Abstract
Objective
Certain cognitive deficits in individuals with schizophrenia have been linked to disturbed GABA and glutamate neurotransmission in the prefrontal cortex (PFC). Thus, it is important to understand how the mechanisms that regulate GABA and glutamate neurotransmission are altered in schizophrenia. For example, group I metabotropic glutamate receptors (mGluR1α and mGluR5) modulate both GABA and glutamate systems. In addition, regulator of G protein signaling 4 (RGS4) reduces intracellular signaling through several different G protein-coupled receptors, including group I mGluR. Finally, the endocannabinoid system plays an important role in regulating GABA and glutamate neurotransmission; the status of endocannabinoid ligands, such as 2-arachidonoylglycerol (2-AG), can be inferred, in part, through measures of diacylglycerol lipase and monoglyceride lipase, which synthesize and degrade 2-AG, respectively.
Methods
We used quantitative PCR to measure mRNA levels for group I mGluR, RGS4, and markers of the endocannabinoid system in PFC area 9 from 42 schizophrenia subjects and matched normal comparison subjects. We conducted similar studies in monkeys chronically exposed to haloperidol, olanzapine, or placebo.
Results
Schizophrenia subjects had higher mRNA levels for mGluR1α and lower mRNA levels for RGS4, and these differences did not appear to be attributable to antipsychotic medications or other potential confounds. In contrast, no differences between subject groups were found in mRNA levels for endocannabinoid synthesizing and metabolizing enzymes.
Conclusion
Together, higher mGluR1α and lower RGS4 mRNA levels may represent a disturbed “molecular hub” in schizophrenia that may disrupt the function of PFC cortical networks, including both GABA and glutamate systems.
Keywords: RGS4, diacylglycerol lipase, monoglyceride lipase, fatty acid amide hydrolase, GABA, cannabinoid receptor interacting protein 1
Introduction
Cognitive impairments, such as deficits in working memory, are among the most disabling and difficult to treat features of schizophrenia (1;2) and appear to be linked to impairments in the circuitry of the prefrontal cortex (PFC) (3). For example, markers of GABA neurotransmission are altered in subpopulations of PFC GABA neurons (4), and evidence of disturbed glutamate neurotransmission, such as NMDA receptor hypofunction (5), has also been reported in the illness. Thus, understanding the mechanisms that might contribute to disturbed GABA and glutamate neurotransmission is essential for developing novel treatments for cognitive impairments in schizophrenia.
Group I metabotropic glutamate receptors (mGluR1α and mGluR5) play an important role in regulating GABA and glutamate neurotransmission in the cortex. For example, activation of group I mGluR initiates an intracellular signaling cascade that leads to suppression of GABA release (6–10) and long-term potentiation of NMDA receptor function (11). Interestingly, one study reported higher protein levels of mGluR1α, but not mGluR5, in the PFC of elderly schizophrenia subjects (12), which may contribute to altered GABA and glutamate neurotransmission in schizophrenia. However, it remains unclear whether group I mGluR alterations are generalizable to most subjects with schizophrenia, including younger individuals with the disorder, and whether alterations in group I mGluR protein levels are attributable to differences in gene expression levels in schizophrenia.
Activation of group I mGluR initiates a G protein signaling pathway, which is inhibited by regulator of G protein signaling 4 (RGS4) (13). RGS4 functions as a GTPase-activating protein which increases the hydrolysis of GTP and reduces the duration of activity of several different G protein-coupled receptors, including group I mGluR (13). RGS4 has been previously identified as a gene of interest in schizophrenia because of genetic association studies (14) and reports of lower expression levels in the PFC in two relatively small cohorts of subjects with schizophrenia (15;16). However, it is unclear if lower RGS4 mRNA levels are commonly found in schizophrenia and if lower RGS4 mRNA levels, which may affect the duration of intracellular signaling from group I mGluR, are present in the same schizophrenia subjects who have higher group I mGluR levels.
The endogenous cannabinoid system is also critically involved in regulating GABA and glutamate neurotransmission in the cortex. For example, the endocannabinoid 2-arachidonoylglycerol (2-AG) is synthesized by diacylglycerol lipase (DAGL) (17) in pyramidal neurons (18;19) and then travels retrogradely to stimulate cannabinoid receptors (CB1R) located in nearby axon terminals from certain inhibitory neurons (20) and, to a lesser extent, other pyramidal neurons (21), leading to a suppression of GABA and glutamate release (21;22). 2-AG activity is then terminated through metabolism by monoglyceride lipase (MGL) (23). Interestingly, the endocannabinoid system also appears to be the primary mediator of the aforementioned suppressive effects on group I mGluR on GABA release. For example, activation of group I mGluR, but not other classes of mGluR, leads to increased synthesis of 2-AG (24) and suppression of GABA release (6–10) in a manner that is dependent upon CB1R (7;9;10). We recently reported lower CB1R mRNA and protein levels in the PFC in individuals with schizophrenia (25). However, fully understanding the pathophysiological state of the endocannabinoid system, and the downstream effects on GABA and glutamate neurotransmission, in schizophrenia also requires knowledge of whether the level of 2-AG is also altered in the disorder. Levels of 2-AG cannot be directly assessed in postmortem human brain tissue (26) but can be inferred, in part, by measures of gene products that regulate 2-AG synthesis (DAGL) and degradation (MGL). To our knowledge, no publications have yet reported the status of endocannabinoid ligands in schizophrenia.
