Abstract
Background
Attention deficit/hyperactivity disorder (ADHD) is a major public health concern. It has been suggested that the brain’s default network may provide a crucial avenue for understanding the neurobiology of ADHD. Evaluations of the default network have increased over recent years with the applied technique of resting-state functional connectivity MRI (rs-fcMRI). These investigations have established that spontaneous activity in this network is highly correlated at rest in young adult populations. This coherence seems to be reduced in adults with ADHD. This is an intriguing finding, as coherence in spontaneous activity within the default network strengthens with age. Thus, the pathophysiology of ADHD might include delayed or disrupted maturation of the default network. If so, it is important to determine whether an altered developmental picture can be detected using rs-fcMRI in children with ADHD.
Methods
The present study utilized the typical developmental context provided previously by Fair et al (1) to examine coherence of brain activity within the default network using rs-fcMRI in children with (n=23) and without ADHD (n=23).
Results
We found that functional connections previously shown as developmentally dynamic in the default network were atypical in children with ADHD - consistent with perturbation or failure of the maturational processes.
Conclusions
These findings are consistent with the hypothesis that atypical consolidation of this network over development plays a role in ADHD.
Keywords: ADHD, Default, Connectivity, Development, fMRI, resting state
Introduction
Attention deficit/hyperactivity disorder (ADHD) is a major public health concern with long-term negative impact on health and education. Moreover, ADHD may serve as a gateway condition for other serious psychiatric problems including conduct disorder (2), depression (3), personality disorders (4), and substance use disorders (5). Existing treatments suppress symptoms but are not curative (6). Thus, a better understanding of ADHD’s neurobiology is essential.
It has recently been suggested that the integrity of the brain’s default network (7- 9) may provide a crucial avenue for understanding the neurobiology of ADHD. The default network refers to a set of regions (Table 2) originally defined by Shulman and colleagues (7). This set of regions is unique in that it consistently decreases in neural activity from a physiologic baseline during most goal-directed tasks (7). With its broad implications for brain functioning, this network has recently become a key focus of neuroscientific and psychiatric research (10).
Table 2.
Seed regions for default network
| Regions | Abbreviations | Coordinates (x,y,z) |
|---|---|---|
| Medial prefrontal cortex (ventral) | mPFC (ventral) | 3, 39, 2 |
| Medial prefrontal cortex (anterior) | mPFC(anterior) | 1, 54, 21 |
| Posterior cingulate cortex | post. cingulate | 2, 36, 37 |
| Left lateral parietal cortex | L lat. parietal | −47, −67, 36 |
| Right lateral parietal cortex | R lat. parietal | 53, −67, 36 |
| Left superior frontal cortex | L sup. frontal | −14, 38, 52 |
| Right superior frontal cortex | R sup. frontal | 17, 37, 52 |
| Left inferior temporal cortex | L inf.temporal | −61, 33, 15 |
| Right inferior temporal cortex | R inf.temporal | 65, 17, 15 |
| Left parahippocampal gyrus | L parahippocampus | −22, 26, 16 |
| Right parahippocamal gyrus | R parahippocampus | 25, 26, 14 |
| Retrosplenial | retrosplenial | 3, 51, 8 |
The importance of the default network to typical brain function remains under discussion (8, 11-16). Recent reports suggest that the functions of the default network are related indirectly (and perhaps directly, see (17, 18)) to executive behavior (19, 20), as well as reward processing (21). Because leading neurocognitive models of ADHD have emphasized executive control as well as reward-related dysfunction (22), the default network has been highlighted as an important investigative target for the pathophysiology of ADHD (9, 23-25).
Evaluations of the default network have been dramatically enabled by the relatively new technique of resting-state functional connectivity MRI (rs-fcMRI). rs-fcMRI measures spontaneous intrinsic correlated blood oxygen level dependent (BOLD) activity while subjects are at rest (i.e., not performing an explicit task). It is based on the discovery that spontaneous low-frequency BOLD signal fluctuations in sometimes-distant brain regions show strong correlations at rest (26).
Resting-state fcMRI analyses have now established that activity in regions of the default network is highly correlated at rest in adulthood (1, 27-30). Correspondingly, rs-fcMRI studies in adults with ADHD (24, 25) suggest reduced coherence in the default network. This finding is intriguing in light of recurrent results indicating that the coherence of the default network strengthens with development (1, 30). For example, Fair et al (1) found reduced coherence in the default network in children compared to adults. A follow-up study suggested that coherence within the default network in children was not only reduce between members, but also that these components were more tightly linked to other brain regions closer in anatomic space (30). Over age, however, these local connections weakened, and gave way to increased integration of the default network members. Considering the reduced coherence in the default network in a prior report on adults with ADHD as well as the increased integration within the default network observed over maturation, it was hypothesized that ADHD may be related to delayed or disrupted brain maturation in the default network. If an abnormality in the consolidation of this network over development is central to the pathophysiology of ADHD, it should be apparent prior to adulthood. Therefore, it is crucial to determine whether an altered developmental picture can be detected using rs-fcMRI in children with ADHD. To that end, the present study utilized the typical developmental context provided by Fair et al (30) as a framework to examine coherence of brain activity within the default network in children with and without ADHD.
