Abstract
Background
There is a need for better management strategies to improve survival and quality of life in patients with biliary tract cancer (BTC).
Aim
To assess prognostic factors for survival in a large, non-selective cohort of patients with BTC.
Method
We compared outcomes in 321 patients with a final diagnosis of BTC (cholangiocarcinoma n=237, gallbladder cancer n=84) seen in a tertiary referral cancer centre between 1998–2007. Survival according to disease stage and treatment category was compared using log-rank testing. Cox regression analysis was used to determine independent prognostic factors.
Results
89 (28%) patients underwent surgical intervention with curative intent, of which 38% had R0-resections. Amongst the 321 patients, 34% were given chemo- and/or radiotherapy, 14% were palliated with photodynamic therapy (PDT) and 37% with biliary drainage procedures alone. The overall median survival was 9 months (3-year-survival 14%). R0-resective surgery conferred the most favourable outcome (3-year-survival 57%). Although patients palliated with PDT had more advanced clinical T-stages, their survival was similar to those treated with attempted curative surgery but who had positive resection margins. On multivariable analysis, treatment modality, serum CA19-9, distant metastasis and vascular involvement were independent prognostic indicators of survival.
Conclusion
In this large UK series of BTC, palliative PDT resulted in similar survival to those with curatively intended R1/R2-resections. Surgery conferred a survival advantage only in patients with R0-resection margins, emphasising the need for accurate pre-operative staging.
Keywords: biliary tract cancer, prognosis, surgical resection, photodynamic therapy
Background
Patients with biliary tract cancer (BTC; defined as cholangiocarcinoma; intrahepatic, perihilar (Klatskin) and extrahepatic, and gallbladder cancer) tend to have advanced disease at presentation, with a median survival of approximately 6–9 months from the time of diagnosis 1. Surgery with clear histological margins (R0 resection) offers a possibility of cure, with published five year survival rates of 24–40%2. Curative surgical resection, however, is only feasible in a minority of patients3, even in the setting of radical hepatic surgery4, including pre-operative ipsilateral portal vein embolisation to increase hepatic reserve5 and, in highly selected cases, liver transplantation6.
The management of patients with BTC is complex and has been changing with the advent of novel treatment options such as photodynamic therapy (PDT; a photosensitising agent, activated by light, exhibits thermal tumour destruction) and the addition of newer chemotherapy agents (e.g. gemcitabine)7. In general, published series have concentrated on the surgical management of cholangiocarcinoma3,8,9,10,11, and there is a paucity of information comparing surgical and modern palliative therapies. We describe a nine year experience of consecutive patients with BTC seen in a large UK cancer centre.
Methods
We undertook a retrospective review of all patients with BTC managed at University College Hospital and Royal Free Hospital between 1998 and 2007. Patients were identified by searching a hepatobiliary database, together with cross-referencing clinic letters from the gastrointestinal and oncology departments and pathology and endoscopy records. Missing information was collected at clinic review and by telephone contact with general practitioners and patients. The census date was set at 1st July 2008 to allow at least one year follow-up in all patients.
The following parameters were evaluated: age, sex, presenting symptoms, timing of symptom onset and first attendance at our centre, as well as baseline levels of serum bilirubin and the tumour markers CA19-9 (carbohydrate-associated antigen 19-9) and CEA (carcinoembryonic antigen). Clinical T-stage (Bismuth-Corlette classification for perihilar [Klatskin] tumours), vascular involvement and, where available, tumour histological grade were also recorded, as were the number and type of diagnostic procedures and therapeutic interventions (including surgery, PDT, chemo- and/or radiotherapy, biliary drainage/stenting). A diagnosis of BTC was confirmed by histological examination of resection or biopsy specimens, positive biliary cytology, and in some cases by multi-disciplinary team consensus and evidence of disease progression during follow-up.
Patients were classified into four groups according to the main treatment modality they received: (i) surgery with curative intent; or palliative management with (ii) PDT, (iii) chemo- and/or radiotherapy or (iv) biliary drainage only. In the case of combination therapies, subgroup analysis was performed to look for any additive effects of combined treatments.
Statistical analysis was done using SPSS software, version 14.0 (SPSS Inc., Chicago, IL). Numeric data were presented as medians with ranges or as mean values with standard errors of the mean (SEM). Inter-group comparisons were performed with the Pearson’s chi-square test, Student t test or the Mann-Whitney U test as appropriate. Survival was determined from the time of diagnosis to death or last follow-up date. The Kaplan-Meier method was used for survival rate estimates, and significance between subgroups was compared with the log-rank test. Variables predicting survival independently were evaluated with the Cox proportional hazards model. A p-value of less than 0.05 was considered significant.
Results
Patient characteristics
Between July 1998 and June 2007, a total of 321 patients were diagnosed with BTC; 237 (74%) with cholangiocarcinoma and 84 (26%) with gallbladder carcinoma. Patient and tumour characteristics, including clinical stage and, for perihilar cholangiocarcinoma, Bismuth-Corlette classification, are summarised in Table 1.
Table 1.