Therefore, in this study, we sought to evaluate the status of several important regulators of GABA and glutamate neurotransmission in the PFC by examining the transcript levels of mGluR1α, mGluR5, RGS4, and endocannabinoid synthesizing (DAGL) and metabolizing (MGL) enzymes in a relatively large (n=42) cohort of schizophrenia subjects. We found elevated mRNA levels for mGluR1α, but not mGluR5, and lower RGS4 mRNA levels in schizophrenia subjects, but transcript levels for DAGL and MGL did not differ between subject groups. Higher mGluR1α and lower RGS4 mRNA levels may have an important impact on GABA and glutamate neurotransmission in schizophrenia.
Methods
Human Subjects
Brain specimens were obtained during routine autopsies conducted at the Allegheny County Medical Examiner’s Office (Pittsburgh, PA) after consent was obtained from the next-of-kin. An independent committee of experienced research clinicians made consensus DSMIV (Diagnosis and Statistical Manual of Mental Disorders, 1994) diagnoses for each subject using the results of structured interviews conducted with family members and review of medical records, as previously described (27). In order to control for experimental variance, subjects with schizophrenia or schizoaffective disorder (n=42) were matched individually to normal comparison subjects for sex, and as closely as possible for age and postmortem interval (PMI) (see Table 1 for summary; see Table S1 in Supplemental Data for demographic details on individual subjects), and samples from both subjects in a pair were processed together throughout all stages of the study. The mean age, PMI, brain pH, RNA integrity number (RIN; as determined from Agilent Bioanalyzer 2100, Agilent Technologies, Walbronn, Germany (4;28)), and tissue storage time did not differ between the subject groups (Table 1; for all t(82)≤1.67, p≥0.10). All procedures were approved by the University of Pittsburgh’s Committee for the Oversight of Research Involving the Dead and Institutional Review Board for Biomedical Research.
Table 1.
Summary of Demographic Characteristics
| Characteristic | Control | Schizophrenia |
|---|---|---|
| N | 42 | 42 |
| Sex | 31M/11F | 31M/11F |
| Race | 34W/8B | 29W/13B |
| Age (years) | 48 ± 13 | 47 ± 13 |
| Postmortem Interval (hours) | 17.8 ± 5.9 | 18.1 ± 8.7 |
| Freezer Storage Time (months) | 97 ±43 | 97 ± 46 |
| Brain pH | 6.8 ± 0.2 | 6.6 ±0.4 |
| RNA Integrity Number | 8.3 ± 0.6 | 8.2 ± 0.7 |
For all, t(82)≤1.67, p ≥0.10. Values are group means ± standard deviation.
Tissue Preparation
The right hemisphere of each brain was blocked coronally, immediately frozen and stored at −80°C (29). Cryostat sections from the anterior-posterior level corresponding to the middle portion of the superior frontal sulcus were cut serially and confirmed to contain PFC area 9 from adjacent Nissl-stained sections using cytoarchitectonic criteria (29). The cortical gray matter was dissected in a manner that excluded white matter contamination and provided excellent RNA preservation, and the gray matter was collected into tubes containing Trizol reagent (Invitrogen, Carlsbad, CA) for RNA isolation (30). Total RNA was isolated from Trizol homogenates and cleaned by RNeasy columns (Qiagen, Valencia, CA).
Real-time Reverse Transcription PCR
cDNA was synthesized from total RNA for each subject using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). All primer pairs (Supplemental Data Table S2) demonstrated: 1) high amplification efficiency (>96%) across a wide range of cDNA dilutions; 2) specific single products in dissociation curve analysis; and 3) melting temperatures similar to those predicted by oligonucleotide software. Quantitative PCR was performed using the comparative threshold cycle (Ct) measurement with Power SYBR Green dye and ABI StepOne Plus Real Time PCR System (Applied Biosystems). Based on their stable level of expression between schizophrenia and normal comparison subjects (30), three reference genes (beta actin, cyclophilin A, and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) were used to normalize the target gene expression levels. The difference in cycle threshold for each target transcript was calculated by subtracting the mean cycle threshold for the three reference genes from the cycle threshold of the target transcript. Because this difference in cycle threshold (dCT) represents the log2-transformed expression ratio of each target transcript to the geometric mean of the three reference genes, the relative expression level of the target transcript is determined as 2−dCT (30;31). Four replicate measures were performed for each gene of interest for each subject with a standard threshold consistently applied for each gene across all subjects. The following transcripts were assessed (with the mean coefficient of variance [±SD] for the relative expression level of each transcript in parentheses): mGluR1α (0.043 ± 0.023), mGluR5 (0.028 ± 0.011), regulator of G protein signaling 4 (RGS4; 0.025 ± 0.018), DAGL isoforms α (0.035 ± 0.017) and β (0.046 ± 0.028) (32), MGL (0.026 ± 0.013), fatty acid amide hydrolase (FAAH; 0.037 ± 0.02), and cannabinoid receptor interacting protein 1 (CRIP1a) (0.030 ± 0.015) (Supplemental Data Table S2). In addition, statistically significant findings were validated by repeating the study in a subset of subject pairs using additional primer sets for non-overlapping regions of the same targets.
CB1R mRNA and protein levels of the first 23 subjects with schizophrenia and RGS4 mRNA levels of the first 8 subjects with schizophrenia included in the current study (Supplemental Data Table S1) were previously shown to be reduced (15;25).
