Abstract
Success of nanoparticle-mediated drug delivery is subject to development of optimal drug release strategies within defined space and time (triggered release). Recently, we reported a novel class of photo-triggerable liposomes prepared from dipalmitoyl phosphatidylcholine (DPPC) and photopolymerizable diacetylene phospholipid (DC8,9PC), that efficiently released entrapped calcein (a water soluble fluorescent dye) upon UV (254 nm) treatment. To develop these formulations for in vivo applications, we have examined phototriggering of these liposomes by visible light, and the effect of released anticancer drugs on cellular toxicity. Sonicated liposomes containing various ratios of DPPC:DC8,9PC and 4 mol% DSPE-PEG2000 were loaded with calcein (Ex/Em, 485/517 nm) or a chemotherapy drug, Doxorubicin (DOX, Ex/Em 490/590 nm). Our initial experiments showed that 514 nm laser treatment of liposomes containing 10 or 20 mol% DC8,9PC for 1–3 minutes resulted in significant release of calcein. Based on these results, we performed studies with DOX-loaded liposomes. First, biophysical properties (including liposome size and stability) and DOX encapsulation efficiency of the liposomes were determined. Subsequently, the effect of 514 nm laser on DOX release, and cellular toxicity by released DOX were examined. Since liposomes using the 86:10:04 mole ratio of DPPC:DC8,9PC:DSPE-PEG2000, showed highest encapsulation of DOX, these formulations were investigated further. We report that (i) Liposomes retained about 70% of entrapped DOX at 37°C in the presence of 0–50% serum. (ii) 514 nm laser treatment resulted in DOX release from liposomes in a wavelength specific manner. (iii) Laser treatment of co-cultures containing DOX-loaded liposomes and cells (Raji and MCF-7) resulted in at least 2–3 fold improved cell-killing as compared to untreated samples. Taken together, the phototriggerable liposomes described here may provide a platform for future drug delivery applications. To our knowledge, this is the first report demonstrating improved cell killing following light-triggered release of an encapsulated anticancer agent from photosensitive liposomes.
Keywords: polymerizable lipids, laser, triggered drug release, diacetylene phospholipids, radiation-sensitive liposomes, lipid modification
Introduction
Several nano-delivery systems that are amenable to release drugs at desired sites have been developed since decades. Among these, intensive research has been conducted using the liposomes as triggerable drug carriers [1]. Various triggering modalities examined to date include local hyperthermia, use of metal ions, pH, enzymes and light (radiation) [2–4].
Electromagnetic radiation-sensitive liposomes present a promising system and rely on strategically designed phospholipid molecules to initiate light-induced trigger [5]. The principle(s) of photo-triggering include photopolymerization of lipids [6], photosensitization by membrane anchored hydrophobic probes [7–10], or photoisomerization of photoreactive lipids [11]. However, none of the formulations developed so far have been successful for in vivo applications presumably due to the lack of adequate photon energy produced by the radiation source(s) or inability of radiation sources used to penetrate into biological tissues [5]. Therefore, alternate methods to develop liposomes sensitive to tissue-penetrating wavelengths are warranted for their successful applications in the clinic.
Photo-triggerable liposome formulations containing wavelength-specific photosensitizers (primarily lipidic and/or hydrophobic in nature) in conjunction with photoactivable lipids are also described. For example, inclusion of the cationic dye, 1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), induces destabilization of DOPE:SorbPC (3:1) liposomal membranes when radiated at 550 nm [12]. In another example, researchers have studied light-sensitive liposomes based on photochemical triggering, such as plasmenylcholine activation by a near infra red (NIR) sensitizer. Plasmenylcholine photooxidation was initiated by different hydrophobic molecules such as Zn-phthalocyanine, octabutoxyphthalocyanine, and bacterio-chlorophyll-a. These sensitizing agents absorb between 630–820 nm inducing membrane phase changes that leads to photo-triggering of liposomes and release of contents [5]. Although these wavelengths have the advantage of deeper tissue penetration depth for clinical therapeutics, dug release experiments were not established [5,13]. An added asset in developing photo-triggerable nanoparticles is the steady improvements in laser-technology yielding greater ability to control laser systems by the light wavelength, intensity, duration and beam diameter [14]
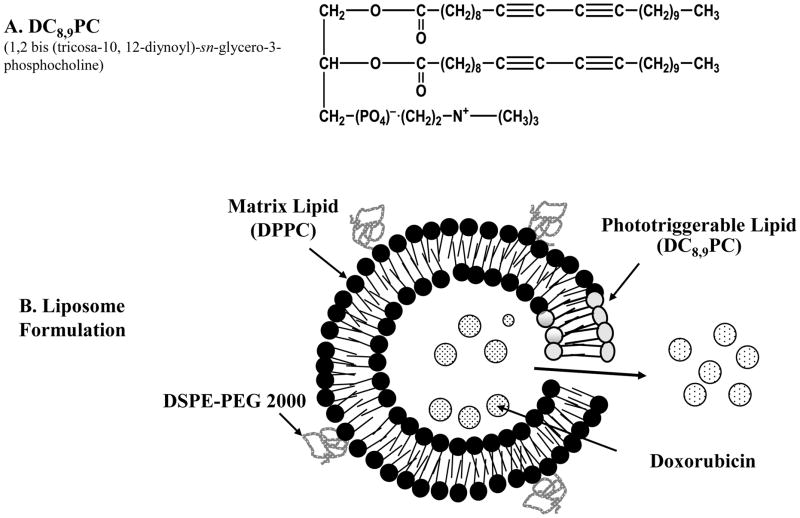
The photopolymerizable phospholipid, (1,2 bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine (DC8,9PC) (Fig. 1A), is a lipid present in lower organisms with unique bilayer packing-self-assembly [15]. Due to highly reactive diacetylene groups uniquely assembled in the lipid bilayer, photopolymerization by UV treatment results in chains of covalently linked lipid molecules within the bilayer [16][17]. Diacetylene groups can be photopolymerized by high energy radiation and are used to monitor high energy radiation released in case of a dirty bomb attack [18]. Liposomes prepared from DC8,9PC alone form tube like structures that are not suitable for drug entrapment, therefore, liposome formulations with lipid mixtures were used in our studies (Fig. 1B). Biological applications of DC8,9PC examined thus far include functionalized polymerized vesicles for vascular targeted molecular imaging [19], candidates for oral vaccine preparations [20], and DNA delivery [21][22]. However, in situ light-triggered drug release potential of DC8,9PC liposomes has not been reported until recently [23].
Figure 1. Design principle of photo-triggerable liposomes.
(A) Chemical structure of DC8,9PC., (B) A diagram showing various components of DPPC:DC8,9PC liposomes including DPPC (matrix lipid), DC8,9PC (photo-triggerable lipid), DSPE-PEG2000 (stabilizing lipid) and entrapped DOX (anticancer drug). The cartoon also shows light- induced defects in the lipid membrane resulting in release of DOX.
Recently, we have demonstrated that liposomes derived from DPPC:DC8,9PC mixture when treated with UV (254 nm) light released their entrapped contents. Since the observed leakage was selective to this lipid mixture, we proposed that the unique packing properties of DC8,9PC in the liposome bilayer were crucial to promote the observed effect (photopolymerization) [6,16]. In this report, we have extended our studies to investigate visible light-triggered drug delivery potential of these liposomes. Our studies show that laser (514 nm) treatment of DPPC:DC8,9PC liposomes results in release of entrapped calcein (Ex/Em, 485/517 nm) but not calcein blue (Ex/Em 360/460 nm) under identical conditions. This observation suggests that visible-light induced solute leakage from the liposomes shown here depends on the spectral properties of entrapped solutes. Since cellular toxicity by liposomal DOX was also enhanced upon laser treatment, our phototriggerable liposomes are likely to form the basis for next-generation of clinically suitable nano-drug delivery tools.
