Abstract
There is a significant need for evidence-based treatments for adolescent smoking cessation. Prior research, though limited, has suggested potential roles for bupropion SR and contingency management (CM), but no previous studies have assessed their combined effect. In a double-blind, placebo-controlled design, 134 adolescent smokers were randomized to receive a 6-week course of bupropion SR + CM, bupropion SR + non-CM, placebo + CM, or placebo + non-CM, with final follow-up at 12 weeks. The primary outcome was 7-day cotinine-verified point prevalence abstinence, allowing for a 2-week grace period. Combined bupropion SR + CM treatment yielded significantly superior abstinence rates during active treatment when compared with placebo + non-CM treatment. Additionally, combined treatment demonstrated greater efficacy at multiple time points than either bupropion SR + non-CM or placebo + CM treatment. Combined bupropion SR and CM appears efficacious, at least in the short term, for adolescent smoking cessation, and may be superior to either intervention alone.
Keywords: Bupropion, Contingency Management, Nicotine, Smoking, Tobacco, Adolescent, Youth, Treatment
1. Introduction
It is clear that nicotine dependence is an illness with origins in adolescence. Almost 90% of adult smokers start smoking before age 21, and 3% of eighth graders, 6% of tenth graders, and 11% of twelfth graders smoke daily (Centers for Disease Control and Prevention, 2007; Johnston et al., 2009; Substance Abuse and Mental Health Service Administration, 2008). Most adolescents who smoke regularly are likely to continue smoking well into adulthood (Backinger et al., 2003; Chassin et al., 1990), though many may be interested in quitting (Carpenter et al., 2009).
Despite the high prevalence and significant health implications of adolescent smoking, little work has been done to develop smoking cessation programs targeting this age group (Sussman et al., 2006). This area of research has almost exclusively focused on psychosocial treatments, yielding discouraging results overall. For example, a meta-analysis of psychosocial treatments showed a mean quit rate of 12% three months following treatment, compared with 7% among control groups (Sussman, 2002). One psychosocial intervention that has demonstrated encouraging but still preliminary results is contingency management (CM), a behavioral treatment based on operant conditioning, in which desired behaviors (such as smoking abstinence) are directly reinforced with rewards (e.g., vouchers, cash). CM has been studied more extensively in adult smokers, but initial within-subjects feasibility (Corby et al., 2000) and subsequent randomized pilot (Cavallo et al., 2007; Correia & Benson, 2006; Krishnan-Sarin et al., 2006; Roll, 2005) studies suggest that CM, alone or when added to cognitive-behavioral therapy (CBT), may be efficacious for smoking cessation in adolescents. One study (n = 28) (Krishnan-Sarin et al., 2006) demonstrated particularly encouraging results, with 53% of participants receiving CM + CBT achieving abstinence at the end of one-month treatment, compared with 0% of those receiving only CBT. Building upon these pilot findings, a recent larger-scale study (n = 110) investigated a three-week, twice-daily CM intervention, alone or combined with motivational enhancement therapy, among non-treatment seeking college student smokers (Tevyaw et al., 2009). Participants receiving CM (escalating monetary rewards based on expired carbon monoxide levels in the first week and on smoking abstinence in the second and third weeks) demonstrated significantly lower carbon monoxide levels and greater abstinence (55% versus 18% of readings) during treatment than those not receiving CM.
A significant advance in adult smoking cessation has been the introduction of pharmacotherapy targeting nicotine withdrawal and craving, since both are highly predictive of relapse during quit attempts (Killen & Fortmann, 1997; United States Department of Health and Human Services, 2000). Nicotine replacement therapy (NRT), bupropion SR, and varenicline are approved by the U.S. Food and Drug Administration (FDA) for smoking cessation in adults. However, despite clear evidence of nicotine withdrawal and craving in adolescents (Jacobsen et al., 2005; Killen et al., 2001; Prokhorov et al., 2001), limited research has focused on pharmacological agents in adolescent smoking cessation.
A non-controlled, open-label trial (n = 101) of nicotine patch, combined with minimal behavioral treatment, demonstrated point prevalence abstinence rates of 11% at end of treatment and 5% at six-month follow-up (Hurt et al., 2000). A subsequent controlled study (n = 100) supported the safety of patch in adolescents when added to CBT and a CM intervention, but revealed no differences in cessation between patch (end of treatment point prevalence abstinence 28%) and placebo (24%) groups (both groups received CBT + CM) (Hanson et al., 2003). Moolchan and colleagues compared patch, gum, and placebo treatment among adolescent smokers also receiving group-based CBT for smoking cessation (n = 120) (Moolchan et al., 2005). Continuous abstinence following a two-week grace period was achieved by 18%, 7% and 3% in the three groups, respectively. The difference between the patch and placebo groups was statistically significant at the end of treatment, but significance was not sustained at six-month follow-up. A recent pilot investigation (n = 40) revealed poor treatment adherence and no difference in cessation outcome between nicotine nasal spray and placebo (Rubinstein et al., 2008).
