Abstract
Transgenic rabbits expressing loss-of-function pore mutants of the human gene KCNQ1 (KvLQT1-Y315S) have a Long QT-Syndrome 1 (LQT1) phenotype. We evaluated for the first time the effect of nicorandil, an opener of ATP-sensitive potassium channels, and of isoproterenol on cardiac action potential duration and heart rate dependent dispersion of repolarisation in transgenic LQT1 rabbits.
In vivo LQT1 and littermate control were subjected to transvenous electrophysiological studies; in vitro monophasic action potentials were recorded from explanted Langendorff-perfused hearts.
In vivo ventricular effective refractory periods (VERP) at the right ventricular base were significantly prolonged in LQT1 as compared to littermate control, resulting in a more pronounced VERP dispersion in LQT1. This difference in VERP dispersion between LQT1 and littermate control disappeared after infusion of nicorandil. In vitro, mean action potential durations (APD75 and APD90) of LQT1 were significantly prolonged compared to littermate control at baseline. Nicorandil decreased APD75 and APD90 in LQT1 and littermate control at all stimulated heart rates. After adding nicorandil, the APD90 at all hearts rates and the APD75 at high heart rates were no longer different. Dispersion of repolarisation (ΔAPD75 and ΔAPD90) was heart rate dependently decreased after nicorandil at all tested stimulation cycle lengths only in LQT1.
We demonstrated phenotypic differences of LQT1 and littermate control in vivo and in vitro. Nicorandil 20 μmol/l improved repolarisation abnormalities and heterogeneities in transgenic LQT1 rabbits.
Keywords: Long QT-Syndrome, polymorphic ventricular tachycardia, transgenic rabbit model, electrophysiological studies, nicorandil, dispersion of repolarisation
1. Introduction
The Long QT-syndrome (LQTS) is a genetically heterogeneous arrhythmogenic disorder with a population prevalence of 1:2000 to 1:5000 (Crotti et al., 2008; Goldenberg et al., 2008). The LQTS is characterised by prolongation of cardiac repolarisation which is associated with increased dispersion of repolarisation and the occurrence of early-after-depolarisations. Patients may present with syncopes or sudden cardiac death caused by fast polymorphic ventricular tachycardia. At least twelve different forms of congenital LQTS have been described (Zareba and Cygankiewicz, 2008).
The underlying cause of LQT1 are loss-of-function mutations in the voltage-gated potassium channel KvLQT1 (α-subunit of the slowly activating delayed rectifier potassium current IKs)(Donger et al., 1997). On a cellular basis, these mutations lead to prolongation of the action potential duration (APD) and increase repolarisation heterogeneities leading to polymorphic ventricular tachycardia. To raise the understanding of mechanisms of arrhythmogenesis in LQTS, pharmacologically-induced LQTS animal models were used (Lu et al., 2001; Shimizu and Antzelevitch, 2000; Fabritz et al., 2003). However, these were models with potential cross-blocking effects of LQTS-inducing and APD-normalizing substances.
We established the first transgenic LQT1 rabbit model by over-expressing a dominant-negative loss-of-function pore mutant of the human gene KCNQ1 (KvLQT1-Y315S, loss of IKs) displaying a LQT1 phenotype (Brunner et al., 2008). The rabbit is a suitable model, because its cardiac ion channels and action potentials bear marked similarities with those of humans.
Currently, the only therapy for LQTS is symptomatic using beta-receptor-antagonists (Moss et al., 2000), implantable cardioverter-defibrillators, and sympathectomy in patients with incessant arrhythmias despite optimized medical therapy (Priori et al., 2001). As no causal therapy for LQTS is available, pharmacological shortening of the pathologically prolonged APD seems to be an attractive option. In a small study of LQT1 patients Shimizu et al. had previously shown that nicorandil, an opener of ATP-sensitive potassium channels, reduces endocardial monophasic action potential duration in vivo (Shimizu et al., 1998b). The same group also showed in a pharmacological LQT1 canine model in vitro, that transmural dispersion of repolarisation may be decreased by potassium channel openers (Shimizu et al., 2000). Furthermore intravenous nicorandil was shown to reduce QT dispersion in humans after percutaneous transluminal coronary angioplasty of the right coronary artery or after acute myocardial infarction, respectively (Ueda et al., 2004a; Ueda et al., 2004b) Nicorandil prevents ventricular tachycardia during acute global ischemia in canine left ventricular wedges (Hirose et al., 2008) and inhibits the increase of QT dispersion in humans (Akagi et al., 2006).
In patients with LQTS, transmural dispersion of repolarisation is likely a key mechanism influencing vulnerability for malignant ventricular arrhythmias (Idriss and Wolf, 2004). The degree of dispersion determines whether a torsade de pointes degenerates to ventricular fibrillation (Vincent, 2003).
We hypothesized that by increasing the net outward potassium currents, nicorandil would improve repolarisation abnormalities and heterogeneities in LQT1. To that end, we characterised the acute physiological effects of nicorandil on APD, on cardiac refractory periods and, with special emphasis, on dispersion of repolarisation.
2. Material and methods
2.1. Rabbits
All experiments were performed in accordance with the local guidelines of the institutions and with the German Law on the Protection of Animals after approval by the appropriate authorities. The transgenic LQT1 New Zealand White rabbits and littermate control were genotyped as described (Brunner et al., 2008). We compared adult LQT1 rabbits (n = 10) and littermate control (n = 9) with a mean weight of 4.0 ± 0.2 kg or 3.9 ± 0.3 kg and aged 14 ± 2 months or 13 ± 2 months, respectively. The LQT1 group consisted of four male and six female rabbits compared to four male and five female rabbits in littermate control.
