Abstract
The TAM receptor tyrosine kinases Tyro3, Axl, and Mer and their ligands Gas6 and Protein S are essential for the phagocytosis of apoptotic cells and membranes in the adult immune, nervous, and reproductive systems. Genetic studies indicate that this receptor-ligand system is central to apoptotic cell engulfment that is triggered by the ‘eat-me’ signal phosphatidylserine. At the same time, TAM signaling is normally activated by Toll-like receptor (TLR) and type I interferon signaling, as part of the innate inflammatory response in dendritic cells and macrophages, where it inhibits this response. Deficiencies in TAM signaling result in human retinal dystrophies and may contribute to lupus and other human autoimmune diseases.
Introduction
Autoimmune diseases arise when the immune system makes the fundamental mistake of confusing self with non-self. Although rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), and other autoimmune disorders have differential presentations, organ and tissue targets, and dependence on cellular versus humoral immunity, each is characterized by chronic inflammation and autoreactivity. SLE is a particularly illustrative example of the challenges of both understanding and treating autoimmune disease. This broad spectrum disorder is characterized prominently by the presence of auto-antibodies directed against nuclear antigens such as ribonucleoproteins and double-stranded DNA. While the etiology and pathogenesis of SLE remain to be defined, it is increasingly evident that impaired clearance of apoptotic cells, enhanced activation of dendritic cells (DCs), and a concomitant type I interferon (IFN) response are associated with, and almost certainly contribute to, autoimmunity [1–3]. The first of these defects represents an especially serious threat, since programmed cell death and the generation of apoptotic cells are central to cellular turnover and tissue homeostasis during adulthood. While in some cases apoptotic cells appear to be shed; e.g., as occurs with intestinal epithelial cells and epidermal keratinocytes, most such cells must be actively cleared by phagocytes. Defects in apoptotic cell clearance can lead to the accumulation of intracellular components and the aberrant (sustained) exposure of nuclear antigens to the immune system. This can lead, in turn, to the activation of autoreactive B cells, autoantibody production, and the formation of immune complexes. Immune complexes of DNA and RNA are known to activate DCs and to trigger the production of type I IFNs, which perpetuate a cycle of immune activation [4]. The pathogenic role of type I IFNs in autoimmunity is highlighted by the observed amelioration of disease in lupus-prone mice that lack the Type I IFN receptor (IFNAR) [5]. Thus, SLE may be driven by the pathologic exposure to self-antigens due to the aberrant clearance of apoptotic cells, which in turn leads to the unabated activation of DCs and the subsequent production of type I IFNs. Recent studies on the function of the TAM family of receptor tyrosine kinases have provided important insights into these two discrete, but tightly linked phenomena - the removal of apoptotic cells and the regulation of DC activation and the type I IFN response. In this review, we summarize findings from these studies, and highlight their potential importance to SLE specifically and to human autoimmune disease in general.