Methods
Participants and Measures
Youth (7-16 years) were recruited with a combination of advertisements, mailings, and clinic outreach. Informed written consent or assent was obtained from parents and children, respectively; all procedures complied with the University Human Investigation Review Board. A total of 23 ADHD participants and 23 age-matched healthy control participants were included. Data for this manuscript were collected as a large collaborative effort at OHSU, and as such slightly different assessment protocols were used for adolescents and children.
For youth aged 10-16, psychiatric diagnoses were evaluated with youth and parent versions of the Diagnostic Interview Schedule for Children (DISC-IV; (31)) and a parent Conners Rating Scale-Revised (32). A clinical review by a child psychiatrist was then used to determine final diagnosis. IQ was estimated with the Wechsler Abbreviated Scale of Intelligence (WASI) (33) (Non-ADHD control youth were given the 2-subtest WASI short-form, i.e., Vocabulary and Matrix Reasoning subtests).
For youth 7- 9 years old, psychiatric diagnoses were evaluated with the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-I; (34)) administered to a parent; a parent and teacher Conners Rating Scale-3rd Edition (35); and a clinical review by a child psychiatrist and neuropsychologist who had to agree on the diagnosis. Intelligence was evaluated with three-subtest short form (Block Design, Vocabulary, and Information) of the Wechsler Intelligence Scale for Children, Fourth Edition ((36)).
Children were excluded if they did not meet criteria for ADHD or non-ADHD groups (i.e. children deemed sub-threshold by the clinicians were excluded). Children were also excluded if a history of neurological illness, chronic medical problems, sensori-motor handicap, autistic disorder, mental retardation, or significant head trauma (with loss of consciousness) was identified by parent report, or if they had evidence of psychotic disorder or bipolar disorder on the structured parent psychiatric interview. Children currently prescribed non-stimulant psychotropic medications (including atomoxetine) were excluded. Children prescribed short-acting stimulant medications were scanned after a minimum washout of five half-lives (i.e., 24-48 hours depending on the preparation). Control children were excluded for presence of conduct disorder, major depressive disorder, or history of psychotic disorder, as well as for presence of ADHD.
Data acquisition and processing
Participants were scanned using a 3.0 Tesla Siemens Magnetom Tim Trio scanner with a twelve-channel head-coil at the OHSU Advanced Imaging Research Center. One high resolution T1-weighted MPRAGE sequence (orientation=sagittal, TE=3.58 ms, TR=2300ms, 256×256 matrix, resolution=13mm, total scan time=9 min 14sec) was collected. Blood-oxygen level dependent (BOLD)-weighted functional imaging data were collected in an oblique plane (parallel to the ACPC) using T2*-weighted echo-planar imaging (TR=2000ms, TE=30ms, flip angle=90°, FOV=240mm, 36 slices covering the whole brain, slice thickness = 3.8mm, in-plane resolution=3.8 × 3.8mm). Steady state magnetization was assumed after 5 frames (~10 s). During rest periods subjects were instructed to stay still, and fixate on a standard fixation-cross in the center of the display. For children aged 10-16 two 5-minute runs were obtained. For children aged 7-9 years, 3 runs of 3.5 minutes each were obtained. Three ADHD and one control subject had 1 run removed due to excess movement (see methods). All children (n=46) had between 420 and 630 seconds of resting state data available for analysis.
Functional images were processed to reduce artifacts (37). These steps included: (i) removal of a central spike caused by MR signal offset, (ii) correction of odd vs. even slice intensity differences attributable to interleaved acquisition without gaps, (iii) correction for head movement within and across runs, and (iv) within-run intensity normalization to a whole brain mode value of 1,000. Atlas transformation of the functional data was computed for each individual via the MPRAGE scan. Each run then was resampled in atlas space (38) on an isotropic 3mm grid, combining movement correction and atlas transformation in one interpolation (39). All subsequent operations were performed on the atlas-transformed volumetric time series.
Connectivity preprocessing followed prior methods (1, 28, 30, 40, 41). These steps included: (i) a temporal band-pass filter (0.009 Hz < f <0.08 Hz) and spatial smoothing (6 mm full width at half maximum), (ii) regression of six parameters obtained by rigid body head motion correction, (iii) regression of the whole brain signal averaged over the whole brain, (iv) regression of ventricular signal averaged from ventricular region of interest (ROI), and (v) regression of white matter signal averaged from white matter ROI. Regression of first order derivative terms for the whole brain, ventricular, and white matter signals were also included in the correlation preprocessing. These preprocessing steps likely reduced spurious variance unlikely to reflect neuronal activity (42).