2. Materials and Methods
2.1 Materials
1,2 bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine,(DC8,9PC) was synthesized at Naval Research Laboratory using published literature procedure [24]. All other phospholipids were purchased from Avanti Polar lipids, Inc. (Alabaster, AL, USA). Calcein and calcein blue were purchased from Fluka-Sigma-Aldrich (St. Louis, MO, USA). Agarose beads, Bio-Gel A0.5m were purchased from Bio-Rad Laboratories, Richmond, CA, USA. Doxorubicin-hydrochloride (DOX-HCl) (Bedford Laboratories, Bedford, OH) was obtained through the NIH Pharmacy, Clinical Center, Bethesda, MD. PD10 columns were from GE Healthcare Lifesciences (Piscataway, NJ). All other reagents and buffers were of reagent grade.
2.2 Cell lines
Lymphoblastoid cells derived from a Burkitt lymphoma (Raji, ATCC CRL-2367) were grown in RPMI media supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (GIBCO® Media, Invitrogen, USA). Human breast adenocarcinoma cell line (MCF7, ATCC HTB-22) received through the NCI-DCTD Tumor/cell line repository (http://dtp.nci.nih.gov/brances/btb/tumor-catalog.pdf), were grown in RPMI media with 10% fetal calf 2 mM L-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (GIBCO® Media, Invitrogen, USA).
2.3 Formation of liposomes
Liposomes were prepared using the probe sonication essentially as described [23]. The following lipid mixtures were used (Table 1): DPPC:DSPE-PEG2000 (96:04 mol%, Formulation I); DPPC:DC8,9PC:DSPE-PEG2000 (86:10:04 mol%, Formulation II); DPPC:DC8,9PC:DSPE-PEG2000 (76:20:04 mol%, Formulation III), and Egg PC:DC8,9PC:DSPE-PEG2000 (86:10:04 mol%, Formulation IV).
Table 1.
Size Analysis and Efficiency of Doxorubicin Loading of Liposomes
| Liposomes* | Size Distribution / DOX loading | ||||
|---|---|---|---|---|---|
| No DOX& | 7°c | 25°c | 37°c | ||
| DPPC:DC8,9PC (96:0) Formulation I | Size1 → | 83.46±2 nm | 101.2±2 nm | 98.14 nm | 117.4±4 nm |
| Liposomal DOX2 → | N.A. | 728 ng | 598 ng | 666 ng | |
| DPPC:DC8,9PC (86:10) Formulation II | Size1 → | 99.84±4 nm | 150.6±6 nm | 159.9±6 nm | 114.6±6 nm |
| Liposomal DOX2 → | N.A. | 1036 ng | 1335 ng | 141 ng | |
| DPPC:DC8,9PC (76:20) Formulation III | Size 1 → | 105.3±2 nm | 184.4±6 nm | 140.4±6 nm | 120.2±2 nm |
| Liposomal DOX2 → | N.A. | 597 ng | 538 ng | 182 ng | |
| Egg PC:DC8,9PC (86:10) Formulation IV | Size1 → | 98.31±4 nm | 101.2±2 nm | 98.31±4 nm | 98.31± 4nm |
| Liposomal DOX 2 → | N.A. | 2223 ng | 1296 ng | 720 ng | |
All formulations included 4 mol% DSPE-PEG2000
Liposomes before loading of DOX
Size was measured by DLS and values represent average diameter of the peak S.D. (from at least 3 measurements)
Liposomal Pi and DOX were measured (Methods section). The DOX values (nanograms) are expressed per nmol Pi.
2.3.1. Encapsulation of calcein or calcein blue in liposomes
To encapsulate calcein or calcein blue, lipid film was reconstituted in HEPES buffer (HBS, 10 mM HEPES, 140 mM NaCl, pH 7.5) containing self-quenched concentration of calcein or calcein blue (0.1M at pH=7.2–7.6). Unilamellar Vesicles were formed by sonication at 4°C for 5–10 min (1 min pulses and 1 min rest) using a Probe Sonicator (W-375 Heat Systems-Ultrasonics, New York, USA). The samples were centrifuged to remove any titanium particles and larger aggregates. Solute-loaded liposomes were separated from unentrapped calcein (or calcein blue) using size exclusion gel chromatography column (Bio Gel A0.5m, 1×40 cm, 40 ml bed volume). Eluted liposomes were collected, liposome–rich fractions were pooled and the total lipid was determined by measuring inorganic phosphorus (Pi) as described [25,26]. Liposomes were filtered through a 0.45 micron filter (Millex-HV 0.45 μm filter unit, Millipore Corporation, Billerica, MA) to maintain their sterility. Solute loading into liposomes was determined by examining a small aliquot for fluorescence before and after addition of Triton X-100 (TX100, 0.02% final concentration). Fluorescence was measured using a fluorescent micro plate reader (SpectraMax M2, Molecular Devices, Sunnyvale CA, USA). Filter sets at Ex/Em 490/530 nm were used for Calcein and sets at Ex/Em 360/460 nm for Calcein blue.
2.3.2 Encapsulation of Doxorubicin in liposomes
To load DOX into liposomes we used (NH4)2SO4 gradient exchange method [26,27]. Liposomes were prepared in the presence of ammonium sulfate (300 mM, pH 7.5) and were fractionated on a Bio Gel A0.5m column pre-equilibrated with HBS buffer. Eluted liposomes were collected, liposome–rich fractions were pooled and the total lipid was determined by measuring inorganic phosphorus (Pi) [25,26]. The liposomes were incubated immediately with DOX.HCL at various temperatures for 12–16 hours, typically using 1 mg of the drug for 3 mg of liposomal lipids.
In our initial studies, we used the Bio Gel A0.5m column to separate liposomes from unentrapped (free) DOX. This chromatography column has a fractionation range of 10,000–500,000 molecular weight and is a suitable matrix to separate antibodies and small molecules from liposomes [26,28]. To determine entrapment efficiency of DOX into liposomes, small aliquots from column fractions were diluted in 2 ml PBS, Triton-X100 (0.02% final concentration) was added, and DOX fluorescence (Ex/Em 485/590 nm) was measured in a Fluorimeter (FluoroMax 3, HORIBA Jobin Yvon, Edison NJ, USA). Next, we determined the suitability of the PD10 column (GE Healthcare, UK) to separate unentrapped DOX from liposomal DOX. The amounts of liposome-encapsulated DOX for a given concentration of liposomal Pi were similar whether the PD10 column or the Bio Gel A0.5m column were used, therefore, we continued with PD10 columns in our subsequent experiments (see below).
2.3.3 DOX Release Assay
To determine serum stability and light-induced leakage of liposomal DOX (see below), the assay to monitor DOX leakage was developed as follows: Test samples were fractionated on the PD10 column and 1 ml (x30) fractions were collected. The fractions were analyzed for DOX fluorescence in the presence of TX100 (see preceding section). DOX leakage from liposomes was calculated based on the formula:
F = florescence)
The total amount of DOX loaded on the column equaled the sum of peak I and peak II representing the area of liposome fractions and free DOX respectively.
2.3.4 Liposome Stability Assay
Stability of liposomes was determined in the presence of PBS supplemented with different concentrations of heat-inactivated fetal bovine serum (FBS). Typically 0.5 ml DOX-loaded liposomes were incubated with the desired concentration of serum or PBS at 37°C for 24 hours. At the end of incubations, liposomes were fractionated on the PD10 column and DOX release was determined as described in the preceding section. Specific conditions are given in Table 2.
Table 2.
Serum Stability of DOX-loaded Liposomes at 37°C*
| Natural Leakage in PBS& | DOX leakage (percent of total entrapped DOX) | |||
|---|---|---|---|---|
| DPPC:DC8,9PC Formulation I^ | DPPC:DC8,9PC Formulation II)^ | DPPC:DC8,9PC Formulation III^ | Egg PC:DC8,9PC Formulation IV^ | |
| 0% FBS | 8.26 | 2.89 | 6.58 | 3.59 |
| 10% FBS | 15.6 | 20.69 | 20.87 | 4.04 |
| 25% FBS | 20.14 | 29.59 | 29.51 | 2.91 |
| 50% FBS | 32.02 | 34.48 | 30.48 | 17.97 |
PBS was supplemented with various concentrations of FBS.