TAM receptors and ligands
The TAM receptor tyrosine kinases, Tyro3, Axl and Mer, were identified as a distinct receptor protein-tyrosine kinase sub-family in 1991 [6•, 7]. The TAMs share a characteristic tandem arrangement of two immunoglobulin-like and two fibronectin type III repeats in their extracellular, ligand-binding domains, which are followed by a single-pass transmembrane domain and a cytoplasmatic protein-tyrosine kinase [8]. The TAM receptors remained orphans, in the sense that their activating ligands were unknown, until 1995. In a study employing a receptor-based affinity purification approach, Stitt and colleagues purified the closely-related Gas6 and Protein S (ProS) proteins as TAM ligands [9•,10–13]. Gas6 and ProS are secreted soluble proteins that carry an N-terminal gamma-carboxylated glutamic acid (GLA) domain, whose glutamic acid residues are carboxylated at the free gamma hydroxyl position, in a vitamin-K-dependent reaction. GLA domains confer the ability to bind phosphatidylserine (PtdSer) to their associated proteins [14]. This phospholipid is strictly confined to the inner leaflet of the plasma membrane bilayer in most normal cells, but in apoptotic cells its compartmentalization is lost, and PtdSer is displayed to the extracellular environment. Extracellular PtdSer serine is in fact one of the most generally recognized signatures of apoptotic cells [3, 15–17]
The GLA domain in Gas 6 and ProS is followed by four epidermal growth factor (EGF)-like domains, and a C-terminal sex hormone binding globulin (SHBG) like module that is both necessary and sufficient for the activation of TAM receptors. The ability of each ligand to activate each TAM receptor has been studied, but mostly in the context of cells expressing a single recombinant TAM receptor. In these studies, Gas6 has been found to activate all three receptors, albeit with differing potency (Axl>Tyro3>>>Mer) [13], while ProS has been found to activate both Tyro3 and Mer [9, 18]. However, TAM ligands can form heterodimers (Rothlin and Lemke, unpublished results), and it is possible that the same holds true for TAM receptors, which are frequently co-expressed [8]. Thus, it will be important to measure the receptor-ligand affinities in the context of heterodimers. ProS also carries a TAM-independent activity as an anticoagulant, and is present at relatively high levels (~300nM) in the circulation. ProS serves as a co-factor for activated Protein C, a protease that inhibits coagulation through the degradation of factor Va and factor VIII [19]. ProS carries a thrombin sensitive region, which is not present in Gas6. While it is known that cleavage by thrombin (which is generated during blood clotting) abolishes ProS anticoagulant co-factor activity [20], the effect of thrombin on ProS bioactivity as a TAM ligand has not been comprehensively assessed. It is interesting to note that ProS is not the only molecule shared between coagulation and inflammation regulatory networks [21]. Indeed, thrombin and activated Protein C can also regulate the inflammatory response [22, 23].
TAM signaling and apoptotic cell clearance
Significant progress in our understanding of the biological role of the TAM pathway was made possible with the generation of mice lacking Tyro3, Axl and Mer [24•, 25•], and both TAM ligands [26–29]. In agreement with the ability of TAM ligands to bind both to PtdSer exposed on the extracellular surface of apoptotic cells and to the TAM receptors expressed by phagocytes [8], a plethora of degenerative phenotypes that result from the inefficient phagocytosis of apoptotic cells and membranes have been described in TAM knock-out (KO) mice. TAM triple KO males, for example, were found to be sterile due to the inefficient removal of apoptotic germ cells (spermatogonia, spermatocytes, and spermatids) and apoptotic cell debris generated during spermatogenesis [24•, 30, 31]. Importantly, this phenotype is degenerative rather than developmental in nature, and is only revealed at ~5 weeks after birth, just after the onset of sexual maturity and active production of sperm. The degeneration phenotype is also cell non-autonomous with respect to the dying germ cells, as it appears to arise from the loss of TAM signaling in Sertoli cells. Sertoli cells, which express all three TAM receptors, phagocytose the ~108 germ cells that normally die by apoptosis during each new meiotic cycle of spermatogenesis. In the complete absence of TAM signaling, apoptotic cell corpses pile up and eventually poison the tubule epithelium.
A remarkably similar phenotype is observed in the retina of Mer KO mice. Adult Mer mutants are blind, due to the nearly complete loss of photoreceptors. Again, this phenotype is degenerative rather than developmental, as the retinae of Mer KO mice develop normally, and at two weeks after birth are indistinguishable histologically from wild-type. However, beginning around three weeks after birth, shortly after the onset of eye opening in the mouse, markers of apoptotic cells are detected specifically in the photoreceptor layer of the Mer mutants. Over the next several weeks, cell death progresses to an almost total degeneration of the photoreceptor (PR) layer by 10 weeks of age [18, 24•, 32]. As for germ cell degeneration in the testes, this photoreceptor death in the retina reflects loss of TAM signaling in a specialized phagocytic cell –the retinal pigmental epithelia (RPE) cell. RPE cells engulf and metabolize the distal ends of PR outer segments, which are the rhodopsin-containing organelles in which light is detected. In Mer KO mice, RPE cells fail to perform the daily phagocytosis of outer segments, which leads to the non-autonomous apoptotic death of all PRs in the retina [18, 32]. Mutations in the Mer gene have also been found to account for a rare form of inherited retinitis pigmentosa in humans [33–37], and for the PR death that occurs in the RCS rat, a long-standing animal model of hereditary retinal degeneration [38, 39].