In light of the modest effect of NRT, some investigators have focused on bupropion SR for adolescent smoking cessation. Our research group conducted an open-label trial (n = 16) of bupropion SR, combined with brief individual cessation counseling, in adolescent smokers, revealing 31% abstinence after four weeks of treatment (Upadhyaya et al., 2004). Killen and colleagues compared combined treatment with bupropion SR 150 mg daily and nicotine patch to nicotine patch alone, each added to a group skills training intervention (n = 211) (Killen et al., 2004). At treatment conclusion, combined treatment yielded 23% abstinence and patch-only treatment yielded 28% abstinence, and 26-week follow-up revealed only 8% and 7% abstinence rates, respectively. In a subsequent large-scale (n = 312), randomized trial, Muramoto and colleagues compared bupropion SR 300 mg/day, bupropion SR 150 mg/day, and placebo treatment, added to brief weekly individual counseling (Muramoto et al., 2007). The bupropion SR 300 mg/day group (but not the 150 mg/day group) demonstrated superior point prevalence abstinence, compared to placebo, at treatment conclusion (15% versus 6%) and at 26-week follow-up (14% versus 10%).
Comparative interpretation of the aforementioned studies is difficult due to the inconsistency in methodology of, and lack of control for, concurrent psychosocial treatments. Without a more rigorous design, it is impossible to evaluate the relative contribution of pharmacological versus psychosocial treatment effects. Given that overall results appear less robust in these adolescent studies when compared with similar adult studies, careful evaluation of each component of treatment may be critical to the development of optimal evidence-based practice. The present study sought to build upon previous preliminary research by evaluating the individual and combined effects of promising psychosocial and pharmacological treatments for adolescent smoking cessation. Treatment-seeking adolescent smokers were randomized to receive bupropion SR and/or CM, each alone or in combination with the other, in a 2×2 6-week controlled trial. We hypothesized that the combined bupropion SR + CM treatment would have better efficacy than either the bupropion SR + non-CM or placebo + CM treatments, and that all active interventions would have better efficacy than the placebo only intervention.
2. Methods
2.1. Participant Eligibility and Recruitment
To be enrolled in the study, adolescents were required to a) be 12 to 21 years old, b) smoke at least 5 cigarettes per day and express interest in quitting, c) have baseline urine cotinine > 100 ng/mL, d) be non-pregnant and use birth control to avoid pregnancy, e) lack current substance abuse/dependence aside from nicotine, f) have no history of serious psychiatric or medical illness, g) have no suicide attempts in the past year or suicidal ideation in the past month, h) have no history of seizures or eating disorders, and i) not be taking current pharmacotherapy for smoking cessation treatment.
Recruitment occurred primarily through local secondary schools, colleges, universities, and community media advertisements. If an initial telephone screen suggested potential eligibility, participants were scheduled for an informed consent and baseline assessment visit. Participant consent was obtained for all adolescents age 18 or older, while parental consent and participant assent were obtained for those below age 18. The procedures followed were approved by the university Institutional Review Board and were in accord with the Helsinki Declaration of 1975.
2.2. Assessments
Comprehensive psychiatric assessment (First et al., 1994; Shaffer et al., 2000), physical examination, laboratory testing (complete blood count, comprehensive metabolic panel, urine pregnancy test, urine drug screen), and electrocardiogram were performed. A thorough smoking history was obtained, and baseline nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991; Nonnemaker & Homsi, 2007).
2.3. Treatment Randomization
Consenting participants who met eligibility requirements were given quit smoking brochures and randomized to a six-week trial of one of four conditions: 1) Bupropion SR + CM, 2) Bupropion SR + Non-CM, 3) Placebo + CM, or 4) Placebo + Non-CM. Throughout active treatment [Week 0 (medication initiation) – Week 6 (end of treatment)], participants were seen (in the university research clinic or the high school health clinic) twice weekly by the research assistant and once weekly by the study physician. Participants were asked to set a quit date any time within 2 weeks of treatment initiation; abstinence outcomes were thus evaluated only at time points beyond this initial 2-week grace period. A post-treatment follow-up visit with the research assistant and study physician was scheduled 6 weeks after the conclusion of treatment (Week 12).
2.4. Bupropion SR/Placebo
Per dosing recommendations, participants weighing ≥ 90 pounds received bupropion SR or matched placebo (double-blinded; encapsulated by the university Investigational Drug Service so that the active and placebo medication appeared identical) 150 mg every morning for three days, titrated to 150 mg every morning and 150 mg every afternoon thereafter, as tolerated. Participants weighing < 90 pounds received bupropion SR or matched placebo 150 mg every morning throughout the trial, as tolerated. Medication was started at Week 0 and discontinued at Week 6. Medication adherence was documented by participants in daily self-report diaries and confirmed with weekly pill counts conducted by research personnel.
2.5. Contingency Management/Compensation
The CM protocol roughly followed precedent from others (Budney & Higgins, 1998; Kamon et al., 2005), with twice-weekly assessments, judged as a feasible frequency for translation to clinical practice. In contrast, some previous studies have involved “higher-dose” CM, with daily (Cavallo et al., 2007; Correia & Benson, 2006; Krishnan-Sarin, 2006; Roll, 2005) or twice-daily (Tevyaw et al., 2009) assessments, often conducted in a school setting. In the present study, participants randomized to receive CM were eligible for an escalating schedule of cash payments, with resets, contingent upon abstinence from smoking, assessed twice weekly starting one week from medication initiation. Thus, participants in the CM groups were eligible for up to 11 smoking-contingent payments (at the end of the first week of treatment and twice weekly thereafter). Cash payments were used instead of vouchers due to the potential for improved participant satisfaction and retention (Festinger et al., 2008). Consistent with prior studies (e.g., Higgins et al., 2004), carbon monoxide breathalyzer-verified abstinence (cutoff: 7 ppm) served as the basis for reward at the end of Week 1 (Jatlow et al., 2008), and reward thereafter was based on self-reported abstinence confirmed with urine cotinine immunoassay test strips (cutoff: ≤ 100 ng/mL) (Schepis et al., 2008). Payment for the initial biologically verified abstinent visit was $10, with subsequent consecutive abstinent visits escalating by $3 ($13, $16, $19, and so on). If a participant relapsed, he/she was not eligible for contingent compensation at that visit, and the contingent reward for the next abstinent visit was re-set to $10 (with eligibility to escalate by $3 at subsequent abstinent visits). Thus, the maximum amount of compensation throughout the 6-week treatment period was $275.