Pre-treatment study group demographics and tumour characteristics
| CCA (n=237) | GBCA (n=84) | |
|---|---|---|
| Age (years)* | 63 (29–102) | 74 (42–87) |
| Gender (female:male) | 112:125 (1:1.1) | 49:35 (1.4:1) |
| Tumour size (mm)* | 35 (7–145) | 30 (5–95) |
| Serum bilirubin (μmol/L)* | 56 (4–900) | 18 (3–607) |
| Serum CA19-9 (IU/L)* | 404 (1–100,000) | 180 (1–222,270) |
| Serum CEA (ng/ml)* | 3 (1–1649) | 5 (1–900) |
| CCA location^ | ||
| Intrahepatic (peripheral) | 13 (6%) | |
| Perihilar (Klatskin) | 189 (81%) | |
| Bismuth IV | 90 (40%) | |
| Bismuth III | 68 (31%) | |
| Bismuth II | 17 (8%) | |
| Bismuth I | 4 (2%) | |
| Extrahepatic (distal) | 31 (13%) | |
| Clinical T-stage$ | ||
| T1 | 3 (1%) | 4 (5%) |
| T2 | 36 (15%) | 13 (15%) |
| T3 | 91 (38%) | 23 (27%) |
| T4 | 69 (29%) | 29 (35%) |
| Unknown | 38 (16%) | 15 (18%) |
| Distant metastasis | ||
| Yes | 50 (21%) | 20 (24%) |
| No | 140 (59%) | 48 (57%) |
| Unknown | 47 (20%) | 16 (19%) |
Values are median with ranges.
Topography data insufficient in four cases; denominator n=233.
Assessment by means of imaging or, if available, pathological T-stage (T=tumour). CCA denotes cholangiocarcinoma, GBCA denotes gallbladder cancer, CA19-9 denotes carbohydrate-associated antigen 19-9, CEA denotes carcinoembryonic antigen.
Diagnosis
Ninety percent (290/321) of the patients had either histological (239/321, 74%) or cytological (51/321, 16%) confirmation of BTC. A positive tissue diagnosis had been made in the referring hospitals prior to referral in 64 (20%) cases. In 252 patients (79%), a median of one additional procedure (range 0–8; mean 1.6, SD 0.99) was performed in our hospitals and provided a tissue diagnosis in 226, corresponding to a local success rate of 226/252 (90%). In 26/321 (8%), the diagnoses were made in a multidisciplinary cancer meeting on radiological and clinical grounds and in five patients (2%) the information was missing. 84% (26/31) of the patients without tissue diagnosis for malignancy showed progressive disease over time on imaging, and there was no difference in survival between BTC patients with tissue diagnosis and those without it. The median time from symptom onset to final diagnosis was 2.0 (range 0–29) months, and from referral to our centre to final diagnosis was 0.5 (0–21) months.
Treatment
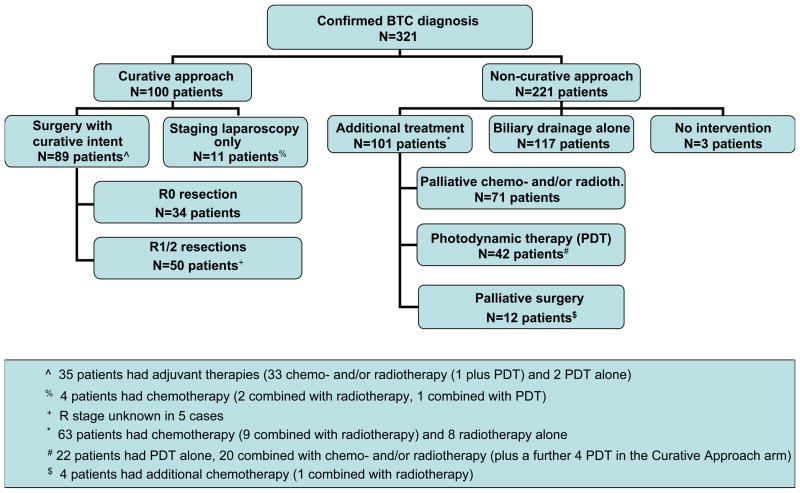
Figure 1 shows the management algorithm for the 321 patients. 89/321 (28%) patients underwent surgery with curative intent (35 with adjuvant therapies), and 12 (4%) patients had primary palliative surgery. 46/321 (14%) patients had PDT (alone in 24, combined with chemotherapy in 22), while 108 (34%) received chemotherapy and/or radiotherapy. 117/321 (37%) patients were managed with biliary drainage procedures alone, 3/321 (1%) were considered unsuitable for any intervention.
Figure 1.
Management algorithm for 321 patients with biliary tract cancer (BTC). R0, curative resection; R1, microscopic infiltration of the resection margins; R2, evidence of macroscopic residual disease.
