Summary
The hypothalamic neuropeptide oxytocin, known for its involvement in social affiliation and bonding in animals, has recently been associated with a host of prosocial behaviors that are beneficial for maintaining positive social relationships in humans. Paradoxically, however, people with high endogenous levels of oxytocin also tend to report relational distress and interpersonal difficulties in their everyday lives. To address these contradictory findings, oxytocin reactivity was measured in response to a well-defined laboratory task in young adult women following recent interpersonal harms. Elevated mean peripheral oxytocin reactivity (but not baseline levels of oxytocin or cortisol reactivity) was associated with increased post-conflict anxiety and decreased levels of forgiveness. These results corroborate previous research implicating oxytocin as a neuroendocrine marker of relational distress, but not general stress, and demonstrate the utility of studying oxytocin in response to naturally occurring relational events.
Keywords: Oxytocin, Relational Distress, Interpersonal Conflict, Cortisol, Anxiety, Forgiveness
Positive social relationships are invaluable for human health and well-being, and yet, damaged relationships are an inevitable part of life. The biological processes that motivate remedial action in response to relationship damage are therefore of tremendous interest. Though previous research has associated baseline oxytocin with relational distress (Taylor et al., 2006; Taylor et al., 2010; Turner et al., 1999), no research to date has demonstrated an association between oxytocin reactivity and relational distress following recent interpersonal harms.
In addition to oxytocin’s role in social attachment and bond formation (Bartz and Hollander, 2006), intranasal administration of oxytocin in humans increases trust (Kosfeld et al., 2005) and constructive communication between romantic partners discussing conflictual issues (Ditzen et al., 2009)— perhaps in part by inhibiting neural systems associated with fear (Baumgartner et al., 2008) and stress (Heinrichs et al., 2003). Laboratory manipulations of affiliative social interaction also increase oxytocin in plasma (Light et al., 2005).
In light of the positive associations between affiliative social contact and oxytocin, it seems paradoxical that naturally occurring tonic levels of plasma oxytocin have been positively associated with interpersonal distress (Taylor, 2006; Taylor et al., 2006; Taylor et al., 2010; Turner et al., 1999) and romantic attachment anxiety (Marazitti et al., 2006). Cyranowski et al. (2008) also recently found that people scoring higher on several self-report measures of interpersonal problems demonstrated higher oxytocin concentrations during a one-hour imagery task. To account for this apparent contradiction, Taylor (2006) surmised that oxytocin— in addition to its role in supporting social behavior during non-stressful circumstances — might also be part of an evolved biobehavioral system whose function is to motivate individuals to seek out affiliation during times of stress.
Interpersonal conflict with social relationship partners represents a significant source of daily stress (Bolger et al., 1989), and the stress, anger, and rumination resulting from interpersonal conflict can negatively impact psychological well-being and physical health (e.g., Lawler et al., 2003; McCullough et al., 2007a; Witvliet et al., 2001). Efforts to increase oxytocin through standard laboratory stress inductions such as the “Trier Social Stress Test” (Kirschbaum et al., 1993), in which participants face evaluative threat from a panel of strangers, have been largely unsuccessful (e.g., Ditzen et al., 2007; Taylor et al., 2006). However, tasks such as these may be better at eliciting threat-related glucocorticoid production (Dickerson and Kemeny, 2004) than affiliation-related oxytocin release, since such tasks have little to do with disruptions in valuable social relationships. In light of the hypothesis that oxytocin release indexes relational distress (Taylor, 2006; Taylor et al., 2006; Taylor et al., 2010), we predicted that participants who (a) were less forgiving of their partners, and (b) experienced lingering anxiety about the transgressions would have higher task-related increases in oxytocin, but not necessarily in cortisol (Dickerson and Kemeny, 2004).
Methods
Participants
Participants were 39 female undergraduate psychology students at the University of Miami.1 The subsample of 39 female participants came from a total sample of 182 participants who completed the protocol over the course of several semesters. This subsample represented all the female participants who consented to the blood sampling procedure. Chi-squared tests were conducted to examine whether the study subsample (n = 35) differed from the total sample (N = 182) on any of the variables of interest. No significant differences were found. Four women were excluded from inferential data analysis in advance due to incorrect administration of the Aprotinin reagent following blood draws. The resulting sample of 35 women (mean age = 19.26 years, SD = 3.6, range = 17–39 years) had encountered a significant interpersonal transgression that they considered both wrong and potentially hurtful approximately 5 days (M = 4.6; SD = 2.75) prior to enrollment. Participants were not accepted into the study if the transgression involved: someone whom they did not know, a petty argument that was quickly resolved, a misunderstanding that was easily cleared up, or something the participant did that they regretted. Introduction to Psychology students received course credit, and all participants signed a written informed consent and were paid between $60 and $100 for completing various aspects of the study.