Antipsychotic-Exposed Monkeys
As described previously (33), experimentally–naïve, young adult, male, macaque monkeys (Macaca fascicularis) were administered oral doses of haloperidol, olanzapine or placebo (n = 6 monkeys per group) twice daily for 17 – 27 months. The final trough drug plasma levels were within the range associated with clinical efficacy in humans (~1.5 ng/ml for haloperidol and ~15 ng/ml for olanzapine) (33). Monkeys were matched by terminal body weight and euthanized in triads (one animal from each group) on the same day. Brains were rapidly removed, and the right frontal lobe was cut into coronal blocks, frozen and stored at −80°C. RNA was isolated from PFC area 9, and qPCR was conducted for the same three reference genes and mGluR1α (Supplemental Data Table S2) with all monkeys from a triad processed together on the same plate. Primers were designed using monkey-specific cDNA sequences, and primer amplification efficiencies were >96% in monkey brain tissue. All animal studies were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Statistical Analysis
Two analysis of covariance (ANCOVA) models (34) were performed to test the effect of diagnosis on relative expression level for each target mRNA (25;30). The first model included relative expression level as the dependent variable, diagnostic group as the main effect, storage time, brain pH, and RIN as covariates, and subject pair as a blocking factor. Subject pairing may be considered an attempt to balance diagnostic groups for sex, age, and postmortem interval and to account for the parallel processing of tissue samples from a pair, and not a true statistical paired design. Therefore, we also utilized a second model without subject pair as a blocking factor that included all pairing factors (age, sex, and PMI), storage time, brain pH, and RIN as covariates. Because these two ANCOVAs produced nearly identical results, only the results from the first model are reported. All statistical tests are two-tailed. The Bonferoni correction for multiple comparisons was employed to set the statistical significance for the seven newly studied target mRNAs in this cohort to α=.007.
The effects of potential confounding variables on relative mRNA expression levels in subjects with schizophrenia were assessed using an ANCOVA model with each potential confounding variable as the main effect and with sex, age, PMI, storage time, pH, and RIN as covariates. An ANOVA model with relative mRNA expression level as the dependent variable, treatment group as the main effect, and triad as a blocking factor was used for the antipsychotic-exposed monkey study.
Results
Group I mGluR in Schizophrenia
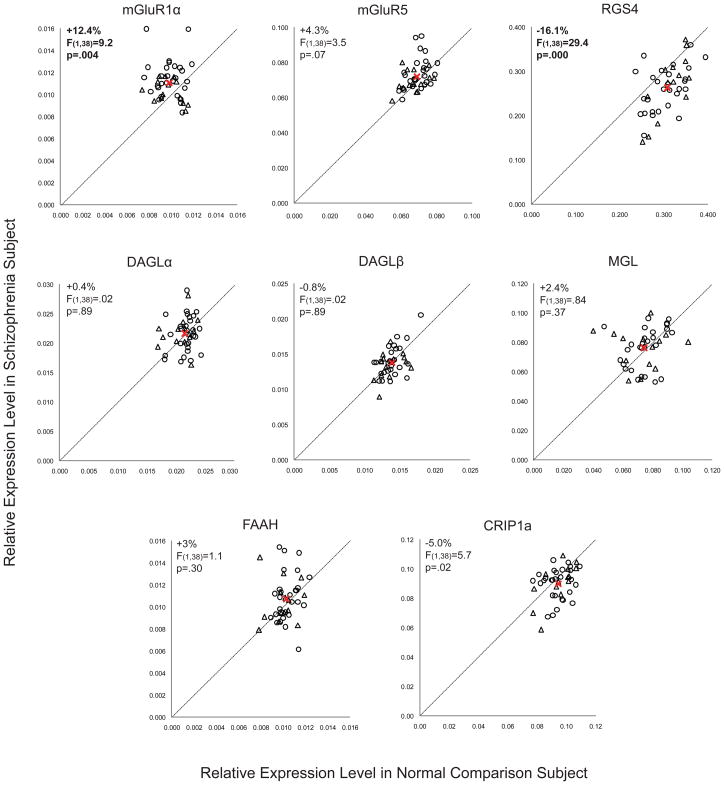
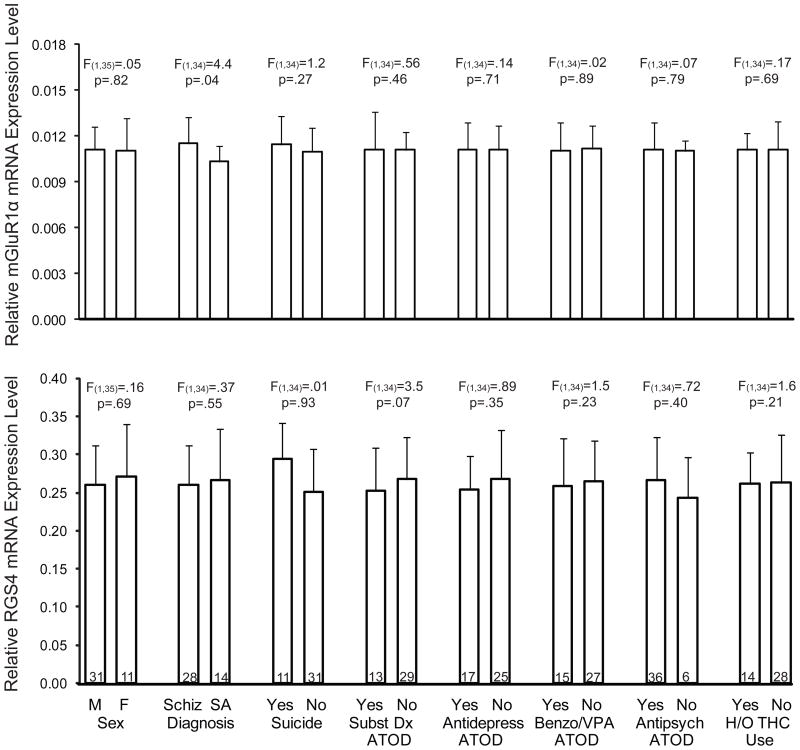
We found elevated mRNA levels for mGluR1α (+12.4%; F(1,38)=9.2, p=.004), but not mGluR5 (F(1,38)=3.5, p=.07), in subjects with schizophrenia (Figure 1). Exclusion of the two subject pairs with the greatest mGluR1α mRNA levels in the schizophrenia subject still resulted in significantly higher mGluR1α mRNA levels in schizophrenia subjects (+10.2%; F(1,36)=7.77, p=.008). The relative mRNA expression level of mGluR1α in schizophrenia subjects did not differ as a function of sex; suicide; diagnosis of substance abuse or dependence current at the time of death; use of antidepressants, benzodiazepines or sodium valproate, or antipsychotics at time of death; or history of cannabis use (all F≤1.24, p≥.273) (Figure 2). However, schizophrenia subjects (n=28) may have a higher level (+11.5%; F(1,35)=4.42, p=.043) of mGluR1α mRNA expression than schizoaffective disorder subjects (n=14). Furthermore, when