All formulations included 4 mol% DSPE-PEG2000
Liposomes were incubated for 24 hr. DOX leakage was measured as described in Methods section.
2.4 Light Treatment
Light-triggered release of entrapped contents from liposomes was evaluated either upon exposure to UV [23] or visible light.
UV (254 nm) light treatment of the liposomes (typically containing 1.5 nmol Pi/μl diluted 1:10 in PBS, in a total volume of 0.2 ml) was done in the 96 well-plates essentially as described [23], using the UV lamp (UVP, SHORT WAVE ASSEMLY 115V, 60Hz).
For visible light treatment, a multiline mode Argon laser (488/514 nm, Lexel Laser, Inc., Freemont, CA) was used. The liposomes were placed in an eppendorf tube (pre-cooled) and irradiated horizontally with a beam of 500 mW and focused on an area of 1 cm2 (166 mW/cm2/min) for 0–7 minutes [29]. Light-triggered release of entrapped calcein or calcein-blue from liposomes was measured as above (section 2.3.1). To monitor light-triggered release of DOX from liposomes, DOX release assay was used (described in section 2.3.3).
2.5 Liposome Characterization
Size and population distribution of liposomes were determined by dynamic light scattering (DLS) measurements using a Malvern instrument (NANO ZS, Malvern Instruments, CA, USA)[28]. For a typical sizing experiment 20 μl of liposome solution in 500 μl HBS buffer were placed into a 1.5 ml microcuvette. Each run consisted of 3 measurements of 12 to 20 acquisitions per sample.
2.6 Cellular Toxicity Assay
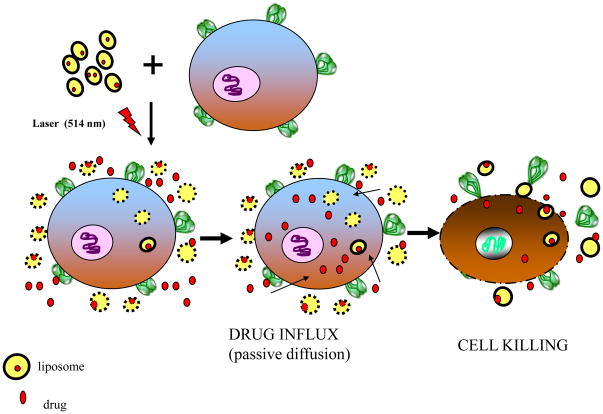
The protocol followed for DOX-mediated cellular toxicity (as a result of light-triggered release of liposomal DOX) is shown in the cartoon (Fig. 5). We examined two cell systems; suspension cells (Raji, B lymphocyte cell line), and adherent cells (MCF-7, a breast cancer cell line) in these cytotoxicity assays. Various concentrations of DOX-loaded liposomes were added to cells in their respective culture media in 1.5 ml eppendorf tubes and the cell-liposome suspensions were treated with 514 nm laser for 0–5 minutes. Subsequently, cells were transferred to 96-well plates (1–5×104 cells per well, triplicate wells per sample) and incubations were continued for 48 hr at 37°C. DOX-mediated cellular toxicity was measured using a cell viability kit (Cell Titer-Blue, Promega, Madison WI USA) at Ex/Em of 560/590 nm using a fluorescent micro plate reader (SpectraMax M2, Molecular Devices, Sunnyvale CA, USA). Untreated cells (without light treatment and without liposomes) were used as positive controls, and the cell viability in these samples was taken as 100% cell survival. Additional controls (both positive and negative) used in our cytotoxicity assays included: (i) similar concentrations of empty liposomes (without DOX) with or without light treatment; (ii) DOX-loaded liposome-cell suspensions without light treatment, and (iii) Equivalent concentrations of free DOX with or without light treatment.
Figure 5. A Schematic representation of a proposed mechanism of laser-triggered release of entrapped solutes from Liposomes.
Light-treatment of DOX-loaded DPPC:DC8,9PC liposomes results in release of DOX. The effective concentration of the free drug is increased in the vicinity of cells. DOX is subsequently taken up by the cells by passive diffusion leading to improved cell killing.
3. Results and Discussion
In our recent studies, we showed that UV-treatment of DPPC liposomes containing various concentrations of DC8,9PC resulted in photopolymerization of DC8,9PC and release of entrapped calcein [23]. However, application of these liposomes in vivo is subject to adaptability to light source(s) that are amenable to tissue-penetrating wavelengths. This study was designed to examine release of entrapped solutes by visible wavelength (514 nm) as a platform to further develop these formulations. The results are presented below.
3.1 Light-induced calcein release from liposomes containing a diacetylene phospholipid
UV (254 nm) triggered calcein release from DPPC:DC8,9PC liposomes [23] suggested that the inclusion of DPPC (but not Egg PC) as the matrix lipid was critical for the observed solute release. Calcein release in this case was preceded by the photopolymerization of DC8,9PC as evidenced by change in chromogenic properties of DC8,9PC [23]. However, the experimental conditions that were used to observe UV-triggered calcein release from these liposomes resulted in substantial damage of cultured cell lines with or without liposomes as expected (data not shown).
To demonstrate that these liposomes may serve as promising candidates for light-triggered release applications in vivo, we rationalized that cell-compatible wavelengths (such as visible or Near Infra Red (NIR)) in combination with an appropriate photosensitizer are likely to produce modifications in the diacetylene groups necessary for release of entrapped contents [5,30]. Our first experiments towards this effort were designed to establish the ability of visible light source(s) to promote leakage of liposome-entrapped molecules. Since DPPC:DSPE-PEG2000 liposomes containing 10 and 20 mol% DC8,9PC are most suitable with respect to the solute entrapment efficiency and stability (our previous study) [23], we have tested these formulations. We used calcein as the model drug as it is a widely used liposome-entrapped aqueous marker for biophysical studies, and is also known to bear photosensitizing properties [31]. The results presented below describe the effect of visible light on the kinetics and extent of calcein release from light-sensitive liposomes.
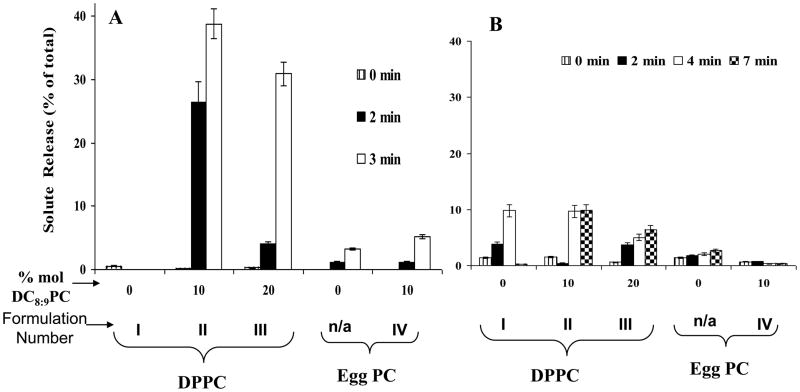
DPPC liposomes containing 0, 10 and 20 mol% DC8,9PC (Formulation I, II&III, Table I) were treated with a 514 nm argon laser for 0–7 minutes at 4°C, and calcein release was measured (Methods section). Controls included Egg PC:DSPE-PEG2000 liposomes (96:04 mole ratios) or Egg PC:DC8,9PC:DSPE-PEG2000 (Formulation IV, Table I). The data presented in Figure 2A show that significant calcein release (up to 40% of total entrapped) occurred from formulation II and III, within 2–3 minutes of laser treatment. Prolonged exposures (>5 minutes) resulted in maximum calcein release; however, fluorescence intensity of released calcein was compromised by laser-induced photodynamic effects of the fluorophore (data not shown). In contrast, liposomes prepared from Egg PC alone or Egg PC: DC8,9PC (Formulation IV) did not promote any substantial calcein release (only ≤ 5%) under identical conditions (Fig. 2A) consistent with our UV-triggered release results [23]. Surprisingly, we did not see an evidence for photopolymerization upon 514 nm laser treatment of formulations II & III (unpublished), although calcein release clearly occurred (Fig. 2A). Therefore, the visible-light induced solute release from these liposomes occurs via a mechanism unrelated to photopolymerization.
