Abstract
Covalent and non-covalent conjugation of proteins to nanoparticles provides access to functional hybrid systems with applications in biotechnology, medicine, and catalysis. The creation of effective conjugates requires the retention of protein structure and function, a challenging task. In this review we discuss successes, challenges and opportunities in the area of protein-nanoparticle bioconjugation.
Introduction
Nanoparticles (NPs) provide a platform for integrating biology and synthetic materials. A wide variety of core materials can be used for fabricating NPs, including metals, metal oxides, semiconductors, and core-shell hybrids [1]. With these systems, the composition of the core material dictates the physical properties of the nanoparticle, including optical and magnetic behavior. Taken together, the diversity of available core materials and properties make nanoparticles pragmatic tools for numerous applications [2••].
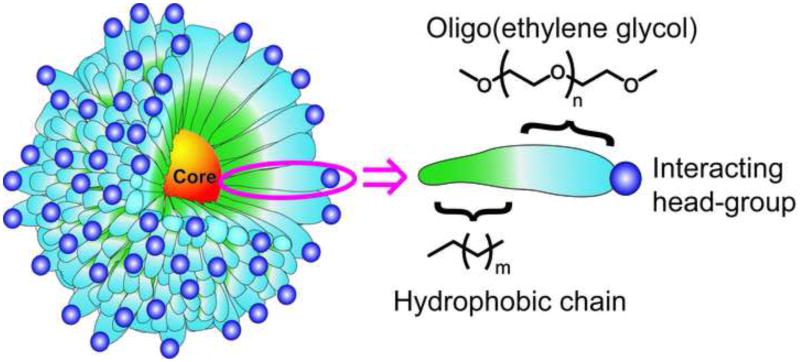
The organic monolayers used to passivate nanoparticles play a key role in their biological applications. Functionally, the solubility and stability of the nanoparticles are dictated by the surface properties of the particle. More importantly, the biointerfacial interactions of nanoparticles are dictated by the chemical and topological nature of the NP coverage. A common motif used for NP stabilization/solublization and biocompatiblization (Figure 1) features a ligand shell incorporating a hydrophobic interior (for micelle-like stabilization of the shell) and an oligo(ethylene glycol) spacer to minimize protein denaturation.
Figure 1. Structure of common nanoparticles.
A general schematic of surface ligands on most of the common NPs used for interaction with biological systems. The oligo(ethylene glycol) linker helps to retain the structure and function of proteins after conjugation.
Two fundamentally different approaches can be used to conjugate proteins to nanoparticles. The first approach uses direct covalent linkage of the protein to the particle surface. The second method uses non-covalent interactions between the particle and protein to generate supramolecular assemblies. Both of these approaches have strengths and limitations, and hence have a place in the bionanotechnology “toolkit”. This review covers only a small subset of the recent literature in both covalent and non-covalent conjugation of proteins to nanoparticles, highlighting both advances and challenges in the area.
Covalent Protein-Nanoparticle Conjugates
Covalent attachment of proteins to NPs provides conjugates that are stable toward dissociation, an important issue for many applications. In particular, the stability and irreversibility of covalent protein-NP conjugates makes this strategy useful for applications in complex biological media with other interfering species. Covalently conjugating proteins to the NP surface also provides control over protein reactivity and NP aggregation.
One strategy for attachment relies on direct interactions between multiple protein sidechains, e.g. lysine and glutamate, and the NP core. This approach has been used by Rotello et al. to generate Fe3O4 particles that were stable in serum for applications in hyperthermic therapy [3]. A common issue with this approach, however, is the loss of protein structure due to multivalent interactions between numerous residues of protein and the NP surface [4]. Conditions that minimize non-specific adsorption and optimize protein structure and activity are desirable for efficient direct conjugation onto NPs. Efforts have been directed to reduce this effect through appropriate NP co-functionalization and through NP loading [5•]. A potentially less disruptive approach is to use a monovalent attachment, such as a single cysteine. However, substantial denaturation can likewise occur with this approach [6••].
The use of engineered ligands for conjugating proteins to NPs has met with much greater success in terms of retaining protein structure (Figure 2). This type of attachment has traditionally been accomplished using standard amine-carboxylate coupling methods [7]. More recently, the ubiquitous alkyne-azide Huisgen “click” reaction has been used to attach proteins to NPs. In the presence of a catalyst, “click” reactions can proceed with high yield and can also allow the site-specific conjugation of the azide or alkyne tagged proteins to the NP partners. A variety of proteins have been attached in functional form using this method, including lipase [8••], horseradish peroxidase [9], and luciferase [10]. Ligand design is crucial in this strategy, with each of these studies employing biocompatible oligo(ethylene glycol) (OEG) spacers between protein and NP. The OEG chains reduce nonspecific interactions as well as provide additional degrees of freedom for the terminal functional groups.