Participants reported transgressions that had been committed by girlfriends/boyfriends (51.4%), friends of the same gender (20%), casual dating partners (11.4%), friends of the other gender (8.6%), “other” (5.7%), or relatives (2.9%). Transgressions involved romantic infidelity (22.9%), rejection or abandonment by a friend or prospective relationship partner (20%), termination of a romantic relationship (17.1%), betrayals of confidence or insults by a friend (14.3%), “other” (11.4%), neglect by a romantic partner, spouse, or ex-romantic partner (8.6%), rejection, neglect or insult by a family member (2.9%), or insults by people other than family or friends (2.9%).
Measures
Relationship-specific questionnaires
Upon enrolling, participants completed several relationship-specific self-report measures (See Table 1). Participants rated their perceptions of closeness and commitment to the offender prior to the transgression using three seven-point Likert-type scales (higher numbers indicated higher levels of closeness and commitment, respectively). Participants also completed Aron, Aron, and Smollan’s (1992) Inclusion of Other in the Self (IOS) Scale. The IOS displays seven Venn diagrams, which include pairs of circles ranging from no overlap to almost complete overlap. Instructions ask participants to circle the pair that best describes their relationship with the transgressor. Scores ranged from 1 (no overlap between the circles) to 7 (extreme overlap). A linear composite of these measures four items was computed (α = .81). In addition, participants indicated how painful they perceived the transgression to be with a single item that read, “How painful was the offense to you right after it happened?” using a 7-point Likert-type scale (0 = not very painful at all, 6= worst pain I ever felt).
Table 1.
List of relationship specific and potential confound variables
| Construct | Citation |
|---|---|
| *Perceived painfulness of the transgression | (Bono et al., 2008) |
| *Closeness/commitment to the transgressor prior to being hurt | (Bono et al., 2008) |
| *Personality inventories | (John et al., 1991; Wiggins et al., 1988) |
| *Perceptions of their transgressors’ personalities | (John et al., 1991; Wiggins et al., 1988) |
| Satisfaction with life | (Diener et al., 1985) |
| Social support when coping | (Winefield et al., 1992) |
| Recent interpersonal strife | (Finch et al., 1999) |
| Vitality | (Reis et al., 2000) |
| Loneliness | (Russell et al., 1980) |
| Psychosomatic symptoms | (Bono et al., 2008) |
Note. Constructs with asterisks were measured upon enrollment, whereas constructs without asterisks were measured following the speech reactivity task.
Approximately 3 days before their laboratory visits (M = 2.78, SD = 3.48, n = 32), participants completed an online self-report measure of forgiveness (McCullough et al., 2006) Forgiveness was measured using the 18-item form of the Transgression-Related Interpersonal Motivations (TRIM) Inventory (McCullough et al., 2006) which conceptualizes forgiveness as a process of reducing negative motivations (avoidance and revenge) toward a transgressor and restoring positive, benevolent motivations regarding the transgressor. The TRIM is composed of three factors: a 7-item subscale that measures motivation to avoid a transgressor (e.g., “I live as if he/she doesn’t exist, isn’t around”), a 5-item subscale that measures motivation to seek revenge (e.g., “I’ll make him/her pay”), and a 6-item subscale that measures benevolence motivation (e.g., “Even though his/her actions hurt me, I have goodwill for him/her”). All subscales have demonstrated high internal consistency and reliability (see McCullough et al., 2003; McCullough and Hoyt, 2002; McCullough et al., 1998). The six benevolence items were reversed scored so that high scores on the TRIM indicated less forgiveness.
Recently, McCullough et al. (2010) demonstrated how the rating scale version of the Rasch model (Andrich, 1978; Fox and Jones, 1998) could be used to extrapolate a single unidimensional measure of forgiveness based on scores from the 18 TRIM items. Using this statistical technique allows one to account for both the rating on the given construct and also the endorsability of each item. In the present study computed Rasch scores from TRIM data were used as our measurement of forgiveness (for further details see McCullough et al., 2010).
Following the speech task, participants rated their current post-conflict anxiety regarding their transgressors with 14 items that they endorsed on a 7-point Likert-type scale (1 = completely disagree, 7 = completely agree). Items were written in both positive (e.g., “He/she doesn’t intend to wrong me again”) and negative (e.g., “I can’t trust their intentions toward me”) forms. Positively worded items were reverse-scored so that higher total scores indicated more post-conflict anxiety (A.D. Cohen, unpublished master’s thesis). The 14 items demonstrated high internal consistency (α= .87), so we used their mean as a measure of post-conflict anxiety. In addition, participants rated their self-reported calmness, happiness, and control during the speech task in addition to several other self-reported variables (See Table 1 for full list).
Procedure
Recruitment
Participants were recruited in undergraduate psychology courses. We discussed our interest in recruiting participants who had encountered interpersonal transgressions within approximately the past 7 days. Over the course of several semesters we continued to visit courses to facilitate enrollment of eligible participants. We provided initial packets that included the measures of perceived transgression painfulness and pre-transgression closeness/commitment. Following the completion of the initial questionnaires, participants returned the information. If they were deemed eligible for further study participation, they were scheduled for future lab visits including the relational laboratory task described below.