2.2. Electrophysiological studies in vivo
Standard twelve lead surface electrocardiograms (ECG) were performed in anesthetized (S-Ketamine/Xylazine 12.5/3.75 mg/kg i.m. and inhaled isoflurane 0.5%–4.0%) and ventilated rabbits (FiO2 0.5, 8cc stroke volume/kg, 40 strokes/min, positive end-expiratory pressure 3 cmH2O) immediately prior to invasive electrophysiological studies. We measured cycle length and QT interval (QTabs) online and offline. Microvoltage T wave alternans (μV-TWA) was acquired using an algorithm developed by GE Healthcare (HEART-Exercise, GE Healthcare, Freiburg, Germany) and approved by the Food and Drug Administration for human use (regulation number: 21 CFR 870.1425, received: October 8, 2002). The algorithm is based on the time-domain modified moving average analysis (Nearing and Verrier, 2002) and was advanced by GE Healthcare with noise and artefact rejection algorithms. As action potential and ECG of humans and rabbits show many similarities, we applied the algorithm to rabbit hearts.
In vivo electrophysiological studies were performed with endotracheal intubated rabbits under general anaesthesia. Anaesthesia and ventilation were performed as above. Briefly, after preparation of the right internal jugular vein, a decapolar 4 F catheter (Inquiry® Steerable, St. Jude Medical) was advanced through the right atrium and placed in the apex of the right ventricle guided by fluoroscopy, ECG signals and pacing thresholds.
Baseline measurements included cycle length, atrial and ventricular effective refractory period (AERP and VERP) and sinus node recovery time (SNRT). AERP and VERP were measured following a standard protocol using a train of eight paced atrial or ventricular impulses (S1, cycle length 240 ms) followed by an extrastimulus (S2). The effective refractory period was defined as the longest coupling interval that fails to initiate an action potential. The SNRT was determined after atrial stimulation with 240 ms cycle length over 30 seconds. After baseline measurements over 20 minutes, nicorandil 200 μg/kg/min (Merck KGaA, Darmstadt, Germany) in isotonic saline was added as phase 1 and the complete electrophysiological study protocol was repeated after 20 min equilibration time. Thereafter, a combined infusion of nicorandil 200 μg/kg/min and the beta-receptor-agonist isoproterenol (Sanofi-Aventis GmbH, Frankfurt, Germany) was given as phase 2. Isoproterenol was titrated to decrease the cycle length by 20%. Thus all parameters of the electrophysiological studies were recorded at baseline, in phase 1 and phase 2. In case of polymorphic ventricular tachycardia or ventricular fibrillation electrical cardioversion or defibrillation (2 Joule/kg) was used to restore sinus rhythm.
2.3. Perfusion of Langendorff in vitro
The LQT1 and littermate control rabbits were heparinised (500 IU/kg i.v.) and anesthetized with midazolam (15 mg i.m.) and thiopental (25 mg i.m. and 40 mg/kg i.v.). After thoracotomy, hearts were excised and immediately immersed in ice-cold modified Krebs-Henseleit solution. The modified Krebs-Henseleit solution contained the following (mM): NaCl 118.0, KCl 4.7, KH2PO4 1.2, NaHCO3 25.0, CaCl2 2.5, MgSO4 1.2, glucose 11.0, Na-Pyruvat 2.0 adjusted to pH 7.4 with HCl (Sigma-Aldrich, Munich, Germany). The solution was sterile filtered through a 0.2μm mesh and continuously aerated with a mixture of 95% O2 and 5% CO2. The solution temperature was maintained at 37°C by a circulating water bath and the rabbit hearts were immersed completely with a surface temperature of 37°C. Temperature, pH and oxygenation were checked repetitively.
Each heart was then retrogradely perfused via the aorta using the method of Langendorff (IH5, Hugo Sachs Electronic – Harvard Apparatus GmbH, Hugstetten, Germany). Cycle length, end diastolic and systolic left ventricular pressure, coronary flow, ΔLVPΔtmax, cardiac index, aortic pressure and APD were continuously recorded. Recording of signals started after a run-in phase of at least 20 min to stabilize the signals.
Six epicardial monophasic action potentials at different epicardial left and right ventricular positions (monophasic action potential 1–6) were derived. These positions were apex of left ventricle (monophasic action potential 1), apical antero-lateral wall of left ventricle (monophasic action potential 2), mid-basal wall of left ventricle (monophasic action potential 3), base of left ventricle (monophasic action potential 4), base of right ventricle (monophasic action potential 5) and apex of right ventricle (monophasic action potential 6). Action potentials were measured at 75% and 90% repolarisation (APD751–6 and APD901–6) at baseline, during perfusion with nicorandil (20 μmol/l) alone and combined infusion of nicorandil (20 μmol/l) and isoproterenol (0.1 μmol/l).
A latex balloon filled with distilled water was inserted into the left ventricle through an incision of the left atrium and via the mitral valve, and end diastolic pressure was adjusted to 2–6 mmHg. All signals were digitized (1 kHz), recordings were archived on hard disc drive and data analysis was performed online and offline. Data recording and analyses were performed with Isoheart® software (Hugo Sachs Electronic, Harvard Apparatus GmbH, Version 1.1.1.218(32), Hugstetten, Germany). All parameters, except VERP, were recorded during external atrial stimulation at physiological heart rates to maintain normal depolarisation of the ventricles and to measure dispersion of repolarisation. To measure VERP, epicardial left ventricular stimulation at the base of the left ventricle was deployed. The applied stimulation cycle lengths were 286 ms (210 bpm), 250 ms (240 bpm), 222 ms (270 bpm) and 200 ms (300 bpm).
In case of polymorphic ventricular tachycardia or ventricular fibrillation, rinsing of the heart with ice cold Krebs-Henseleit solution was used to electrically inactivate the heart, while maintaining perfusion via the coronaries. After rewarming the sinus rhythm was restored.
2.4. Genotyping
Genotyping was performed as previously described (Brunner et al., 2008).
2.5. Data recording and statistics
All data analysis was performed blinded to the genotype. All data are expressed as mean ± standard error of the mean. One-way analysis of variance (ANOVA) and two-tailed Student's t-test (paired and unpaired) were used for statistical analysis of normally distributed values (significance * P<0.05). All statistics and curve fittings were performed using GraphPad Prism (Version 4.03, GraphPad software, San Diego, CA, USA).