the schizophrenia cohort is subdivided into schizophrenia subjects and schizoaffective disorder subjects, schizophrenia subjects have a significantly higher level of mGluR1α mRNA expression compared to their matched normal comparison subjects (+16.3%; F(1,24)=9.7, p=.005), while schizoaffective disorder subjects do not (+5.4%; F(1,10)=1.2, p=.30). Similarly, but to a lesser extent, schizophrenia subjects may also have a higher level of mGluR5 mRNA expression than schizoaffective disorder subjects (+5.9%; F(1,35)=5.31, p=.027). However, after excluding schizoaffective disorder subjects, mGluR5 mRNA levels still do not differ between schizophrenia subjects and matched normal comparison subjects (+5.1%; F(1,24)=2.49, p=.127). Finally, higher mGluR1α mRNA expression levels in the PFC in schizophrenia were validated in a subset of subject pairs using additional qPCR primer sets designed against non-overlapping regions of mGluR1α cDNA (Supplemental Figure S1 and Supplemental Data Table S2).
Figure 1. Relative expression levels of transcripts in schizophrenia subjects compared to matched normal comparison subjects.
mRNA expression levels for schizophrenia subjects compared to matched normal comparison subjects are indicated by open black circles, and mRNA expression levels for schizoaffective disorder subjects compared to matched normal comparison subjects are indicated by open triangles. Data points to the left of the unity line indicate higher levels of mRNA expression in the affected subject relative to the normal comparison subject and vice versa. The average mRNA expression levels for both diagnostic groups are indicated by the red “X” symbol. Statistically significant differences (α=.007) in mRNA expression levels in schizophrenia subjects were found for mGluR1α (+12.4%; F(1,38)=9.2, p=.004) and RGS4 (−16.1%; F(1,38)=29.4, p=.000).
Figure 2. The effects of potentially confounding variables on mGluR1α and RGS4 mRNA expression levels in schizophrenia subjects.
Sex, suicide, diagnosis of substance abuse or dependence current at the time of death, antidepressant medication use at time of death, use of benzodiazepines or sodium valproate at time of death, antipsychotic medication use at time of death, and history of cannabis use (THC; tetrahydrocannabinol) did not significantly affect mGluR1α mRNA or RGS4 mRNA expression levels. However, schizophrenia subjects may have a higher level of mGluR1α mRNA expression than schizoaffective disorder (SA) subjects (+11.5%; F(1,34)=4.42, p=.043) with no difference in RGS4 mRNA expression levels. Numbers within bars indicate the sample size for that subgroup of schizophrenia subjects.
RGS4 in Schizophrenia
We also studied mRNA levels for regulator of G protein signaling 4 (RGS4), which have previously been reported to be reduced in the PFC in schizophrenia (15;16), including in the first 8 schizophrenia subjects (15) of the current cohort of 42 subjects with schizophrenia (Supplemental Data Table S1). We found lower RGS4 mRNA expression levels in the entire cohort of 42 schizophrenia subjects compared to matched normal comparison subjects (−16.1%; F(1,38)=29.4, p=.000) (Figure 1) and also in the previously untested 34 subject pairs alone (−16.3%; F(1,30)=22.0, p=.000). The relative mRNA expression level of RGS4 in schizophrenia subjects did not differ as a function of sex; diagnosis of schizophrenia versus schizoaffective disorder; suicide; diagnosis of substance abuse or dependence current at the time of death; use of antidepressants, benzodiazepines or sodium valproate, or antipsychotics at time of death; or history of cannabis use (all F≤3.5, p≥.07; Figure 2).