Participants randomized to non-CM groups received fixed compensation ($10 per visit) for attending the twice-weekly treatment visits.
Participants in all groups were eligible for a weekly bonus payment of $5 throughout active treatment for completion of study materials, including daily smoking diaries. Additionally, all participants received $30 for completing the initial assessment visit, $20 for completing the initial medication management visit, and $20 for completing the final post-treatment follow-up visit.
2.6. Smoking Outcome Measures
Biologically verified 7-day point prevalence abstinence was assessed weekly, beginning on Week 3 (i.e., the week following the two-week grace period), throughout treatment (Weeks 4–6) and again at the post-treatment follow-up visit (Week 12). Abstinence was measured by participant self-report (smoking diary) and confirmed by urine cotinine ≤ 100 ng/mL. Participants reporting minimal smoking “slips” (≤ 2 cigarettes on ≤ 2 days in a given week) were considered abstinent if supported by biological verification (Hughes et al., 2003). If a treatment visit was missed or if a urine cotinine value was unavailable at any visit, the participant was considered to have relapsed.
2.7. Safety Monitoring
Adverse events were assessed during weekly medication management visits with the study physician, via detailed interview and structured review of systems (Kalachnik, 2001). Vital sign measurements, urine pregnancy tests, and suicidality assessments were conducted weekly, and follow-up laboratory tests (complete blood count and comprehensive metabolic panel) and electrocardiograms were performed at Week 4 of treatment.
2.8. Statistical Analysis
Efficacy and intent-to-treat comparisons are reported for all randomized participants (regardless of protocol failures or study status). Sample size estimation was based on preliminary data suggesting an estimated 32% abstinence rate in the bupropion SR group and between 9 and 19% in the placebo group. It was estimated that 54 subjects were needed in each of the four treatment groups (Bupropion SR + CM, Bupropion SR + Non-CM, Placebo + CM, and Placebo + Non-CM) to attain a power of between 0.77 and 0.87 to detect this difference at an alpha level of 0.05 using a two-tailed χ2 statistic. Baseline demographic and smoking history variables were analyzed with the Kruskal-Wallis test statistic for continuous variables and the Chi-Square test statistic for categorical variables. All baseline tests were 2-sided and were analyzed at an alpha level of 0.05. The effects of bupropion SR and CM on 7-day point prevalence abstinence rates were assessed by logistic regression models (Agresti, 2007), where smoking status was the dependent variable of interest. Post hoc cell comparisons of interest were made following analysis of the main effects when significant effects were noted. Adverse event frequencies and medication adherence were compared across treatment groups with a chi-square test statistic. The Mantel-Cox (log rank) test was used to determine differential retention effects of both bupropion SR and CM while overall treatment completion was compared with logistic regression. For all analyses, statistical significance was measured at a 2-sided alpha level of 0.05, marginal significance was measured at an alpha level of 0.10, and all reported p values were not adjusted for multiple comparisons. SAS software (Version 9.1.3, SAS Institute, Cary, NC) was used in all statistical analyses.
3. Results
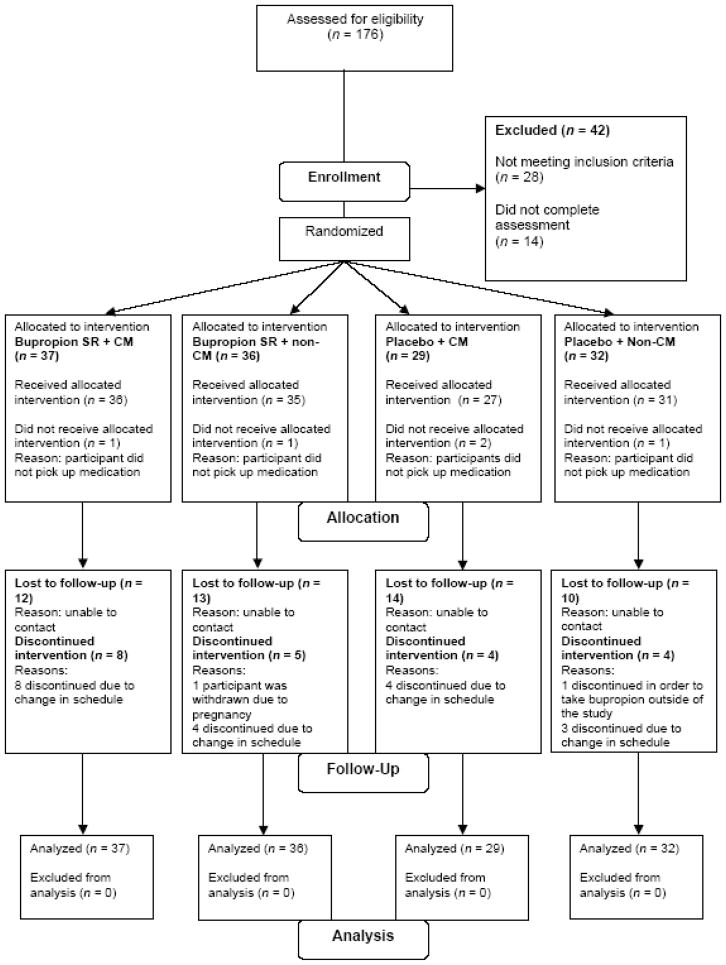
Between March 2004 and June 2008, 134 adolescent smokers were randomized into one of four treatment groups (37 in Bupropion SR + CM, 36 in Bupropion SR + Non-CM, 29 in Placebo + CM, and 32 in Placebo + Non-CM) (see Figure 1). The groups did not differ significantly in demographics, baseline characteristics, or smoking history (see Table 1).