3. Results
3.1. Electrophysiological studies in vivo
Full in vivo electrophysiological studies in anesthetized rabbits were performed in 10 LQT1 and 9 littermate control rabbits of similar age and weight. Female and male rabbits were equally represented comparing LQT1 and littermate control.
The in vivo spontaneous baseline cycle length of LQT1 was significantly longer than of littermate control under general anaesthesia (Tab. 1). A longer baseline cycle length is described in humans (children) with LQT1 (Schwartz et al., 1993). However, as previously published, in awake and free-moving rabbits no differences of baseline cycle lengths between LQT1 and littermate control were seen (Brunner et al., 2008). Whereas nicorandil infusion had no significant effect on the cycle length, combined infusion of nicorandil plus isoproterenol decreased the cycle lengths of both. After adding nicorandil (with or without isoproterenol), there were no significant differences of the cycle length left comparing LQT1 and littermate control. Similar to our previous studies with isoflurane (Odening et al., 2008), the QTabs values were not significant different comparing LQT1 and littermate control, but shorter by trend in littermate control (Tab. 1).
Table 1.
In vivo data of electrophysiological studies (in ms): cycle length and QTabs were determined at intrinsic heart rates, and VERPbase, VERPapex and ΔVERP while stimulating at 240ms cycle length.
| cycle length | QTabs | VERPbase | VERPapex | ΔVERP | ||
|---|---|---|---|---|---|---|
| littermate control | baseline | 312.5 ± 11.4 | 196.6 ± 5.6 | 151.6 ± 2.8 | 150.9 ± 6.4 | 0.7 ± 6.0 |
| nicorandil | 307.6 ± 12.0 | 200.4 ± 5.2 | 155.3 ± 4.7 | 148.4 ± 6.6 | 7.0 ± 5.6 | |
| nicorandil+isoproterenol | 271.6 ± 8.3 | 185.7 ± 4.9 | 138.2 ± 4.9 | 139.0 ± 7.1 | 2.0 ± 6.1 | |
| LQT1 | baseline | 354.5 ± 12.2 | 215.0 ± 9.5 | 172.8 ± 5.9 | 154.2 ± 4.0 | 16.9 ± 4.0 |
| nicorandil | 343.7 ± 18.4 | 225.0 ± 8.7 | 172.2 ± 6.5 | 157.6 ± 6.5 | 12.0 ± 4.1 | |
| nicorandil+isoproterenol | 289.2 ± 13.1 | 212.3 ± 10.4 | 171.8 ± 6.5 | 161.6 ± 6.0 | 8.4 ± 3.8 | |
| intrinsic heart rate | stimulation at 240 ms cycle length | |||||
Baseline right ventricular endocardial VERP (VERPbase) was significantly prolonged in the right ventricular base in LQT1 as compared to littermate control; the endocardial VERP (VERPapex) in the right ventricular apex was not different (Tab. 1). The VERP dispersion (ΔVERP), defined as the absolute value of the difference between VERPbase and VERPapex, was significantly larger in LQT1 compared to littermate control (Tab. 1, last row). The combined infusion of nicorandil plus isoproterenol significantly decreased VERP in the right ventricular base of littermate control but not in LQT1. Nicorandil infusion, with or without isoproterenol, abolished the difference of ΔVERP between LQT1 and littermate control.
During determining VERP by programmed stimulation in vivo, in one out of ten LQT1 rabbits ventricular fibrillation was induced at baseline measurements by S1S2 stimulation. Defibrillation was used for termination. Ventricular fibrillation did not occur in littermate control rabbits during in vivo experiments.
Analysing the μV-TWA measurements, neither in LQT1 nor in littermate control a significant μV-TWA was observed at baseline, after nicorandil alone or the combination of nicorandil with isoproterenol. SNRT at baseline was not significantly different comparing LQT1 and littermate control in vivo. SNRT of littermate control was significantly influenced neither by nicorandil nor by nicorandil plus isoproterenol. Whereas nicorandil alone had no significant effect on SNRT of LQT1, the combined infusion of nicorandil and isoproterenol decreased the SNRT significantly. AERP was not influenced by phenotype or nicorandil/isoproterenol infusion.
3.2. Electrophysiological studies in vitro, Perfusion of Langendorff
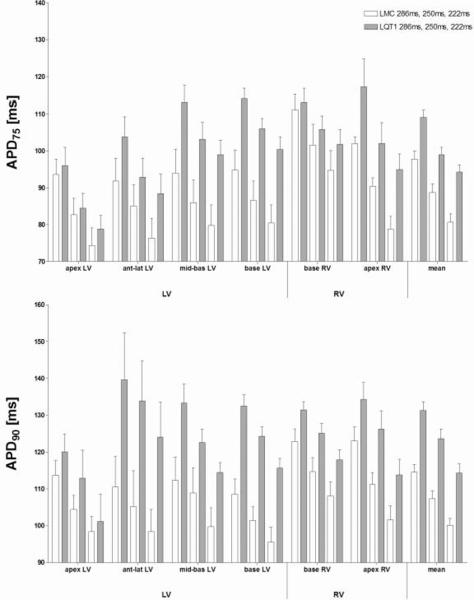
The mean baseline APD75 and APD90 of LQT1 were prolonged compared to littermate control at all cycle lengths (Fig. 1, last row). Regarding each position at the left and right ventricle separately, the APD75 and APD90 values were significantly different comparing LQT1 and littermate control at all positions except at the apex of the left and at the base of the right ventricle (Fig. 1). The difference of monophasic action potential duration between the genotypes was most pronounced at the basal region of the left ventricle.
Figure 1.
In vitro APD measured at 75% (APD75) or 90% (APD90) repolarisation, respectively. Four left and two right ventricular positions are displayed, the last row shows mean values.