It is interesting to note that the testis and the retina are shielded from the blood stream by blood-organ barriers, and thus rely on resident TAM-dependent phagocytes, Sertoli and RPE cells, to handle an extraordinary level of apoptotic cell and membrane turnover. For those apoptotic cells generated in organs and tissues that are not separated by a blood-organ barrier, macrophages are central players in their removal. Loss of Mer in macrophages has been shown to lead to impaired removal of apoptotic thymocytes induced by dexamethasone administration [40]. More recent studies have extended this finding to the other members of the TAM receptor family, and suggest that all of them contribute to the phagocytosis of apoptotic cells by DCs and macrophages in vitro, albeit to different degrees [41].
Linking TAM-dependent apoptotic cell clearance to TAM regulation of the inflammatory response
In addition to playing an essential role in the turnover of apoptotic cells and membranes in adult tissues, TAM signaling has been found to play a fundamental role in the regulation of the innate immune response. Camenisch and colleagues were the first to describe the excessive production of TNFα in response to LPS administration together with an associated increased sensitivity to LPS-induced endotoxic shock in Mer KO mice [25]. Consistent with this finding, the activation of murine antigen presenting cells (APCs) in vitro by TLR ligands was found to be potently inhibited by recombinant Gas6 and ProS [42••, 43]. The production of multiple cytokines, including type I IFNs, by mouse DCs was found to be suppressed in the presence of these TAM ligands, and the ability of Gas6 to inhibit DC activation has been recently extended to human DCs [44]. Biochemical analyses revealed that TAM receptors function as negative regulators of TLR3, TLR4 and TLR9 pathways in DCs. Intriguingly, these TLR pathways are known to trigger type I IFN responses, which further drive the maturation of APCs [45, 46]. A closer analysis of the molecular mechanism of TAM-mediated inhibition of TLR signaling revealed a physiological and physical association between TAMs and the type I IFN receptor (IFNAR). First, the TAM receptor Axl was found to be markedly upregulated upon treatment of DCs with type I IFNs [42••, 44, 47]. This finding suggests that engagement of the TAM immunoregulatory axis occurs as a normal consequence of the activation of the immune response [42••]. Second, activation of the TAM receptors was found to usurp IFNAR and lead to the induction of well-known inhibitors of TLR and Cytokine receptor pathways - the suppressor of cytokine signaling 1 (SOCS1) and SOCS3 [42••]. SOCS proteins have for many years been known to be induced by cytokine receptor activation and to account for the negative regulation of both cytokine receptor and TLR pathways [48–50]. Interestingly, the induction of SOCS proteins by IFNAR activation was found to proceed through and to be dependent on TAM receptors [42••]. Taken together, these findings revealed a central role of the TAM pathway as negative regulators of the inflammatory response.
Thus, the TAM pathway functions in two discrete phenomena: the phagocytosis of apoptotic corpses and the regulation of the innate immune response. It is likely that, at least in macrophages and DCs, these pathways are functionally linked. Indeed, evidence for such an integrative view of TAM signaling was provided by Tisch and colleagues. The addition of irradiated apoptotic thymocytes to DCs in culture was found to suppress LPS-induced NF-κB activation and production of TNF and IL-12 in a Mer dependent manner [51••, 52].
TAM signaling and autoimmunity
Perhaps not surprisingly, the delayed clearance of apoptotic cells and the loss of regulation of the inflammatory response are associated with the development of a lupus-like syndrome in TAM KO mice [53••, 54••]. TAM triple knock out (TKO) mice display profound lymphoproliferation and features of systemic autoimmunity [54••]. The peripheral lymphoid organs of these mice become grossly enlarged, due to the expansion of both myeloid and lymphoid cell populations [42••, 54••]. In addition, high titers of circulating auto-antibodies, including anti-chromatin, anti-double and single stranded DNA, and anti-phospholipid antibodies, can be detected in the sera of TAM KO mice [53••, 54••]. Again, this phenotype appears to be cell non-autonomous with respect to lymphocytes, in that T cells and resting B cells do not express TAMs.