RGS4 reduces the duration of intracellular signaling from group I mGluR (13). Therefore, we further examined the relationship between RGS4 and mGluR1α mRNA levels in this subject cohort. Pearson correlation analysis revealed that RGS4 and mGluR1α mRNA levels were not correlated in individual subjects (r=−.14, p=0.21), and differences in RGS4 and mGluR1α mRNA levels between schizophrenia and matched control subjects were not correlated in subject pairs (r=−.01, p=.94). However, a significant number of subject pairs (n=27 out of 42 pairs) showed both higher mGluR1α and lower RGS4 mRNA levels in the schizophrenia subject compared to the matched normal comparison subject (χ2(df=3,n=42)=37.6, p=.000). In contrast, only 5 subject pairs showed higher levels of both mGluR1α and RGS4 mRNA in the schizophrenia subject compared to the matched normal comparison subject, 9 subject pairs showed lower levels of both mGluR1α and RGS4 mRNA in the schizophrenia subject compared to the matched normal comparison subject, and 1 subject pair showed lower levels of mGluR1α and higher levels of RGS4 mRNA in the schizophrenia subject compared to the matched normal comparison subject.
Synthesizing and Metabolizing Enzymes for Endocannabinoids in Schizophrenia
We quantified mRNA expression levels for both isoforms of the synthesizing enzyme for 2-AG (DAGLα and DAGLβ), the metabolizing enzyme for 2-AG (MGL), and fatty acid amide hydrolase (FAAH), which is the metabolizing enzyme for another cortical endocannabinoid, anandamide. Relative mRNA expression levels for DAGLα, DAGLβ, MGL, or FAAH did not differ between subject groups (for all transcripts: maximum difference in group means ≤ ±3%; F(1,38)≤1.1, p≥.30) (Figure 1). We also studied mRNA levels for cannabinoid receptor interacting protein 1a (CRIP1a), which has been reported to bind to and inhibit the functioning of CB1R (35). We found a small difference in CRIP1a mRNA expression levels in schizophrenia subjects (−5%; F(1,38)=5.7, p=.022) that was not statistically significant after correction for multiple comparisons (α=.007; see Statistical Analysis section) (Figure 1). In contrast to group I mGluR, transcript levels for DAGLα, DAGLβ, MGL, FAAH, and CRIP1a did not show a difference between schizophrenia and schizoaffective disorder subjects (F(1,35)≤1.37, p≥0.25).
Antipsychotic-Exposed Monkeys
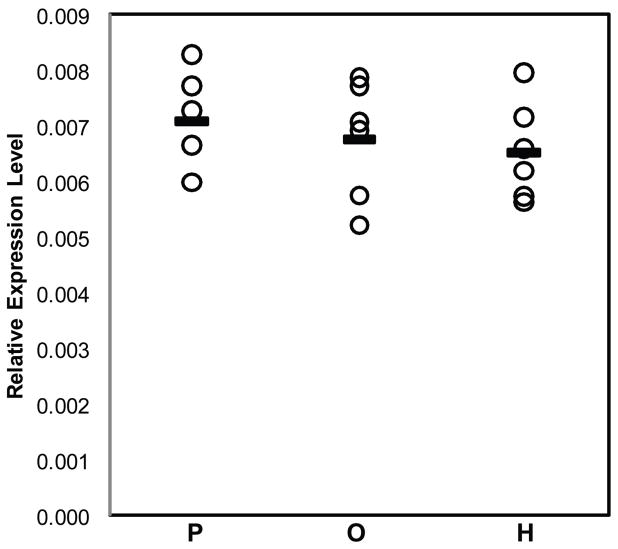
Prior studies in antipsychotic-exposed monkeys have provided evidence suggesting that lower RGS4 mRNA levels in the PFC in schizophrenia do not appear to be attributable to antipsychotic treatment (15). However, the effects of antipsychotic treatment on mGluR1α in the PFC have not been established. In order to further assess whether alterations in mGluR1α mRNA expression in schizophrenia are confounded by treatment with antipsychotic medications, we used qPCR to determine relative mRNA expression levels of mGluR1α in monkeys chronically exposed to either haloperidol, olanzapine, or placebo. In contrast to our findings in schizophrenia, the relative expression level of mGluR1α mRNA was slightly lower in haloperidol-exposed (−7.7%) and in olanzapine-exposed monkeys (−4.7%) compared to placebo-exposed monkeys (Figure 3). However, these differences did not achieve statistical significance (F(2,15)=0.53, p=.60). When the haloperidol- and olanzapine-exposed monkeys were combined into a single group, the relative expression level of mGluR1α mRNA was still slightly, but not statistically significantly, lower in the antipsychotic-exposed monkeys compared to placebo-exposed monkeys (−6.2%; F(1,5)=0.83, p=.41).
Figure 3. Relative mRNA expression levels for mGluR1α in antipsychotic medication-exposed monkeys.
No statistically significant differences were found in mRNA expression for mGluR1α (F(2,15)=1.14, p=.346) in monkeys chronically exposed to either olanzapine (O) or haloperidol (H) compared to placebo (P).
Discussion
In this study, we found that mRNA expression levels for mGluR1α, but not mGluR5, are significantly higher in the PFC in schizophrenia subjects. Consistent with prior reports (15;16), we also found lower mRNA levels for RGS4, which reduces the duration of G protein-mediated intracellular signaling from mGluR1α activation (13); lower RGS4 mRNA levels were commonly found in the same schizophrenia subjects who had higher mGluR1α mRNA levels. In contrast, no differences were found in mRNA levels for the synthesizing and metabolizing enzymes for 2-AG, DAGL (both α and β isoforms) and MGL, respectively, or for FAAH, the metabolizing enzyme of the other cortical endocannabinoid, anandamide, in schizophrenia subjects. Taken together, these data suggest that altered mGluR1α and RGS4 mRNA levels may be common in schizophrenia subjects and may represent a disturbed “molecular hub” that has an important impact on multiple components of neuronal communication in the PFC.