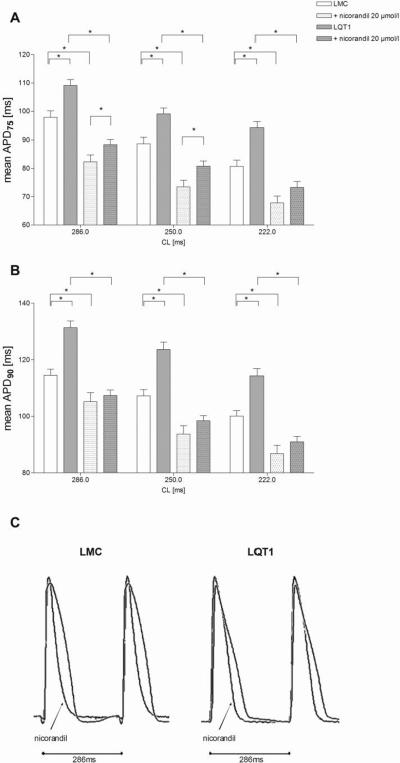
Perfusion with nicorandil significantly decreased mean APD75 and mean APD90 in LQT1 and littermate control at all stimulated heart rates as exemplary displayed in Fig. 2C. Nicorandil shortened the mean APD75 of LQT1 by 18.2 to 21.2 ms and of littermate control by 13.0 to 15.5 ms depending on the stimulation cycle lengths (Fig. 2A). Similarly, nicorandil decreased the mean APD90 of LQT1 by 23.3 to 25.2 ms and of littermate control by 9.3 to 13.5 ms (Fig. 2B). Notably, after adding nicorandil, the APD90 at all heart rates and the APD75 at high heart rates (222 ms) were no longer different between LQT1 and littermate control. Comparing littermate control at baseline and LQT1 with nicorandil, the APD75 and APD90 of LQT1 were decreased by nicorandil below the level of littermate control at baseline. In our pilot experiments with a lower concentration of nicorandil (2 μmol/l, data not shown), the APD of LQT1 were still significantly longer than in littermate control at baseline.
Figure 2.
In vitro effect of nicorandil 20 μmol/l on APD75 (A) and APD90 (B) of LQT1 and littermate control (* P<0.05). (C) Exemplary section of original monophasic action potential 4 curves (base of left ventricle) under atrial stimulation (cycle length 286ms) depicting the genotypic difference and the effect of nicorandil 20 μmol/l.
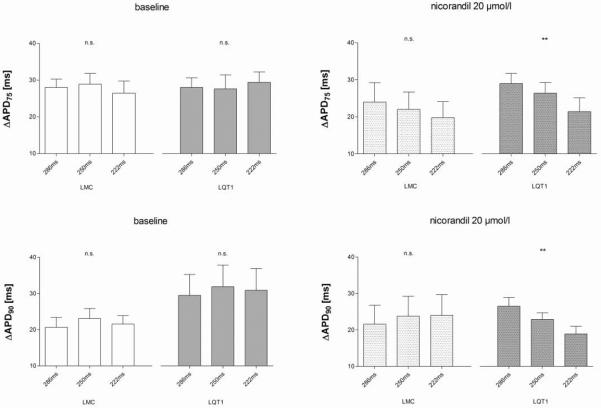
Dispersion of repolarisation across the heart, defined as the difference between shortest and longest of APD75 1–6 (ΔAPD75) or APD90 1–6 (ΔAPD90), respectively, was heart rate dependently decreased after nicorandil in LQT1 but not in littermate control (Fig. 3). When comparing the effect of nicorandil in LQT1 at apex (monophasic action potential 1) and base (monophasic action potential 4) of the left ventricle, nicorandil had greater absolute effects at the base (e.g. APD90 at 286 ms stimulation cycle length: apexbaseline 120.1 ± 4.8 ms, apexnicorandil 107.6 ± 5.3 ms, P = n.s.; basebaseline 132.5 ± 3.0 ms, basenicorandil 102.7 ± 4.7 ms, P<0.01). Whereas the apical APD were not influenced by genotype or nicorandil, both had an impact on repolarisation at the base. The decrease of the APD by nicorandil at the base was more pronounced in LQT1. These effects at the base of the left ventricle particularly contributed to the decrease of ΔAPD75 and ΔAPD90 in LQT1 under nicorandil. Nicorandil did not affect contractility (ΔLVPΔtmax).
Figure 3.
Dispersion of repolarisation: ΔAPD75 and ΔAPD90. The dispersion was decreased by nicorandil with ascending heart rates only in LQT1 (** P<0.01 (ANOVA), n.s. non-significant).
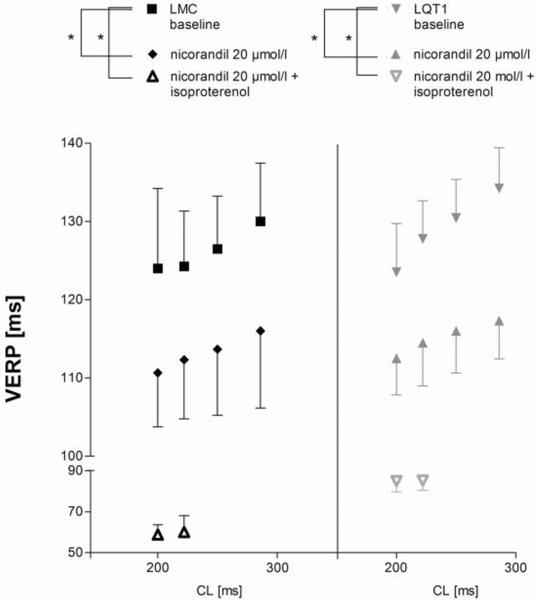
The epicardial left ventricular VERP of LQT1 and littermate control under basal ventricular stimulation with different cycle lengths were similar at baseline (Fig. 4). In both LQT1 and littermate control the VERP was significantly decreased by nicorandil and also by the combined infusion of nicorandil and isoproterenol. The latter data were only determinable at stimulation cycle lengths of 222 ms and 200 ms, because of increased heart rates under isoproterenol infusion.
Figure 4.
In vitro VERP measured under ventricular stimulation at the base of the left ventricle at four different stimulation cycle lengths (* P<0.05).