Relational laboratory task
Approximately 27 days (M = 26.9, SD = 4.67, n = 35) after enrollment, participants completed a 90-minute laboratory session consisting of baseline blood draws, a speech reactivity task, and subsequent post-task blood draws and questionnaires. Participants arrived between 6:00pm and 7:30pm to minimize diurnal fluctuations in hormone levels (Ditzen et al., 2007). A licensed phlebotomist fitted consenting participants with a plastic intravenous catheter into their nondominant arm. Baseline blood samples were drawn into two 6 ml vacutainer tubes with EDTA (these tubes were used for all subsequent draws). The catheter remained in each participant’s arm for the remainder of the procedure. Ten minutes later, following a relaxation period intended for catheter habituation, a second baseline was drawn into one 6 ml tube.
Participants were then given 4 minutes to prepare a short speech that they would have liked to give to their transgressor as if the video camera were the person who harmed them. Participants received the following scripted instructions:
For this task, we really want you to relax and “get into” the task so that you can express your feelings to this person without holding anything back — as if you were really talking to this person… Specifically, we would like you to spend a few minutes preparing some thoughts about what you would say to the person who hurt you, focusing on:
What you would like to say about the hurtful event
How you are currently feeling about the individual who harmed you as a person
How you feel like acting toward that individual
You will have four minutes to prepare anything that you would like. Feel free to take notes if you would be more comfortable. After the preparation time, you will be asked to give this speech into the video recorder.
Following a 2-minute break, participants delivered the 4-minute speech. Two, five, seven, and ten minutes following the conclusion of the speech, four additional 6 ml tubes of blood were drawn. Based on findings associating rumination and elevated cortisol (Denson et al., 2009; McCullough et al., 2007b), we considered the 4 minutes of preparation time to be the beginning of stressor onset. The time intervals for blood draws represented 12 minutes, 15 minutes, 17 minutes, and 20 minutes after the start of the preparation period, and therefore enabled us to capture slower peaking task-induced cortisol increases (Dickerson and Kemeny, 2004) as well as faster pulsatile oxytocin increases (Amico et al., 1987; Cyranowski et al., 2008; Gimpl and Fahrenholz, 2001; Light et al., 2005). After the fourth post-speech blood draw, the catheter was removed, and participants completed questionnaires.
Biological assessment
After blood samples were collected, 0.38 ml of Aprotinin reagent was added to each tube. Tubes were gently rocked, submerged into an ice bath, and centrifuged at 1600 g at 4 degrees C for 20 minutes within 1 hour. Plasma was then frozen at −80 degrees C until time of assay. All samples were analyzed in duplicate. Plasma oxytocin was extracted by solid phase chromatography using Sep-Pak columns (Peninsula Laboratories, San Carlos, CA) and assayed by radioimmunoassay (RIA) (RK-051-01) from Phoenix Pharmaceuticals (Burlingame, CA) following the manufacturer's methods. The limit of detection was 1.0 pg/tube and intra- and inter-assay coefficients of variability (CV) were 7% and 15% as reported by the manufacturer. In the current study, on average across time points, 43% of the samples fell below the level of detection, which is consistent with recent evidence that measuring extracted plasma oxytocin via RIA is subject to low sensitivity (A. Szeto et al., unpublished data).
To confirm that the large number of non-detectable values resulted primarily from limitations of the RIA’s sensitivity, rather than from assay error — in combination with the fact that some participants had very low plasma levels of oxytocin — we correlated the number of measurements for which participants’ oxytocin values were measured by the RIA assay as undetectable with the mean of participants’ detectable oxytocin values, reasoning here that participants with very low mean oxytocin values would be the participants who would also be most likely to have relatively high numbers of undetectable values. These two variables were highly correlated, Spearman's rho = −.87, p < .01, n = 31. Thus, it appeared to be the case that non-detectability was driven predominantly by the fact that some participants had very low levels of plasma oxytocin — so low, in fact, that they hovered around or below the lower limit of the assay’s sensitivity.
Based on this conclusion, we set all undetectable values to 0 pg/ml. When we re-ran the analyses reported below with the undetectable values treated as missing values rather than arbitrarily set to zero, results were unchanged. The two resultant plasma oxytocin values for each measurement point were then averaged to create a single estimate of plasma oxytocin for each of the six measurement points for each individual.