Figure 2. Nanoparticle-protein complexation through covalent conjugation.
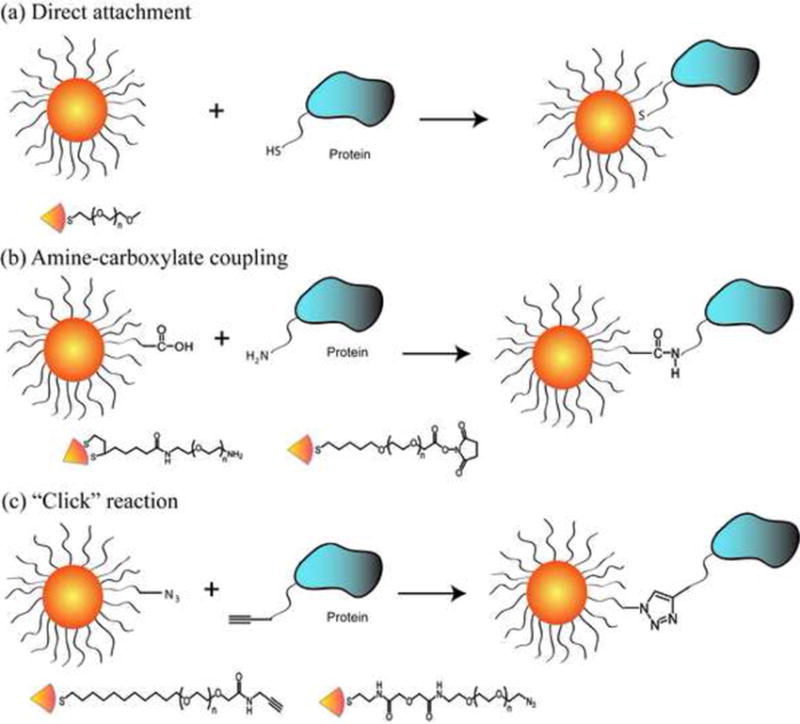
(a) The direct attachment of proteins to NPs using a thiol group on the protein surface. (b) Amine-carboxylate coupling using carboxylate-presenting NPs and amine groups on protein surface. (c) “Click” reaction using azide tagged NP and alkyne tagged protein. Representative ligands used to modify the NP surface are presented under each strategy.
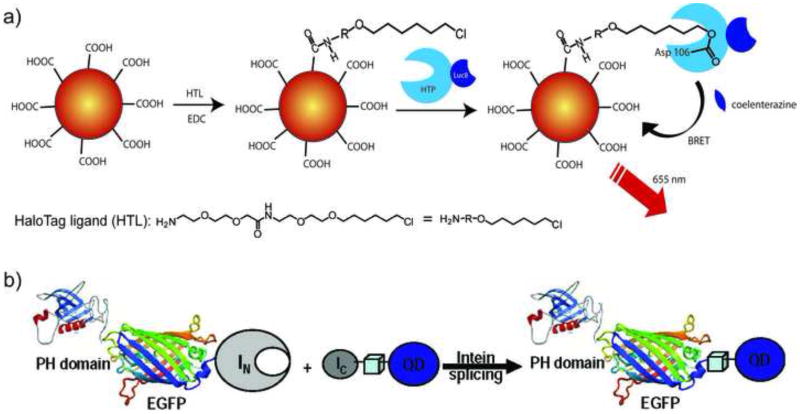
Obtaining both site specificity and protein activity remains a challenge in NP conjugation. One particularly clever approach to this end was developed by Rao et al., who conjugated quantum dots (QDs) to “HaloTag” proteins based on a modified dehalogenase that reacts specifically and irreversibly with appropriate halogenated substrates (Figure 3a). The HaloTag ligand was designed with an amino ethylene glycol group to orient the ligand away from the surface of the QD and also reduce the steric hindrance between the HaloTag and QD. The versatility of the approach was provided through the use of fusion proteins, allowing the facile attachment of luciferase functionality to the particle for biolouminescence resonance energy transfer (BRET) applications [11•].
Figure 3. Site-specific conjugation of active proteins.
a) Conjugation of a HaloTag protein to QDs mediated by the appropriate alkyl chloride ligand. b) Schematic representation of site-specific intein-mediated conjugation of QD to target protein (reproduced with permission from ref [12]).
