada R, Miranda MA, Borrás D, Calvo JH, et al. Modelling the distributions and spatial coincidence of bluetongue vectors Culicoides imicola and Culicoides obsoletus group throughout the Iberian Peninsula. Med Vet Entomol. 2008;22:124–134. doi: 10.1111/j.1365-2915.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- 17.Meiswinkel R. Discovery of a Culicoides imicola-free zone in South Africa: preliminary notes and potential significance. Onderstepoort J Vet Res. 1997;64:81–86. [PubMed] [Google Scholar]
- 18.Conte A, Goffredo M, Ippoliti C, Meiswinkel R. Influence of biotic and abiotic factors on the distribution and abundance of Culicoides imicola and the Obsoletus Complex in Italy. Vet Parasitol. 2007;150:333–344. doi: 10.1016/j.vetpar.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Meiswinkel R, Venter GJ, Nevill EM. Vectors: Culicoides spp. In: Goetzer JAW, Tustin RC, editors. Infectious diseases of livestock, Vol. I. Oxford: Oxford University Press; 1994. pp. 93–136. [Google Scholar]
- 20.Kirkeby C, Bødker R, Stockmarr A, Enøe C. Association between land cover and Culicoides (Diptera: Ceratopogonidae) breeding sites on four Danish cattle farms. Entomol Fennica. 2010;20:228–232. [Google Scholar]
- 21.Meiswinkel R. The 1996 outbreak of African horse sickness in South Africa – the entomological perspective. Arch. 1998;Virol(Suppl 14):69–83. doi: 10.1007/978-3-7091-6823-3_8. [DOI] [PubMed] [Google Scholar]
- 22.Wittmann EJ, Mellor PS, Baylis M. Using climatic data to map the potential distribution of Culicoides imicola (Diptera: Ceratopogonidae) in Europe. Rev Sci Tech Off Int Epiz. 2001;20:731–740. doi: 10.20506/rst.20.3.1306. [DOI] [PubMed] [Google Scholar]
- 23.Epstein PR. Is global warming harmful to health? Sci Am. 2000;283:50–57. doi: 10.1038/scientificamerican0800-50. [DOI] [PubMed] [Google Scholar]
- 24.González C, Wang O, Strutz SE, González-Salazar C, Sánchez-Cordero V, et al. Climate change and risk of Leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Neglect Trop D. 2010;4(1):e585. doi: 10.1371/journal.pntd.0000585. doi: 10.1371/journal.pntd.0000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecol Lett. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 26.Conte A, Gilbert M, Goffredo M. Eight years of entomological surveillance in Italy show no evidence of Culicoides imicola geographical range expansion. J Appl Ecol. 2009;46:1332–1339. [Google Scholar]
- 27.Márquez AL, Real R, Olivero J, Estrada A. Climatic Change. In press; 2010. Combining climate with other influential factors for modelling the impact of climate change on species distribution. [Google Scholar]
- 28.Ruiz-Fons F, Gilbert L. The role of deer as vehicles to move ticks Ixodes ricinus between contrasting habitats. Int J Parasitol. 2010;40:1013–1020. doi: 10.1016/j.ijpara.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Real R, Barbosa M, Porras D, Kin MS, Márquez AL, et al. Relative importance of environment, human activity and spatial situation in determining the distribution of terrestrial mammal diversity in Argentina. J Biogeogr. 2003;30:939–947. [Google Scholar]
- 30.Font I. Salamanca: Ediciones Universidad de Salamanca; 2000. Climatología de España y Portugal.422 [Google Scholar]
- 31.Calvete C, Miranda MA, Estrada R, Borrás D, Sarto V, et al. Spatial distribution of Culicoides imicola, the main vector of the Bluetongue virus, in Spain. Vet Rec. 2006;158:130–131. doi: 10.1136/vr.158.4.130. [DOI] [PubMed] [Google Scholar]