Ventricular fibrillation was more often induced by programmed ventricular S1S2 stimulation in vitro compared to in vivo experiments: four of ten LQT1 and four of nine littermate control rabbits developed ventricular fibrillation, one littermate control rabbit displayed asystole in the course of the experiment. These tachyarrhythmias occurred both with and without nicorandil or isoproterenol, respectively.
4. Discussion
Prolongation of repolarisation itself is not a sufficient criterion to promote polymorphic ventricular tachycardia, but together with an enhanced dispersion of repolarisation as an additional requirement, it represents a pro-arrhythmic substrate that facilitates the initiation of polymorphic ventricular tachycardia (Smith et al., 1988; Frazier et al., 1989; Shimizu and Antzelevitch, 1998a; Vincent, 2003). Pharmacological interventions aiming at influencing the APD have been in use for many years, such as class III antiarrhythmics that prolong the APD. It is well known that this pharmacological APD-prolongation can have proarrhythmic effects (Taira et al., 2009). Unlike the prolongation of the APD, the pharmacological shortening of a pathologically prolonged APD has been investigated only in very few studies. The pharmacological shortening of a prolonged APD may enhance regional differences in repolarisation or decrease dispersion of repolarisation. Nicorandil, an approved antianginal agent (administered intravenously 2–14 mg as well as sublingually and orally in single doses of 10–60 mg), that opens ATP-sensitive potassium channels (Chibana et al., 1991; Treese et al., 1992; Taira, 1987), has been shown to shorten the QT interval only slightly when administered orally at a dose of 15 or 30 mg/day, respectively (Aizawa et al., 1998). Moreover, in a small human study of six patients with LQT1, endocardial monophasic action potentials showed a shortening of the APD during combined infusion of nicorandil and isoproterenol (Shimizu et al., 1998b; Shimizu et al., 1998a). Nicorandil was also shown to successfully shorten the QT interval in an infant with incessant ventricular tachycardia and suspected LQTS (Chang et al., 2002). In an experimental study using pharmacological LQTS models, nicorandil reduced the transmural dispersion of repolarisation in LQT1 in the left ventricular wall (Shimizu et al., 2000).
Our transgenic rabbit model with LQT1 is a new model for a genetically determined APD-prolongation. Therefore we sought to investigate the effect of nicorandil on the APD in vivo and in vitro with special emphasis on the effects at physiological heart rates and on regional dispersion of repolarisation. Unlike other groups which ablate the AV node, we analysed the dispersion of repolarisation in both, spontaneously beating hearts (sinus rhythm) and during atrial pacing to maintain the physiological order of ventricular de- and repolarisation.
In the present study, differences of QTabs between LQT1 and littermate control were small in vivo, likely caused by the IKs blocking effect of isoflurane, which was necessary for maintenance of anaesthesia (Odening et al., 2008). Interestingly, isoflurane also activates ATP-sensitive potassium channels, but it only influences vascular ATP-sensitive potassium channels (type Kir6.1/SUR2B) and not the cardiac ATP-sensitive potassium channels (type Kir6.2/SUR2A)(Fujita et al., 2006).
In vivo LQT1 rabbits showed a significant higher ΔVERP at baseline than littermate control. Corresponding to prolonged QT intervals in free-moving LQT1 rabbits (Brunner et al., 2008), the VERPbase in vivo was prolonged in LQT1 compared to littermate control . The ΔVERP-effects were mainly promoted by changes of VERPbase. The values of VERPapex were not different between LQT1 and littermate control. It is known that in mammalian hearts potassium channels are heterogeneously distributed along the wall from apex to base with higher IKs at the base in rabbits. This regionally different expression of potassium currents leads to different APD at the apex compared to the base not only in rabbits (Choi et al., 2002), but also in guinea pigs (Salama et al., 1987; Efimov et al., 1996) and mice (Baker et al., 2000). Additionally these differences are described across the ventricular wall from endocardial to epicardial layers of the tissue (Shimizu et al., 1998a). However, it is not known whether there is heterogeneity of the overexpression of the dominant-negative loss-of-function pore mutant in LQT1 rabbit hearts or whether this mutant is directly sensitive to nicorandil. The difference of ΔVERP between LQT1 and littermate control disappeared under nicorandil. Given the importance of a reasonably small dispersion of repolarisation, a decrease of ΔVERP in LQT1 might be regarded as an anti-arrhythmic homogenisation of repolarisation.
Macrovoltage TWA has been shown to precede episodes of polymorphic ventricular tachycardia in patients with LQTS (Schwartz et al., 1975), and the presence of macrovoltage TWA at rest seems to be an adverse prognostic factor (Cruz Filho et al., 2000; Fujimoto et al., 1999; Motoyasu et al., 1992; Shimizu et al., 1996). In another study, μV-TWA was only infrequently found and no significant differences were detectable comparing patients with or without LQTS (Kaufman et al., 2001). In our study, we could not detect macro- or μV-TWA both before and after nicorandil or under the combined infusion of both substances in LQT1 or in littermate control rabbits. Clearly, one limitation of our TWA-analyses is the use of sedated animals, which might influence TWA. Ideally μV-TWA recorded in awake, free moving LQTS rabbits in vivo over a longer period of time would be interesting to clarify this issue.
All in vitro parameters, except VERP, were recorded during atrial stimulation and intact AV node function to maintain normal depolarisation of the ventricles. In vitro we observed significant differences of baseline APD75 and APD90 between LQT1 and littermate control. A significant shortening of the mean APD75 and APD90 by nicorandil was demonstrated in both experimental groups at all tested stimulation rates and additionally the phenotypic differences disappeared for APD90 at all and for APD75 at high heart rates. Considering the significant decrease of APD in LQT1 by nicorandil (20 μmol/l) below the level of littermate control at baseline, an optimal concentration of nicorandil is expected between 2 μmol/l and 20 μmol/l.