Figure 1.
Participant enrollment flow chart.
Table 1.
Baseline demographics, characteristics, and smoking history
| Variable | Bupropion SR + CM (n = 37) | Bupropion SR + Non-CM (n = 36) | Placebo + CM (n = 29) | Placebo + Non-CM (n = 32) | p-value* |
|---|---|---|---|---|---|
| Age | 18.4 (1.9) | 18.4 (1.9) | 18.1 (1.9) | 19.0 (1.8) | 0.26 |
| % Male | 56.8 | 61.1 | 62.1 | 53.1 | 0.89 |
| % White | 94.6 | 86.1 | 89.7 | 84.4 | 0.55 |
| % with history of ADHD | 32.4 | 38.9 | 34.5 | 34.4 | 0.95 |
| % in school | 67.6 | 75.0 | 69.0 | 68.8 | 0.90 |
| % that live with a smoker | 70.3 | 66.7 | 62.1 | 62.5 | 0.90 |
| Prior Quit Attempts (SD) | 3.3 (4.2) | 2.6 (2.0) | 1.8 (1.3) | 3.1 (2.6) | 0.20 |
| Years of Smoking | 4.2 (2.3) | 3.7 (2.1) | 4.1 (2.3) | 4.2 (2.0) | 0.73 |
| Baseline Cigarettes per Day | 11.3 (6.3) | 11.5 (8.1) | 11.2 (6.4) | 9.1 (4.5) | 0.61 |
| FTND (Nicotine Dependence) Score (range 0 – 10) | 4.0 (2.3) | 4.1 (2.3) | 4.1 (2.0) | 4.5 (2.3) | 0.89 |
| Baseline Expired Carbon Monoxide Level (ppm) | 7.8 (5.4) | 7.9 (5.0) | 9.2 (8.3) | 8.8 (6.9) | 0.99 |
| Baseline Urine Cotinine Level (ng/mL) | 1023.9 (911.8) | 1056.3 (659.6) | 964.9 (627.6) | 1059.4 (774.2) | 0.89 |
all p-values are 2-sided Kruskal-Wallis p-values or Fisher Exact Values
3.1. Abstinence
Seven-day point prevalence abstinence rates (self-report confirmed by urine cotinine) at weekly treatment visits and at the post-treatment follow-up visit are reported in Table 2. Table 3 lists the Odds Ratios as well as the corresponding 95% Confidence Interval for each main (pooled) effect (Bupropion SR, CM) and all post hoc treatment group comparisons (Parzen et al, 2002). Bupropion SR treatment (Bupropion SR + CM and Bupropion SR + Non-CM groups) was superior to placebo treatment (Placebo + CM and Placebo + Non-CM groups) at Weeks 3 and 4 (p < 0.01 and p < 0.05, respectively) and marginally superior at Week 5 (p = 0.07). CM treatment (Bupropion SR + CM and Placebo + CM groups) was marginally superior to non-CM treatment (Bupropion SR + Non-CM and Placebo + Non-CM groups) at Week 6 (p = 0.08). Although interactions were tested, the study was not powered to detect such an effect and the bupropion SR × CM interaction was insignificant at all time points (p = 0.42 to 0.97). When comparing individual treatment arms, combined bupropion SR + CM yielded significantly superior abstinence rates relative to placebo + non-CM treatment at Week 3, Week 4, and Week 5 (p = 0.01, p < 0.05, and p < 0.05, respectively), and a marginally superior rate at Week 6 (p = 0.07). Compared with medication-only treatment (Bupropion SR + Non-CM), combined treatment (Bupropion SR + CM) was marginally superior at Week 3 (p = 0.09) and significantly superior at Weeks 4 and 6 (p < 0.05 for both weeks). Compared with CM-only treatment (Placebo + CM), combined treatment (Bupropion SR + CM) was superior at Weeks 3 and 4 (p = 0.02 for both weeks) and marginally superior at Week 5 (p = 0.08) and at Week 12 post-treatment follow-up (p < 0.10).
Table 2.
Cotinine-confirmed 7-day point prevalence abstinence [n (%)] by treatment group
| Week | Bupropion SR + CM (n = 37) | Bupropion SR + Non-CM (n = 36) | Placebo + CM (n = 29) | Placebo + Non-CM (n = 32) |
|---|---|---|---|---|
| 3† | 14 (37.8) | 7 (19.4) | 3 (10.3) | 3 (9.4) |
| 4 | 14 (37.8) | 6 (16.7) | 3 (10.3) | 5 (15.6) |
| 5 | 11 (29.7) | 5 (13.9) | 3 (10.3) | 3 (9.4) |
| 6 (End of Treatment) | 10 (27.0) | 3 (8.3) | 3 (10.3) | 3 (9.4) |
| 12 (Follow-Up) | 4 (10.8) | 2 (5.6) | 0 (0.0) | 2 (6.3) |
following initial 2-week grace period
Table 3.