Figure 2. Effect of visible light treatment on release of liposome-entrapped calcein.
Calcein-loaded liposomes were prepared from DPPC or Egg PC (as matrix lipids) and 0–20 mol% of DC8,9PC. All formulations included 4 mol% DSPE-PEG2000. The liposomes were treated with 514 nm laser (0–7 minutes) and calcein release was measured. Kinetics and extents of Light-induced Calcein (A) and Calcein blue (B) release from liposomes are presented and the numbers represent fraction of calcein released from liposomes taking liposomes treated with TX100 as 100% total calcein. The data are reproducible from at least three independent experiments.
Since calcein release occurs in the absence of photopolymerization in laser-treated formulations II & III, we surmise that the photosensitizing properties of entrapped calcein may play a role in destabilizing the liposomes. Therefore, our next experiments were designed to evaluate the contribution of liposome-entrapped calcein on photo-induced leakage by testing an alternate fluorescent marker, calcein blue (Ex/Em 360/460 nm). Calcein-blue loaded liposomes were treated with 514 nm laser as above and the data are presented in Figure 2B. Interestingly, treatment of calcein-blue loaded liposomes (Formulation II&III) with 514 nm laser under identical conditions did not result in any significant leakage of calcein blue above background (Fig. 2B). However, laser treatments for longer times (4–7 minutes) resulted in 5–10% release of calcein blue from formulation II and III. Taken together (Fig. 2A and Fig. 2B), our results support the notion that a wavelength-specific tunable photosensitizer (calcein in this case) is important for photodestabilization of DC8,9PC formulations in the visible wavelength. We are currently pursuing this hypothesis by examining the nature of chemical modifications in DC8,9PC upon laser treatment in the absence or presence of calcein (work in progress).
The next experiments aimed to evaluate the potential biological application of our light-sensitive liposomes are described below. This experimental design includes studies with liposomes loaded with an anticancer agent DOX in vitro (section 3.2) and the ability of released DOX to promote cytotoxicity (section 3.3).
3.2 Doxorubicin-loaded liposomes: Biophysical Studies
For the last several decades, DOX has been used as a key chemotherapy component in the treatment of various cancers [32]. Side effects associated with DOX, including irreversible cardio-toxicity, have led to investigations into approaches for drug carriers to achieve better therapeutic index of DOX. Liposome formulations are well-studied examples as therapeutic tools for DOX delivery in a variety of cancer treatments [33,34]. DOX loaded liposome formulations, (such as Doxil [35]) are known to improve liposome stability and prolong circulation time in vivo, but they lack the ability to release entrapped drugs within defined space and time. Light-triggered release of liposomal fluorescent molecules (either encapsulated in the aqueous compartment or incorporated into the lipid counter part) has been reported previously [2,5], however, no data is available on the release of liposome-encapsulated therapeutic drugs in response to light. Here we have used DOX as a model anticancer agent to demonstrate the feasibility of DPPC:DC8,9PC liposomes for light-triggered drug delivery. The following section includes results on DOX encapsulation efficiency (section 3.2.1), stability of DOX-loaded liposomes (section 3.2.2), visible light-mediated release of DOX from liposomes (section 3.2.3), and the effect of DOX on cytotoxicity following light-triggered release from liposomes (3.3).
3.2.1 Entrapment Efficiency of Doxorubicin into DPPC:DC8,9PC Liposomes
It is evident that optimal drug loading in the liposome formulations is an important parameter for successful in vivo applications. Our DOX loading protocol is based on the well-established ammonium sulfate gradient assay that has proven to be a viable method for achieving payload of weak bases such as DOX in the liposomes [27]. We examined efficiency of DOX loading using various lipid formulations at 7, 25 and 37°C for 12–16 hr. Concurrently, the effect of DOX loading on average size distribution was also determined (Methods section). The results are presented in Table 1. Quantitation of liposomal DOX was done by measuring fluorescence (Methods section) [26] and the values are presented as ng/nmol phospholipid (determined by inorganic phosphorus (Pi) analysis [25], data not shown).
Our results show that DPPC liposomes (Formulation I) entrapped ≈500–700 ng DOX/nmol phospholipid. The incubations at various temperatures did not have a significant effect on DOX loading for Formulation I (Table I). However, DPPC:DC8,9PC:DSPE-PEG2000 liposomes (Formulation II) loaded maximum concentration of DOX at 25°C (≈1300 ng/nmol Pi), while loading efficiency was dramatically reduced at 37°C (≈140 ng/nmol Pi) (Table 1). Similarly, DOX loading for Formulation III (Table 1) was also optimal at 7°C or 25°C (≈500 ng/nmol Pi), and was significantly decreased at 37°C (180 ng/nmol Pi). The efficiency of DOX loading was at a given temperature optimal for Formulation II as compared to that of Formulation III. Therefore, we used Formulation II in our cytotoxicity assays (see below section 3.3). Formulation IV (containing Egg PC) showed maximum loading at 7°C, presumably due to the lower phase transition temperature of Egg PC[36]. It is also evident from Table 1, that there was a slight increase in average size distribution of DPPC liposomes (Formulation I), whereas DPPC:DC8,9PC liposomes (formulation II and III) showed approximately 1.5 fold increase in size following loading of DOX. In contrast, we did not observe such an effect on the size distribution of Egg PC:DC8,9PC liposomes (Formulation IV) after DOX loading (Table 1). A detailed analysis of the morphological parameters is needed to understand this effect.
3.2.2 Stability of DOX-loaded DPPC:DC8,9PC liposomes in serum
Liposomes are known to interact with serum components and hence destabilize the lipid bilayer [37,38] limiting their successful application as drug delivery vehicles in vivo [39,40]. Therefore, we tested the DOX leakage from the liposomes at various concentrations of serum in PBS for 24 hr at 37°C. The formulations that entrapped highest DOX (see Table 1) were used for stability experiments. Although the DOX-loaded liposomes remained stable at 25°C for 21 days (data not shown), DOX leakage occurred in the presence of serum at 37°C. Results are presented in Table 2. We observed only a slight increase in DOX release above original background in the absence of FBS at 37°C for all formulations tested (Table II). Incubations in the presence of FBS at 37°C showed an effect on the release DOX from liposomes. As expected, increase in serum concentration from 0 to 50% resulted in more pronounced DOX release from liposomes. We found that ≈70% DOX remained associated with formulations I, II and III in the presence of 50 % serum (a concentration that mimics serum concentration in vivo). The formulation IV (Egg PC) showed ≈80% DOX associated with the liposomes under similar conditions. Therefore, our liposomes serve as candidates for the proof of principle experiments to validate our proposed light-triggered drug delivery technology (described below, see section 3.2.3 & 3.3).
3.2.3 Light-induced leakage of entrapped Doxorubicin from DPPC:DC8,9PC liposomes
We have previously demonstrated that UV (254 nm)-induced release of entrapped solutes [23] from DPPC:DC8,9PC liposomes occurs via the photopolymerization reaction of DC8,9PC molecules in the lipid bilayer. UV-triggered release is not dictated by physical and chemical properties of entrapped molecules. In contrast, visible light (514 nm)-triggered release of calcein (this study) from these liposomes occurs via an alternate mechanism (unrelated to photopolymerization) and depends on the properties of entrapped molecules (Fig. 2A, calcein, Fig. 2B, calcein blue). Based on these observations, DOX (Ex/Em 490/590 nm) that possesses photoreactive properties can serve as a suitable model drug for these studies. First, it was important to establish that phototriggering of DPPC:DC8,9PC liposomes resulted in release of liposomal DOX, similar to the observed release calcein.