In vitro the epicardial dispersion of repolarisation across the heart was decreased only in LQT1 by nicorandil, which corresponds to a change of ΔVERP (in the right ventricle) by nicorandil in vivo. This reduction of repolarisation gradients across the ventricles might indicate an anti-arrhythmic effect, as an increased dispersion of repolarisation is a key mechanism of arrhythmias (Killeen et al., 2008; Brunner et al., 2008). Patients with LQT1 often develop arrhythmias during physical exercise. In our in vitro experimental setup, the dispersion of repolarisation was decreased by nicorandil in LQT1 with ascending heart rates. In other studies, using different agents, it was shown that the over-expression of ATP-sensitive potassium channels in the hearts of mice, or the pharmacological opening of ATP-sensitive potassium channels in rabbit hearts had proarrhythmic effects (Flagg et al., 2007; Milberg et al., 2007). However, these studies aimed to shorten an already normal APD, and not a prolonged APD as seen in our experiments. Although we could not show a significant effect of nicorandil on the VERP in vivo (likely due to high variability), basal VERP in vitro was significantly decreased by nicorandil in both LQT1 and littermate control. The differing stimulation sites (endocardial right ventricle in vivo vs. epicardial left ventricle in vitro) might be responsible for these small but significant aberrances.
In mouse and rabbit models of genetic arrhythmias (Nerbonne and Guo, 2002; Odening et al., 2008; Brunner et al., 2001) induction of ventricular tachycardia during electrophysiological studies using extrastimuli was unspecific. In our experiments induction of ventricular tachycardia in both LQT1 and littermate control suggests the same lack of specificity as in humans (DiCarlo, Jr. et al., 1985). This is consistent with the current recommendations, concerning the diagnostic pathway of LQTS, where electrophysiological studies are not regarded as helpful for clinical decision making in most patients (Krahn et al., 2009). Therefore it is not surprising, that we could not demonstrate a beneficial effect of nicorandil concerning the frequency of ventricular tachycardia. However, in vivo electrophysiological studies remain the best available clinical tool to evaluate refractoriness, AV-conduction and -adaptation to different heart rates.
Currently only beta-receptor-antagonists are routinely used for pharmacological treatment of patients with LQT1. Based on the small scale patient study of Shimizu et al. (Shimizu et al., 1998b) and our data, it would be interesting to study the long term effects of appropriate doses of nicorandil in larger cohorts of LQT1 patients. Unfortunately, current oral dose regimens fail to achieve sufficiently sustained plasma levels of nicorandil, which limits its use for long-term studies. The intravenous dosages used in studies on antiarrhythmic effects are markedly higher than those used for ischemia/reperfusion studies (Imagawa et al., 1998).
In conclusion, in this transgenic LQT1 model nicorandil 20 μmol/l shortened the APD at physiological heart rates, abolished the difference of ΔVERP between LQT1 and littermate control, and reduced the dispersion of repolarisation (ΔAPD) heart rate dependently in LQT1. Nicorandil improved repolarisation abnormalities and heterogeneities in transgenic LQT1 rabbits.
Acknowledgments
This work was supported by the Max-Schaldach-Forschungsstipendium of the German Cardiac Society. We thank Willi Kaiser (GE Healthcare Information Technologies, Freiburg, Germany) for supporting the analyses of μV-TWA and QT intervals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest The authors declare that they have no conflict of interest.
Reference List
- Aizawa Y, Uchiyama H, Yamaura M, Nakayama T, Arita M. Effects of the ATP-sensitive K channel opener nicorandil on the QT interval and the effective refractory period in patients with congenital long QT syndrome. Investigator Group for K-Channel Openers and Arrhythmias. J. Electrocardiol. 1998;31:117–123. doi: 10.1016/s0022-0736(98)90042-5. [DOI] [PubMed] [Google Scholar]
- Akagi T, Sarazawa K, Inai Y, Kitagawa M, Takahashi N, Hamanaka I, Yamazaki T, Takebe M, Hama N, Hiraoka Y, Ueda K, Nakazawa K, Matsumoto N. Continuous administration of nicorandil decreases QT dispersion during the chronic phase of acute myocardial infarction. Int. Heart J. 2006;47:351–361. doi: 10.1536/ihj.47.351. [DOI] [PubMed] [Google Scholar]
- Baker LC, London B, Choi BR, Koren G, Salama G. Enhanced dispersion of repolarization and refractoriness in transgenic mouse hearts promotes reentrant ventricular tachycardia. Circ. Res. 2000;86:396–407. doi: 10.1161/01.res.86.4.396. [DOI] [PubMed] [Google Scholar]
- Brunner M, Guo W, Mitchell GF, Buckett PD, Nerbonne JM, Koren G. Characterization of mice with a combined suppression of I(to) and I(K,slow) Am. J. Physiol Heart Circ. Physiol. 2001;281:H1201–H1209. doi: 10.1152/ajpheart.2001.281.3.H1201. [DOI] [PubMed] [Google Scholar]
- Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR, Mathur R, Hajjiri M, Odening KE, Steinberg E, Folco EJ, Pringa E, Centracchio J, Macharzina RR, Donahay T, Schofield L, Rana N, Kirk M, Mitchell GF, Poppas A, Zehender M, Koren G. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J. Clin. Invest. 2008;118:2246–2259. doi: 10.1172/JCI33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang IK, Shyu MK, Lee CN, Kau ML, Ko YH, Chow SN, Hsieh FJ. Prenatal diagnosis and treatment of fetal long QT syndrome: a case report. Prenat. Diagn. 2002;22:1209–1212. doi: 10.1002/pd.475. [DOI] [PubMed] [Google Scholar]