Another attractive approach is intein-mediated site specific conjugation of nanomaterials to proteins. This approach allows the covalent conjugation of any nanostructure to proteins with retained structure and activity, and the reaction is specific in the cellular environment as well. Skourides et al. genetically tagged EGFP to pleckstrin-homology (PH) domain with the N-terminal half of a split intein and conjugated the C-terminal half to QDs (Figure 3b). In vivo intein-splicing in Xenopus embryos showed a fully functional QD-PH conjugate that could be tracked in real time [12].
Non-Covalent Protein-Nanoparticle Conjugation
On a fundamental level, understanding the supramolecular interactions between proteins and nanoparticles is central to the applications of NPs in vivo. A number of studies have explored the kinetics and thermodynamics of binding between individual proteins and NPs [13], demonstrating that properly functionalized NPs interact with proteins in analogous fashion to protein-protein interactions [14••]. The biophysical properties, such as binding affinity, residence time, binding cooperativity of NP and the common serum proteins, have been quantitatively characterized in buffer medium to gain fundamental understanding of the behavior of proteins on the NP surfaces [15,16]. More recently, efforts have been directed towards understanding the interactions of NPs with complex protein mixtures, e.g. serum [17••,18], an important issue for drug delivery and sensing (vide infra). An evolution from a loosely bound to irreversibly attached protein “corona” around the NPs over time has been observed in cell culture media containing serum, a process that potentially correlates the cellular uptake and other biological processes with NP-protein complexes [19,20].
Pragmatically, non-covalent conjugation of proteins to NPs provides a complementary strategy to covalent attachment, enabling applications in sensing and delivery. As with covalent conjugation, denaturation of the protein on the surface is quite rapid [21] unless appropriately engineered ligands (almost universally OEG-based) are used for particle functionalization [22,23]. The simplest way to produce non-covalent NP-protein conjugates is through complementary electrostatic interactions between the nanoparticle and the protein, a particularly useful approach when specificity is not required [22]. To overcome the inherent non-specificity of this approach, the NP surface has been strategically modified by varying its charge and hydrophobicity, enabling regiospecific interaction [24]. Metal-mediated interactions provide a useful alternative that imparts selectivity to the NP-protein conjugation process. A particularly facile method is through nickel-mediated interactions with His-tagged proteins [25,26], a technique that also provides effective control of NP-protein stoichiometry [27]. In a particularly elegant implementation of His-tag binding, Mattoussi, Medintz et al. used the affinity of hexa-His peptides to directly conjugate fluorescent proteins (FPs) to QD surfaces to create caspase sensors (Figure 4) [28]. In this system the His-tag and the peptide linker were long enough to minimize the denaturation observed above with cysteine-based immobilization.
Figure 4. Metal-mediated non-covalent conjugation and its application.
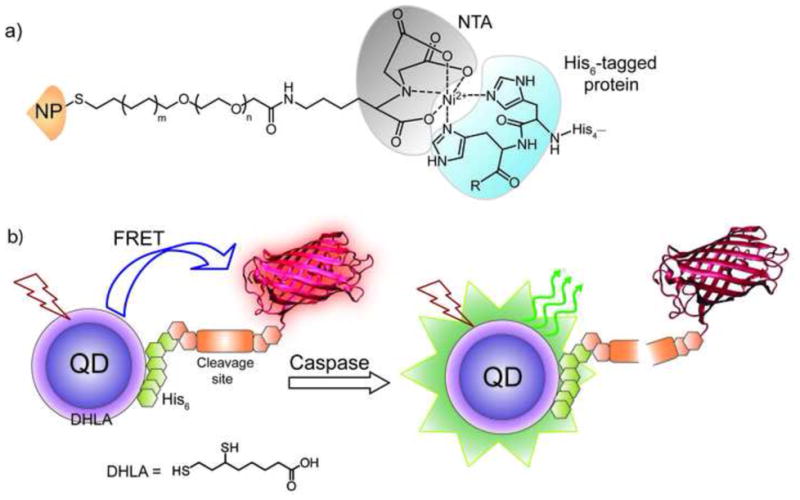
a) Depiction of metal-mediated high affinity conjugation of hexa-His tagged protein to nitrilotriacetic acid (NTA) functionalized NP. b) Schematic of the QD-fluorescent protein sensor constructed through non-covalent conjugation between DHLA coated QD and mCherry protein with an N-terminal linker expressing the caspase 3 cleavage site and a His6 sequence. The specific cleavage of the linker by caspase 3 removes FRET to enable protein sensing.