Increased mGluR1α mRNA levels appear to be specific to the disease process of schizophrenia, or at least not attributable to factors frequently associated with the illness. For example, treatment with antipsychotic medications does not appear to affect mGluR1α mRNA levels since mGluR1α mRNA levels were similar in schizophrenia subjects on and off medications at time of death. In addition, in contrast to our findings in schizophrenia subjects, mGluR1α mRNA levels were slightly lower in antipsychotic-exposed monkeys, although these differences did not achieve statistical significance. Furthermore, differences in sex, suicide as a cause of death, diagnosis of substance abuse or dependence current at the time of death, use of antidepressants, benzodiazepines or sodium valproate at the time of death, or a history of cannabis use did not appear to affect mGluR1α levels in schizophrenia subjects. Interestingly, mGluR1α mRNA levels and mGluR5 mRNA levels appeared to be higher in schizophrenia subjects than in schizoaffective disorder subjects; however, no other markers showed a difference between schizophrenia and schizoaffective disorder subjects, including CB1R (25).
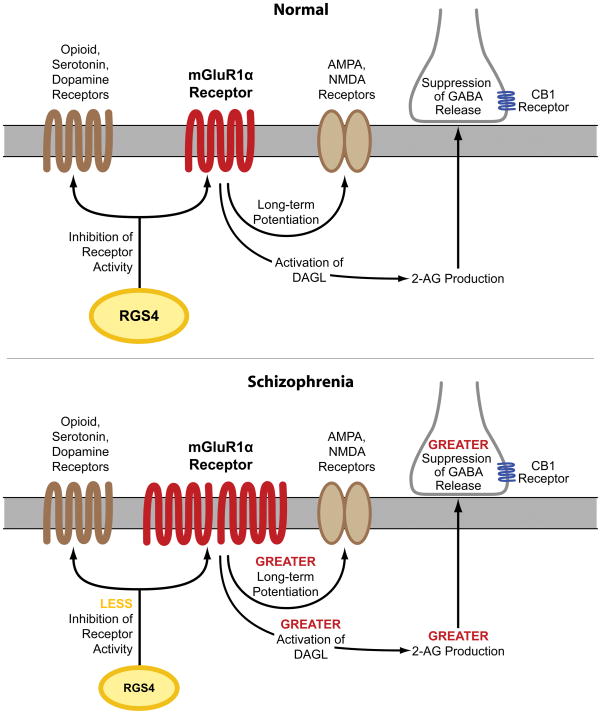
Several lines of evidence suggest that the capacity for mGluR1α activation may be increased in the PFC in schizophrenia. First, our findings of elevated mGluR1α mRNA levels parallel a previous report of higher mGluR1α protein levels in the PFC in schizophrenia (12), suggesting that higher levels of mGluR1α mRNA expression may lead to translation of more protein, although this hypothesis needs to be directly tested in the same subjects. Second, RGS4 reduces the duration of intracellular signaling from mGluR1α (13). Consistent with evidence that RGS4 also regulates G protein coupling for multiple other receptors (36–39), RGS4 and mGluR1α mRNA levels were not correlated in individual subjects and differences in RGS4 and mGluR1α mRNA levels between schizophrenia and matched normal comparison subjects were not correlated in subject pairs. However, out of the 42 subject pairs, 27 pairs showed both higher mGluR1α and lower RGS4 mRNA levels in the schizophrenia subject compared to the matched normal comparison subject. Taken together, these data suggest that higher mGluR1α and lower RGS4 mRNA levels are generally found in the same schizophrenia subjects and, if accompanied by changes in levels of the corresponding proteins, may lead to a greater capacity for, and a longer duration of intracellular signaling from, mGluR1α activation in the PFC in schizophrenia (Figure 4).
Figure 4. Potential downstream effects of alterations in mGluR1α and RGS4 mRNA levels on neurotransmitter systems in schizophrenia.
Top figure: Under normal conditions, activation of mGluR1α results in long-term potentiation of NMDA and AMPA receptors (11) and activation of DAGL which leads to synthesis of 2-AG (24) and suppression of GABA release from nearby inhibitory axon terminals that contain the CB1 receptor (6–10). In addition, RGS4 reduces signaling through several different G protein-coupled receptors, including group I mGluR, opioid, serotonin, and dopamine receptors (13;36–39). Bottom figure: In schizophrenia, higher mGluR1α and lower RGS4 mRNA levels suggest the presence of enhanced signaling through mGluR1α. Higher mGluR1α signaling may have diverse effects on multiple components of neural transmission in schizophrenia (red font), including greater long-term potentiation of NMDA and AMPA receptors, enhanced 2-AG synthesis, and greater suppression of GABA release from inhibitory axon terminals that contain the CB1 receptor. In addition, lower RGS4 levels may result in less inhibition of several classes of G protein-coupled receptors (yellow font). However, additional studies characterizing the cell-type specificity of transcript and protein level alterations in mGluR1α and RGS4 are needed to clarify the nature of PFC circuitry disturbances in the illness.