- Chibana T, Nagamine F, Sunagawa R, Oshiro K, Nakada Y, Shimabukuro M, Shinzato T, Murakami K, Mimura G, Sakanashi M. Comparison of the acute hemodynamic and coronary vasodilating effects between nicorandil and glyceryl trinitrate. Arzneimittelforschung. 1991;41:591–594. [PubMed] [Google Scholar]
- Choi BR, Nho W, Liu T, Salama G. Life span of ventricular fibrillation frequencies. Circ. Res. 2002;91:339–345. doi: 10.1161/01.res.0000031801.84308.f4. [DOI] [PubMed] [Google Scholar]
- Crotti L, Celano G, Dagradi F, Schwartz PJ. Congenital long QT syndrome. Orphanet. J. Rare. Dis. 2008;3:18. doi: 10.1186/1750-1172-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Filho FE, Maia IG, Fagundes ML, Barbosa RC, Alves PA, Sa RM, Boghossian SH, Ribeiro JC. Electrical behavior of T-wave polarity alternans in patients with congenital long QT syndrome. J. Am. Coll. Cardiol. 2000;36:167–173. doi: 10.1016/s0735-1097(00)00694-x. [DOI] [PubMed] [Google Scholar]
- DiCarlo LA, Jr., Morady F, Schwartz AB, Shen EN, Baerman JM, Krol RB, Scheinman MM, Sung RJ. Clinical significance of ventricular fibrillation-flutter induced by ventricular programmed stimulation. Am. Heart J. 1985;109:959–963. doi: 10.1016/0002-8703(85)90235-2. [DOI] [PubMed] [Google Scholar]
- Donger C, Denjoy I, Berthet M, Neyroud N, Cruaud C, Bennaceur M, Chivoret G, Schwartz K, Coumel P, Guicheney P. KVLQT1 C-terminal missense mutation causes a forme fruste long-QT syndrome. Circulation. 1997;96:2778–2781. doi: 10.1161/01.cir.96.9.2778. [DOI] [PubMed] [Google Scholar]
- Efimov IR, Ermentrout B, Huang DT, Salama G. Activation and repolarization patterns are governed by different structural characteristics of ventricular myocardium: experimental study with voltage-sensitive dyes and numerical simulations. J. Cardiovasc. Electrophysiol. 1996;7:512–530. doi: 10.1111/j.1540-8167.1996.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Fabritz L, Kirchhof P, Franz MR, Eckardt L, Monnig G, Milberg P, Breithardt G, Haverkamp W. Prolonged action potential durations, increased dispersion of repolarization, and polymorphic ventricular tachycardia in a mouse model of proarrhythmia. Basic Res. Cardiol. 2003;98:25–32. doi: 10.1007/s00395-003-0386-y. [DOI] [PubMed] [Google Scholar]
- Flagg TP, Patton B, Masia R, Mansfield C, Lopatin AN, Yamada KA, Nichols CG. Arrhythmia susceptibility and premature death in transgenic mice overexpressing both SUR1 and Kir6.2[DeltaN30,K185Q] in the heart. Am. J. Physiol Heart Circ. Physiol. 2007;293:H836–H845. doi: 10.1152/ajpheart.00011.2007. [DOI] [PubMed] [Google Scholar]
- Frazier DW, Wolf PD, Wharton JM, Tang AS, Smith WM, Ideker RE. Stimulus-induced critical point. Mechanism for electrical initiation of reentry in normal canine myocardium. J. Clin. Invest. 1989;83:1039–1052. doi: 10.1172/JCI113945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y, Morita H, Fukushima KK, Ohe T. Nicorandil abolished repolarisation alternans in a patient with idiopathic long QT syndrome. Heart. 1999;82:e8. doi: 10.1136/hrt.82.5.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Ogura T, Tamagawa M, Uemura H, Sato T, Ishida A, Imamaki M, Kimura F, Miyazaki M, Nakaya H. A key role for the subunit SUR2B in the preferential activation of vascular KATP channels by isoflurane. Br. J. Pharmacol. 2006;149:573–580. doi: 10.1038/sj.bjp.0706891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg I, Zareba W, Moss AJ. Long QT Syndrome. Curr. Probl. Cardiol. 2008;33:629–694. doi: 10.1016/j.cpcardiol.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Hirose M, Tsujino N, Nakada T, Yano S, Imamura H, Yamada M. Mechanisms of preventive effect of nicorandil on ischaemia-induced ventricular tachyarrhythmia in isolated arterially perfused canine left ventricular wedges. Basic Clin. Pharmacol. Toxicol. 2008;102:504–514. doi: 10.1111/j.1742-7843.2008.00242.x. [DOI] [PubMed] [Google Scholar]
- Idriss SF, Wolf PD. Transmural action potential repolarization heterogeneity develops postnatally in the rabbit. J. Cardiovasc. Electrophysiol. 2004;15:795–801. doi: 10.1046/j.1540-8167.2004.03622.x. [DOI] [PubMed] [Google Scholar]
- Imagawa J, Baxter GF, Yellon DM. Myocardial protection afforded by nicorandil and ischaemic preconditioning in a rabbit infarct model in vivo. J. Cardiovasc. Pharmacol. 1998;31:74–79. doi: 10.1097/00005344-199801000-00011. [DOI] [PubMed] [Google Scholar]
- Kaufman ES, Priori SG, Napolitano C, Schwartz PJ, Iyengar S, Elston RC, Schnell AH, Gorodeski EZ, Rammohan G, Bahhur NO, Connuck D, Verrilli L, Rosenbaum DS, Brown AM. Electrocardiographic prediction of abnormal genotype in congenital long QT syndrome: experience in 101 related family members. J. Cardiovasc. Electrophysiol. 2001;12:455–461. doi: 10.1046/j.1540-8167.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- Killeen MJ, Sabir IN, Grace AA, Huang CL. Dispersions of repolarization and ventricular arrhythmogenesis: lessons from animal models. Prog. Biophys. Mol. Biol. 2008;98:219–229. doi: 10.1016/j.pbiomolbio.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Krahn AD, Healey JS, Chauhan V, Birnie DH, Simpson CS, Champagne J, Gardner M, Sanatani S, Exner DV, Klein GJ, Yee R, Skanes AC, Gula LJ, Gollob MH. Systematic assessment of patients with unexplained cardiac arrest: Cardiac Arrest Survivors With Preserved Ejection Fraction Registry (CASPER) Circulation. 2009;120:278–285. doi: 10.1161/CIRCULATIONAHA.109.853143. [DOI] [PubMed] [Google Scholar]