Surgical Interventions
98/112 (87%) surgical interventions took place in our institutions (11 patients underwent staging laparoscopies only). Of the 89 surgeries performed with curative intent, negative (R0) histological margins were achieved in 34 (38%; positive microscopic (R1) and positive macroscopic (R2) resection margins in 33% and 24%, respectively). In the curative-intent surgical group, there were 33 cholecystectomies (24 with gallbladder fossa resection), 27 hemi-hepatectomies, 12 pancreaticoduodenectomies, 9 common bile duct resections, two liver transplants (both in patients with primary sclerosing cholangitis who were found to have incidental cholangiocarcinoma in the explant livers) and six operations converted to palliative procedures. In the group with planned palliative surgery, the most common procedures were duodenal (n=6) and biliary bypasses (n=3), all before 2003. The 30-day mortality rate in patients undergoing surgery with curative intent was 7% (6/89); 3% (1/34) in the subgroup with R0 resections and 9% (5/55) in those with positive resection margins.
35/89 (39%) patients undergoing surgery with curative intent received additional therapies of PDT (n=3) or chemo- and/or radiotherapy (n=33; one combined with PDT). Of the 34 patients with R0 resections, 1 (3%) received neoadjuvant chemotherapy and 8 (24%) adjuvant chemotherapy.
Non-surgical interventions
Photodynamic therapy
From January 2003, PDT with porfimer sodium was offered to patients with locally advanced BTC (non-resectable disease or unfit for surgery), in the context of two prospective non-randomised phase II studies. A total of 46 patients received PDT in combination with biliary stenting (42 in the non-curative approach arm; 4 in the curative approach arm, three with tumour recurrence after curatively intended surgery). PDT was applied once in 32 patients, twice at approximately four-monthly intervals in 12 and three times in two patients.
Chemo- and/or radiotherapy
A total of 108 patients received chemo- and/or radiotherapy: 96 patients received chemotherapy, of which 20 were in combination with radiotherapy, and 12 patients received radiotherapy alone (three of them as brachytherapy). The most frequently given chemotherapy agent was gemcitabine (in 60 patients), usually in the setting of national randomised phase II (ABC-0112) and recently completed phase III (ABC-0213) studies.
Biliary drainage procedures
Fifty-three percent (117/221) of patients in the palliative group were managed by biliary drainage procedures alone, via endoscopic and/or percutaneous approaches. For the whole study population (n=321), a median of three drainage interventions (mean 3.9, range 0–14) per patient were performed. Stenting at endoscopic retrograde cholangiopancreatography successfully relieved biliary obstruction in 48% of recorded cases with a median of two (range 1–10) procedures; the remaining 52% required percutaneous biliary drainage with a median of one additional procedure (mean 1.2, range 1–6). A total of 42% of patients had self-expanding metal stents inserted, endoscopically or radiologically.
Survival analysis
Complete follow-up data were available in 293 (91%) patients, with missing data in five surgical patients and in 23 in the non-surgical group, who were excluded from the survival analysis. The overall median survival was 9 months (range 0–83, 95% confidence interval 7–11 months) with 1-, 2-, 3- and 5-year survival rates for all patients of 40%, 23%, 14% and 6%, respectively (Figure 2). There was no survival difference between patients with cholangiocarcinoma and gallbladder carcinoma (9 vs. 8 months, p=0.39).
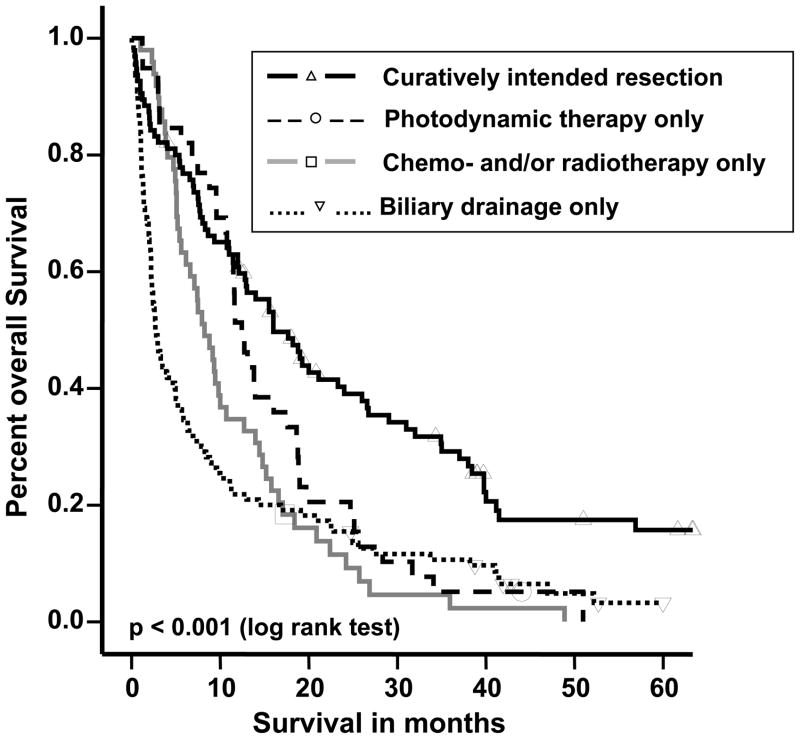
Figure 2.