Specificity of binding can be imparted via conjugation of the particle with biomolecular ligands, exploiting the ease of functionalization inherent to many NP materials. The biotin-(strept)avidin interaction is widely used for the conjugation of NP systems [29]; Zheng and Huang fabricated a biotin group or glutathione capped onto the surface of gold nanoparticles protected by tri(ethylene glycol) thiols [30]. They were able to show specific binding of either streptavidin or glutathione-S-transferase to their respective capped nanoparticles. The glutathione approach has since been applied to the creation of glutathione-S-tranferase fusion proteins [31]. Another popular conjugation strategy is through carbohydrate-lectin interactions [32], with a particularly efficient method being that of Yan et al. using the photochemical attachment of unmodified sugars to the NP surface [33]. The conjugation of carbohydrate-coated magnetic particles to proteins by means of the carbohydrate-lectin specificity provides a powerful tool for rapid detection of pathogens along with utility in separation of different bacterial strains [34].
While most NP-protein conjugates are intended for applications in solution, nanocomposite assemblies provide access to systems with interesting and potentially useful properties. Initial studies of protein-NP composite assemblies focused on the use of proteins as building blocks for control of interparticle spacing and morphology [35,36], exploiting the size, charge and shape of the protein to control composite structure [37•, 38]. Building upon the structural control provided by these composites, electronically-active multilayer systems have been developed using biotin-streptavidin assembly of gold nanoparticles [39]. In recent studies the magnetic properties of a protein (ferritin) have been integrated with those of FePt nanoparticles through electrostatic assembly to generate 3-dimensional assemblies featuring novel magnetic behavior [40]. NP-protein assembly has also been explored at 2-D oil-water interfaces. Through appropriate choice of OEG-containing ligand, β-galactosidase ( -gal, pI 4.6) has been assembled with cationic nanoparticles on oil-in-water emulsion interfaces, with high retention (>70%) of -gal activity [41].
The reversible nature of non-covalent NP-protein assemblies enables their application in areas including sensing and delivery. On the sensing front, non-covalent gold NP-green fluorescent protein (GFP) complexes have been employed in an array-based “chemical nose” fashion to identify five of the more abundant human serum proteins (i.e. human serum albumin, immunoglobulin G, transferrin, fibrinogen and – antitrypsin, Figure 5a) in human serum (overall protein concentration ~1 mM), obtaining a limit of detection of 500 nM. [42••]. In more recent studies an analogous approach has been used for cell surface sensing, with NP-GFP complexes able to differentiate between healthy and cancerous isogenic cells [43]. An array-based approach using magnetic NPs with magnetic resonance imaging (MRI) transduction has been likewise used to identify cell types and states (Figure 5c) based on carbohydrate-lectin selectivity [44•].
Figure 5. GFP-NP “chemical nose” sensor.
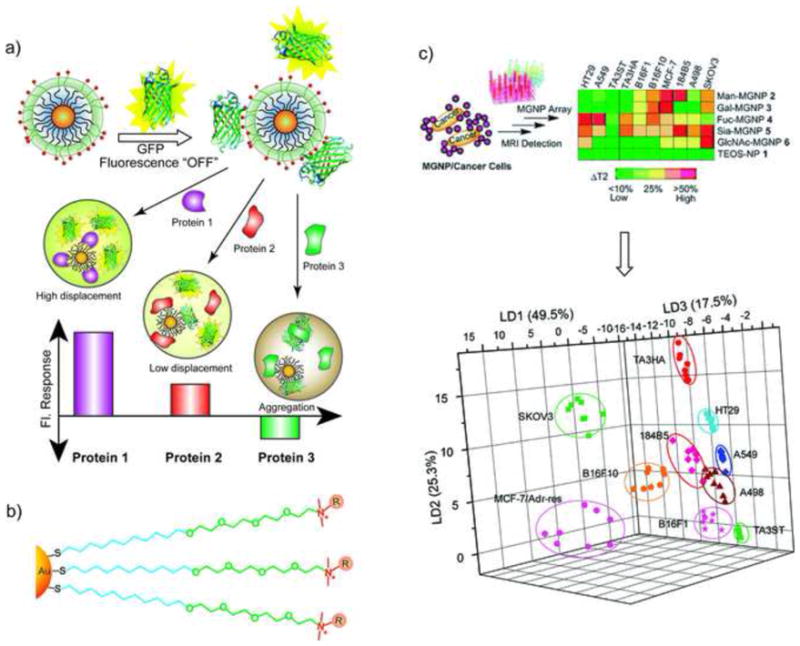
a) Schematic illustration showing the competitive binding between proteins and quenched gold NP-GFP complexes, whose aggregation leads to fluorescence “turn on” or further quenching using a library of cationic nanoparticles. b) Chemical structure of cationic gold NPs. c) Percentage changes of T2 relaxation time (% T2) obtained upon incubating MGNPs with 10 cell lines (105 cells/mL) and the LDA plots for the first three LDs of T2 patterns (reproduced with permission from [44•].