- Lu HR, Remeysen P, Somers K, Saels A, De Clerck F. Female gender is a risk factor for drug-induced long QT and cardiac arrhythmias in an in vivo rabbit model. J. Cardiovasc. Electrophysiol. 2001;12:538–545. doi: 10.1046/j.1540-8167.2001.00538.x. [DOI] [PubMed] [Google Scholar]
- Milberg P, Tegelkamp R, Osada N, Schimpf R, Wolpert C, Breithardt G, Borggrefe M, Eckardt L. Reduction of dispersion of repolarization and prolongation of postrepolarization refractoriness explain the antiarrhythmic effects of quinidine in a model of short QT syndrome. J. Cardiovasc. Electrophysiol. 2007;18:658–664. doi: 10.1111/j.1540-8167.2007.00813.x. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, Vincent GM, Locati EH, Priori SG, Napolitano C, Medina A, Zhang L, Robinson JL, Timothy K, Towbin JA, Andrews ML. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- Motoyasu M, Nishikawa H, Shimizu Y, Aoki T, Ono N, Unno M, Kakuta Y, Yazu T, Kasai A, Yamakado T. A case of congenital long QT syndrome associated with T wave alternans. Kokyu. To Junkan. 1992;40:195–198. [PubMed] [Google Scholar]
- Nearing BD, Verrier RL. Modified moving average analysis of T-wave alternans to predict ventricular fibrillation with high accuracy. J. Appl. Physiol. 2002;92:541–549. doi: 10.1152/japplphysiol.00592.2001. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Guo W. Heterogeneous expression of voltage-gated potassium channels in the heart: roles in normal excitation and arrhythmias. J. Cardiovasc. Electrophysiol. 2002;13:406–409. doi: 10.1046/j.1540-8167.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- Odening KE, Hyder O, Chaves L, Schofield L, Brunner M, Kirk M, Zehender M, Peng X, Koren G. Pharmacogenomics of anesthetic drugs in transgenic LQT1 and LQT2 rabbits reveal genotype-specific differential effects on cardiac repolarization. Am. J. Physiol Heart Circ. Physiol. 2008;295:H2264–H2272. doi: 10.1152/ajpheart.00680.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori SG, Bloise R, Crotti L. The long QT syndrome. Europace. 2001;3:16–27. doi: 10.1053/eupc.2000.0141. [DOI] [PubMed] [Google Scholar]
- Salama G, Lombardi R, Elson J. Maps of optical action potentials and NADH fluorescence in intact working hearts. Am. J. Physiol. 1987;252:H384–H394. doi: 10.1152/ajpheart.1987.252.2.H384. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. Circulation. 1993;88:782–784. doi: 10.1161/01.cir.88.2.782. An update. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Periti M, Malliani A. The long Q-T syndrome. Am. Heart J. 1975;89:378–390. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the long-QT syndrome: effects of beta-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation. 1998a;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Effects of a K(+) channel opener to reduce transmural dispersion of repolarization and prevent torsade de pointes in LQT1, LQT2, and LQT3 models of the long-QT syndrome. Circulation. 2000;102:706–712. doi: 10.1161/01.cir.102.6.706. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Kamakura S, Arakaki Y, Kamiya T, Shimomura K. T wave alternans in idiopathic long-QT syndrome: insight from body surface mapping. Pacing Clin. Electrophysiol. 1996;19:1130–1133. doi: 10.1111/j.1540-8159.1996.tb03426.x. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Kurita T, Matsuo K, Suyama K, Aihara N, Kamakura S, Towbin JA, Shimomura K. Improvement of repolarization abnormalities by a K+ channel opener in the LQT1 form of congenital long-QT syndrome. Circulation. 1998b;97:1581–1588. doi: 10.1161/01.cir.97.16.1581. [DOI] [PubMed] [Google Scholar]
- Smith JM, Clancy EA, Valeri CR, Ruskin JN, Cohen RJ. Electrical alternans and cardiac electrical instability. Circulation. 1988;77:110–121. doi: 10.1161/01.cir.77.1.110. [DOI] [PubMed] [Google Scholar]
- Taira CA, Opezzo JA, Mayer MA, Hocht C. Cardiovascular Drugs Inducing QT Prolongation: Facts and Evidence. Curr. Drug Saf. 2009 doi: 10.2174/157488610789869229. [DOI] [PubMed] [Google Scholar]
- Taira N. Similarity and dissimilarity in the mode and mechanism of action between nicorandil and classical nitrates: an overview. J. Cardiovasc. Pharmacol. 1987;10(Suppl 8):S1–S9. [PubMed] [Google Scholar]
- Treese N, Erbel R, Meyer J. Acute hemodynamic effects of nicorandil in coronary artery disease. J. Cardiovasc. Pharmacol. 1992;20(Suppl 3):S52–S56. doi: 10.1097/00005344-199206203-00010. [DOI] [PubMed] [Google Scholar]
- Ueda H, Hayashi T, Tsumura K, Yoshimaru K, Nakayama Y, Yoshikawa J. Intravenous nicorandil can reduce QT dispersion and prevent bradyarrhythmia during percutaneous transluminal coronary angioplasty of the right coronary artery. J. Cardiovasc. Pharmacol. Ther. 2004a;9:179–184. doi: 10.1177/107424840400900305. [DOI] [PubMed] [Google Scholar]
- Ueda H, Nakayama Y, Tsumura K, Yoshimaru K, Hayashi T, Yoshikawa J. Intravenous nicorandil can reduce the occurrence of ventricular fibrillation and QT dispersion in patients with successful coronary angioplasty in acute myocardial infarction. Can. J. Cardiol. 2004b;20:625–629. [PubMed] [Google Scholar]
- Vincent GM. The long-QT syndrome--bedside to bench to bedside. N. Engl. J. Med. 2003;348:1837–1838. doi: 10.1056/NEJMp030039. [DOI] [PubMed] [Google Scholar]
- Zareba W, Cygankiewicz I. Long QT syndrome and short QT syndrome. Prog. Cardiovasc. Dis. 2008;51:264–278. doi: 10.1016/j.pcad.2008.10.006. [DOI] [PubMed] [Google Scholar]