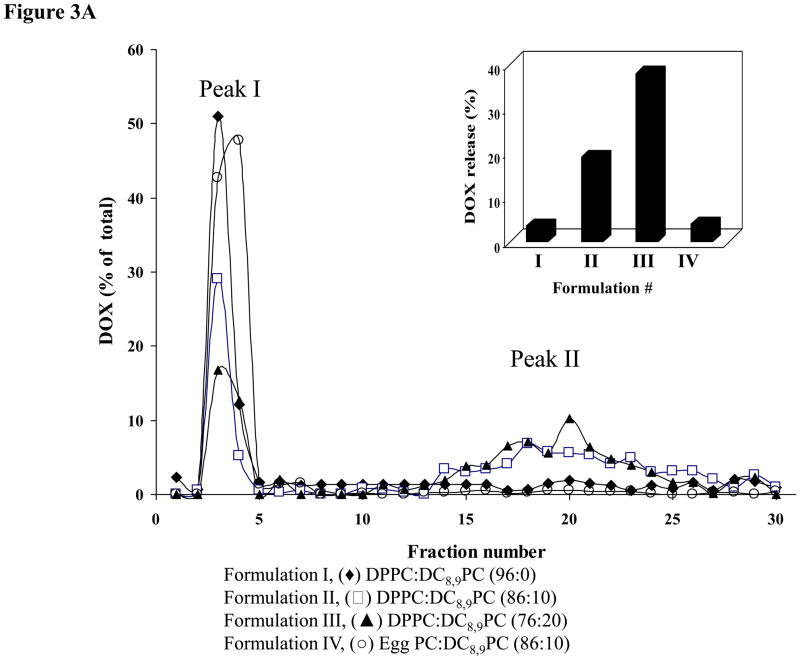
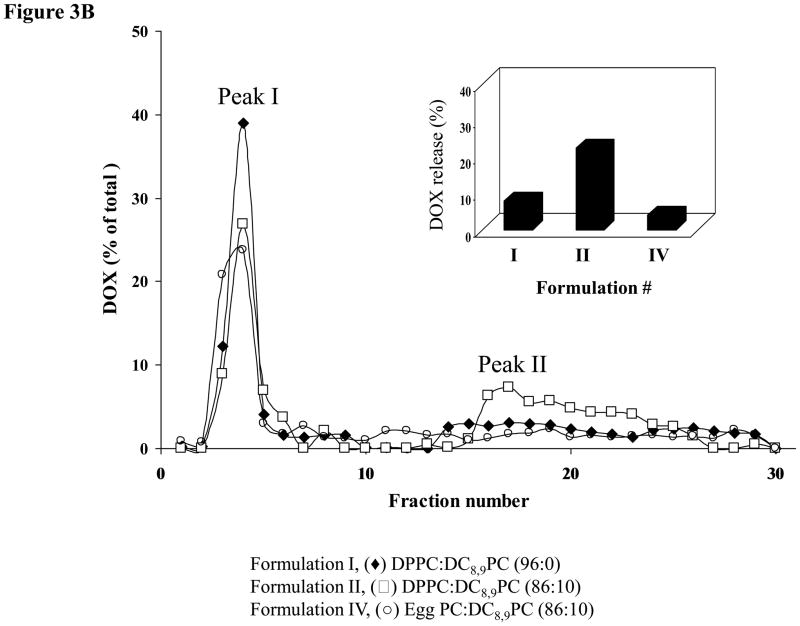
DOX-loaded liposomes were treated with either 514 or 254 nm and effects of these treatments on release of DOX were monitored (Fig. 3A&B). Liposome-associated DOX was separated from free DOX on a PD10 column (Methods section). DOX fluorescence in peak I (liposomes) and peak II (free DOX) was assayed (Methods section), and total DOX for a given sample equaled to the sum of peaks I and II. We also calculated the extent of DOX release for a given sample (shown in insets Fig. 3A&B), and the values are presented as percent of total DOX per sample where higher numbers indicate a higher amount of free DOX.
Figure 3. Effect of UV or visible light treatment on release of liposomal Doxorubicin.
DOX-loaded liposomes were prepared from DPPC or Egg PC (as matrix lipids) and 0–20 mol% of DC8,9PC. All formulations included 4 mol% DSPE-PEG2000. Liposomes were either treated with UV or visible light and leakage of entrapped DOX was measured after separation of liposomes from free DOX on a PD10 column (Methods section).
3A. DOX release by UV treatment (254 nm, 30 min): (◆) Formulation I (DPPC:DC8,9PC (96:0); (□), Formulation II (DPPC:DC8,9PC (86:10)); (▲) Formulation III (DPPC:DC8,9PC (76:20)); (●) Formulation IV (Egg PC:DC8,9PC (86:10)). Inset shows the percent of DOX leakage calculated as the ratio of peak II (representing free/leaked DOX) divided by total DOX (the sum of peak I, representing the liposome fractions and peak II, representing free/leaked DOX).
3B. DOX release by laser treatment (514 nm, 5 minutes): (◆)Formulation I (DPPC:DC8,9PC ((96:0)); (□) Formulation II (DPPC:DC8,9PC (86:10)); (●) Formulation IV (Egg PC:DC8,9PC (86:10)). Inset shows the percent of DOX leakage calculated as the ratio of peak II (representing free/leaked DOX) divided by total DOX (the sum of peak I, representing the liposome fractions and peak II, representing free/leaked DOX). Data were reproducible from at least two independent experiments.
After UV treatment the extent of DOX release from liposomes was as follows: ≈20% (Formulation II), and ≈40% (Formulation III) and ≈4% (Formulation I) suggesting that our formulation may be useful for treatment of cancer (Fig. 3A). These results are consistent with our previously published calcein release data [23]. We have previously demonstrated that UV-triggered release of calcein was specific to DPPC:DC8,9PC formulations, and replacement of DPPC by Egg PC in these liposomes obliterated this effect that correlated with lack of photopolymerization [23]. To validate specificity of UV-mediated DOX release from DPPC:DC8,9PC liposomes, we performed similar studies with Egg PC:DC8,9PC liposomes. After UV treatment of Egg PC liposomes containing 10 mol% DC8,9PC (Formulation IV), majority of DOX remained associated with the liposome fraction (≈4 % DOX released). Hence the effect of UV treatment was specific to DPPC:DC8,9PC formulations (Fig. 3A, Inset). Formulation III showed higher DOX release upon UV treatment when compared with Formulation II. However, the amount of DOX encapsulated in Formulation II was significantly higher (≈1300 ng/nmol Pi) in comparison to Formulation III (≈500 ng/nmol Pi) (Table I). Therefore, we focused on Formulation II for our further studies (see below 514 nm laser-induced leakage and subsequent cell toxicity experiments).
Next, we examined the effect of 514 nm laser on release of DOX from DPPC:DC8,9PC liposomes. The results presented in Figure 3B showed similarity to the UV-mediated effect. We observed a significant increase in DOX release from 514 nm–treated Formulation II (≈22%) (Fig. 3B, Inset). Under identical conditions, Formulation I and Formulation IV showed only a slight DOX release (≈8% and 7% respectively, Fig. 3B, Inset), consistent with lack of calcein release from liposomes that do not contain DC8,9PC (Fig. 2A). Although visible light-induced release of fluorescent molecules from phototriggerable liposomes have been reported earlier, release of a drug (such as DOX shown in this study) in response to visible light is not shown previously until this report.
3.3 Visible light treatment increases efficiency of cell killing by liposomal Doxorubicin
To evaluate the drug delivery potential of our liposomes, we examined the effect of phototriggering on DOX-mediated cellular toxicity using a variety of cell lines. Our first experiments in this series were conducted using Raji cells (a B-lymphocyte cell line). In a typical experiment, various concentrations of Formulation II (see Table I) were mixed with Raji cells (5×104 per sample) and the liposome-cell suspensions were treated with 514 nm laser for 5 minutes. Subsequently the cell viability (cell survival) was measured 48 hr post-incubations at 37°C (Methods section). These incubation conditions were selected because we did not observe any non-specific effects of the laser on cell viability in the absence of DOX and/or liposomes (not shown). Moreover, 5 min treatments with the laser resulted in optimal DOX release from the liposomes (Figure 3B). Results presented in Table 3, show that the laser treatment in co-cultures of Formulation II with Raji cells reduced cell survival by 40–60%, and the extents were dependent on the initial input of liposomes.