Plasma concentrations of cortisol were determined using a solid phase RIA (Siemens Medical Solutions Diagnostics). The antibody employed in the kit has high specificity for cortisol and the minimal detectable level was 0.2 μg/ml (5.5nmol/l). The intra-assay CV was 5.1% and the inter-assay CV was 4.0% as reported by the manufacturer. Baseline plasma levels of progesterone and estradiol were also assessed via RIA method (Siemens Medical Solutions Diagnostics). The limit of detection for progesterone was 0.02 ng/ml, the intra-assay CV was 4.0%, and the inter-assay CV was 5.7%; the limit of detection for estradiol was 8.0 pg/ml, the intra-assay CV was 4.3%, and the inter-assay CV was 6.8% as reported by the manufacturer. In the current study, four samples of estradiol fell below the level of detection and were therefore set to 0 pg/ml. Our analyses were re-run with the undetectable values treated as missing values rather than arbitrarily set to zero, and results were unchanged. All sample extractions and assays were performed at the Diabetes Research Institute at the University of Miami’s Miller School of Medicine.
Statistical Analysis
Baseline levels of all hormones were estimated from the mean of the values from the two baseline draws. Four measurements of changes in plasma cortisol and oxytocin concentrations after the speech task were estimated by subtracting baseline levels of each respective hormone from the concentrations measured at 2, 5, 7, and 10 minutes following completion of the speech. To account for the pulsatile nature of oxytocin secretion (Amico et al., 1987; Cyranowski et al., 2008), mean oxytocin reactivity was computed by taking the mean of the four change scores. Prior to correlation and regression analyses, raw cortisol values were base-10 log transformed (McCullough et al., 2007b), and raw oxytocin values were square-root transformed (Light et al., 2005) to better approximate normal distributions. Analyses were conducted using repeated measures ANOVA (using the Greenhouse-Geiser correction when appropriate), paired samples t-tests, and Pearson product-moment correlations. All analyses were all based on two-tailed tests of significance.
Results
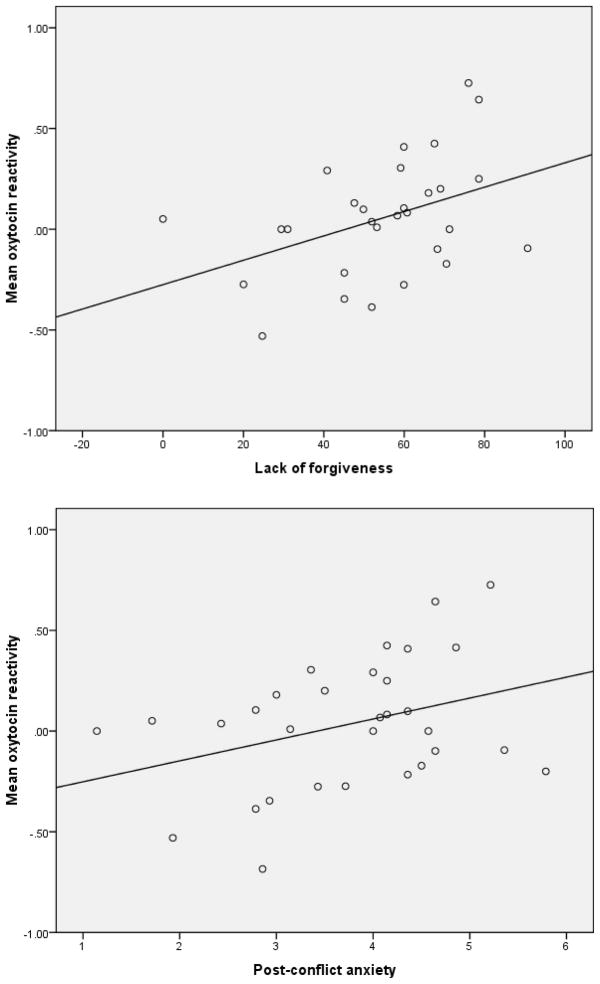
Means and standard deviations for major study variables appear in Table 2. A one-way (square-root transformed oxytocin [time]) repeated measures ANOVA revealed that the relational stress task did not significantly induce a change in oxytocin over time F(4, 104) = .42, p = .80, n = 27. In addition, as shown in Table 3, baseline oxytocin was not significantly associated with post-conflict anxiety (r = .12, p = .52, n = 33) or lack of forgiveness (r = −.09, p = .64, n = 32). However, as Figure 1 shows, task-related oxytocin reactivity (calculated by subtracting the mean of two baseline values from the mean of four post-task values) was positively associated with participants’ self-reported lack of forgiveness (r = .41, p < .05, n = 29), and post-conflict anxiety, (r = .35, p = .05, n = 31). Thus, oxytocin reactivity, but not baseline oxytocin, in this imagery task was associated with lingering concerns about recently conflictual relationships (Taylor, 2006; Taylor et al., 2006; Taylor et al., 2010). Oxytocin reactivity was also marginally associated with participants’ ratings of closeness/commitment to their transgressors (r = −.33, p = .08, n = 30).
Table 2.