Kaplan-Meier survival estimates of patients in the four treatment groups. Individual patients still alive during the follow-up period are indicated by marks on the curves. The median survival time difference between the four treatment groups was statistically significant (p<0.001). Comparing the treatment groups’ survival times individually, the significance levels were as follows: curatively intended surgery vs. photodynamic therapy (PDT), p=0.012; surgery vs. chemo- and/or radiotherapy, p<0.001; surgery vs. biliary drainage procedure, p<0.001; PDT vs. chemo- and/or radiotherapy, p=0.06; PDT vs. biliary drainage procedure, p=0.019; chemo- and/or radiotherapy vs. biliary drainage procedure, p=0.136.
There were significant (p<0.001) differences in survival between treatment groups. In patients undergoing attempted curative surgery, the median survival time was 19 (range 0–83) months, compared with 12 (1–51) months for PDT, 8 (1–49) months for chemo- and/or radiotherapy, and 3 (0–60) months for those patients who received no additional treatment other than biliary drainage procedures. The 1-year survival was 69%, 51%, 37% and 20% in the curatively intended surgery, PDT, chemo- and/or radiotherapy and biliary drainage only groups, respectively.
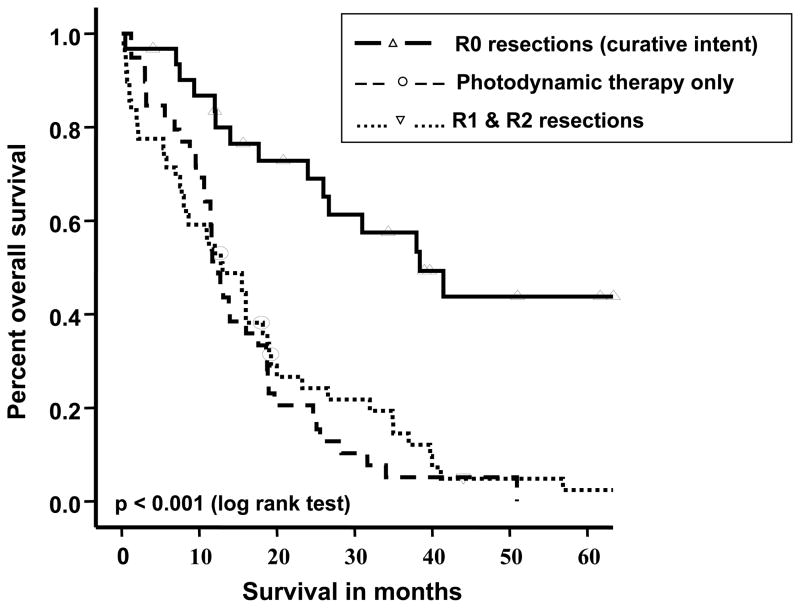
Figure 3 shows the survival graphs for curatively intended surgery, divided into negative (R0) and positive (R1/R2) resection margins and compared with the survival of those who had palliative PDT as the mainstay of treatment. Although PDT patients had a more advanced clinical T-stage than those undergoing surgery (93% vs. 63% T3/T4 stage, p<0.001; for perihilar cholangiocarcinoma trend towards higher Bismuth grades, p=0.08) and tended to be older (mean age 64 vs. 60 years, p=0.05), their survival did not differ from those treated with curatively intended surgery who had positive resection margins (PDT vs. R1, p=0.13 and PDT vs. R2, p=0.32; no survival difference between R1 and R2 resections, p=0.09). PDT treated and R1/R2 resected patients did not differ with regards to serum bilirubin (p=0.99), CA19-9 (p=0.18), CEA (p=0.64), distant metastasis (p=0.87) and vascular involvement (p=0.83). The 1-year survival rates were 87%, 55% and 51% following curative resections (R0), non-curative resections (R1/R2) and PDT, respectively (R0 vs. R1/R2 or PDT, p<0.001 and R1/R2 vs. PDT, p=0.52). There was no early mortality in patients given PDT, whereas the 30-day mortality was 9% (5/55) following non-curative resection. PDT-treated patients had significantly better survival than those who had biliary drainage procedures alone (1-year survival 51% vs. 20%, respectively, p=0.01; T3/T4 stage in 93% vs. 85%, respectively, p=0.18), and there was a trend towards better survival compared to those who received chemo- and/or radiotherapy alone (1-year survival 51% vs. 37%, p=0.07; T3/T4 stage in 93% vs. 89%, respectively, p=0.45). There was a significant (p=0.03) difference in survival rates between the R1/R2 resection group and chemo- and/or radiotherapy alone group with 1-year survival rates of 55% and 37%, respectively. There was no difference in survival between patients who received PDT alone vs. patients who received PDT and chemo- and/or radiotherapy combined (p=0.35).
Figure 3.
Kaplan-Meier survival estimates comparing patients with curatively intended surgeries (with R0 and R1/R2 outcome) with patients receiving photodynamic therapy (PDT). Survival times differed significantly between R0 resection and PDT (p<0.001), as well as R0 and R1/2 resections (p<0.001), but there was no difference between PDT and R1/2 resections (p=0.52).