As noted above, motion was corrected and quantified using an analysis of head position based on rigid body translation and rotation. The data derived from these adjustments needed to realign head movement on a frame-by-frame basis were calculated as root mean square (RMS) values for translation and rotation in the x, y, and z planes in millimeters. Total RMS values were calculated on a run-by-run basis for each participant. Participant’s BOLD runs with movement exceeding 2mm RMS were removed. Movement was relatively low in both groups (see Table 1), and not significantly different (see Table 1).
Table 1.
| Variable | Control group | ADHD group | p | ||
|---|---|---|---|---|---|
| Mean | Std.Dev. | Mean | Std.Dev. | ||
| Age | 10.04 | 2.58 | 10.57 | 2.86 | 0.47 |
| FullscaleIQ | 116.18 | 9.77 | 106.83 | 13.00 | 0.01 |
| Movement | 0.38 | 0.27 | 0.50 | 0.43 | 0.29 |
| Scantime | 588.17 | 101.33 | 569.74 | 110.25 | 0.56 |
| ConnersParent | |||||
| Cognitiveproblems/inattention | 46.14 | 7.01 | 71.65 | 6.44 | 0.00 |
| Hyperactivity | 44.75 | 5.10 | 68.18 | 14.34 | 0.00 |
| PDS | 2.85 | 0.71 | 2.83 | 0.62 | 0.94 |
|
| |||||
| % | N | % | N | ||
|
| |||||
| Gender | |||||
| Male | 52.17 | 12 | 69.57 | 16 | |
| Female | 47.83 | 11 | 30.43 | 7 | |
| ADHDsubtype | |||||
| Combined | - | - | 91.30 | 21 | |
| Inattentive | - | - | 8.70 | 2 | |
PDS:PubertalDevelopmentalScale
Computation of group mean correlation matrices for analysis
For graph theoretical analyses, twelve a priori ROIs (12mm diameter spheres) were selected based on their involvement in the default network identified in a previous study (28)(Note: Fair et al (1, 30) used the same regions identified in Fox et al (28)). One previously identified cerebellar region was not included, due to incomplete cerebellar coverage across all subjects. The resting-state BOLD time series were correlated region by region for each participant across the full length of the resting time series, creating 46 square correlation matrices (12 × 12) (1, 30, 41, 43). For each group, the correlation coefficients (r) were combined across subjects by using the Schmidt–Hunter method for meta-analyses of r-values (41, 43, 44). Only positive correlations were examined.
Direct comparison between youth with ADHD and control youth
We performed between group two-sample two-tailed t tests (assuming unequal variance; P < 0.05) on all potential connections represented in the 12 × 12 correlation matrices. Fisher z transformation was applied. To account for multiple comparisons, the Benjamini and Hochberg False Discovery Rate (45) was applied. However, connections depicted in Figure 2 are uncorrected, as no connections for this particular analysis passed this correction. Connections that were significantly different between groups, but r < 0.1 in both groups, were not displayed. Connection density (K) is defined as the number of present connections for a given threshold divided by the total number of connections possible, and was implemented using a toolbox generously provided by Sporns, Rubinov, Kotter, and Hagmann (46). Connection weights (i.e. strength of correlation) were ignored for connection density analysis but were otherwise examined. Bootstrap verification of connection density was implemented in Matlab 7.2 and was based on methods highlighted in Wilke et al (47) among others (48, 49). One hundred bootstrap replicates were created by randomly sampling from the pool of 23 individual subject correlation matrices with replacement for each group (see (48, 49). The bootstrap replicates, which consisted of 23 correlation matrices per group, were converted to a single mean correlation matrix. This procedure increases the stability of the connection density index by limiting the potential effects of outlier subjects (47).
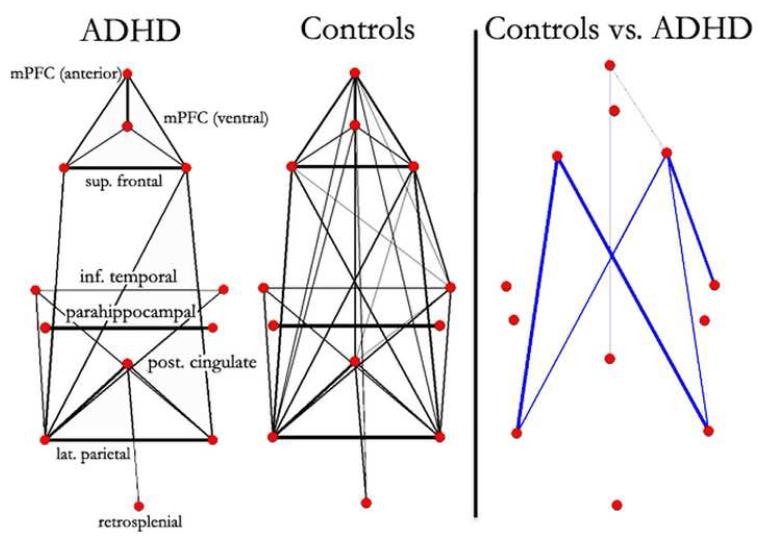
Fig. 2.