Impaired clearance of apoptotic cells has been detected in the germinal centers (GC) of the lymph nodes of patients with SLE and has been proposed to be involved in the pathogenesis of this disease [1]. A specialized macrophage in the GC, known as the tingible body macrophage (TBM), engulfs low-affinity or self-reactive apoptotic B cells that are generated through negative selection [55]. This appears to be fundamental to preventing the activation of nearby auto-reactive B cells, and the consequent development of autoimmunity. This requirement is nicely illustrated by the phenotype that develops in mice that carry loss-of-function mutations for MFG-E8 - an opsonin that promotes clearance of apoptotic cells through interaction with integrins expressed on the surface of phagocytes. MFG-E8 KO mice display impaired removal of B cell corpses in lymph node GCs, and a lupus-like autoimmune disease [56]. These disease phenotypes are especially interesting with respect to TAM signaling, since an essential association of Mer with integrin-based engulfment systems has also been demonstrated. In RPE cells, for example, MFG-E8 stimulation of photoreceptor outer segment phagocytosis through αvβ5 integrins has been demonstrated to require a physical association and physiological integration with Mer [57]. At the same time, Mer has been found to be expressed in TBMs [58], and it will be interesting to assess whether loss of Mer in TBMs leads to the accumulation of apoptotic bodies in GCs and an increase susceptibility to lupus-like autoimmune disease.
More well-established in TAM KO mice is the hyperproliferation and hyperactivation of DCs and macrophages accounting from the loss of negative regulation of inflammation [42••, 54••]. Future studies employing cell-type specific KOs and genetic approaches that dissociate the phagocytosis from the anti-inflammatory effect will further illuminate the function of this receptor tyrosine kinase family in specific steps leading to autoimmunity. In this respect, a study by Birge and colleagues shows that the phagocytosis and anti-inflammatory effects mediated by Mer appear to rely on the phosphorylation of different tyrosine residues and may indeed be dissociable events [43].
Are defects in TAM signaling associated with autoimmunity in humans? It is particularly interesting that deficiencies on the TAM ligand ProS have been a fairly consistent observation in various autoimmune diseases. There is an anecdotal medical literature that has tied reduced levels of ProS to ulcerative colitis and other inflammatory bowel diseases. A far more substantial literature, however, points to a clear association between reduced ProS levels in the circulation and SLE [59–62••]. A recent analysis of a large cohort of SLE patients found that levels of free protein S – but not Gas6 - were significantly lower in SLE patients with a history of serositis, neurologic disorder, hematologic disorder and immunologic disorder [62••]. These results have been interpreted to suggest that reduced TAM signaling may contribute to the development of SLE.
Concluding Remarks and Future Directions
TAM signaling has been found to be a central player in the phagocytosis of the outer segments of PR and apoptotic germ cells. Yet, the specificity of the TAM pathway in the phagocytosis mediated by professional phagocytes, which are known to express a variety of phagocytic receptors, remains unknown. As proposed by Medzhitov [63], apoptotic cells may produce signals that are associated with the type of death that they have undergone, which might in turn determine the type of phagocytic receptors to be engaged together with the outcome of their removal. Thus, while phagocytosis of apoptotic cells generated by infection is expected to induce a host-defence response in macrophages, apoptotic cells that are generated during inflammatory or immune responses might be removed by pathways, such as the TAM, that are associated with anti-inflammatory or immunosuppressive responses.