Protein delivery presents a second area where reversible interactions would be desirable, ultimately providing the delivery of free native protein to the cell. Recently, the intracellular delivery of the large protein β-gal (465 kD) was demonstrated using cationic nanoparticles having a short peptide conjugated to a tetra(ethylene glycol) unit (Figure 6). Significantly, the enzymatic activity of the β-gal was retained in the cell, and the β-gal escaped from the endosome and into the cytosol [45•]. Non-covalent conjugation has been employed in delivering proteins by transmucosal and oral pathways. Amino acid capped gold NPs were used to carry insulin in active form with sustained release profile [46]. There are certain advantages of increased selectivity of non-covalent approaches in delivery applications as well. Specific carbohydrate-lectin conjugation enables uptake via receptor-mediated endocytosis. The preferential uptake of sugar-capped PEGylated QDs by specific asialo-gycoprotein has been demonstrated to be effective for in vivo targeting and imaging [47].
Figure 6. Delivery of protein cargo via non-covalent conjugation.
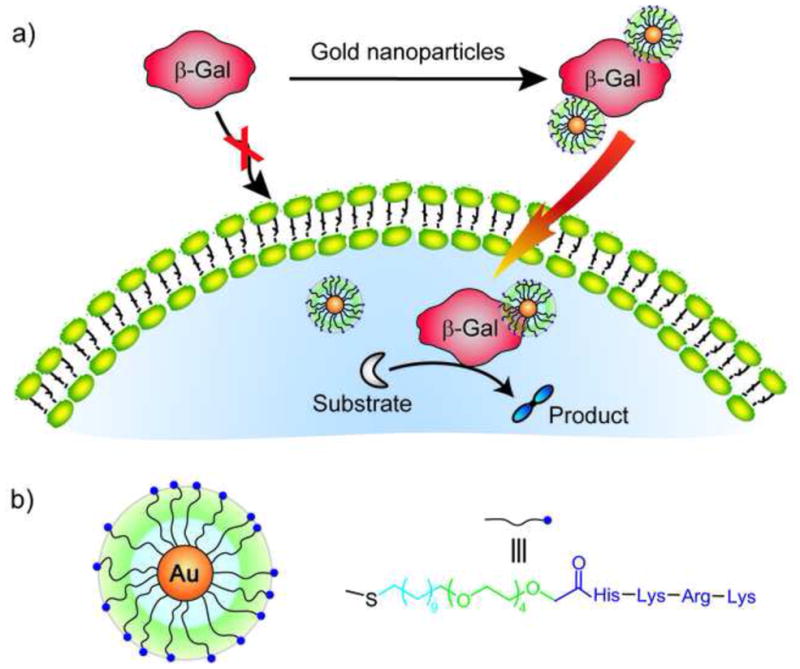
a) Schematic representation of the complexation between cationic NP and anionic protein (β-Gal), leading to efficient permeation through the cell membrane compared to uncomplexed proteins. b) Structure of the cationic nanoparticle and the surface ligands.
Summary and Outlook
Substantial progress has been made in recent years on the formation of both covalent and non-covalent protein-NP conjugates. Perhaps the most important lesson learned is that the protein-NP interface is crucial, and that the most straightforward way to provide stability is through OEG-containing ligands. Efforts to fine-tune these interfaces are ongoing, as there is still no completely reliable means of conjugating proteins to NPs. An additional challenge is provided by systems where intimate contact between protein and NP is desirable, e.g. optical and electronic systems. As seen above, direct conjugation of NPs to proteins remains an open challenge. Finally, as these issues become resolved, the potential for synergistic engineering of NPs and proteins presents the possibility for new bionanoconjugates featuring behavior unique from those of either component.