Graph visualization of group correlation matrices represented in a pseudo-anatomical organization. Default regions (28) are weakly connected in youth with ADHD. In age matched control youth, the default regions appear much consolidated. Only connections with r > 0.25 are shown on the left (line width is proportional to the connection strength). Statistically significant differences in functional connectivity between ADHD youth and controls are highlighted on the right. Blue lines represent significantly greater functional connectivity (r) in typically developing youth. For the direct comparison, line width is proportional to the significance level (i.e., increased level of statistical significance; Note: connections for this particular analysis (as opposed to Figure 1) did not pass multiple comparisons corrections, with all lines meeting p < 0.05 uncorrected. Thus, the subsequent analyses in Figures 3&4 help confirm these findings).
For the voxelwise (whole brain) maps, a correction based on Monte Carlo simulation was implemented (50). To achieve p < 0.05 corrected for voxel clusters, a threshold of 53 contiguous voxels with a z value of > 2.25 was used. We also compared groups with ANOVA. This additional analysis allowed us to examine the differences in sample means between the two groups, while examining the effects of gender and also IQ.
Atypical patterns of development were also assessed on 14 predefined connections of interest (COIs). These COIs were obtained from a previous study examining the development of rs-fcMRI within the default network (1). Specific connections that significantly changed over age were used. The sign test (51) was applied across all of the connections, considering a “success” when a connection that was previously found stronger in adults (see (1)) was stronger in controls, or when a connection that was previously found stronger in children than adults (1) was stronger in ADHD than non-ADHD youth.
RESULTS
Sample overview
Participant characteristics are summarized in Table 1 (also see Supplement: Table S1). Intellectual functioning was in the normal range for both groups, but as is commonly found, the ADHD group had a lower estimated full scale IQ than the control group. Parent Conners scores were in the clinical range for the ADHD group, but in the normal range for the typically developing group. Symptom scores did not vary with age across groups (Hyperactivity; r = 0.13, p = 0.39, Inattention; r = 0.01, p = 0.94) and pubertal status measured via Pubertal Development Sale (52) was equivalent between groups (see Table 1 and Supplement: Table S1).
Primary test of hypothesized default network effect on ADHD
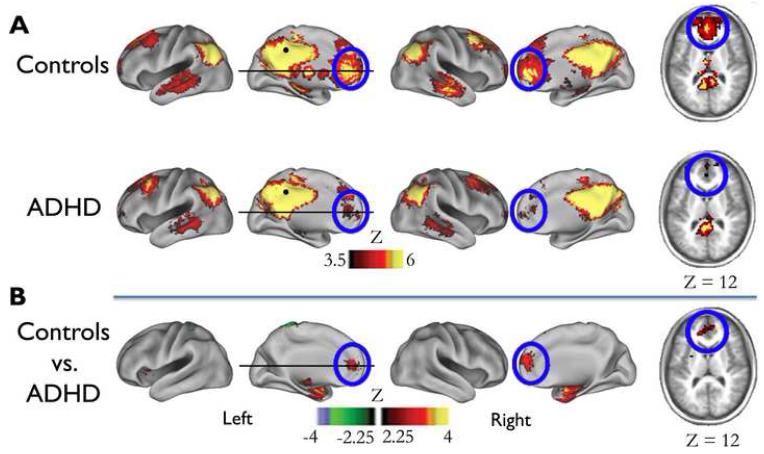
Voxelwise functional connectivity mapping and graph theoretical analysis strategies were employed to examine differences in resting-state functional connectivity between children with and without ADHD. The voxelwise maps resulted from the cross-correlation of the BOLD signal time series for a core default network region of interest (posterior cingulate cortex - pCC: −2,−36, 37; defined by (28) – see also (24, 25)). This method produced three statistical maps: ADHD, typically developing, and direct statistical comparison between groups. The results of the voxelwise approach are displayed in Figure 1. In the control group, a significant functional connection was observed between pCC and frontal default regions (e.g., medial prefrontal cortex - mPFC). In contrast, in ADHD, the pCC was weakly correlated to mPFC regions. This between-group difference in connection strength was confirmed in the direct comparison (Figure 1).
Fig. 1.
Voxel-wise resting state functional connectivity maps for a seed region (filled black circle) in pCC ( −2, −36, 37). A) Qualitatively, the rs-fcMRI map for the PCC seed region (black circle) reveals a commonly observed connectivity pattern of the default network (27-29). The connectivity map in ADHD population deviates from the age-matched control group. Functional connections with regions in the medial prefrontal cortex (highlighted with blue open circles) are weaker in ADHD compared to controls. B) These qualitative differences between youth with and without ADHD are confirmed by the direct comparison between the groups (random effects, corrected for multiple comparisons).