The mechanisms that lead to the activation of the TAM anti-inflammatory pathway in vivo remain ill-defined. TAM signaling has been recently found to be an integral component of potent immunosuppressive pathways. Several broad-spectrum immunosuppressive drugs that are used to treat the chronic inflammation that is associated with autoimmune disease may function by regulating the TAM pathway. The well-known ability of immunosuppressive drugs such as glucocorticoids, liver X receptors (LXR) and PPARγ/δ agonists to stimulate macrophage phagocytosis of apoptotic cells has been tied to their ability to up-regulate Mer expression. Glucocorticoids, for example, have been shown to induce ProS-dependent phagocytosis of apoptotic cells by macrophages; and this glucocorticoid induction appears to be Mer-dependent [64]. Similarly, activation of LXRs in macrophages stimulates the phagocytosis of apoptotic thymocytes in a Mer-dependent fashion [65]. The integration of the TAM pathway with well-known immunosuppressive pathways notwithstanding, the comparative ability of each of the TAM ligands to trigger this anti-inflammatory pathway in vivo is currently unknown. The analysis of genetically modified mice lacking the expression of Gas6 and ProS is bound to yield insights into the in vivo specificity and cellular source of each ligand in TAM mediated regulation of the innate immune response.
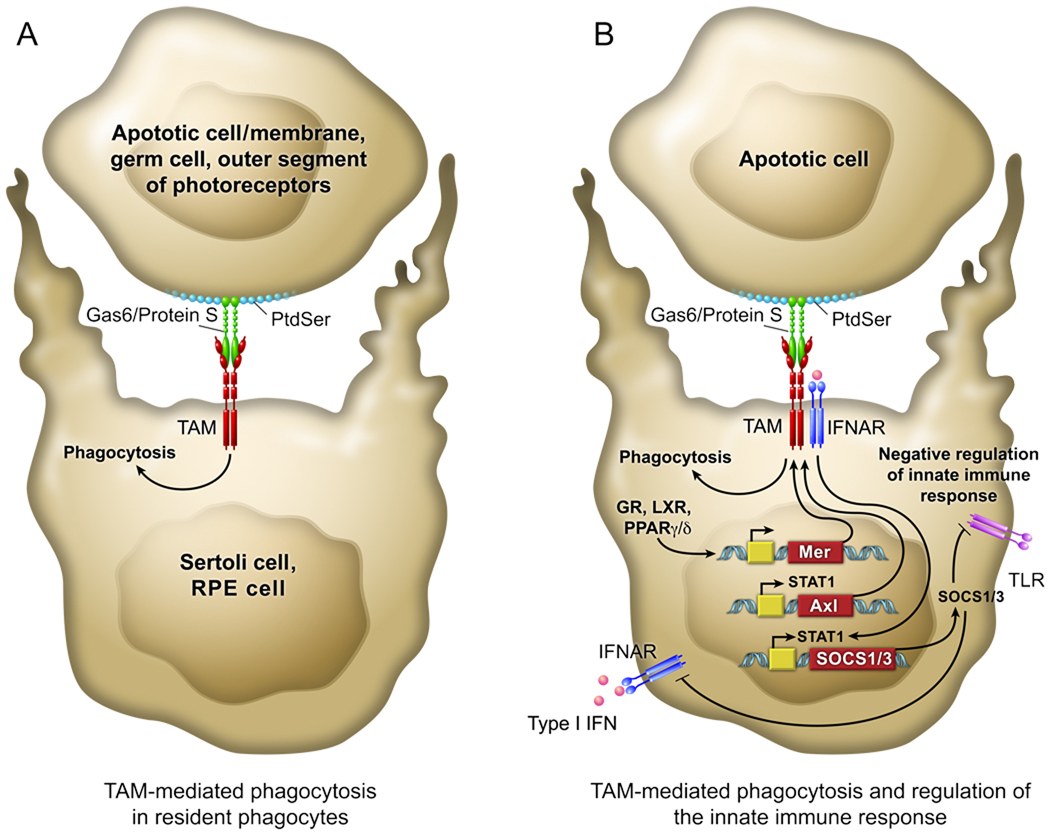
Figure 1. Integrative view of TAM signaling in both the phagocytic removal of apoptotic cells (A/left) and the regulation of the inflammatory response (B/right).