As mentioned above, alterations in RGS4 mRNA levels in schizophrenia may have additional effects beyond modifying the intracellular signaling efficacy of mGluR1α. First, RGS4 is a GTPase-activating protein that reduces the duration of activity of several different G protein-coupled receptors in addition to metabotropic glutamate receptors, including dopamine, serotonin, and opioid receptors (36–39). Thus, lower RGS4 mRNA levels, if also present at the protein level (16), may enhance the functioning of an array of receptors in schizophrenia (15) (Figure 4). Second, some, though not all (40), case control and family based association studies have identified single nucleotide polymorphisms (SNPs) of the RGS4 gene as susceptibility factors for schizophrenia, although different alleles of these SNPs have been associated with schizophrenia in different subject populations (14;41). Some of the risk haplotypes for RGS4 are also associated with smaller volume of the PFC (42) and altered activation of the PFC during working memory tasks (43) in schizophrenia subjects. Thus, alterations in RGS4 at the genomic and transcript level may have a broad impact on diverse neurotransmitter systems and on functional properties of the PFC in schizophrenia.
Higher mRNA levels for mGluR1α and lower mRNA levels for RGS4, if accompanied by altered levels of the corresponding proteins (12;16), are consistent with a higher capacity for mGluR1α-mediated neurotransmission in schizophrenia, which may have a diverse and complicated impact on multiple components of neural transmission in the disorder. For example, immunoreactivity for mGluR1α and RGS4 is found in both pyramidal neurons and GABA neurons in primate PFC (44;45). An important limitation of our study is that a tissue level analysis of mRNA levels does not allow a determination of a potential cell-type specificity of higher mGluR1α and lower RGS4 mRNA levels in schizophrenia; thus, follow up studies are needed to determine whether altered levels of mGluR1α and RGS4 are predominantly found in pyramidal neurons or GABA neurons or both. With this caveat in mind and for the purpose of discussion, we will focus on the potential downstream impact of greater mGluR1α-mediated neurotransmission on the NMDA receptor, endocannabinoid, and GABA systems in schizophrenia. For example, activation of group I mGluR results in long-term potentiation of NMDA receptor function (11), and NMDA receptor function appears to be impaired in schizophrenia (5). Thus, these lines of evidence invite speculation that in schizophrenia, greater mGluR1α activity may have a partially compensatory effect for NMDA receptor hypofunction. In addition, activation of group I mGluR has been reported to increase synthesis of 2-AG (24) and suppress GABA release (6–10). mGluR1α-mediated activation of 2-AG synthesis has been reported to occur through a G protein coupled mechanism that leads to increased levels of substrate (i.e. diacylglycerol) for DAGL (46). Thus, while mRNA levels for the synthesizing and metabolizing enzymes for 2-AG are not altered in schizophrenia, higher mGluR1α activity may increase the substrate-dependent activity of DAGL, even in the absence of changes in DAGL enzyme level. Thus, higher mGluR1α-mediated activation of 2-AG synthesis may further suppress GABA release, which may worsen GABAergic deficits in schizophrenia. Higher mGluR1α mRNA levels and lower RGS4 mRNA levels may also affect additional components of neural transmission, including AMPA, dopamine, serotonin, and opioid receptors (11;36–39), (Figure 4). Taken together, these data suggest that altered mGluR1α and RGS4 mRNA levels may represent a disturbed “molecular hub” that has an important impact on multiple neurotransmitter systems in schizophrenia. Additional studies characterizing the cell-type specificity of transcript and protein level alterations in mGluR1α and RGS4 may provide greater insight into their downstream effects on PFC circuitry, and potentially their relationship to cognitive impairments, in schizophrenia.
Supplementary Material
Acknowledgments
Disclosures and Acknowledgments: David A. Lewis currently receives investigator-initiated research support from the BMS Foundation, Bristol-Myers Squibb, Curridium Ltd and Pfizer and in 2007–2009 served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, BioLine RX, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck, Neurogen, and SK Life Science. All other authors report no competing interest. The authors thank Elizabeth Sengupta for technical assistance.
Grant Support: Supported by NIH grants MH-084018 (Dr. Volk) and MH-043784, MH-084053 and a NARSAD Distinguished Investigator Award (Dr. Lewis).