When comparing patients treated in the first 4.5 years (1998–2002; n=92) with those treated in the second 4.5 years of the study (2003–2007; n=200), there was no difference in median survival (8.3 vs. 9.0 months; p=0.63).
In the univariable analysis, the following parameters, apart from treatment category, were predictors for survival: clinical T-stage (p<0.001), distant metastases (p<0.001), serum CA19-9 (p<0.001), CEA (p<0.001) and bilirubin (p=0.002), vascular involvement (p=0.002) and tumour size (p=0.044) on imaging (Table 2).
Table 2.
Univariable analysis of prognostic factors
| Parameters(n) | Survival (%) |
Median survival in months (95% CI) | p-value | ||
|---|---|---|---|---|---|
| 1-yr | 2-yr | 3-yr | |||
| Age (years) | |||||
| <65 (147) | 42 | 22 | 12 | 10 (7–13) | 0.949 |
| ≥65 (146) | 38 | 22 | 15 | 8 (6–10) | |
| Gender | |||||
| Female (142) | 39 | 27 | 17 | 10 (7–12) | 0.130 |
| Male (151) | 40 | 18 | 11 | 8 (6–10) | |
| Bilirubin (μmol/L)# | |||||
| <20 (68) | 54 | 24 | 20 | 16 (8–24) | 0.002 |
| ≥20 (134) | 34 | 16 | 11 | 7 (5–9) | |
| CA19-9 (IU/L)# | |||||
| <27 (29) | 64 | 57 | 42 | 28 (11–46) | 0.000 |
| ≥27 (187) | 38 | 18 | 11 | 8 (6–10) | |
| CEA (ng/ml)# | |||||
| <5 (99) | 55 | 33 | 23 | 14 (11–18) | 0.000 |
| ≥5 (61) | 25 | 13 | 7 | 5 (3–7) | |
| Tumour size (mm)^ | |||||
| <35 (65) | 51 | 26 | 15 | 14 (9–19) | 0.044 |
| ≥35 (71) | 37 | 17 | 15 | 8 (5–10) | |
| Clinical T-stage* | |||||
| T1 (6) | 100 | 53 | 53 | 33 (11–70) | 0.000 |
| T2 (41) | 58 | 46 | 23 | 24 (12–36) | |
| T3 (109) | 49 | 24 | 15 | 12 (9–15) | |
| T4 (95) | 23 | 8 | 2 | 5 (3–8) | |
| Differentiation grade | |||||
| Well (29) | 43 | 22 | 15 | 11 (6–17) | 0.708 |
| Moderate (106) | 46 | 27 | 20 | 11 (6–15) | |
| Poor (29) | 44 | 27 | 4 | 10 (5–15) | |
| Topography (for CCA) | 0.175 | ||||
| Intrahepatic (8) | 46 | 46 | 24 | 26 (0–56) | |
| Bismuth type I (3) | 67 | 67 | 0 | 27 | |
| type II (16) | 25 | 25 | 0 | 5 (0–10) | |
| type III (62) | 47 | 19 | 9 | 12 (7–16) | |
| type IV (85) | 30 | 14 | 7 | 7 (5–10) | |
| Extrahepatic (distal) (31) | 48 | 25 | 21 | 12 (3–21) | |
| Treatment category | |||||
| Surgery (84) | 69 | 46 | 33 | 19 (11–27) | 0.000 |
| Photodynamic therapy (39) | 51 | 21 | 5 | 12 (11–14) | |
| Chemo- ± radiotherapy (52) | 37 | 11 | 2 | 8 (6–10) | |
| Biliary stenting alone (118) | 20 | 14 | 10 | 3 (2–3) | |
| Distance metastasis^ | |||||
| No (228) | 48 | 28 | 18 | 12 (9–14) | 0.000 |
| Yes (65) | 12 | 2 | 0 | 3 (2–4) | |
| Vascular involvement^ | |||||
| No (104) | 51 | 29 | 23 | 13 (8–18) | 0.002 |
| Yes (71) | 32 | 18 | 8 | 2 (4–11) | |
On imaging (CT or MRI).
At the time of diagnosis.
Clinical T-stage, unless pathological T-stage available.
CI denotes confidence interval, CA19-9 denotes carbohydrate-associated antigen 19-9, CEA denotes carcinoembryonic antigen, CCA denotes cholangiocarcinoma.
To identify independent factor(s) related to prognosis, a stepwise multivariable Cox’s proportional hazards regression analysis was carried out in patients who had definitive therapy (omitting patients with biliary drainage alone). Independent variables predicting patient survival were as follows: treatment modality (p<0.001), serum CA19-9 (p=0.001), distant metastasis (p=0.002) and vascular involvement (p=0.034) (Table 3). Restricting the analysis to patients with curatively intended surgeries, R-stage became the only independent (hazard ratio 4.12, confidence interval 1.28–13.25; p=0.017) predictor of survival.
Table 3.