The second analysis examined connectivity between all nodes of the default network using graph theory (46). For the graph analyses, the resting-state BOLD time series for each of the predefined 12 default regions, in each participant, was correlated with the resting-state BOLD time series of every other default region (1, 28). Composite matrices for each of the two clinical groups are presented as graphs in Figure 2. Regions with correlations greater than r > .25 (chosen, in part, secondary to subsequent analyses below) were considered connected. The qualitative side-by-side visual comparison (Fig 2) revealed what appeared to be stronger integration of the default network in the control youth. The direct between-group comparisons in Figure 2 (p < 0.05; uncorrected) revealed that the default network is more integrated in the control sample.
Recall that, although the results of the voxel-wise analysis displayed in Figure 1 passed multiple comparisons correction, the results from Figure 2 did not. Therefore, additional analyses were conducted (below) to further examine the robustness of the findings.
Confirming that the Group Effect is not due to confounders, outliers, or thresholding
To control for group differences in gender, we repeated the group comparisons with ANOVA using gender as an additional factor. This gender analyses revealed no connection differences between boys and girls (all p > .05). In addition, none of the connections identified as significantly different in Figure 2 showed a Gender x ADHD status interaction effect. The same analysis was applied using IQ as a factor. As with gender the IQ × ADHD ANOVA revealed that no connections identified in Figure 2 interacted with IQ, suggesting that the current findings were not influenced by IQ discrepancies between groups.
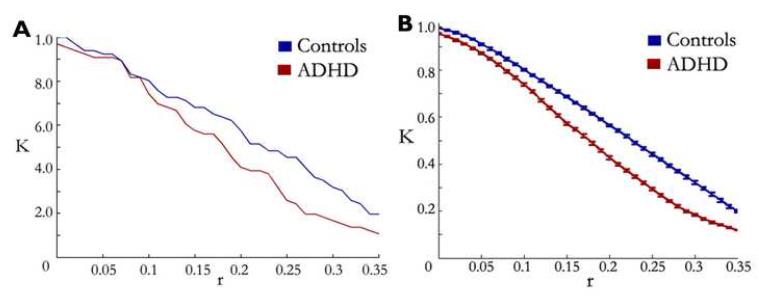
The preceding graph analytic analysis relies on thresholding of r-values. Generally, thresholding is a necessary step in the derivation of graphs. The choice of threshold is therefore a critical decision point in the analytical process. For example, a choice of r approaching 1.0 will generate very sparse graphs, whereas a choice of r close to 0.0 will generate densely connected graphs. To evaluate whether our results were dependent on a particular threshold, we calculated connection density (K) as a function of threshold for each of the two groups. That result, shown in Figure 3A, indicated that the groups differed irrespective of threshold chosen. This confirms that weaker connectivity in ADHD youth within the default network is not due to threshold selection.
Fig. 3.
Connection density is decreased in ADHD youth compared to age-matched controls independent of threshold. Connection density (K) represents the number of connections surpassing a given threshold over the total number of connections possible. A) Connection density on average matrices for ADHD youth (red line) and age-matched control youth (blue) line. Despite the threshold, connection density is consistently higher in the control population. B) Connection density of bootstrapping samples across thresholds. As in (A), connection density is consistently higher in the control sample versus ADHD youth, suggesting that results in A are not due to outlier subjects (standard errors are also plotted for the bootstrapping samples).
To evaluate the potential influence of outliers, we applied a bootstrapping procedure to the data (see methods). We created 100 random data sets by drawing from the subject pool (with replacement) as explained in Methods, thus reducing effects of potential outliers (47). Results of the bootstrapping procedure (Figure 3B) confirmed the ADHD versus non-ADHD difference, and highlight the main result in Figure 3A as robust to outliers.
Evaluation of developmentally significant connections of interest
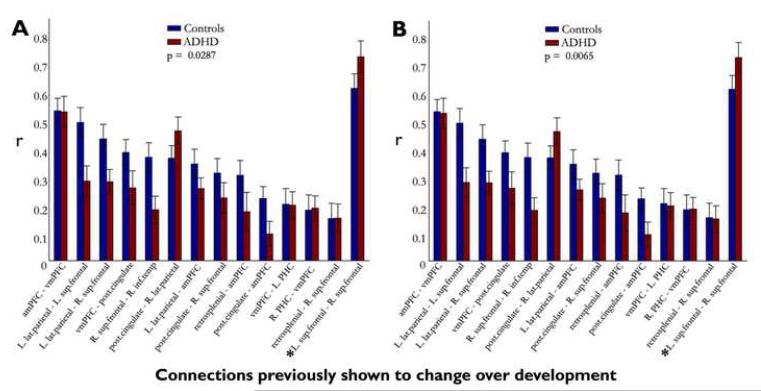
To further examine the potential match of the data pattern to what would be predicted based on atypical developmental patterns within the default network in ADHD, we examined 14 a priori connections of interest (COI) that were identified as being developmentally dynamic in an unrelated sample (1). If ADHD is characterized by failure of circuits to integrate and segregate normally, then connections that get stronger over age should be weaker in the ADHD than control group (failure to integrate), whereas connections that get weaker over age should be stronger in the ADHD than control group (failure to segregate). Figure 4A displays the correlation values in ADHD and non-ADHD groups for these 14 COIs. Eleven of 14 connections had patterns in the hypothesized direction. That is, 10 of the 13 connections that typically increase in strength over age are indeed stronger in the control group, and the one connection identified as ‘growing down’ over age (1) is stronger in the ADHD group. To quantitatively assess this overall pattern, the sign test (51) was applied to examine the hypothesis that connection differences between groups would be in a specific direction (i.e. non-random). The atypical pattern of default network connectivity was indeed non-random (p=0.0287). To confirm that outliers did not influence this result, we reanalyzed the sign test after applying the previously mentioned bootstrapping procedure. The bootstrap analysis confirmed the result (see Figure 4B) (Sign Test: p = 0.0065).