(A) Resident phagocytes, such as Sertoli cells of the testis, RPE cells of the eye, and tissue macrophages (bottom cell), use the TAM receptors (red) to recognize and engulf apoptotic cells and membranes (top cell). This is achieved through binding of the amino-terminal domains of the two TAM ligands – Gas6 and ProS (green) – to the phosphatidylserine (PtdSer; light blue) that is expressed on the surface of apoptotic cells. The carboxy-terminal domains of these ligands then bind to and activate the TAM receptors on the phagocyte. This triggers a signal transduction cascade that results in mobilization of the actin cytoskeleton, and the phagocytosis of the apoptotic cell. (B) This same scheme of engagement, when it occurs in the context of the joint expression of TAM and type I IFN receptors (IFNARs; dark blue) in macrophages and dendritic cells (DCs), also inhibits the inflammatory response of the innate immune system through induction of the genes encoding the cytokine suppressors SOCS1 and 3. In this case, apoptotic cells, whose binding to DCs is generally immunosuppressive, serve as ‘presentation platforms’ for Gas6 and/or ProS, which together with a type I IFN (pink), trigger a STAT1-dependent cascade that leads to SOCS1/3 expression. These SOCS proteins inhibit signaling downstream of both IFNARs and Toll-like receptors (TLRs, violet). Increasing evidence suggests that activation of nuclear receptors (e.g., GR, LXR, PPARγ/δ) - by glucocorticoids, oxysterols, and other immunosuppressive hormones - leads to the up-regulation of Mer, and that this Mer up-regulation is required for hormone stimulation of the phagocytosis of apoptotic cells by macrophages.
Acknowledgements
Work in the authors’ laboratories is supported by grants from the NIH (AI089824 to CVR and AI077058 to GL), the American Heart Association (0835404N to CVR), the Crohns and Colitis Foundation of America (2686 to CVR), DTRA (08-1-0009 to GL), and the Ipsen Foundation (to GL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
•• of outstanding interest
- 1.Gaipl US, et al. Clearance of apoptotic cells in human SLE. Curr Dir Autoimmun. 2006;9:173–187. doi: 10.1159/000090781. [DOI] [PubMed] [Google Scholar]
- 2.Ronnblom L, Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus. 2008;17(5):394–399. doi: 10.1177/0961203308090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140(5):619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 5.Santiago-Raber ML, et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197(6):777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6(5):691–704. doi: 10.1016/0896-6273(91)90167-x.. •This study was the first to identify the TAM receptors as a distinct receptor protein tyrosine kinase subfamily.
- 7.O'Bryan JP, et al. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11(10):5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8(5):327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stitt TN, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80(4):661–670. doi: 10.1016/0092-8674(95)90520-0.. • This paper reports the biochemical identification of two secreted proteins, Gas6 and ProS, as ligands for the TAM receptors.
- 10.Godowski PJ, et al. Reevaluation of the roles of protein S and Gas6 as ligands for the receptor tyrosine kinase Rse/Tyro 3. Cell. 1995;82(3):355–358. doi: 10.1016/0092-8674(95)90424-7. [DOI] [PubMed] [Google Scholar]
- 11.Mark MR, et al. Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. J Biol Chem. 1996;271(16):9785–9789. doi: 10.1074/jbc.271.16.9785. [DOI] [PubMed] [Google Scholar]
- 12.Varnum BC, et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995;373(6515):623–626. doi: 10.1038/373623a0. [DOI] [PubMed] [Google Scholar]
- 13.Nagata K, et al. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271(47):30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 14.Huang M, et al. Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat Struct Biol. 2003;10(9):751–756. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- 15.Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148(7):2207–2216. [PubMed] [Google Scholar]
- 16.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7(12):964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 17.Lemke G, Burstyn-Cohen T. TAM receptors and the clearance of apoptotic cells. Proc. N. Y. Acad. Sci. 2010 doi: 10.1111/j.1749-6632.2010.05744.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad D, et al. TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci. 2006;33(1):96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Rezende SM, Simmonds RE, Lane DA. Coagulation, inflammation, and apoptosis: different roles for protein S and the protein S-C4b binding protein complex. Blood. 2004;103(4):1192–1201. doi: 10.1182/blood-2003-05-1551. [DOI] [PubMed] [Google Scholar]
- 20.Walker FJ. Regulation of vitamin K-dependent protein S. Inactivation by thrombin. J Biol Chem. 1984;259(16):10335–10339. [PubMed] [Google Scholar]
- 21.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131(4):417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 22.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006;32 Suppl 1:49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 23.Niessen F, et al. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452(7187):654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 24. Lu Q, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398(6729):723–728. doi: 10.1038/19554.. •This is the first study to report a complete set of (single, double, and triple) loss-of-function mutants in all three mouse TAM genes, and the resulting degeneration of germ cells in the testis that occurs in the absence of TAM signaling.