Cotinine-confirmed 7-day point prevalence abstinence logistic regression results, summary of treatment comparisons [Odds Ratio (95% Confidence Interval)]
| Week | Pooled Effects | Pair Wise Treatment Comparisons | ||||||
|---|---|---|---|---|---|---|---|---|
| Bupropion SR (n = 73) | CM (n = 66) | Bupropion SR + Non-CM vs. Placebo + Non-CM | Placebo + CM vs. Placebo + Non-CM | Bupropion SR + CM vs. Placebo + Non-CM | Bupropion SR + CM vs. Bupropion SR + Non-CM | Bupropion SR + CM vs. Placebo + CM | Bupropion SR + Non-CM vs. Placebo + CM | |
| 3† | 3.7** (1.4 – 10.0) | 2.0 (0.8 – 4.9) | 2.3 (0.6 – 9.9) | 1.1 (0.2 – 6.0) | 5.9** (1.5 – 23.0) | 2.5* (0.9 – 7.3) | 5.3** (1.3 – 20.7) | 2.1 (0.5 – 8.9) |
| 4 | 2.5** (1.0 – 6.2) | 1.8 (0.8 – 4.2) | 1.1 (0.3 – 4.0) | 0.6 (0.1 – 2.9) | 3.3** (1.0 – 10.5) | 3.0** (1.0 – 9.1) | 5.3** (1.3 – 20.7) | 1.7 (0.4 – 7.6) |
| 5 | 2.6* (0.9 – 7.1) | 2.0 (0.8 – 5.2) | 1.6 (0.3 – 7.1) | 1.1 (0.2 – 6.0) | 4.1** (1.0 – 16.3) | 2.6 (0.8 – 8.5) | 3.7* (0.9 – 14.7) | 1.4 (0.3 – 6.4) |
| 6 (End of Treatment) | 2.0 (0.7 – 5.6) | 2.5* (0.9 – 7.1) | 0.9 (0.2 – 4.7) | 1.1 (0.2 – 6.0) | 3.6* (0.9 – 14.4) | 4.1** (1.0 – 16.3) | 3.2 (0.8 – 13.0) | 0.8 (0.1 – 4.2) |
| 12 (Follow-Up) | 2.6 (0.5 –13.6) | 1.0 (0.2 – 4.2) | 0.9 (0.1 – 6.7) | 0.2§ (0.6 – 3.8) | 1.8 (0.3 – 10.7) | 2.1 (0.4 – 12.0) | 10.5*§ (0.8 – 23.4) | 5.3§ (0.2–13.9) |
Calculated using Modified Median Unbiased Estimator of Odds Ratio and Bootstrapped 95% Confidence Interval (Parzen et al., 2002) due to cells with count zero
following initial two-week grace period
p < 0.05
p < 0.10
There was no significant effect of gender on smoking outcome (p > 0.60), and there was no gender × bupropion SR interaction effect (p > 0.25). Participants without history of ADHD were marginally more likely to be abstinent than those with history of ADHD at Weeks 5 and 6 (p < 0.10).
3.2. Safety/Adverse Events
A total of 76 (57%) participants experienced at least one adverse event. There was no significant difference between treatment groups in the number of participants reporting at least one adverse event (68% in Bupropion SR + CM, 61% in Bupropion SR + Non-CM, 48% in Placebo + CM, and 47% in Placebo + Non-CM; p = 0.24). Adverse events were marginally more common in the bupropion SR groups (pooled) than in the placebo groups (64% vs. 48%; p = 0.05). Headaches, insomnia, irritability, and dream disturbances were reported by more than 20% of participants in at least one treatment group. Headache and irritability occurred in the Bupropion SR + Non-CM group most often while insomnia and dream disturbances occurred most often in the Bupropion SR + CM group. Of the nine reported dream disturbances, all were in active medication groups. No seizures or suicidal behaviors were reported.
All randomized participants weighed at least 90 pounds at initial assessment, and were thus titrated to 300 mg per day bupropion SR or placebo. Over the course of study enrollment, 5 participants (3 in Bupropion SR + CM and 2 in Bupropion SR + Non-CM) reduced from 300 mg to 150 mg/day and 6 participants (1 in Bupropion SR + CM, 2 in Bupropion SR + Non-CM, 2 in Placebo + CM, and 1 in Placebo + Non-CM) discontinued medication due to adverse effect/tolerability issues.
3.3. Participant Retention/Medication Adherence
Participants receiving bupropion SR took 85% of dispensed doses, and those receiving placebo took 83% of dispensed doses. There were no significant between-group differences in medication adherence.
Of the 134 participants randomized, 41 (31%) completed all treatment visits, and 30 (22%) completed all study visits (treatment + follow-up). The Bupropion SR + CM group had the highest rate of treatment visit completion (38%) followed by the Bupropion SR + Non-CM group (36%), the Placebo + Non-CM group (28%), and the Placebo + CM group (17%). There was no significant difference in retention between the four treatment groups or between those receiving CM treatment and those not. Treatment completion was marginally higher among participants in bupropion SR groups (pooled) than among those in placebo groups (37% vs. 23%; OR = 2.0, p = 0.08). Participants in active medication groups had a slightly higher probability of being retained in the study for a longer period of time (median 38 days) than those in placebo groups (24 days; log rank p = 0.09).