Means and standard deviations for major study variables
| Measure | N | M | SD |
|---|---|---|---|
| Lack of forgiveness (TRIM-18) | 32 | 56.22 | 23.23 |
| Post-conflict anxiety | 33 | 3.67 | 1.09 |
| Oxytocin Baseline (pg/ml) | 35 | 1.61 | 2.78 |
| Oxytocin Time 1 (pg/ml) | 30 | 1.71 | 2.89 |
| Oxytocin Time 2 (pg/ml) | 30 | 1.45 | 2.21 |
| Oxytocin Time 3 (pg/ml) | 29 | 1.30 | 2.04 |
| Oxytocin Time 4 (pg/ml) | 30 | 1.48 | 2.60 |
| Cortisol Baseline (μg/dl) | 35 | 17.78 | 7.52 |
| Cortisol Time 1 (μg/dl) | 33 | 19.41 | 8.94 |
| Cortisol Time 2 (μg/dl) | 31 | 19.65 | 8.53 |
| Cortisol Time 3 (μg/dl) | 31 | 19.43 | 8.88 |
| Cortisol Time 4 (μg/dl) | 30 | 18.99 | 9.17 |
| Progesterone Baseline (ng/ml) | 35 | 1.28 | 2.25 |
| Estradiol Baseline (pg/ml) | 35 | 44.6 | 55.34 |
| Closeness and commitment to the transgressor | 33 | 4.77 | .92 |
| Painfulness of transgression | 33 | 5.03 | .88 |
| Psychosomatic symptoms | 35 | 1.85 | .49 |
Note. TRIM = Transgression Related Interpersonal Motivations Inventory.
Table 3.
Associations among major study variables
| Lack of forgiveness | Post-conflict anxiety | Closeness/commitment | |
|---|---|---|---|
| Baseline Oxytocin | −.09 | .12 | −.13 |
| Mean Oxytocin reactivity | .41* | .35* | −.33 |
| * Mean Oxytocin reactivity (controlling for psychosomatic symptoms) | .47** | .39* | −.21 |
| * Mean Oxytocin reactivity (controlling for self-reported calmness during the speech) | .41* | .39* | −.21 |
| * Mean Oxytocin reactivity (controlling for baseline estradiol) | .43* | .34a | −.27 |
| * Mean Oxytocin reactivity (controlling for baseline progesterone) | .41* | .38* | −.32 |
| Baseline Cortisol | .09 | −.01 | −.08 |
| Mean Cortisol reactivity | −.13 | −.08 | −.11 |
Note. Correlations in rows with asterisks represent partial correlations. N = 26 – 33.
p = .08,
p < .05,
p = .01
Figure 1.
Correlations of mean oxytocin reactivity with: (a) lack of forgiveness (n = 29), and (b) post-conflict anxiety (n = 31).
*Note. Raw values of plasma oxytocin were square-root transformed to approximate a normal distribution. Mean oxytocin reactivity values were computed using the transformed values.
We evaluated several potential confounds (Table 1) and found that current degree of psychosomatic symptoms (e.g., “runny/congested nose,” “depressed mood,” “chest pain”), and perceived painfulness of the transgression were significantly associated with mean oxytocin reactivity (r = −.36, p < .05, n = 32; r = −.54, p < .01, n = 30). In addition, perceived calmness during the speech task was marginally associated (r = −.33, p = .06, n = 32) with oxytocin reactivity. Controlling for psychosomatic symptoms, and then for calmness during the speech task, did not substantially alter the main results (Table 3). Because perceived painfulness of the transgression was not significantly associated with post-conflict anxiety (r = −.28, p = .13, n = 31) or forgiveness (r = −.06, p = .75, n = 30), we did not evaluate it further as a potential confound. In agreement with previous research (Light et al., 2005), the non-significant correlations of baseline levels of oxytocin with baseline levels of progesterone (r = −.05, p = .80; n = 35), and estradiol (r = .14, p =.43, n = 35) suggested that menstrual cycle variation and oral contraceptive use did not significantly alter the present results. To further assess the influence of menstrual cycle variation and oral contraceptive use as potential confounds, we controlled for baseline levels of progesterone and estradiol in major analyses. These controls did not substantially alter the main results (Table 3) 2.