Table 3.
Effect of 514 nm Laser Treatment on Liposomal DOX-mediated Cytotoxicity
| Liposomes | Light Treatment | Cell Survival (% of Control)* | ||
|---|---|---|---|---|
| 80# | 160# | 200# | ||
| Formulation II, DPPC:DC8,9PC (86:10) | None | 99.5 8.5 | 83.33 10.5 | 70.46 10.5 |
| Laser | 64.26 12.68 | 51.21 13.1 | 49.26 12.68 | |
| Laser** | 100.17 5.5 | 99.1.12 5.5 | 101.12 5.5 | |
| Formulation IV, Egg PC:DC8,9PC (86:10) | None | 101.04 2.3 | 97.35 8.6 | 93.04 7.5 |
| Laser | 104.56 11.5 | 94.22 8.4 | 80.59 10.5 | |
| Laser** | 102.77 6.2 | 100.97 1.5 | 105.57 6.2 | |
Raji cells and liposomes were mixed, treated with 514 nm laser (5 min), subsequently incubated for 48 hr at 37°C and cell survival was measured (Methods Section). Controls included cell-liposome mixtures without laser treatment or empty liposome-cell suspensions treated with laser under identical conditions. The data are expressed as percent cell survival as compared to the cell viability of cells (without liposomes and without laser treatment) as 100%. The values are expressed as SD from triplicate samples within a single experiment. The results were reproducible from at least three independent experiments.
Empty Liposomes (without any DOX loading)
Nanogram liposomal Doxorubicin added per sample.
Lower doses of liposomal DOX (80 ng, Formulation II) did not affect the viability of cells in the absence of laser treatment. However, the cell viability was reduced in laser-treated samples at this concentration (64% cell survival, Table 3). When higher concentrations of liposomal DOX were used (160&200 ng, Formulation II) in these experiments, we observed a 20–30% reduction in cell viability in the absence of laser treatment. Laser treatment of these samples (160&200 ng) resulted in a further reduction in cell viability (≈50% cell survival, Table 3). To determine the non-specific effects mediated by liposomal lipids at the higher concentrations (unrelated to DOX-mediated cell killing), we incubated equivalent amounts of empty liposomes (not loaded with DOX) and examined cell viability before and after laser treatment. We did not observe any effect of lipids on cell viability at all the doses tested (data are only shown for laser-treated samples, Table 3).
As Formulation IV (containing Egg PC as the matrix lipid) did not promote a significant DOX release upon treatment with 514-nm laser (Fig. 3B), we used this formulation as an additional control in our cell survival experiments. We did not see any decrease in cell survival when Formulation IV was used (with or without laser treatment) up to 160 ng liposomal DOX (Table 3). At highest dose (200 ng), there was only a slight decrease in cell viability (5–10%). Furthermore, laser pretreatment in these samples did not improve cell killing, confirming the effects seen for Formulation II were mediated by released DOX following laser treatment. In the absence of laser treatment, slight reduction in cell survival at high doses of Formulation II corroborates with our DOX leakage data in the presence of serum (Table 2). For example, the incubation of Raji cells with Formulation II (160 ng) reduced cell survival to 80% whereas at the same concentration of Formulation IV we did not observe any significant reduction (97% cell survival). We attribute this effect to the natural leakage of DOX from Formulation II (≈20%) as compared to only slight leakage of DOX from formulation IV (≈4%).
We also examined the effect of laser treatment on improved cell killing by liposomal DOX using another suspension cell line, Sup-T1 (T lymphocytes) and obtained similar results (data not shown). Although we have established that DPPC;DC8,9PC formulations are viable photo-triggerable drug delivery carriers for suspension cells, (preceding experiments, this section) we wished to determine if improved cell killing by laser treatment could be applied to other cancer cell lines, that are adherent in nature. The similar experiments were performed using MCF7 cells (breast cancer epithelial cells), and the results are presented below.
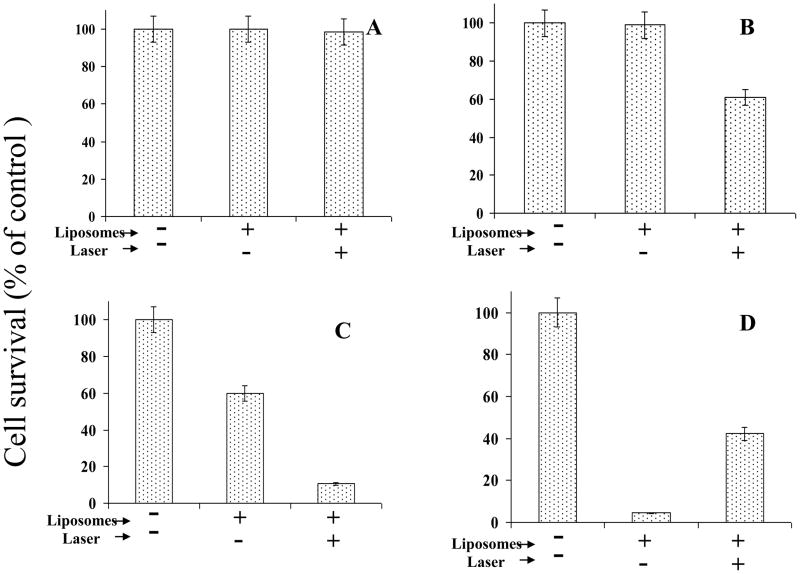
Co-cultures of MCF7 cells (1×104 per sample) and Formulation II (see Table I,) were treated with 514 laser as above. Following the laser treatment, the cell-liposome suspensions were plated on 96-well clusters (see Methods section), and incubations were continued for 48 hours at 37°C. The cytotoxicty was determined, and results are presented in Figure 4(A–C). The values are presented as % cell survival taking MCF-7 cells alone as 100%. First data set (Figure 4A) shows results on our control experiment to establish any non-specific effects of laser and/or liposomal lipids on the viability of MCF7 cells. Incubation of empty liposomes (without DOX) at the corresponding highest dose of liposomal lipids ( 0.12 nmol Pi/sample) did not result in any cellular toxicity with or without laser treatment. Therefore, the total lipid and/or laser treatments per se did not cause any non-specific effects on cell viability. In addition, there was no effect of laser treatment on viability of MCF7 cells in the absence of liposomes (data not shown). Figure 4B shows that the incubation of Formulation II at lower DOX concentration (80 ng DOX per sample) with MCF7 cells did not reduce cell viability. However, when the samples were treated with laser (in paired experiments), we observed a noticeable reduction in cell survival (40% of controls, Fig. 4B), suggesting that DPPC:DC8,9PC liposomes are likely to be valid candidates for light-triggered drug delivery. In our next experiments, we incubated liposomes with MCF7 cells at high DOX dose (160 ng DOX per sample). We observed 40% cell killing in samples incubated with DOX-loaded Formulation II, without laser treatment (Fig. 4C). Pretreatment of MCF7/Formulation II suspension with the laser led to a further significant decrease in cell viability (90% cell killing, Fig. 4C). Therefore phototriggering of DPPC:DC8,9PC formulations improves cell-killing due to the release of DOX in the incubation medium, for a variety of target cell lines (Raji or MCF7). Our drug delivery carriers described here are likely to have implications for treatment of multiple tumor types. The observed cytotoxicity at high doses of Formulation II (in the absence of photo-triggering) was due to natural leakage of DOX from the liposomes. Therefore, it is critical to modify liposomes (without compromising their light-sensitivity) for in vivo applications. . Our future studies are aimed at developing stable liposomes adaptable to in vivo light-triggered drug delivery. (Work in progress).
Figure 4. Effect of 514 nm laser treatment on liposomal DOX-mediated cytotoxicty of MCF7 cells.