- 32.Miranda MA, Rincón C, Borrás D. Seasonal abundance of Culicoides imicola and C. obsoletus in the Balearic Islands. Vet Ital. 2004;40:292–295. [PubMed] [Google Scholar]
- 33.Baylis M, El Hasnaoui H, Bouayoune H, Touti J, Mellor PS. The spatial and seasonal distribution of African horse sickness and its potential Culicoides vectors in Morocco. Med Vet Entomol. 1997;11:203–212. doi: 10.1111/j.1365-2915.1997.tb00397.x. [DOI] [PubMed] [Google Scholar]
- 34.Ortega MD, Holbrook FR, Lloyd JE. Seasonal distribution and relationship to temperature and precipitation of the most abundant species of Culicoides in five provinces of Andalusia, Spain. J Am Mosq Control Assoc. 1999;15:391–399. [PubMed] [Google Scholar]
- 35.Veronesi E, Venter GJ, Labuschagne K, Mellor PS, Carpenter S. Life-history parameters of Culicoides (Avaritia) imicola Kieffer in the laboratory at different rearing temperatures. Vet Parasitol. 2009;163:370–373. doi: 10.1016/j.vetpar.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 36.Legendre P. Spatial autocorrelation: trouble or new paradigm? Ecology. 1993;74:1659–1673. [Google Scholar]
- 37.Soberón J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett. 2007;10:1115–1123. doi: 10.1111/j.1461-0248.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 38.Gutiérrez-Illán J, Gutiérrez D, Wilson RW. The contributions of topoclimate and land cover to species distributions and abundance: fine-resolution tests for a mountain butterfly fauna. Global Ecol Biogeogr. 2010;19:15–173. [Google Scholar]
- 39.Eastman JR. Clark Labs, Clark University; 2004. Idrisi Kilimanjaro GIS, User Guide and Software. [Google Scholar]
- 40.Nakicenovic N, Alcamo J, Davis G, de Vries B, Fenhann J, et al. Cambridge: Cambridge University Press; 2000. IPCC Special Report on Emissions Scenarios.599 [Google Scholar]
- 41.Brunet M, Casado MJ, de Castro M, Galán P, Lopez JA, et al. Madrid: Ministerio de Medio Ambiente y Medio Rural y Marino; 2007. Generación de escenarios de cambio climático para España.158 [Google Scholar]
- 42.Real R, Márquez AL, Olivero J, Estrada A. Are species distribution models in climate warming scenarios useful for informing emission policy planning? An uncertainty assessment using fuzzy logic. Ecography. 2010;33:304–314. [Google Scholar]
- 43.Ortega MD, Mellor RS, Rawlings P, Pro MJ. The seasonal and geographical distribution of Culicoides imicola, C. pulicaris group and C. obsoletus biting midges in central and southern Spain. Arch Virol. 1998;14:85–91. doi: 10.1007/978-3-7091-6823-3_9. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Fons F, Reyes-García AR, Alcaide V, Gortázar C. Spatial and temporal evolution of bluetongue virus in wild ruminants, Spain. Emerg Infect Dis. 2008;14:951–953. doi: 10.3201/eid1406.071586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García I, Napp S, Casal J, Perea A, Allepuz A, et al. Bluetongue epidemiology in wild ruminants from Southern Spain. Eur J Wildlife Res. 2009;55:173–178. [Google Scholar]
- 46.Rodríguez-Sánchez B, Gortázar C, Ruiz-Fons F, Sánchez-Vizcaíno JM. Bluetongue virus serotypes 1 and 4 in red deer, Spain. Emerg Infect Dis. 2010;16:518–520. doi: 10.3201/eid1603.090626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Real R, Barbosa AM, Vargas JM. Obtaining environmental favourability functions from logistic regression. Environ Ecol Stat. 2006;13:237–245. [Google Scholar]
- 48.Real R, Barbosa AM, Rodríguez A, García FJ, Vargas JM, et al. Conservation biogeography of ecologically interacting species: the case of the Iberian lynx and the European Rabbit. Divers Distrib. 2009;5:390–400. [Google Scholar]