Acknowledgments
The support of the NIH (GM077173) is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
[•] of special interest
[••] of outstanding interest
- 1.Burda C, Chen X, Narayanan R, El-Sayed MA. Chemistry and properties of nanocrystals of different shapes. Chem Rev. 2005;105:1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 2[••].De M, Ghosh PS, Rotello VM. Applications of nanoparticles in biology. Adv Mat. 2008;20:4225–4241. This review discusses nanoparticle-biomolecular interactions and also provides various biological applications of nanoparticles, including sensing, delivery and imaging. [Google Scholar]
- 3.Samanta B, Yan H, Fischer NO, Shi J, Jerry DJ, Rotello VM. Protein-passivated Fe3O4 nanoparticles: low toxicity and rapid heating for thermal therapy. J Mat Chem. 2008;18:1204–1208. doi: 10.1039/b718745a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubin-Tam ME, Zhou H, Hamad-Schifferli K. Structure of cytochrome c at the interface with magnetic CoFe2O4 nanoparticles. Soft Matter. 2008;4:554–559. doi: 10.1039/b711937b. [DOI] [PubMed] [Google Scholar]
- 5[•].Bajaj A, Samanta B, Yan H, Jerry DJ, Rotello VM. Stability, toxicity and differential cellular uptake of protein passivated-Fe3O4 Nanoparticles. J Mater Chem. 2009;19:6328–6331. The mechanism and differential uptake of BSA coated Fe3O4 nanoparticles were explored in different cancerous and isogenic cell types. [Google Scholar]
- 6[••].Aubin-Tam ME, Hwang W, Hamad-Schifferli K. Site-directed nanoparticle labeling of cytochrome c. Proc Natl Acad Sci USA. 2009;106:4095–4100. doi: 10.1073/pnas.0807299106. The authors controlled specific attachment between cytochrome c and gold nanoparticles by using cysteine mutations of surface residues. It showed that the choice of labeling site affects the protein structure in the conjugate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drechsler U, Fischer NO, Frankamp BL, Rotello VM. Highly efficient biocatalysts via covalent immobilization of Candida rugosa lipase on ethylene glycol modified gold-silica nanocomposites. Adv Mat. 2004;16:271–274. [Google Scholar]
- 8[•].Brennan JL, Hatzakis NS, Tshikhudo TR, Dirvianskyte N, Razumas V, Patkar S, Vind J, Svendsen A, Nolte RJM, Rowan AE, Brust M. Bionanoconjugation via click chemistry: The creation of functional hybrids of lipases and gold nanoparticles. Bioconjugate Chem. 2006;17:1373–1375. doi: 10.1021/bc0601018. A simple and versatile conjugation method using a click reaction between nanoparticle and enzyme was developed. The activity of the enzyme was retained after conjugation. [DOI] [PubMed] [Google Scholar]
- 9.Zhang MX, Huang BH, Sun XY, Pang DW. Clickable gold nanoparticles as the building block of nanobioprobes. Langmuir. 2010;26:10171–10176. doi: 10.1021/la100315u. [DOI] [PubMed] [Google Scholar]
- 10.Kim YP, Daniel WL, Xia ZY, Xie HX, Mirkin CA, Rao JH. Bioluminescent nanosensors for protease detection based upon gold nanoparticle-luciferase conjugates. Chem Commun. 2010;46:76–78. doi: 10.1039/b915612g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11[•].Zhang Y, So MK, Loening AM, Yao HQ, Gambhir SS, Rao JH. HaloTag protein-mediated site-specific conjugation of bioluminescent proteins to quantum dots. Angew Chem Int Ed. 2006;45:4936–4940. doi: 10.1002/anie.200601197. A specific conjugation between a HaloTag protein and QDs was generated. These bioluminescent protein-QDs conjugates presented the self illuminating properties. [DOI] [PubMed] [Google Scholar]
- 12.Charalambous A, Andreou M, Skourides PA. Intein-mediated site-specific conjugation of quantum dots to proteins in vivo. J Nanobiotech. 2009;7:1–9. doi: 10.1186/1477-3155-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You C-C, Agasti S, Rotello VM. Isomeric control of protein recognition with amino acid- and dipeptide-functionalized gold nanoparticles. Chem Eur J. 2008;14:143–150. doi: 10.1002/chem.200701234. [DOI] [PubMed] [Google Scholar]
- 14[••].De M, You C-C, Srivastava S, Rotello VM. Biomimetic interactions of proteins with functionalized nanoparticles: A thermodynamic study. J Am Chem Soc. 2007;129:10747–10753. doi: 10.1021/ja071642q. This study characterizes the thermodynamic parameters of binding between amino acid functionalized nanoparticles and proteins using isothermal titration calorimetry. The quantitative measurements indicate the complexation thermodynamically mimics natural protein-protein interactions. [DOI] [PubMed] [Google Scholar]