Fig. 4.
Connection strength is consistently ‘delayed’ in connections of interest identified as being developmentally dynamic in a previous study (1). Correlation coefficients for fourteen connections of interest identified from a previous study shown to significantly mature over development are shown her for both ADHD youth and age-matched controls. (A) Eleven of 14 connections are consistent with a potential ‘delayed’ developmental dynamic. Ten of 13 connections, shown to ‘grow up’ with age, were weaker in the ADHD group compared to controls, and the one connection previously shown to ‘grow down’ with age (L. sup frontal – R. sup frontal, highlighted with ‘*’) was stronger in the ADHD group compared to controls. The hypothesized pattern was significant, measured via the sign test (p = 0.0287). (B) After applying the bootstrapping procedure as in Fig. 3, similar patterns emerged. Eleven of 13 connections previously shown to strengthen with age were decreased in the ADHD group, and the connection previously shown to weaken with age was stronger in the ADHD group (Sign test: p = 0.0065). Standard errors are also plotted for both A and B.
DISCUSSSION
We found that correlated spontaneous activity within the default network is reduced in children with ADHD. We further showed that specific functional connections, previously shown as developmentally dynamic (1), were also atypical in children with ADHD. These findings are consistent with perturbation in the typical developmental trajectory of circuit consolidation, and suggest that the consolidation of this network over development may play a central role to the pathophysiology of ADHD.
We note that while our ADHD and control groups were well matched for age and pubertal status, it will be of interest in future reports to relate our findings to pubertal changes across development. We also note that findings in Figure 2 of this report were illustrative and did not survive correction; however, the supporting analyses illustrated in the remaining figures provides strong evidence of atypical connectivity in the default network in ADHD youth. Last, we should point out that 6 of our 23 ADHD participants had co-morbid ODD. While it is unlikely that this small sample was driving the effects observed in this report, it will be important in future work to identify the atypical pathophysiology that accompanies this, and other, comorbid conditions of ADHD. Nonetheless, assuming that the atypical default network connectivity described here does, indeed, contribute to the pathophysiology of ADHD, the question then becomes: What is the neurobiological underpinnings of this atypical connectivity, and how does it relate to the behavioral manifestations of the disease?
Atypical connectivity and its relation to the neural substrate
One potential contributor to altered functional connectivity is altered myelination within the default network. rs-fcMRI is related to the level of myelination connecting brain regions (53, 54). Thus, continued maturation of myelin through young adulthood (55) may contribute to changes in functional connectivity over typical development (1, 30, 41). It is plausible that some of our findings regarding childhood ADHD may reflect reduced myelination along fibers running between regions of the default network. Work in ADHD using diffusion tensor imaging, showing atypical white matter microstructure, lends credence to this view (56).
On the other hand, whereas aberrant myelination may account for some of the observed differences in rs-fcMRI between ADHD and healthy youth, the connectivity signal measured via rs-fcMRI is not purely a representation of monosynaptic anatomical connectivity (53, 57). Thus, other explanations must be considered. Changes in network dynamics can, and likely do, occur in the absence of changes in the underlying neural substrate or myelination. In fact, Fair et al (30) found decreases in connectivity over development between brain areas thought to increase in myelination. Such data suggest that there is likely a complex interaction between the underlying neural substrate and Hebbian interactions of large-scale brain networks that account for some atypical resting connectivity measures (see (30) for further discussion).
Atypical connectivity in ADHD may relate to delayed or disrupted maturation
Fair et al (1) reported that correlated spontaneous intrinsic activity within the default network strengthens with maturity (1, 30, 58)). The present findings, which show reduced connectivity within this network in ADHD compared to age matched controls is, in part, consistent with, and may renew assertions that ADHD is a disorder of delayed neural development (59-62). Indeed, while numbers vary and largely depend on diagnostic criteria, many children with ADHD appear to improve with age (63). Even so, because of the sparsity of data across the wide age range used here, we do not know whether the pattern seen here will prove to be a true delay (with the potential for later “catch up” (64)) or an aberrant developmental trajectory when examined longitudinally. The fact remains that while some patients with ADHD do appear to recover with maturation, others do not (with atypical default network connectivity persisting in this ADHD population (65)). Thus, a simple ‘delay only’ portrayal will likely be insufficient to fully characterize ADHD populations. Further, data on IQ (64) indicate that even when a developmental brain trajectory “catches up” the phenotype may still be different; that is, the timing of brain maturation may be as important as its end point. It will be quite interesting in future reports to examine and characterize all of these possibilities in larger cross-sectional and longitudinal cohorts.