Multivariable analysis of prognostic factors
| Parameters | Hazard ratio + 95% confidence interval | p-value |
|---|---|---|
| Distant metastasis^ | 4.62 (1.78–12.02) | 0.002 |
| CA19-9# (27 IU/L cut-off) | 3.22 (1.59–6.53) | 0.001 |
| Treatment modality | 2.10 (1.44–3.07) | <0.001 |
| Vascular involvement^ | 1.80 (1.05–3.10) | 0.034 |
| Bilirubin# (20 μmol/L cut-off) | 1.31 (0.73–2.37) | 0.370 |
| CEA# (5 ng/ml cut-off) | 1.29 (0.58–2.84) | 0.535 |
| Clinical T-stage* | 1.09 (0.70–1.71) | 0.702 |
On imaging (CT or MRI).
At the time of diagnosis.
Clinical T-stage, unless pathological T-stage available.
CA19-9 denotes carbohydrate-associated antigen 19-9, CEA denotes carcinoembryonic antigen.
Discussion
There are few large series or randomised controlled trials14 comparing survival following surgical treatment with modern palliative therapies, including chemotherapy and photodynamic therapy, in patients with BTC. In this study, we describe the management of a large cohort of consecutive, non-selected patients with BTC referred to a tertiary referral centre. Palliative photodynamic therapy in BTC patients appeared to result in a survival outcome similar to curatively intended surgery but positive (R1/R2) resection margins.
A particular strength of the present study is that the diagnosis of BTC was confirmed by positive histology or cytology in 90% of cases, so that we are confident that the cohort did not include a significant number of patients with benign disease. Cytological or histological confirmation of malignancy in BTC is difficult, due in part to the small volume and desmoplastic nature of tumours, and this is reflected in recent series from the UK9,15, Korea16, and Germany10, in which at most 50–70% of patients had pathological confirmation of malignancy. Recent reports suggest that up to 17% of patients undergoing surgery with curative intent for cholangiocarcinoma have benign disease, and that almost half of the benign cases have features of an autoimmune cholangiopathy, possibly IgG4-associate17, emphasising the need for accurate diagnosis.
Surgery was performed on 32% of patients, 88% of whom underwent resections with curative intent, and 38% of this latter group had negative (R0) resection margins (11% of the total). Previous studies have reported R0 resection rates of 32–39% (13–28% of total patients with cholangiocarcinoma)3,8,10 with 46–56% R0 rates in highly selected, exclusively surgical cohorts11,18. Consistent with our own data, several studies have shown that achieving an R0 resection improves survival in comparison to R1 or R2 resection3,19, 20, 21; which may be explained in part by earlier diagnosis (with less advanced disease for R0 resections) and lead-time bias. The importance of achieving an R0 resection has resulted in concomitant liver resection becoming the standard of care 22 and protocols being developed to treat cholangiocarcinoma with liver transplantation23. In patients who underwent surgery with curative intent, R0 resection was the only independent predictor of improved survival, although well differentiated tumours24 and negative lymph node status11 have also been identified by other groups as predicting a better outcome.
A survival advantage of palliative resection (R1/R2) over biliary stenting alone has been reported in some studies10,19,20,21,25, including our own, and challenged in others3,26,27. Comparisons of treatments tend to be hampered by dissimilar patient groups, with ‘biliary stenting only’ being usually reserved for patients with more advanced disease and poorer performance status, a finding also seen in our study. However, an important finding of our study was that survival for patients with curatively intended surgery but positive resection margins did not differ from those who had PDT. PDT is an emerging treatment for cholangiocarcinoma28, which in combination with plastic biliary stenting has been shown in two small randomised studies to improve survival over stent placement alone29,30. The issue of whether this treatment improves survival in patients who have already had successful biliary stenting needs further study31 and is being investigated by our group in a multi-centre randomised trial of PDT plus stenting vs. stenting alone (Photostent 2, ClinicalTrials.gov number, NCT00513539), which is currently recruiting patients. A recent analysis of a German cohort of 184 patients with hilar cholangiocarcinoma has also demonstrated no significant difference in median survival between R1/R2 resection (n=18; 12.2 months) and palliative PDT plus stenting (n=68; 12.0 months)10. As attempted surgical resection is associated with high morbidity and mortality rates of up to 10%32, palliative PDT may be a good alternative for patients at high risk of non-curative resections. In order to select such patients for PDT, improvements in the accuracy of current preoperative staging are needed33; for example MRI/MRCP can under-stage the disease in up to 20% of cases34. Positron-emission tomography (PET, incl. PET-CT) has been shown to be highly sensitive for detecting metastatic deposits35, but has relatively low specificity. Whether new diagnostic tools like intraductal cholangioscopy will improve diagnostic accuracy remains to be established.
Endoscopic stenting alone relieved malignant biliary obstruction in 48% of patients in our study. The published range of effective endoscopic biliary drainage in BTC is very wide (21–97%)9,15,16,36,37, depending on stricture location, endoprothesis used and different definitions of success (e.g. technical endoprosthesis insertion rate vs. successful drainage rate38). Self-expanding metal stents, which have a larger internal diameter than plastic stents, were used in almost half of our patients. There is little consensus, however, as to the optimum approach (endoscopic vs. percutaneous), stent type (metal vs. plastic) or stent number (unilateral vs. bilateral) that should be initially used to palliate patients with hilar cholangiocarcinoma39, due in part to the lack of high quality randomised data in this area.