- 15.Rocker C, Potzl M, Zhang F, Parak WJ, Nienhaus GU. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat Nanotech. 2009;4:577–580. doi: 10.1038/nnano.2009.195. [DOI] [PubMed] [Google Scholar]
- 16.Lacerda SHP, Park JJ, Meuse C, Pristinski D, Becker ML, Karim A, Douglas JF. Interaction of gold nanoparticles with common human blood proteins. ACS Nano. 2010;4:365–379. doi: 10.1021/nn9011187. [DOI] [PubMed] [Google Scholar]
- 17[••].Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. Different approaches were developed to study the rates, affinities, stoichiometries of protein association/dissociation on nanoparticle surfaces in plasma and simple model systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci USA. 2008;105:14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes V. Time evolution of the nanoparticle protein corona. ACS Nano. 2010;4:3623–3632. doi: 10.1021/nn901372t. [DOI] [PubMed] [Google Scholar]
- 20.Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. What the cell “sees” in bionanoscience. J Am Chem Soc. 2010;132:5761–5768. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- 21.You C-C, De M, Rotello VM. Contrasting effects of exterior and interior hydrophobic moieties in the complexation of amino acid-functionalized gold clusters with -chymotrypsin. Org Lett. 2005;7:5685–5687. doi: 10.1021/ol052367k. [DOI] [PubMed] [Google Scholar]
- 22.Hong R, Fischer NO, Verma A, Goodman CM, Emrick TS, Rotello VM. Control of protein structure and function through surface recognition by tailored nanoparticle scaffolds. J Am Chem Soc. 2004;126:739–743. doi: 10.1021/ja037470o. [DOI] [PubMed] [Google Scholar]
- 23.Yildiz I, McCaughan B, Cruickshank SF, Callan JF, Raymo FM. Biocompatible CdSe-ZnS core-shell quantum dots coated with hydrophilic polythiols. Langmuir. 2009;25:7090–7096. doi: 10.1021/la900148m. [DOI] [PubMed] [Google Scholar]
- 24.Bayraktar H, You C-C, Rotello VM, Knapp MJ. Facial Control of Nanoparticle Binding to Cytochrome c. J Am Chem Soc. 2007;129:2732–2733. doi: 10.1021/ja067497i. [DOI] [PubMed] [Google Scholar]
- 25.Abad JM, Mertens SFL, Pita M, Fernandez VM, Schiffrin DJ. Functionalization of thioctic acid-capped gold nanoparticles for specific immobilization of histidine-tagged proteins. J Am Chem Soc. 2005;127:5689–5694. doi: 10.1021/ja042717i. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Valencia CA, Liu RH, Lin WB. Highly-efficient purification of native polyhistidine-tagged proteins by multivalent NTA-modified magnetic nanoparticles. Bioconjugate Chem. 2007;18:333–341. doi: 10.1021/bc060195l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De M, Rana S, Rotello VM. Nickel-ion-mediated control of the stoichiometry of His-tagged protein/nanoparticle interactions. Macromol Biosci. 2009;9:174–178. doi: 10.1002/mabi.200800289. [DOI] [PubMed] [Google Scholar]
- 28.Boeneman K, Mei BC, Dennis AM, Bao G, Deschamps JR, Mattoussi H, Medintz IL. Sensing caspase 3 activity with quantum dot-fluorescent protein assemblies. J Am Chem Soc. 2009;131:3828–3829. doi: 10.1021/ja809721j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Susumu K, Uyeda HT, Medintz IL, Pons T, Delehanty JB, Mattoussi H. Enhancing the stability and biological functionalities of quantum dots via compact multifunctional ligands. J Am Chem Soc. 2007;129:13987–13996. doi: 10.1021/ja0749744. [DOI] [PubMed] [Google Scholar]
- 30.Zheng M, Huang XY. Nanoparticles comprising a mixed monolayer for specific bindings with biomolecules. J Am Chem Soc. 2004;126:12047–12054. doi: 10.1021/ja047029d. [DOI] [PubMed] [Google Scholar]
- 31.Chen CT, Chen WJ, Liu CZ, Chang LY, Chen YC. Glutathione-bound gold nanoclusters for selective-binding and detection of glutathione S-transferase-fusion proteins from cell lysates. Chem Commun. 2009;45:7515–7517. doi: 10.1039/b916919a. [DOI] [PubMed] [Google Scholar]