Another question that remains is whether the atypical developmental picture seen here is specific to ADHD. Work in Tourette Syndrome has shown a similarly delayed trajectory via rs-fcMRI (66). Recent work in autistic patients using rs-fcMRI has also shown decreased integration between brain regions (67) that typically increase their strength of interaction over age (41). This appears to be particularly true for default network connectivity (e.g. in ADHD (65), Tourette syndrome (66), and autism (68) where the vmPFC-PCC connection is atypical). Lastly, other connectivity work in childhood ADHD has identified different, perhaps more distinct, atypical connections than what has been found here (69-72). Thus, it remains unclear whether the pattern of altered connectivity seen here is disorder-specific, or rather a generalized principle that links many developmental neuropsychiatric disorders. Studies which include multiple clinical samples that allow for direct between group comparison have yet to be performed, but will assist in informing such issues.
Relation to models of ADHD
Note that we did not attempt to differentiate between sub-classifications of ADHD (i.e., DSM-IV subtypes). This decision was partly based on inadequate power, and partly on growing questions about the stability and validity of the DSM-IV subtypes (73). However, it is likely that ADHD is a heterogeneous disorder, particularly at the level of neurobiology, and therefore likely to have multiple mechanistic subtypes (22).
For example, our findings regarding atypical connectivity with the vmPFC (i.e., vmPFC-PCC) tracks closely with ‘motivational’ or reward based theories of one pathway to ADHD(74). Importantly, the functional neuroanatomy associated with reward processing and shown to be atypical in ADHD (75) often include regions of the default network – notably, vmPFC (sometimes referred to as rostral ACC) (21). These theories suggest that some children with ADHD are unable to correctly explore, anticipate, and value outcomes between present action and future rewards (74), with recent behavioral data providing some support for this picture (76). Importantly, similar functions have been linked directly to the default network as a whole, but not necessarily to a specific region (10)).
Conclusion and potential approach for future study
In this report we have shown the atypical nature of the default network in ADHD children and young adolescents. The effects were consistent with failure of normal circuit maturation in this network. Subsequent connectivity investigations with large sample sizes and multiple diagnostic groups should not only enable better characterizing of subtypes within diagnostic groups (i.e., heterogeneity), but also aid in identifying atypical developmental principles that track across multiple syndromes versus those that may be specific to ADHD.
Supplementary Material
Acknowledgments
Research was supported by the Oregon Clinical and Translational Research Institute (Fair), Medical Research Foundation (Fair), UNCF Merck postdoctoral fellowship (Fair), Ford Foundation (Fair), R01 MH59105 (Nigg), K08 NS52147 (Nagel), Portland Alcohol Research Center pilot funds (P60 AA010760 – Nagel), and the OHSU Neuropsychiatric Institute (Nigg).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson GR, DeGarmo DS, Knutson N. Hyperactive and antisocial behaviors: comorbid or two points in the same process? Dev Psychopathol. 2000;12:91–106. doi: 10.1017/s0954579400001061. [DOI] [PubMed] [Google Scholar]
- 3.Daviss WB. A review of co-morbid depression in pediatric ADHD: etiology, phenomenology, and treatment. J Child Adolesc Psychopharmacol. 2008;18:565–571. doi: 10.1089/cap.2008.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller TW, Nigg JT, Faraone SV. Axis I and II comorbidity in adults with ADHD. J Abnorm Psychol. 2007;116:519–528. doi: 10.1037/0021-843X.116.3.519. [DOI] [PubMed] [Google Scholar]
- 5.Cumyn L, French L, Hechtman L. Comorbidity in adults with attention-deficit hyperactivity disorder. Can J Psychiatry. 2009;54:673–683. doi: 10.1177/070674370905401004. [DOI] [PubMed] [Google Scholar]
- 6.Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 8.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 11.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 12.Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2006 [Google Scholar]
- 13.Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: the default network and stimulus-independent thought”. Science. 2007;317:43. doi: 10.1126/science.317.5834.43. author reply 43. [DOI] [PubMed] [Google Scholar]
- 14.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckner RL, Vincent JL. Unrest at rest: the importance of default activity and spontaneous network correlations. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.01.010. duplicate. [DOI] [PubMed] [Google Scholar]
- 16.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert SJ, Spengler S, Simons JS, Frith CD, Burgess PW. Differential Functions of Lateral and Medial Rostral Prefrontal Cortex (Area 10) Revealed by Brain-Behavior Associations. Cereb Cortex. 2006 doi: 10.1093/cercor/bhj113. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: The default network and stimulus-Independent thought”. Science. 2007;317:43. doi: 10.1126/science.317.5834.43. [DOI] [PubMed] [Google Scholar]