- 49.Palomo LJ, Gisbert J, Blanco JC. Madrid: Dirección General para la Biodiversidad-SECEM-SECEMU; 2007. Atlas y Libro Rojo de los Mamíferos Terrestres de España.586 [Google Scholar]
- 50.Purse BV, Tatem AJ, Caracappa S, Rogers DJ, Mellor PS, et al. Modelling the distributions of Culicoides bluetongue virus vectors in Sicily in relation to satellite-derived climate variables. Med Vet Entomol. 2004;18:90–101. doi: 10.1111/j.0269-283X.2004.00492.x. [DOI] [PubMed] [Google Scholar]
- 51.Nicholson SE, Farrar FJ. The influence of Soil Type on the Relationships between NDVI, Rainfall, and Soil Moisture in Semiarid Botswana: NDVI Response to Rainfall. Remote Sens Environ. 1994;50:107–120. [Google Scholar]
- 52.European Environmental Agency. Nature/land cover information package. Luxemburg: European Environment Agency; 2000. NATLAN. [Google Scholar]
- 53.IGME. Explicación de los mapas de lluvia útil, de reconocimiento hidrogeológico y de síntesis de los sistemas acuíferos, 2nd edn. Madrid, Spain: Instituto Geológico y Minero de España; 1979. Mapa hidrogeológico nacional. [Google Scholar]
- 54.Jiménez-Valverde A, Lobo JM. Distribution Determinants of Endangered Iberian Spider Macrothele calpeiana (Araneae, Hexathelidae). Environ Entomol. 2006;35:1491–1499. [Google Scholar]
- 55.Ruiz-Fons F, Vidal D, Vicente J, Acevedo P, Fernández-de-Mera IG, et al. Epidemiological risk factors of Aujeszky's disease in wild boars (Sus scrofa) and domestic pigs in Spain. Eur J Wildlife Res. 2008;54:549–555. [Google Scholar]
- 56.Hilbe JM. Cambridge, UK: Cambridge University Press; 2007. Negative Binomial Regression. ISBN 9780521857727. [Google Scholar]
- 57.Boyce MS, Mackenzie DI, Manly BFJ, Haroldson MA, Moody D. Negative binomial models for abundance estimation of multiple closed populations. J Wildlife Manage. 2001;65:498–509. [Google Scholar]
- 58.Pearce J, Ferrier S. The practical value of modelling relative abundance of species for regional conservation planning: a case study. Biol Conserv. 2001;98:33–43. [Google Scholar]
- 59.Fowler J, Cohen L, Jarvis P. New York: John Wiley & Sons; 1998. Practical statistics for field biology, 2nd edn.259 [Google Scholar]
- 60.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 61.Calvete C, Estrada R, Miranda MA, Borrás D, Calvo JH, et al. Ecological correlates of bluetongue virus in Spain: Predicted spatial occurrence and its relationship with the observed abundance of the potential Culicoides spp. Vector. Vet J. 2009;182:235–243. doi: 10.1016/j.tvjl.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 62.Borcard D, Legendre P, Drapeau P. Partialling out the spatial component of ecological variation. Ecology. 1992;73:1045–1055. [Google Scholar]
- 63.Legendre P, Legendre L. Amsterdam: Elsevier Science; 1998. Numerical ecology. Second English edition.853 [Google Scholar]
- 64.Hortal J, Rodríguez J, Nieto-Díaz M, Lobo JM. Regional and environmental effects on the species richness of mammal assemblages. J Biogeogr. 2008;35:1202–1214. [Google Scholar]
- 65.Diniz-Filho JAF, Bini LM, Rangel TF, Loyola RD, Hof C, et al. Partitioning and mapping uncertainties in ensembles of forecasts of species turnover under climate change. Ecography. 2009;32:897–906. [Google Scholar]
- 66.Barbosa AM, Real R, Vargas JM. Transferability of environmental favourability models in geographic space: The case of the Iberian desman (Galemys pyrenaicus) in Portugal and Spain. Eco Model. 2009;220:747–754. [Google Scholar]
- 67.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods in Ecology & Evolution. 2010;1:3–14. [Google Scholar]