- 25. Camenisch TD, et al. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162(6):3498–3503.. •This is the first study to report an increased response to LPS in the absence of TAM signaling in vivo. Employing an LPS induced endotoxic shock, the authors show that Mer deficient mice are more susceptible to succumb to LPS injection and that is largely due to the overt production of TNFα
- 26.Burstyn-Cohen T, Heeb MJ, Lemke G. Lack of Protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J Clin Invest. 2009 doi: 10.1172/JCI39325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angelillo-Scherrer A, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7(2):215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 28.Saller F, et al. Generation and phenotypic analysis of protein S-deficient mice. Blood. 2009;114(11):2307–2314. doi: 10.1182/blood-2009-03-209031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanagita M, et al. Essential role of Gas6 for glomerular injury in nephrotoxic nephritis. J Clin Invest. 2002;110(2):239–246. doi: 10.1172/JCI14861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong W, et al. Gas6 and the Tyro 3 receptor tyrosine kinase subfamily regulate the phagocytic function of Sertoli cells. Reproduction. 2008;135(1):77–87. doi: 10.1530/REP-07-0287. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, et al. Functions of TAM RTKs in regulating spermatogenesis and male fertility in mice. Reproduction. 2009;138(4):655–666. doi: 10.1530/REP-09-0101. [DOI] [PubMed] [Google Scholar]
- 32.Duncan JL, et al. An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest Ophthalmol Vis Sci. 2003;44(2):826–838. doi: 10.1167/iovs.02-0438. [DOI] [PubMed] [Google Scholar]
- 33.Gal A, et al. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26(3):270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 34.Shahzadi A, et al. Nonsense mutation in MERTK causes autosomal recessive retinitis pigmentosa in a consanguineous Pakistani family. Br J Ophthalmol. 2010 doi: 10.1136/bjo.2009.171892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackay DS, et al. Novel mutations in MERTK associated with childhood onset rod-cone dystrophy. Mol Vis. 2010;16:369–377. [PMC free article] [PubMed] [Google Scholar]
- 36.Charbel Issa P, et al. Characterisation of severe rod-cone dystrophy in a consanguineous family with a splice site mutation in the MERTK gene. Br J Ophthalmol. 2009;93(7):920–925. doi: 10.1136/bjo.2008.147397. [DOI] [PubMed] [Google Scholar]
- 37.Brea-Fernandez AJ, et al. Novel splice donor site mutation in MERTK gene associated with retinitis pigmentosa. Br J Ophthalmol. 2008;92(10):1419–1423. doi: 10.1136/bjo.2008.139204. [DOI] [PubMed] [Google Scholar]
- 38.D'Cruz PM, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9(4):645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 39.Nandrot E, et al. Homozygous deletion in the coding sequence of the c-mer gene in RCS rats unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol Dis. 2000;7(6 Pt B):586–599. doi: 10.1006/nbdi.2000.0328. [DOI] [PubMed] [Google Scholar]
- 40.Scott RS, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411(6834):207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 41.Seitz HM, et al. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178(9):5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 42. Rothlin CV, et al. TAM Receptors Are Pleiotropic Inhibitors of the Innate Immune Response. Cell. 2007;131(6):1124–1136. doi: 10.1016/j.cell.2007.10.034.. ••This study reveals the molecular mechanism by which the TAM receptors negatively regulate TLR and cytokine receptor signaling. Activation of the TAM receptors in conjunction with the Type I interferon receptor is reported to lead to the induction of the suppression of cytokine and TLR receptor signaling, SOCS1 and SOCS3.