Participants in bupropion SR groups (pooled) attended more abstinence assessment visits following medication initiation (6 weekly during treatment + 1 follow-up) than those in placebo groups (3.8 ± 2.7 [mean ± SD] attended in bupropion SR groups vs. 2.8 ± 2.6 in placebo groups; p = 0.02). There was no difference in the number of abstinence assessments attended across CM treatment status (3.2 ± 2.7 attended in CM groups vs. 3.6 ± 2.7 in non-CM groups; p = 0.28). Fifty-five participants (41% of the 134 randomized; 15 in Bupropion SR + CM, 10 in Bupropion SR + Non-CM, 16 in Placebo + CM, 14 in Placebo + Non-CM) did not attend any visits following the two-week grace period and were assumed to be smoking at all remaining time points.
4. Discussion
The present study is the first 2×2 investigation of pharmacological and psychosocial treatment for adolescent smoking cessation. Results indicate that combined treatment with bupropion SR and CM is superior to placebo and non-CM throughout active treatment. Moreover, findings suggest that combined treatment may be superior to either bupropion SR or CM alone, at least during treatment.
Comparison of end-of-treatment cotinine-confirmed point prevalence abstinence between the present study and the findings of Muramoto and colleagues (Muramoto et al., 2007) is warranted. Our study revealed abstinence rates of 27% for combined bupropion SR + CM, 8% for bupropion SR + non-CM, 10% for placebo + CM, and 9% for placebo + non-CM, while Muramoto and colleagues reported rates of 15% for bupropion SR 300 mg/day, 11% for bupropion SR 150 mg/day, and 6% for placebo (all added to brief individual counseling). While we were unable to replicate the finding of superior efficacy with bupropion SR alone, our results indicate that the combination of bupropion SR and CM enhances end of treatment abstinence four-fold when compared with bupropion SR alone. Indeed, CM appeared most powerful in our study when used to augment active pharmacotherapy. CM yielded only trend level main effects on abstinence at end-of-treatment, but when added to bupropion SR was associated with increased odds of abstinence at multiple time points. It is likely that a more intensive CM schedule, with more frequent assessments and opportunities for reward, would exert a stronger main effect, in line with other recent studies (Krishnan-Sarin et al., 2006; Tevyaw et al., 2009).
Our results are compromised by limitations in power, owing in large part to challenges with recruitment and retention, which are known to be common in adolescent smoking cessation research (Kealey et al., 2007). Additionally, our reported abstinence rates may be underestimates, as we conservatively opted to consider participants to be non-abstinent at all missed visits. Our treatment completion rate, 30% inclusive of all randomization groups, was somewhat lower than those in prior large-scale adolescent smoking cessation pharmacotherapy studies, which have ranged roughly from 40% to 60% (Hanson et al., 2003; Moolchan et al., 2005; Muramoto et al., 2007). We encountered several potential contributing factors. While adolescent smoking is common, numbers diminish when narrowing adolescent smokers to the eligibility criteria imposed for this study. Furthermore, in light of the extraordinary recent caution around adolescent pharmacotherapy (Cheung et al., 2008; Nemeroff et al., 2007), many adolescents and families may be unwilling to consent to an investigational treatment for smoking cessation, particularly one carrying a U.S. FDA “black box warning.” Another likely contributing factor, particularly to poor retention, was adolescent/family difficulty with transportation and scheduling to participate in a two-hour assessment/screening visit and adhere to twice weekly treatment visits at a downtown academic medical center during business hours. Over the course of the study, we undertook several measures to enhance recruitment and retention. Among the most beneficial was establishing after-school research clinics in local high schools, allowing students to participate in the study without additional transportation and scheduling requirements. We recommend that, at the outset, future studies should incorporate strategies, such as in-school or at-home (Reynolds et al., 2008) treatment, to overcome recruitment and retention barriers.
Contrary to our expectations, CM did not enhance study retention. We suspect that the choice of CM design, while based on prior studies (Budney & Higgins, 1998; Kamon et al., 2005), may not have been a sufficiently powerful reinforcer of treatment adherence to convey retention effects. Participants in the CM groups who had difficulty achieving abstinence or who relapsed were not eligible for contingent reinforcement, and may thus have been inadvertently discouraged from attending treatment visits. A 2-tiered escalating reinforcement CM model, separately reinforcing study retention and abstinence, may be a means of addressing this issue (Carroll et al., 2006). More frequent CM reinforcement opportunities, as used in other studies (Cavallo et al., 2007; Krishnan-Sarin et al., 2006; Roll, 2005; Tevyaw et al., 2009), may additionally improve retention.
Adolescent smoking cessation remains a particular challenge with considerable public health implications. In addition to the inherent difficulties with treatment recruitment and retention, there has been a dearth of solid evidence to indicate a “go-to” treatment approach. Psychosocial treatments, often regarded as first-line due to low potential for risks and the perception that pharmacotherapy may not be indicated early in the process of nicotine dependence, have had overall limited efficacy (Sussman, 2002). Pharmacotherapy, while less extensively studied, has also yielded mixed results in adolescents, with cessation outcomes often lagging behind those in adults (Richmond & Zwar, 2003). Contrary to commonly held assumptions, nicotine dependent adolescents may require more intensive cessation treatments than adults. Combining treatment approaches with distinct and potentially complementary mechanisms of action may be indicated. Results of the present study suggest that carefully selected approaches combining pharmacotherapy and psychosocial treatment could be used to enhance treatment outcomes.