- 68.Montgomery DC, Peck EA. New York: Wiley; 1992. Introduction to Linear Regression Analysis. [Google Scholar]
- 69.Watson DM, Peterson AT. Determinants of diversity in a naturally fragmented landscape: humid montane forest avifaunas of Mesoamerica. Ecography. 1999;22:582–589. [Google Scholar]
- 70.Barbosa AM, Segivia JM, Vargas JM, Torres J, Real R, et al. Predictors of red fox (Vulpes vulpes) helminth parasite diversity in the provinces of Spain. Wildl Biol Pract. 2005;1:3–14. [Google Scholar]
- 71.Baylis M, Mellor PS, Wittmann EJ, Rogers DJ. Prediction of areas around the Mediterranean at risk of bluetongue by modelling the distribution of its vector using satellite imaging. Vet Rec. 2001;149:639–643. [PubMed] [Google Scholar]
- 72.Savini L, Ippoliti C, Conte A, Parisse M, Dall'Acqua F, et al. Development of a web-based geographic information system for the epidemiological surveillance of bluetongue in the Balkans and eastern Mediterranean countries. Vet Ital. 2006;42:201–207. [PubMed] [Google Scholar]
- 73.Beaumont LJ, Hughes L, Pitman AJ. Why is the choice of future climate scenarios for species distribution modelling important? Ecol Lett. 2008;11:1135–1146. doi: 10.1111/j.1461-0248.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- 74.Woodward FI, Beerling DJ. The dynamics of vegetation change: health warnings for equilibrium ‘dodo’ models. Global Ecol Biogeogr Lett. 1997;6:413–418. [Google Scholar]
- 75.Davis AJ, Jenkinson LS, Lawton JH, Shorrocks B, Wood S. Making mistakes when predicting shifts in species range in response to global warming. Nature. 1998;391:783–786. doi: 10.1038/35842. [DOI] [PubMed] [Google Scholar]
- 76.Buisson L, Thuiller W, Casajus N, Lek S, Grenouillet G. Uncertainty in ensemble forecasting of species distribution. Global Change Biol. 2010;16:1145–1157. [Google Scholar]
- 77.Acevedo P, Ruiz-Fons F, Vicente J, Reyes-García AR, Alzaga V, et al. Estimating red deer abundance in a wide range of management situations in Mediterranean habitats. J Zool. 2008;276:37–47. [Google Scholar]
- 78.Tatem AJ, Baylis M, Mellor PS, Purse BV, Capela R, et al. Prediction of bluetongue vector distribution in Europe and north Africa using satellite imagery. Vet Microbiol. 2003;97:13–29. doi: 10.1016/j.vetmic.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 79.Braverman Y, Galun R, Ziv M. Breeding sites of some Culicoides species (Diptera: Ceratopogonidae) in Israel. Mosquito News. 1974;34:303–308. [Google Scholar]
- 80.Heikkinen RK, Luoto M, Kuussaari M, Pöyry J. New insights into butterfly–environment relationships using partitioning methods. Proc R Soc B. 2005;272:2203–2210. doi: 10.1098/rspb.2005.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Capela R, Purse BV, Pena I, Wittmann EJ, Margarita Y, et al. Spatial distribution of Culicoides species in Portugal in relation to the transmission of African horse sickness and bluetongue viruses. Med Vet Entomol. 2003;17:165–177. doi: 10.1046/j.1365-2915.2003.00419.x. [DOI] [PubMed] [Google Scholar]
- 82.Purse BV, Brown HE, Harrup L, Mertens PPC, Rogers DJ. Invasion of bluetongue and other orbivirus infections into Europe: the role of biological and climatic processes. Rev Sci Tech Off Int Epiz. 2008;27:427–442. [PubMed] [Google Scholar]
- 83.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 84.Slenning BD. Global climate change and implications for diseases emergence. Vet Pathol. 2010;47:28–33. doi: 10.1177/0300985809354465. [DOI] [PubMed] [Google Scholar]