MCF7 cells were harvested using the cell-dissociation buffer and mixed with DOX-loaded liposomes (Formulation II), treated with laser for 5 minutes and the cell-liposome mixtures were transferred to 96-well plates (1×104 cells/well in triplicate). Cell toxicity was measured 48 hr post-incubations at 37°C using the Cell Titer Blue assay (Methods section). Control samples included cells without liposomes or cell/liposome suspension without laser treatment. Values are presented as cell survival taking the cell viability of untreated cells as 100% cell survival. ±S.D. represents average of three samples within a single experiment.
A: Empty liposomes (without DOX) corresponding to the 0.12 nmol liposomal Pi per well .
B: DOX-loaded liposomes (Formulation II) at 80 ng liposomal DOX (containing 0.06 nmol Pi) per well.
C: DOX-loaded liposomes (Formulation II) at 160 ng liposomal DOX (containing 0.12 nmol Pi) per well.
D: Effect of Laser treatment on efficiency of cell killing by free Doxorubicin.
Free DOX was added to MCF7 cells to a final concentration of 500 ng/well and the samples were treated with laser for 7 minutes. Controls were without liposomes or without laser treatment.
Previous studies show that free DOX is prone to photodynamic damage when exposed to laser, and intracellular accumulation of DOX minimizes this photo-induced damage [30,41]. Therefore, it was important to examine the biological activity of free DOX in our experimental set up. Initially, we optimized the dose of free DOX for killing of MCF7 cells; 500 ng DOX was sufficient to kill >95% of the cells, and, therefore this concentration was used for our experiments (not shown). Free DOX was added to MCF7 cells, the samples were treated with 514 nm laser, incubated at 37°C for 48 hr, and cellular toxicity was determined. The results are presented in Figure 4D. Incubation of MCF7c ells with free DOX resulted in >95% cytotoxicty as expected. When the samples were pretreated with laser, the cytotoxic effect of free DOX was substantially decreased (40% cell survival). We attribute this effect to the photodynamic damage of free DOX upon laser treatment. In contrast, DOX encapsulation into liposomes minimizes the direct photo-induced damage on DOX [30,41], suggesting that our formulation may have an advantage for cancer treatment.
Visible-light triggered release of liposome-entrapped contents has been reported using the light-sensitive formulations including various phototriggerable lipids [1, 5]. Although calcein has been extensively used as an aqueous model solute, light-induced activation was also achieved by lipophilic photosensitizers. Nonetheless, the most of the light-triggerable liposome systems reported thus far are limited to in vitro assays. Here, for the first time, we have used DC8,9PC for phototriggering, and to our knowledge, this is the first report to demonstrate light-triggered release of an anticancer agent (DOX) and the effect of released drug on the biological activity of cells. Moreover, our formulations are based on photosensitizing properties of molecules that are entrapped in the aqueous core and not in the lipidic environment. Hence DPPC:DC8,9PC liposomes are likely to be suitable in vivo drug delivery applications for multiple drugs candidates provided the drug can be encapsulated into liposomes at high concentrations. Further work is being performed in our lab to characterize the visible light triggered chemical reactions that result in destabilization of DPPC:DC8,9PC liposomes.
Use of visible light sources, such as a laser, could be a powerful tool to improve release of encapsulated drugs from liposomes with minimal tissue damage. It has been proposed earlier that use of a laser source matched to the absorption spectrum of DOX can create photoactivation that will electronically excite molecules and increase cytotoxicity [30,41]. Published data showed that Argon ion laser radiation at 488 or 514 nm can photoactivate intracellular free DOX and dramatically increase DOX cytotoxicity in L929 cells. Our results (Table 3, Figs. 4 and 5) are in agreement with the literature findings that photo-induced damage will be reduced when DOX is its encapsulated form.
Light-regulated drug delivery has become a useful therapeutic tool with our enhanced understanding of the photo-regulation and the properties of photoactivated materials. Treatments based on the UV-triggered release are restricted to the areas such as skin or mucosa [5], due to restriction in deep tissue penetration of UV light. Near infra red light wavelengths penetrate through tissues without damaging DNA [42]. Therefore, NIR photosensitizers may find applications as external drug delivery devices and as imaging tools in animal models [14].
Concluding remarks
An important requirement for effective drug delivery is the precise spatial and temporal release of therapeutic agents at the target site. Development of nano-carriers with triggered release mechanism(s) will reduce potential toxicity and lead to more effective therapy. To date, various photo-triggerable liposome formulations are described in literature as candidates for localized drug delivery. However, none of these studies have demonstrated light-triggered delivery of cytotoxic agents to improve cell killing.
The focus of the present work was to demonstrate that light-triggered release of an anticancer agent (DOX) from light-sensitive liposomes (containing the photosensitive phospholipid DC8,9PC) promotes cell-killing. The model presented in Figure 6 summarizes the advantages of light-triggerable drug delivery vehicles for cancer therapeutics. Although nanoparticles are preferentially taken up by the leaky vasculature of tumors, we are improving liposome binding by developing DPPC:DC8,9PC liposomes formulations that include agents for specific targeting to tumor cells. The triggerable liposomes described here also bear the potential to adapt to electromagnetic radiations (such as X-rays or NIR) lasers for future in vivo applications.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- Egg PC
L-α-Phosphatidylcholine (Egg, Chicken)
- DC89PC
(1,2 bis (tricosa-10, 12-diynoyl)-sn-glycero-3-phosphocholine)
- DSPE-PEG2000 (18: 0 PEG2 PE)
1,2-Distearoyl-sn-Glycero-3 Phosphoethanolamine-N-[Methoxy(Polyethylene glycol)-2000] (Ammonium Salt)
- HEPES buffer (HBS)
10 mM HEPES, 140 mM NaCl (pH 7.5)
- PBS
2.66 mM KCl, 1.47 mM KH2PO4, 138 mM NaCl, 8.06 mM Na2HPO4-7H2O (pH 7.1)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.varez-Lorenzo C, Bromberg L, Concheiro A. Light-sensitive Intelligent Drug Delivery Systems. Photochemistry and Photobiology. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 2.Andresen TL, Jensen SS, Jorgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Torchilin VP, Smirnov VN. [Use of liposomes for drug targeting] Ukr Biokhim Zh. 1984;56:339–345. [PubMed] [Google Scholar]
- 4.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 5.Shum P, Kim JM, Thompson DH. Phototriggering of liposomal drug delivery systems. Adv Drug Deliv Rev. 2001;53:273–284. doi: 10.1016/s0169-409x(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 6.Regen SL, Singh A, Oehme G, Singh M. Polymerized phosphatidyl choline vesicles. Stabilized and controllable time-release carriers. Biochem Biophys Res Commun. 1981;101:131–136. doi: 10.1016/s0006-291x(81)80020-4. [DOI] [PubMed] [Google Scholar]