Since the cause of death in BTC after successful stenting is commonly due to recurrent biliary obstruction and intra-biliary sepsis, a key aim of palliative therapy is that of control of locally progressive disease. Thirty percent of our patients received chemotherapy and/or radiotherapy. Oncological opinion supports the use of palliative chemotherapy, but until recently there has been no agreement on regimen or proven survival benefit over biliary drainage alone14. However, a recent meta-analysis of 104 trials involving 2810 patients reported a beneficial effect of chemotherapy with a pooled (complete and partial) response rate of 23%, particularly when using gemcitabine and platinum-based regimens40. Furthermore, results of the UK phase III ABC-02 trial of gemcitabine, alone or in combination with cisplatin in 410 patients with locally advanced or metastatic BTC, reported a median survival of 11.7 vs. 8.2 months (log rank p=0.002) with gemcitabine and cisplatin over gemcitabine alone, This is the largest ever study in advanced biliary tract cancer and demonstrated a clear advantage for gemcitabine and cisplatin without added clinically significant toxicity, setting a new international standard of care13.
In conclusion, biliary tract cancer survival increases with successful R0 resection. PDT appears to be a promising palliative measure for non-R0 resectable disease, but needs further evaluation in conjunction with chemotherapy agents and targeted therapies in phase II/III trials. The concept of neo-adjuvant therapies to achieve higher rates of clear resection margins appears worthy of further study, although improvements in preoperative staging of BTC are also needed.
Acknowledgments
This study was supported in part by National Institute of Health (NIH) grant PO1CA84203. The work was undertaken at UCLH/UCL, which receives a proportion of funding from the Department of Health’s National Institute for Health Research (NIHR) Biomedical Research Centres funding scheme.
Abbreviations used in this paper
- BTC
biliary tract cancer
- CT
computer tomography
- MRI/MRCP
magnetic resonance imaging/magnetic resonance cholangiopancreatography
- PDT
photodynamic therapy
References
- 1.Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WMC, Taylor-Robinson SD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(supplVI):i1–i9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seyama Y, Makuuchi M. Current surgical treatment for bile duct cancer. World J Gastroenterol. 2007;13:1505–1515. doi: 10.3748/wjg.v13.i10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BSJ, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neuhaus P, Jonas S, Settmacher U, Thelen A, Benckert C, Lopez-Hänninen E, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg. 2003;388:194–200. doi: 10.1007/s00423-003-0383-5. [DOI] [PubMed] [Google Scholar]
- 5.Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rea DJ, Rosen CB, Nagorney DM, Heimbach JK, Gores GJ. Transplantation for cholangiocarcinoma: when and for whom? Surg Oncol Clin N Am. 2009;18:325–337. doi: 10.1016/j.soc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Malhi H, Gores GJ. Review article: the modern diagnosis and therapy of cholangiocarcinoma. Aliment Pharmacol Ther. 2006;23:1287–1296. doi: 10.1111/j.1365-2036.2006.02900.x. [DOI] [PubMed] [Google Scholar]
- 8.Silva MA, Tekin K, Aytekin F, Bramhall SR, Buckels JA, Mirza DF. Surgery for hilar cholangiocarcinoma; a 10 year experience of a tertiary referral centre in the UK. Eur J Surg Oncol. 2005;31:533–539. doi: 10.1016/j.ejso.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Mansfield SD, Barakat O, Charnley RM, Jaques BC, O’Suilleabhain CB, Atherton PJ, et al. Management of hilar cholangiocarcinoma in the North of England: Pathology, treatment and outcome. World J Gastroenterol. 2005;11:7625–7630. doi: 10.3748/wjg.v11.i48.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witzigmann H, Berr F, Ringel U, Caca K, Uhlmann D, Schoppmeyer K, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg. 2006;244:230–239. doi: 10.1097/01.sla.0000217639.10331.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valle JW, Wasan H, Johnson P, Bridgewater J, Maraveyas A, Jones E, et al. Gastrointestinal Cancers Symposium. Orlando: American Society of Clinical Oncology; 2006. Gemcitabine, alone or in combination with cisplatin, in patients with advanced or metastatic cholangiocarcinoma (CC) and other biliary tract tumors: A multicenter, randomized, phase II (the UK ABC-01) study. [Google Scholar]
- 13.Valle JW, Wasan HS, Palmer DD, Cunningham D, Anthoney DA, Maraveyas A, et al. Gemcitabine with or without cisplatin in patients with advanced or metastatic biliary tract cancer: Results of a multicenter, randomized, phase III trial (the UK ABC-02 trial) [accessed Oct 17, 2009];J Clin Oncol. 2009 27:15s. (abstr 4503). (Abstract available online at www.abstract.asco.org/AbstView_65_30777.html.