- 32.Jiang XZ, Housni A, Gody G, Boullanger P, Charreyre MT, Delair T, Narain R. Synthesis of Biotinylated alpha-D-Mannoside or N-Acetyl beta-D-Glucosaminoside Decorated Gold Nanoparticles: Study of Their Biomolecular Recognition with Con A and WGA Lectins. Bioconjugate Chem. 2010;21:521–530. doi: 10.1021/bc900431p. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Ramstrom O, Yan MD. A photochemically initiated chemistry for coupling underivatized carbohydrates to gold nanoparticles. J Mater Chem. 2009;19:8944–8949. doi: 10.1039/B917900C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Boubbou K, Gruden C, Huang X. Magnetic Glyco-nanoparticles: A unique tool for rapid pathogen detection, decontamination, and strain differentiation. J Am Chem Soc. 2007;129:13392–13393. doi: 10.1021/ja076086e. [DOI] [PubMed] [Google Scholar]
- 35.Verma A, Srivastava S, Rotello VM. Modulation of the interparticle spacing and optical behavior of nanoparticle ensembles using a single protein spacer. Chem Mat. 2005;17:6317–6322. [Google Scholar]
- 36.Wei G, Pan C, Reichert J, Jandt KD. Controlled assembly of protein-protected gold nanoparticles on noncovalent functionalized carbon nanotubes. Carbon. 2010;48:645–653. [Google Scholar]
- 37[•].De M, Miranda OR, Rana S, Rotello VM. Size and geometry dependent protein-nanoparticle self-assembly. Chem Commun. 2009;45:2157–2159. doi: 10.1039/b900552h. Isothermal calorimetry is a powerful tool to study NP-protein interactions that has been employed in the present study to demonstrate size and geometry dependent protein-NP self-assembly. [DOI] [PubMed] [Google Scholar]
- 38.Hu M, Qian L, Brinas RP, Lymar ES, Hainfeld JF. Assembly of nanoparticle – protein binding complexes: from monomers to ordered arrays. Angew Chem Int Ed. 2007;46:5111–5114. doi: 10.1002/anie.200701180. [DOI] [PubMed] [Google Scholar]
- 39.Azzaroni O, Alvarez M, Abou-Kandil AI, Yameen B, Knoll W. Tuning the unidirectional electron transfer at interfaces with multilayered redox-active supramolecular bionanoassemblies. Adv Funct Mater. 2008;18:3487–3496. [Google Scholar]
- 40.Srivastava S, Samanta B, Jordan BJ, Hong R, Xiao Q, Tuominen MT, Rotello VM. Integrated magnetic bionanocomposites through nanoparticle-mediated assembly of ferritin. J Am Chem Soc. 2007;129:11776–11780. doi: 10.1021/ja073163x. [DOI] [PubMed] [Google Scholar]
- 41.Samanta B, Yang XC, Ofir Y, Park MH, Patra D, Agasti S, Miranda OR, Mo ZH, Rotello VM. Catalytic microcapsules through assembly of enzyme-nanoparticle conjugates at oil-water interfaces. Angew Chem Int Ed. 2009;48:5341–5344. doi: 10.1002/anie.200901590. [DOI] [PubMed] [Google Scholar]
- 42[••].De M, Rana S, Akpinar H, Miranda OR, Arvizo RR, Bunz UHF, Rotello VM. Sensing of proteins in human serum using conjugates of nanoparticles and green fluorescent protein. Nature Chem. 2009;1:461–465. doi: 10.1038/nchem.334. This work demonstrates the ability of the “chemical nose” method to detect proteins in complex biofluids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bajaj A, Rana S, Miranda OR, Yawe JC, Jerry DJ, Bunz UHF, Rotello VM. Cell surface-based differentiation of cell types and cancer states using a gold nanoparticle-GFP based sensing array. Chem Sci. 2010;1:134–138. [Google Scholar]
- 44[•].El-Boubbou K, Zhu DC, Vasileiou C, Borhan B, Prosperi D, Li W, Huang XF. Magnetic Glyco-Nanoparticles: A Tool To Detect, Differentiate, and Unlock the Glyco-Codes of Cancer via Magnetic Resonance Imaging. J Am Chem Soc. 2010;132:4490–4499. doi: 10.1021/ja100455c. Using an array of magnetic nanoparticles bearing carbohydrate ligands, the authors detected and differentiated different cell states through their magnetic resonance image (MRI) signature. [DOI] [PubMed] [Google Scholar]
- 45[•].Ghosh P, Yang X, Arvizo R, Zhu ZJ, Mo Z, Rotello VM. Intracellular delivery of a membrane-impermeable enzyme in active form using functionalized gold nanoparticles. J Am Chem Soc. 2010;132:2642–2645. doi: 10.1021/ja907887z. The authors demonstrated the cellular delivery of membrane impermeable proteins through electrostatic conjugation with cationic nanoparticles displaying a short peptide. The delivery is an effective method owing to the endosomal escape of the cargo with preserved conformation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joshi HM, Bhumkar DR, Joshi K, Pokharkar V, Sastry M. Gold nanoparticles as carriers for efficient transmucosal insulin delivery. Langmuir. 2006;22:300–305. doi: 10.1021/la051982u. [DOI] [PubMed] [Google Scholar]
- 47.Kikkeri R, Lepenies B, Adibekian A, Laurino P, Seeberger PH. In vitro imaging and in vivo liver targeting with carbohydrate capped quantum dots. J Am Chem Soc. 2009;131:2110–2112. doi: 10.1021/ja807711w. [DOI] [PubMed] [Google Scholar]