- 7.Bisby RH, Mead C, Morgan CG. Active uptake of drugs into photosensitive liposomes and rapid release on UV photolysis. Photochem Photobiol. 2000;72:57–61. doi: 10.1562/0031-8655(2000)072<0057:auodip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Bisby RH, Mead C, Morgan CG. Wavelength-programmed solute release from photosensitive liposomes. Biochem Biophys Res Commun. 2000;276:169–173. doi: 10.1006/bbrc.2000.3456. [DOI] [PubMed] [Google Scholar]
- 9.Chandra B, Mallik S, Srivastava DK. Design of photocleavable lipids and their application in liposomal “uncorking”. Chem Commun (Camb ) 2005:3021–3023. doi: 10.1039/b503423j. [DOI] [PubMed] [Google Scholar]
- 10.Lavi A, Weitman H, Holmes RT, Smith KM, Ehrenberg B. The depth of porphyrin in a membrane and the membrane's physical properties affect the photosensitizing efficiency. Biophys J. 2002;82:2101–2110. doi: 10.1016/S0006-3495(02)75557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan CG, Bisby RH, Johnson SA, Mitchell AC. Fast solute release from photosensitive liposomes: an alternative to 'caged' reagents for use in biological systems. FEBS Lett. 1995;375:113–116. doi: 10.1016/0014-5793(95)01193-i. [DOI] [PubMed] [Google Scholar]
- 12.Miller CR, Clapp PJ, O'Brien DF. Visible light-induced destabilization of endocytosed liposomes. FEBS Lett. 2000;467:52–56. doi: 10.1016/s0014-5793(00)01122-4. [DOI] [PubMed] [Google Scholar]
- 13.Gerasimov OV, Boomer JA, Qualls MM, Thompson DH. Cytosolic drug delivery using pH- and light-sensitive liposomes. Adv Drug Deliv Rev. 1999;38:317–338. doi: 10.1016/s0169-409x(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 14.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 15.Pakhomov S, Hammer RP, Mishra BK, Thomas BN. Chiral tubule self-assembly from an achiral diynoic lipid. Proc Natl Acad Sci U S A. 2003;100:3040–3042. doi: 10.1073/pnas.0030051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhodes DG, Blechner SL, Yager P, Schoen PE. Structure of polymerizable lipid bilayers. I--1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine, a tubule-forming phosphatidylcholine. Chem Phys Lipids. 1988;49:39–47. doi: 10.1016/0009-3084(88)90062-x. [DOI] [PubMed] [Google Scholar]
- 17.Singh A, Markowitz MA, Tsao LI, Deschamps J. Enzyme Immobilization on Polymerizable Phospholipid Assemblies. Diagnostic Biosensor Polymers. 1994;556:252–263. [Google Scholar]
- 18.Mod Ali N, Tucker CE, Smith FA. Consideration of radiation-induced polymerization of diacetylene LB films for dosimetry. Thin Solid Films. 1996;289:267–271. [Google Scholar]
- 19.Li KC, Bednarski MD. Vascular-targeted molecular imaging using functionalized polymerized vesicles. J Magn Reson Imaging. 2002;16:388–393. doi: 10.1002/jmri.10174. [DOI] [PubMed] [Google Scholar]
- 20.Alonso-Romanowski S, Chiaramoni NS, Lioy VS, Gargini RA, Viera LI, Taira MC. Characterization of diacetylenic liposomes as carriers for oral vaccines. Chem Phys Lipids. 2003;122:191–203. doi: 10.1016/s0009-3084(02)00190-1. [DOI] [PubMed] [Google Scholar]
- 21.Zarif L. Elongated supramolecular assemblies in drug delivery. J Control Release. 2002;81:7–23. doi: 10.1016/s0168-3659(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 22.Chiaramoni NS, Speroni L, Taira MC, Alonso SV. Liposome/DNA systems: correlation between association, hydrophobicity and cell viability. Biotechnol Lett. 2007;29:1637–1644. doi: 10.1007/s10529-007-9454-y. [DOI] [PubMed] [Google Scholar]
- 23.Yavlovich A, Singh A, Tarasov S, Capala J, Blumenthal R, Puri A. Design of liposomes containing photopolymerizable phospholipids for triggered release of contents. Journal of Thermal Analysis and Calorimetry. 2009;98:97–104. doi: 10.1007/s10973-009-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh A. An efficient synthesis of phosphatidylcholines. J Lipid Res. 1990;31:1522–1525. [PubMed] [Google Scholar]
- 25.Ames BN, Dubin DT. The Role of Polyamines in the Neutralization of Bacteriophage Deoxyribonucleic Acid. Journal of Biological Chemistry. 1960;235:769–775. [PubMed] [Google Scholar]
- 26.Loomis K, Smith B, Feng Y, Garg H, Yavlovich A, Campbell-Massa R, Dimitrov DS, Blumenthal R, Xiao X, Puri A. Specific targeting to B cells by lipid-based nanoparticles conjugated with a novel CD22-ScFv. Exp Mol Pathol. 2010 doi: 10.1016/j.yexmp.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 28.Puri A, Kramer-Marek G, Campbell-Massa R, Yavlovich A, Tele SC, Lee SB, Clogston JD, Patri AK, Blumenthal R, Capala J. HER2-specific affibody-conjugated thermosensitive liposomes (Affisomes) for improved delivery of anticancer agents. J Liposome Res. 2008;18:293–307. doi: 10.1080/08982100802457377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raviv Y, Viard M, Bess J, Jr, Blumenthal R. Quantitative measurement of fusion of HIV-1 and SIV with cultured cells using photosensitized labeling. Virology. 2002;293:243–251. doi: 10.1006/viro.2001.1237. [DOI] [PubMed] [Google Scholar]
- 30.Gao JP, Lanks KW, Rosen M, Lai BT. Mechanism of action and spectrum of cell types susceptible to doxorubicin photochemotherapy. Cancer Chemother Pharmacol. 1997;40:138–142. doi: 10.1007/s002800050638. [DOI] [PubMed] [Google Scholar]
- 31.Beghetto C, Renken C, Eriksson O, Jori G, Bernardi P, Ricchelli F. Implications of the generation of reactive oxygen species by photoactivated calcein for mitochondrial studies. Eur J Biochem. 2000;267:5585–5592. doi: 10.1046/j.1432-1327.2000.01625.x. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda M, Yamaguchi S, Ohta T, Nakayama Y, Ogata H, Shimizu K, Nishikawa T, Adachi Y, Fukuma E. Combination therapy for advanced breast cancer: cyclophosphamide, doxorubicin. UFT, and tamoxifen, Oncology (Williston Park) 1999;13:77–81. [PubMed] [Google Scholar]
- 33.Gabizon AA. Stealth liposomes and tumor targeting: One step further in the quest for the magic bullet. Clinical Cancer Research. 2001;7:223–225. [PubMed] [Google Scholar]
- 34.Lasic DD, Martin FJ, Gabizon A, Huang SK, Papahadjopoulos D. Sterically Stabilized Liposomes - A Hypothesis on the Molecular-Origin of the Extended Circulation Times. Biochimica Et Biophysica Acta. 1991;1070:187–192. doi: 10.1016/0005-2736(91)90162-2. [DOI] [PubMed] [Google Scholar]
- 35.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 36.Kim JC, Bae SK, Kim JD. Temperature-sensitivity of liposomal lipid bilayers mixed with poly(N-isopropylacrylamide-co-acrylic acid) Journal of Biochemistry. 1997;121:15–19. doi: 10.1093/oxfordjournals.jbchem.a021558. [DOI] [PubMed] [Google Scholar]
- 37.Kirby C, Gregoriadis G. Plasma-induced release of solutes from small unilamellar liposomes is associated with pore formation in the bilayers. Biochem J. 1981;199:251–254. doi: 10.1042/bj1990251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirby C, Clarke J, Gregoriadis G. Cholesterol content of small unilamellar liposomes controls phospholipid loss to high density lipoproteins in the presence of serum. FEBS Lett. 1980;111:324–328. doi: 10.1016/0014-5793(80)80819-2. [DOI] [PubMed] [Google Scholar]
- 39.Ishida T, Harashima H, Kiwada H. Interactions of liposomes with cells in vitro and in vivo: opsonins and receptors. Curr Drug Metab. 2001;2:397–409. doi: 10.2174/1389200013338306. [DOI] [PubMed] [Google Scholar]
- 40.Ishida T, Harashima H, Kiwada H. Liposome clearance. Biosci Rep. 2002;22:197–224. doi: 10.1023/a:1020134521778. [DOI] [PubMed] [Google Scholar]
- 41.Lanks KW, Gao JP, Sharma T. Photodynamic enhancement of doxorubicin cytotoxicity. Cancer Chemother Pharmacol. 1994;35:17–20. doi: 10.1007/BF00686279. [DOI] [PubMed] [Google Scholar]
- 42.Tang Y, McGoron AJ. Combined effects of laser-ICG photothermotherapy and doxorubicin chemotherapy on ovarian cancer cells. J Photochem Photobiol B. 2009;97:138–144. doi: 10.1016/j.jphotobiol.2009.09.001. [DOI] [PubMed] [Google Scholar]