A one-way (base-10 log transformed cortisol [time]) repeated measures ANOVA showed that the relational stress task induced a significant change in cortisol over time, F(1.93, 55.84) = 3.57, p < .05, n = 30; Greenhouse-Geiser correction. Paired t-tests showed significant differences between cortisol at 5 minutes post-speech task (Time 2; M = 2.86; SD = .47, n = 33) and baseline cortisol (M = 2.75; SD = .43, n = 33), t(32) = 2.19, p < .05, as well as cortisol at 7 minutes post-speech task (Time 3; M = 2.85; SD = .48, n = 32) and baseline cortisol (M = 2.75; SD = .42; n = 32), t(31) = 2.19, p < .05. Although the relational stress task induced significant changes in cortisol, we found no significant associations of forgiveness, post-conflict anxiety, or closeness/commitment to the transgressor with baseline cortisol, mean task-related cortisol reactivity, or cortisol reactivity at any single time point, which suggests that cortisol reactivity did not index lingering relational problems among our participants (Table 1). 3
Discussion
Despite recent research linking exogenous administrations of oxytocin to increased trust (Kosfeld et al., 2005) and decreased aversion to betrayal (Baumgartner et al., 2008), our results suggest that naturally occurring oxytocin reactivity in response to an interpersonally relevant laboratory task serves, as Taylor and colleagues (2006; 2006; 2000; 2010) surmised, as a biomarker for relational distress in women. Previous research has associated relational distress (Taylor et al., 2006; Taylor et al., 2010; Turner et al., 1999) and romantic attachment anxiety (Marazitti et al., 2006) with higher tonic levels of oxytocin. Interestingly, in the present study baseline oxytocin was not significantly correlated with post-conflict anxiety or forgiveness. We believe that this may result from the present study’s focus on a recently incurred interpersonal transgression, rather than the assessment of general levels of relational distress (Marazitti et al., 2006; Taylor et al., 2006; Taylor et al., 2010; Turner et al., 1999). However, the present study builds on previous research demonstrating task-related elevated levels of mean oxytocin release (Cyranowski et al., 2008), and our study is the first of which we are aware to reveal that the problematic qualities of a single relationship upon which participants have been asked to focus their attention during a well-defined laboratory task also predicted task-related changes in mean peripheral oxytocin.
The fact that our measures of forgiveness and post-conflict anxiety were not associated with task-related increases in cortisol, but were associated with elevated mean peripheral oxytocin reactivity, suggests furthermore that our results may not be the byproduct of a stress response mediated by the hypothalamic-pituitary-adrenal (HPA) axis. As further confirmation of the dissociation between oxytocin and the HPA response to stress (i.e., cortisol), a third one-way (base-10 log transformed cortisol [time]) repeated measures ANCOVA using mean oxytocin reactivity as a covariate revealed a non significant interaction effect for change in cortisol over time by mean oxytocin reactivity, F(1.90, 51.20) = .38, p = .68, n = 29; Greenhouse-Geiser correction. This latter dissociation is particularly important because both central and peripheral oxytocin have been associated with the HPA response to stress (Bartz and Hollander, 2006).
But if the interpersonally relevant oxytocin response documented here is not a byproduct of oxytocin’s effect on the HPA stress response, what function does it serve? This is a more difficult question to answer. These increases in plasma oxytocin could be exerting effects at a variety of sites throughout the periphery (Gimpl and Fahrenholz, 2001), and may not reflect oxytocin increases within the central nervous system (Bartz and Hollander, 2006; Gimpl and Fahrenholz, 2001). Importantly, in addition to the present findings associating oxytocin and relational distress following a recent interpersonal harm, additional results suggest that oxytocin reactivity during this task might be indexing relational sensitivity, rather than just relational distress (i.e., oxytocin reactivity was marginally associated with participants’ ratings of closeness/commitment before the transgression occurred and self-reported calmness during the relational laboratory task). Indeed, in addition to oxytocin’s associations with prosocial behaviors such as affiliation (Bartz & Hollander, 2006) and trust (Kosfeld et al., 2005), oxytocin administration has recently been associated with increased envy and gloating (Shamay-Tsoory et al., 2009), decreased cooperation in an economic game with no prior social contact with potential game partners (Declerck et al., in press), and enhanced neural activation for both positive and negatively valenced emotional stimuli in women (Domes et al., 2010). Domes and colleagues (2010) and Shamay-Tsoory (2010) propose that oxytocin may enhance general social salience rather than only attenuate negative social stimuli or enhance approach to positive social stimuli. Similarly, Declerck et al. (in press) suggests that oxytocin may increase people’s general awareness of social interaction depending on environmental context and cues. The present findings, along with previous studies associating oxytocin and relational distress or anxiety (Marazitti et al., 2006; Taylor et al., 2006; Taylor et al., 2010; Turner et al., 1999), provide additional evidence for the role of oxytocin in enhancing social salience.
In contrast to previous studies associating oxytocin administration and attenuated amygdala activity in men (Baumgartner et al., 2008; Domes et al., 2007; Petrovic et al., 2008), Domes et al. (2010) suggested that the sexually dimorphic patterns of oxytocin may have evolutionary roots in female threat-avoidance and safety seeking behaviors. This coincides with Taylor and colleagues (2006; 2000) conceptualization of the female specific biobehavioral response to stress. However, as Taylor et al. (2010) discussed, research has not yet demonstrated how peripheral oxytocin may help to mobilize effective responses to social distress in humans or animals.