- 14.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Seminar: Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 15.Connor S, Barron E, Redhead DN, Ireland H, Madhavan KK, Parks RW, et al. Palliation for suspected unresectable hilar cholangiocarcinoma. Eur J Surg Oncol. 2007;33:341–345. doi: 10.1016/j.ejso.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Paik WH, Park YS, Hwang JH, Lee SH, Yoon CJ, Kang SG, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55–62. doi: 10.1016/j.gie.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Erdogan D, Kloek JJ, ten Kate FJ, Rauws EA, Busch OR, Gouma DJ, et al. Immunoglobulin G4-related sclerosing cholangitis in patients resected for presumed malignant bile duct strictures. Br J Surg. 2008;95:727–734. doi: 10.1002/bjs.6057. [DOI] [PubMed] [Google Scholar]
- 18.Sano T, Shimada K, Sakamoto Y, Esaki M, Kosuge T. Changing trends in surgical outcomes after major hepatobiliary resection for hilar cholangiocarcinoma: a single-center experience over 25 years. J Hepatobiliary Pancreat Surg. 2007;14:455–462. doi: 10.1007/s00534-006-1194-1. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–671. doi: 10.1097/00000658-199911000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–699. doi: 10.1097/01.sla.0000160701.38945.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito F, Agni R, Rettammel RJ, Been MJ, Cho CS, Mahvi DM, et al. Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg. 2008;248:273–279. doi: 10.1097/SLA.0b013e31817f2bfd. [DOI] [PubMed] [Google Scholar]
- 23.Heimbach JK, Haddock MG, Alberts SR, Nyberg SL, Ishitani MB, Rosen CB, et al. Transplantation for hilar cholangiocarcinoma. Liver Transpl. 2004;10(Suppl 2):S65–S68. doi: 10.1002/lt.20266. [DOI] [PubMed] [Google Scholar]
- 24.Yubin L, Chihua F, Zhixiang J, Jinrui O, Zixian L, Jianghua Z, et al. Surgical management and prognostic factors of hilar cholangiocarcinoma: experience with 115 cases in China. Ann Surg Oncol. 2008;15:2113–2119. doi: 10.1245/s10434-008-9932-z. [DOI] [PubMed] [Google Scholar]
- 25.Pichlmayr R, Weimann A, Klempnauer J, Oldhafer KJ, Maschek H, Tusch G, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg. 1996;224:628–638. doi: 10.1097/00000658-199611000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rea DJ, Munoz-Juarez M, Farnell MB, Donohue JH, Que FG, Crownhart B, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139:514–523. doi: 10.1001/archsurg.139.5.514. [DOI] [PubMed] [Google Scholar]
- 27.Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228:385–394. doi: 10.1097/00000658-199809000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayaru L, Bown SG, Pereira SP. Photodynamic therapy for pancreatic and biliary tract carcinoma. Int J Gastrointest Cancer. 2005;35:1–13. doi: 10.1385/IJGC:35:1:001. [DOI] [PubMed] [Google Scholar]
- 29.Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426–2430. doi: 10.1111/j.1572-0241.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 31.Gores GJ. A spotlight on cholangiocarcinoma. Gastroenterology. 2003;125:1536–1538. doi: 10.1016/j.gastro.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. doi: 10.1053/j.gastro.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 33.Zervos EE, Osborne D, Goldin SB, Villadolid DV, Thometz DP, Durkin A, et al. Stage does not predict survival after resection of hilar cholangiocarcinomas promoting an aggressive operative approach. Am J Surg. 2005;190:810–815. doi: 10.1016/j.amjsurg.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 34.Zidi SH, Prat F, Le Guen O, Rondeau Y, Pelletier G. Performance characteristics of magnetic resonance cholangiography in the staging of malignant hilar strictures. Gut. 2000;46:103–106. doi: 10.1136/gut.46.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breitenstein S, Apestegui C, Clavien PA. Positron emission tomography (PET) for cholangiocarcinoma. HPB (Oxford) 2008;10:120–121. doi: 10.1080/13651820801992583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Palma GD, Pezzullo A, Rega M, Persico M, Patrone F, Mastantuono L, et al. Unilateral placement of metallic stents for malignant hilar obstruction: a prospective study. Gastrointest Endosc. 2003;58:50–53. doi: 10.1067/mge.2003.310. [DOI] [PubMed] [Google Scholar]
- 37.Sherman S. Endoscopic drainage of malignant hilar obstruction: is one biliary stent enough or should we work to place two? Gastrointest Endosc. 2001;53:681–684. doi: 10.1067/mge.2001.114714. [DOI] [PubMed] [Google Scholar]
- 38.Liu CL, Lo CM, Lai EC, Fan ST. Endoscopic retrograde cholangiopancreatography and endoscopic endoprostheses insertion in patients with Klatskin tumors. Arch Surg. 1998;133:293–296. doi: 10.1001/archsurg.133.3.293. [DOI] [PubMed] [Google Scholar]
- 39.Chahal P, Baron TH. Endoscopic palliation of cholangiocarcinoma. Curr Opin Gastroenterol. 2006;22:551–560. doi: 10.1097/01.mog.0000239872.12081.a4. [DOI] [PubMed] [Google Scholar]
- 40.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]