- 19.Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 21.Luhmann CC, Chun MM, Yi DJ, Lee D, Wang XJ. Neural dissociation of delay and uncertainty in intertemporal choice. J Neurosci. 2008;28:14459–14466. doi: 10.1523/JNEUROSCI.5058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57:1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, et al. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Castellanos FX, Margulies DS, Kelly AMC, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 27.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Br Mapping. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Epstein JN, Conners CK, Erhardt D, March JS, Swanson JM. Asymmetrical hemispheric control of visual-spatial attention in adults with attention deficit hyperactivity disorder. Neuropsychology. 1997;11:467–473. doi: 10.1037/0894-4105.11.4.467. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler abbreviated scale of intelligence. Third ed The Psychological Corporation; San Antonio: 1999. [Google Scholar]
- 34.Puig-Antich J, Ryan N. The schedule for affective disorders and schizophrenia for school-age children. Western Psychiatric Institute and Clinic; Pittsburg: 1986. [Google Scholar]
- 35.Conners CK. Conners 3rd Edition Manual. Multi-Health Systems Inc.; Toronto: 2008. [Google Scholar]
- 36.Wechsler D. Wechsler Intelligence Scale for Children Technical and Interpretive Manual. Fourth ed The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- 37.Miezin F, Maccotta L, Ollinger J, Petersen S, Buckner R. Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- 38.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers, Inc; New York: 1988. [Google Scholar]
- 39.Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. A Modality-Independent Approach to Spatial Normalization of Tomographic Images of the Human Brain. Hum Br Mapping. 1995;3:209–223. [Google Scholar]
- 40.Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 43.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Field AP. Meta-analysis of correlation coefficients: a Monte Carlo comparison of fixed- and random-effects methods. Psychol Methods. 2001;6:161–180. doi: 10.1037/1082-989x.6.2.161. [DOI] [PubMed] [Google Scholar]
- 45.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 46.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33:522–530. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 48.Davison AC, Hinkley DV. Bootrap Methods and their Application. Cambridge Cambridge Univ Press; 1997. [Google Scholar]
- 49.Hesterberg T, Moore DS, Monaghan S, Clipson A, Epstein R. Bootstrap Methods and Permutation Tests. In: Moore DS, McCabe GP, editors. Introduction to the Practice of Statistics. WH Freeman and Co; 2005. pp. 1–70. [Google Scholar]
- 50.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 51.Armitage P, Berry G, Mathews JNS. Statistical Methods in Medical Research. Blackwell Science LTD; Oxford: 2002. [Google Scholar]
- 52.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 53.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 56.Pavuluri MN, Yang S, Kamineni K, Passarotti AM, Srinivasan G, Harral EM, et al. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:586–593. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anesthetized monkey brain. Nature. 2007;447:46–47. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 58.Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of Anterior Cingulate Functional Connectivity from Late Childhood to Early Adulthood. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 59.El-Sayed E, Larsson JO, Persson HE, Santosh PJ, Rydelius PA. “Maturational lag” hypothesis of attention deficit hyperactivity disorder: an update. Acta Paediatr. 2003;92:776–784. [PubMed] [Google Scholar]
- 60.Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. Structural development of the basal ganglia in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Psychiatry Res. 2009;172:220–225. doi: 10.1016/j.pscychresns.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Rubia K. Neuro-anatomic evidence for the maturational delay hypothesis of ADHD. Proc Natl Acad Sci U S A. 2007;104:19663–19664. doi: 10.1073/pnas.0710329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Epstein JN, Delbello MP, Adler CM, Altaye M, Kramer M, Mills NP, et al. Differential patterns of brain activation over time in adolescents with and without attention deficit hyperactivity disorder (ADHD) during performance of a sustained attention task. Neuropediatrics. 2009;40:1–5. doi: 10.1055/s-0029-1220686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 64.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Church JA, Fair DA, Dosenbach NU, Cohen AL, Miezin FM, Petersen SE, et al. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain. 2008 doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2009 doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, et al. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2006;400:39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 70.Cao C, Slobounov S. Alteration of Cortical Functional Connectivity as a Result of Traumatic Brain Injury Revealed by Graph Theory, ICA and sLORETA Analyses of EEG Signals. IEEE Trans Neural Syst Rehabil Eng. 2009 doi: 10.1109/TNSRE.2009.2027704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu CZ, Zang YF, Cao QJ, Yan CG, He Y, Jiang TZ, et al. Fisher discriminative analysis of resting-state brain function for attention-deficit/hyperactivity disorder. Neuroimage. 2008;40:110–120. doi: 10.1016/j.neuroimage.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 72.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV Subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- 74.Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 75.Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- 76.Martel MM, Nigg JT, von Eye A. How do trait dimensions map onto ADHD symptom domains? J Abnorm Child Psychol. 2009;37:337–348. doi: 10.1007/s10802-008-9255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.