The present findings were independent of dozens of potential biological and psychological confounds, and our method of studying real-life transgressions rather than hypothetical transgressions, or transgressions between strangers that can be engineered in the laboratory, improves external validity. Nonetheless, several limitations should be noted. First, these associations were correlational and small in magnitude, with our measures of forgiveness and post-conflict anxiety accounting for only 12–16% of the variation in oxytocin reactivity. In addition, we conducted our study in a relatively small sample of undergraduate women. However, the present study’s sample size was comparable to those of several other recent studies in which natural levels of plasma oxytocin were measured in humans (Cyranowski et al., 2008; Turner et al., 1999). The use of an undergraduate female sample does not allow us to comment on the generalizability of these associations to men, or to women in other phases of the life course, although a previous study found similar relationships between relational stress and increased levels of oxytocin in older women (Taylor et al., 2006). Lastly, future work may benefit from longitudinal measurement of relational distress, and extending blood sampling further in order to assess cortisol reactivity over a greater length of time, as well as cortisol recovery (Dickerson and Kemeny, 2004).
The present investigation builds on previous research associating relational distress and baseline oxytocin in humans (Taylor et al., 2006; Taylor et al., 2010; Turner et al., 1999) by demonstrating task-related increases in oxytocin reactivity in the context of naturally occurring interpersonal difficulties. We view these results as an important step toward a more rigorous search for evolved biobehavioral circuitry designed to coordinate adaptive responses to social stressors.
Footnotes
Male participants who underwent the blood sampling procedure (n = 17) were excluded from the sample based on the unique biobehavioral relevance of oxytocin to females (Cyranowski et al., 2008; Taylor et al., 2006; Taylor et al., 2000).
Baseline estradiol (skewness = 2.7, kurtosis = 5.8) and progesterone (skewness = 2.6, kurtosis = 9.9) were non-normally distributed and were therefore natural log-transformed to better approximate a normal distribution. Results reported here remained unchanged when we controlled estradiol and progesterone using untransformed values.
As further confirmation, a one-way (base-10 log transformed cortisol [time]) repeated measures ANCOVA using forgiveness as a covariate revealed a non-significant interaction effect for change in cortisol over time by forgiveness, F(1.91, 49.60) = .41, p = .66, n = 28; Greenhouse-Geiser correction. A second one-way (base-10 log transformed cortisol [time]) repeated measures ANCOVA using post-conflict anxiety as a covariate also revealed a non-significant interaction effect for change in cortisol over time by post-conflict anxiety, F(1.90, 49.27) = .10, p = .90, n = 28; Greenhouse-Geiser correction.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Benjamin A. Tabak, Department of Psychology and Behavioral Medicine Research Center, University of Miami, Coral Gables, FL
Michael E. McCullough, Department of Psychology and Behavioral Medicine Research Center, University of Miami, Coral Gables, FL
Angela Szeto, Department of Psychology and Behavioral Medicine Research Center, University of Miami, Coral Gables, FL.
Armando J. Mendez, Diabetes Research Institute at the University of Miami Miller School of Medicine, Miami, FL
Philip M. McCabe, Department of Psychology and Behavioral Medicine Research Center, University of Miami, Coral Gables, FL
References
- Amico J, Ulbrecht J, Robinson A. Clearance studies of oxytocin in humans using radioimmunoassay measurements of the hormone in plasma and urine. J Clin Endocrinol Metab. 1987;64:340–345. doi: 10.1210/jcem-64-2-340. [DOI] [PubMed] [Google Scholar]
- Andrich D. A rating formulation for ordered response categories. Psychometrika. 1978;43:561–573. [Google Scholar]
- Aron A, Aron EN, Smollan D. Inclusion of Other in the Self Scale and the structure of interpersonal closeness. J Pers Soc Psychol. 1992;63:596–612. [Google Scholar]
- Bartz JA, Hollander E. The neuroscience of affiliation: Forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. 2006;50:518–28. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bolger N, DeLongis A, Kessler RC, Schilling EA. Effects of Daily Stress on Negative Mood. J Pers Soc Psychol. 1989;57:808–818. doi: 10.1037//0022-3514.57.5.808. [DOI] [PubMed] [Google Scholar]
- Bono G, McCullough ME, Root LM. Forgiveness, feeling connected to others, and well-being: Two longitudinal studies. Pers Soc Psychol Bull. 2008;34:182–195. doi: 10.1177/0146167207310025. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens BA, Frank E, Seltman H, Cai H, Amico J. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: The modulating role of incentives and social information. Horm Behav. doi: 10.1016/j.yhbeh.2010.01.006. in press. [DOI] [PubMed] [Google Scholar]