Footnotes
Previous Presentations: Presented in part at the International Congress of Schizophrenia Research, San Diego, CA, March 28 – April 1, 2009 and the American College of Neuropsychopharmacology, Hollywood, Florida, December 6 – 10, 2009
References
- 1.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- 3.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morishita W, Kirov SA, Alger BE. Evidence for metabotropic glutamate receptor activation in the induction of depolarization-induced suppression of inhibition in hippocampal CA1. J Neurosci. 1998;18:4870–4882. doi: 10.1523/JNEUROSCI.18-13-04870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci. 2002;15:953–961. doi: 10.1046/j.1460-9568.2002.01929.x. [DOI] [PubMed] [Google Scholar]
- 9.Edwards DA, Kim J, Alger BE. Multiple mechanisms of endocannabinoid response initiation in hippocampus. J Neurophysiol. 2006;95:67–75. doi: 10.1152/jn.00813.2005. [DOI] [PubMed] [Google Scholar]
- 10.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 11.Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Gupta DS, McCullumsmith RE, Beneyto M, Haroutunian V, Davis KL, Meador-Woodruff JH. Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse. 2005;57:123–131. doi: 10.1002/syn.20164. [DOI] [PubMed] [Google Scholar]
- 13.Saugstad JA, Marino MJ, Folk JA, Hepler JR, Conn PJ. RGS4 inhibits signaling by group I metabotropic glutamate receptors. J Neurosci. 1998;18:905–913. doi: 10.1523/JNEUROSCI.18-03-00905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdari KV, Mirnics K, Semwal P, Wood J, Lawrence E, Bhatia T, Deshpande SN, BKT, Ferrell RE, Middleton FA, Devlin B, Levitt P, Lewis DA, Nimgaonkar VL. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum Mol Genet. 2002;11:1373–1380. doi: 10.1093/hmg/11.12.1373. [DOI] [PubMed] [Google Scholar]
- 15.Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry. 2001;6:293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- 16.Erdely HA, Tamminga CA, Roberts RC, Vogel MW. Regional alterations in RGS4 protein in schizophrenia. Synapse. 2006;59:472–479. doi: 10.1002/syn.20265. [DOI] [PubMed] [Google Scholar]
- 17.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- 23.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- 25.Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palkovits M, Harvey-White J, Liu J, Kovacs ZS, Bobest M, Lovas G, Bago AG, Kunos G. Regional distribution and effects of postmortal delay on endocannabinoid content of the human brain. Neuroscience. 2008;152:1032–1039. doi: 10.1016/j.neuroscience.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 28.Imbeaud S, Graudens E, Boulanger V, Barlet X, Zaborski P, Eveno E, Mueller O, Schroeder A, Auffray C. Towards standardization of RNA quality assessment using user-independent classifiers of microcapillary electrophoresis traces. Nucleic Acids Res. 2005;33 (e56):1–12. doi: 10.1093/nar/gni054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:34.1–34.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di MV, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharm. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 34.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. 4. Vol. 1. Boston: McGraw-Hill; 1996. [Google Scholar]
- 35.Niehaus JL, Liu Y, Wallis KT, Egertova M, Bhartur SG, Mukhopadhyay S, Shi S, He H, Selley DE, Howlett AC, Elphick MR, Lewis DL. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol Pharmacol. 2007;72:1557–1566. doi: 10.1124/mol.107.039263. [DOI] [PubMed] [Google Scholar]
- 36.Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gisubfamily of G protein alpha subunits. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 37.Ghavami A, Hunt RA, Olsen MA, Zhang J, Smith DL, Kalgaonkar S, Rahman Z, Young KH. Differential effects of regulator of G protein signaling (RGS) proteins on serotonin 5-HT1A, 5-HT2A, and dopamine D2 receptor-mediated signaling and adenylyl cyclase activity. Cell Signal. 2004;16:711–721. doi: 10.1016/j.cellsig.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Yan Y, Chi PP, Bourne HR. RGS4 inhibits Gq-mediated activation of mitogen-activated protein kinase and phosphoinositide synthesis. J Biol Chem. 1997;272:11924–11927. doi: 10.1074/jbc.272.18.11924. [DOI] [PubMed] [Google Scholar]
- 39.Leontiadis LJ, Papakonstantinou MP, Georgoussi Z. Regulator of G protein signaling 4 confers selectivity to specific G proteins to modulate mu- and delta-opioid receptor signaling. Cell Signal. 2009;21:1218–1228. doi: 10.1016/j.cellsig.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, Burrell GJ, Rice JP, Nertney DA, Olincy A, Rozic P, Vinogradov S, Buccola NG, Mowry BJ, Freedman R, Amin F, Black DW, Silverman JM, Byerley WF, Crowe RR, Cloninger CR, Martinez M, Gejman PV. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry. 2008;165:497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- 41.Talkowski ME, Seltman H, Bassett AS, Brzustowicz LM, Chen X, Chowdari KV, Collier DA, Cordeiro Q, Corvin AP, Deshpande SN, Egan MF, Gill M, Kendler KS, Kirov G, Heston LL, Levitt P, Lewis DA, Li T, Mirnics K, Morris DW, Norton N, O’Donovan MC, Owen MJ, Richard C, Semwal P, Sobell JL, St Clair D, Straub RE, Thelma BK, Vallada H, Weinberger DR, Williams NM, Wood J, Zhang F, Devlin B, Nimgaonkar VL. Evaluation of a susceptibility gene for schizophrenia: genotype based meta-analysis of RGS4 polymorphisms from thirteen independent samples. Biol Psychiatry. 2006;60:152–162. doi: 10.1016/j.biopsych.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasad KM, Chowdari KV, Nimgaonkar VL, Talkowski ME, Lewis DA, Keshavan MS. Genetic polymorphisms of the RGS4 and dorsolateral prefrontal cortex morphometry among first episode schizophrenia patients. Mol Psychiatry. 2005;10:213–219. doi: 10.1038/sj.mp.4001562. [DOI] [PubMed] [Google Scholar]
- 43.Buckholtz JW, Meyer-Lindenberg A, Honea RA, Straub RE, Pezawas L, Egan MF, Vakkalanka R, Kolachana B, Verchinski BA, Sust S, Mattay VS, Weinberger DR, Callicott JH. Allelic variation in RGS4 impacts functional and structural connectivity in the human brain. J Neurosci. 2007;27:1584–1593. doi: 10.1523/JNEUROSCI.5112-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muly EC, Maddox M, Smith Y. Distribution of mGluR1alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol. 2003;467:521–535. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- 45.Paspalas CD, Selemon LD, Arnsten AF. Mapping the regulator of G protein signaling 4 (RGS4): presynaptic and postsynaptic substrates for neuroregulation in prefrontal cortex. Cereb Cortex. 2009;19:2145–2155. doi: 10.1093/cercor/bhn235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.