- 43.Tibrewal N, et al. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-kappaB transcriptional activation. J Biol Chem. 2008;283(6):3618–3627. doi: 10.1074/jbc.M706906200. [DOI] [PubMed] [Google Scholar]
- 44.Scutera S, et al. Survival and migration of human dendritic cells are regulated by an IFN-alpha-inducible Axl/Gas6 pathway. J Immunol. 2009;183(5):3004–3013. doi: 10.4049/jimmunol.0804384. [DOI] [PubMed] [Google Scholar]
- 45.Asselin-Paturel C, et al. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201(7):1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gautier G, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201(9):1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharif MN, et al. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med. 2006;203(8):1891–1901. doi: 10.1084/jem.20051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wormald S, Hilton DJ. The negative regulatory roles of suppressor of cytokine signaling proteins in myeloid signaling pathways. Curr Opin Hematol. 2007;14(1):9–15. doi: 10.1097/00062752-200701000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimura A, et al. Negative regulation of cytokine signaling and immune responses by SOCS proteins. Arthritis Res Ther. 2005;7(3):100–110. doi: 10.1186/ar1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mansell A, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7(2):148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 51. Sen P, et al. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood. 2007;109(2):653–660. doi: 10.1182/blood-2006-04-017368.. ••This is the first study to report a molecular integration between TAM mediated phagocytosis of apoptotic cells and anti-inflammatory response. The authors show that the removal of apoptotic thymocytes and the associated inhibition of TLR responses in dendritic cells are dependent on the TAM receptor Mer.
- 52.Wallet MA, et al. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205(1):219–232. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen PL, et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196(1):135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293(5528):306–311. doi: 10.1126/science.1061663.. ••These studies (53 and 54) demonstrate that loss of TAM signaling in mice leads to severe lymphoproliferation and an autoimmunity that resembles a lupus-like disease
- 55.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9(12):845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 56.Hanayama R, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304(5674):1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 57.Finnemann SC, Nandrot EF. MerTK activation during RPE phagocytosis in vivo requires alphaVbeta5 integrin. Adv Exp Med Biol. 2006;572:499–503. doi: 10.1007/0-387-32442-9_69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shao WH, et al. The Mer receptor tyrosine kinase is expressed on discrete macrophage subpopulations and mainly uses Gas6 as its ligand for uptake of apoptotic cells. Clin Immunol. 2009;133(1):138–144. doi: 10.1016/j.clim.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meesters EW, et al. The inflammation and coagulation cross-talk in patients with systemic lupus erythematosus. Blood Coagul Fibrinolysis. 2007;18(1):21–28. doi: 10.1097/01.mbc.0000256022.01900.c2. [DOI] [PubMed] [Google Scholar]
- 60.Brouwer JL, et al. The contribution of inherited and acquired thrombophilic defects, alone or combined with antiphospholipid antibodies, to venous and arterial thromboembolism in patients with systemic lupus erythematosus. Blood. 2004;104(1):143–148. doi: 10.1182/blood-2003-11-4085. [DOI] [PubMed] [Google Scholar]
- 61.Song KS, Park YS, Kim HK. Prevalence of anti-protein S antibodies in patients with systemic lupus erythematosus. Arthritis Rheum. 2000;43(3):557–560. doi: 10.1002/1529-0131(200003)43:3<557::AID-ANR11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 62. Suh CH, et al. TAM receptor ligands in lupus: Protein S but not Gas6 levels reflect disease activity in systemic lupus erythematosus. Arthritis Res Ther. 2010;12(4):R146. doi: 10.1186/ar3088.. ••This is the first study to report an association between a component of the TAM pathway and disease activity in a large cohort of patients with SLE. The levels of free ProS, but not Gas6, were found to be significantly lower in a subset of SLE patients with clinical manisfestations of serositis, neurologic, hematologic and immunologic disorders.
- 63.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 64.McColl A, et al. Glucocorticoids induce protein S-dependent phagocytosis of apoptotic neutrophils by human macrophages. J Immunol. 2009;183(3):2167–2175. doi: 10.4049/jimmunol.0803503. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez NA, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31(2):245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]