- Denson TF, Fabiansson EC, Creswell JD, Pedersen WC. Exerpimental effects of rumination styles on salivary cortisol responses. Motiv Emotion. 2009;33:42–48. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Neumann ID, Bodenmann G, von Dawans B, Turner RA, Ehlert U, Heinrichs M. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32:565–74. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–31. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–90. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Finch JF, Okun MA, Pool GJ, Ruehlman LS. A comparison of the influence of conflictual and supportive social interactions on psychological distress. J Pers. 1999;67:581–621. doi: 10.1111/1467-6494.00066. [DOI] [PubMed] [Google Scholar]
- Fox CM, Jones JA. Uses of Rasch modeling in counseling psychology research. J Couns Psychol. 1998;45:30–45. [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–98. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- John OP, Donahue EM, Kentle RL. The “Big Five” Inventory-Versions 4a and 54. University of California; Berkeley: Institute of Personality and Social Research; Berkeley: 1991. [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test - A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;44:527–531. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lawler KA, Younger JW, Piferi RL, Billington E, Jobe R, Edmondson K, Jones WH. A change of heart: cardiovascular correlates of forgiveness in response to interpersonal conflict. J Behav Med. 2003;26:373–93. doi: 10.1023/a:1025771716686. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol Psychol. 2005;69:5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Marazitti D, Dell’osso B, Baroni S, Mungai F, Catena M, Rucci P, Albanese F, Giannaccini G, Betti L, Fabbrini L, Italiani P, Del Debbio A, Lucacchini A, Dell'Osso L. A relationship between oxytocin and anxiety of romantic attachment. Clin Pract Epidemol Ment Health. 2006;2:28. doi: 10.1186/1745-0179-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ME, Bono G, Root LM. Rumination, emotion, and forgiveness: Three longitudinal studies. J Pers Soc Psychol. 2007a;92:490–505. doi: 10.1037/0022-3514.92.3.490. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Fincham FD, Tsang JA. Forgiveness, forbearance, and time: The temporal unfolding of transgression-related interpersonal motivations. J Pers Soc Psychol. 2003;84:540–57. doi: 10.1037//0022-3514.84.3.540. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Hoyt WT. Transgression-related motivational dispositions: Personality substrates of forgiveness and their links to the Big Five. Pers Soc Psychol Bull. 2002;28:1556–1573. [Google Scholar]
- McCullough ME, Luna LR, Berry JW, Tabak BA, Bono G. On the form and function of forgiving: Modeling the time-forgiveness relationship and testing the valuable relationships hypothesis. Emotion. 2010;10:358–376. doi: 10.1037/a0019349. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Orsulak P, Brandon A, Akers L. Rumination, fear, and cortisol: An in vivo study of interpersonal transgressions. Health Psychol. 2007b;26:126–132. doi: 10.1037/0278-6133.26.1.126. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Rachal KC, Sandage SJ, Worthington EL, Jr, Brown SW, Hight TL. Interpersonal forgiving in close relationships: II. Theoretical elaboration and measurement. J Pers Soc Psychol. 1998;75:1586–1603. doi: 10.1037//0022-3514.75.6.1586. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Root LM, Cohen AD. Writing about the benefits of an interpersonal transgression facilitates forgiveness. J Consult Clin Psychol. 2006;74:887–97. doi: 10.1037/0022-006X.74.5.887. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis HT, Sheldon KM, Gable SL, Roscoe J, Ryan RM. Daily well-being: The role of autonomy, competence, and relaedness. Pers Soc Psychol Bull. 2000;26:419–435. [Google Scholar]
- Russell D, Peplau LA, Cutrona CE. The revised UCLA loneliness scale: Concurrent and discriminant validity evidence. J Pers Soc Psychol. 1980;39:472–480. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. One hormonal system for love and envy: A reply to Tops. Biol Psychiatry. 2010;67:e7. [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Hariri AR, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biol Psychiatry. 2009;66:864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Tend and befriend: Biobehavioral bases of affiliation under stress. Curr Dir Psychol Sci. 2006;15:273–277. [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom Med. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–29. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair bond relationships? Psychol Sci. 2010;21:3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Turner RA, Altemus M, Enos T, Cooper B, McGuinness T. Preliminary research on plasma oxytocin in normal cycling women: investigating emotion and interpersonal distress. Psychiatry. 1999;62:97–113. doi: 10.1080/00332747.1999.11024859. [DOI] [PubMed] [Google Scholar]
- Wiggins JS, Trapnell P, Phillips N. Psychometric and geometric characteristics of the Revised Interpersonal Adjective Scales (IAS-R) Multivar Behav Res. 1988;23:517–530. doi: 10.1207/s15327906mbr2304_8. [DOI] [PubMed] [Google Scholar]
- Winefield HR, Winefield AH, Tiggemann M. Social support and psychological well-being in young adults: The Multi-Dimensional Support Scale. J Pers Assess. 1992;58:198–213. doi: 10.1207/s15327752jpa5801_17. [DOI] [PubMed] [Google Scholar]
- Witvliet CVO, Ludwig TE, Vander Laan KL. Granting forgiveness or harboring grudges: Implications for emotions, physiology, and health. Psychol Sci. 2001;121:117–123. doi: 10.1111/1467-9280.00320. [DOI] [PubMed] [Google Scholar]