Conclusions
Taking the findings in the context of limitations, the present results provide support for the use of combined bupropion SR and CM for adolescent smoking cessation. Combined treatment was well tolerated and acutely efficacious, more than tripling the odds of abstinence throughout active treatment. However, further work is needed to determine the long-term efficacy of this treatment approach. Future studies should consider incorporating a larger sample size, a longer course of active treatment, adjustment of CM reinforcement schedules, and added techniques to enhance participant retention in order to attain adequate power for long-term treatment comparisons.
Acknowledgments
Funding was provided by National Institute on Drug Abuse (NIDA) grants R01 DA17460 (HPU, KMG), K12 DA000357 (KMG), K23 DA020482 (MJC), and R25 DA020537 (ALL), by Eunice Kennedy Shriver National Institute of Child Health & Human Development grant K12 HD055885 (KJH), and by United States Public Health Service grant M01 RR01070 (MUSC CTRC).
The authors wish to thank Erin Klintworth, Ashley Leinbach, Erin EuDaly, Christine Horne, Robert F. Woolson, Ph.D., the Charleston County School District, the MUSC Clinical and Translational Research Center (CTRC) staff, the MUSC Investigational Drug Service, LRADAC, and all participants for invaluable contributions to this study.
Footnotes
Clinical Trials Registry: Combined Pharmaco/Behavior Therapy in Adolescent Smokers; NCT00330187; http://clinicaltrials.gov/ct2/show/study/NCT00330187
A summary of study findings was presented at the College on Problems of Drug Dependencse 2009 Annual Meeting (abstract/oral presentation) and the American Academy of Child & Adolescent Psychiatry 2009 Annual Meeting (abstract/poster presentation).
Financial Disclosures
Dr. Gray has received research support from Pfizer, Inc. (medication and placebo supply for NIDA funded research). Dr. Hartwell has received grant support through Global Research Awards for Nicotine Dependence (GRAND), an independent competitive grants program supported by Pfizer, Inc. Dr. Hiott is a past speakers’ bureau member of Bristol-Myers Squibb and Abbott Labs. Dr. Deas has been an advisory board and speakers’ bureau member of Eli Lilly and Company. Dr. Upadhyaya is a past consultant and/or advisory board member of Eli Lilly and Company and Shire Pharmaceuticals. Dr. Upadhyaya is an ex-stockholder of New River Pharmaceutical Company, is a past speakers’ bureau member of Shire Pharmaceuticals and Pfizer, Inc., and has received research support from Cephalon, Inc., Eli Lilly and Company, and Pfizer, Inc. Dr. Upadhyaya recently became an employee of, and is a holder of stock in, Eli Lilly and Company. The other investigators deny any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agresti A. An Introduction to Categorical Data Analysis. Hoboken, NJ: John Wiley & Sons; 2007. [Google Scholar]
- Backinger CL, Fagan P, Matthews E, Grana R. Adolescent and young adult tobacco prevention and cessation: Current status and future directions. Tobacco Control. 2003;12:46–53. doi: 10.1136/tc.12.suppl_4.iv46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST. A Community Reinforcement Plus Vouchers Approach: Treating Cocaine Addiction. Bethesda, MD: National Institute on Drug Abuse; 1998. [Google Scholar]
- Carpenter MJ, Garrett-Mayer E, Vitoc C, Cartmell K, Biggers S, Alberg AJ. Adolescent nondaily smokers: Favorable views of tobacco yet receptive to cessation. Nicotine & Tobacco Research. 2009;11:348–355. doi: 10.1093/ntr/ntp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, Rounsaville BJ. The use of contingency management and motivational/skills—building therapy to treat young adults with marijuana dependence. Journal of Consulting and Clinical Psychology. 2006;74:955–966. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo DA, Cooney JL, Duhig AM, Smith AE, Liss TB, McFetridge AK, Babuscio T, Nich C, Carroll KM, Rounsaville BJ, Krishnan-Sarin S. Combining cognitive behavioral therapy with contingency management for smoking cessation in adolescent smokers: A preliminary comparison of two different CBT formats. American Journal on Addictions. 2007;16:468–474. doi: 10.1080/10550490701641173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. [Accessed January 18, 2010];Targeting Tobacco Use: The Nation’s Leading Cause of Preventable Death. 2007 Available online at: http://www.cdc.gov/nccdphp/publications/aag/pdf/osh.pdf.
- Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking. Health Psychology. 1990;9:701–716. doi: 10.1037//0278-6133.9.6.701. [DOI] [PubMed] [Google Scholar]
- Cheung A, Sacks D, Dewa CS, Pong J, Levitt A. Pediatric prescribing practices and the FDA Black-box warning on antidepressants. Journal of Developmental & Behavioral Pediatrics. 2008;29:213–215. doi: 10.1097/DBP.0b013e31817bd7c9. [DOI] [PubMed] [Google Scholar]
- Corby EA, Roll JM, Ledgerwood DM, Schuster CR. Contingency management interventions for treating the substance abuse of adolescents: A feasibility study. Experimental and Clinical Psychopharmacology. 2000;8:371–376. doi: 10.1037//1064-1297.8.3.371. [DOI] [PubMed] [Google Scholar]
- Correia CJ, Benson TA. The use of contingency management to reduce cigarette smoking among college students. Experimental and Clinical Psychopharmacology. 2006;14:171–179. doi: 10.1037/1064-1297.14.2.171. [DOI] [PubMed] [Google Scholar]
- Festinger DS, Marlowe DB, Dugosh KL, Croft JR, Arabia PL. Higher magnitude cash payments improve research follow-up rates without increasing drug use or perceived coercion. Drug and Alcohol Dependence. 2008;96:128–135. doi: 10.1016/j.drugalcdep.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for Axis I DSM-IV-TR Axis 1 Disorders-Non Patient Edition (SCID-I/NP, November 2002 revision) New York, New York: New York State Psychiatric Institute; 1994. [Google Scholar]
- Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine & Tobacco Research. 2003;5:515–526. doi: 10.1080/14622200307243. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, Lynch ME, Badger GJ. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine & Tobacco Research. 2004;6:1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measurements of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Hurt RD, Croghan GA, Beede SD, Wolter TD, Croghan IT, Patten CA. Nicotine patch therapy in 101 adolescent smokers. Archives of Pediatrics and Adolescent Medicine. 2000;154:31–37. [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jatlow P, Toll BA, Leary V, Krishnan-Sarin S, O’Malley SS. Comparison of expired carbon monoxide and plasma cotinine as markers of cigarette abstinence. Drug and Alcohol Dependence. 2008;98:203–209. doi: 10.1016/j.drugalcdep.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Smoking continues gradual decline among U.S. teens, smokeless tobacco threatens a comeback. Ann Arbor, MI: University of Michigan News Service; 2009. Dec 14, [Accessed January 18, 2010]. Available online at: http://www.monitoringthefuture.org. [Google Scholar]
- Kalachnik JE. Standardized Monitoring for Psychopharmacologic Medication Side Effects. Manual for the Monitoring of Side Effects Scale (MOSES) Columbia, SC: University of South Carolina, School of Medicine, Department of Pediatrics, Center for Disability Resources, Columbia, SC, and the South Carolina Department of Disabilities and Special Needs; 2001. [Google Scholar]
- Kamon J, Budney A, Stanger C. A contingency management intervention for adolescent marijuana use and conduct problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:513–521. doi: 10.1097/01.chi.0000159949.82759.64. [DOI] [PubMed] [Google Scholar]
- Kealey KA, Ludman EJ, Mann SL, Marek PM, Phares MM, Riggs KR, Peterson AV., Jr Overcoming barriers to recruitment and retention in adolescent smoking cessation. Nicotine & Tobacco Research. 2007;9:257–270. doi: 10.1080/14622200601080315. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: Findings from three prospective studies. Experimental and Clinical Psychopharmacology. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Killen JD, Ammerman S, Rohas N, Varady J, Haydel F, Robinson TN. Do adolescent smokers experience withdrawal effects when deprived of nicotine? Experimental and Clinical Psychopharmacology. 2001;9:176–182. doi: 10.1037//1064-1297.9.2.176. [DOI] [PubMed] [Google Scholar]
- Killen JD, Robinson TN, Ammerman S, Hayward C, Rogers J, Stone C, Samuels D, Levin SK, Green S, Schatzberg AF. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. Journal of Consulting and Clinical Psychology. 2004;72:729–735. doi: 10.1037/0022-006X.72.4.729. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Duhig AM, McKee SA, McMahon TJ, Liss T, McFetridge A, Cavallo DA. Contingency management for smoking cessation in adolescent smokers. Experimental and Clinical Psychopharmacology. 2006;14:306–310. doi: 10.1037/1064-1297.14.3.306. [DOI] [PubMed] [Google Scholar]
- Moolchan ET, Robinson ML, Ernst M, Cadet JL, Pickworth WB, Heishman SJ, Schroeder JR. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics. 2005;115:e407–e414. doi: 10.1542/peds.2004-1894. [DOI] [PubMed] [Google Scholar]
- Muramoto ML, Leischow SJ, Sherrill D, Matthews E, Strayer LJ. Randomized, double-blind, placebo-controlled trial of 2 dosages of sustained-release bupropion for adolescent smoking cessation. Archives of Pediatrics and Adolescent Medicine. 2007;161:1068–1074. doi: 10.1001/archpedi.161.11.1068. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Kalali A, Keller MB, Charney DS, Lenderts SE, Cascade EF, Stephenson H, Schatzberg AF. Impact of publicity concerning pediatric suicidality data on physician practice patterns in the United States. Archives of General Psychiatry. 2007;64:466–472. doi: 10.1001/archpsyc.64.4.466. [DOI] [PubMed] [Google Scholar]
- Nonnemaker JM, Homsi G. Measurement properties of the Fagerström Test for nicotine dependence adapted for use in an adolescent sample. Addictive Behaviors. 2007;32:181–186. doi: 10.1016/j.addbeh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Parzen M, Lipsitz S, Ibrahim J, Klar N. An estimate of the odds ratio that always exists. Journal of Computational and Graphical Statistics. 2002;11:420–436. [Google Scholar]
- Prokhorov AV, Hudmon KS, de Moor CA, Kelder SH, Conroy JL, Ordway N. Nicotine dependence, withdrawal symptoms, and adolescents’ readiness to quit smoking. Nicotine & Tobacco Research. 2001;3:151–155. doi: 10.1080/14622200110043068. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Dallery J, Shroff P, Patak M, Leraas K. A web-based contingency management program with adolescent smokers. Journal of Applied Behavioral Analysis. 2008;41:597–601. doi: 10.1901/jaba.2008.41-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug and Alcohol Review. 2003;22:203–220. doi: 10.1080/09595230100100642. [DOI] [PubMed] [Google Scholar]
- Roll JM. Assessing the feasibility of using contingency management to modify cigarette smoking by adolescents. Journal of Applied Behavioral Analysis. 2005;38:463–467. doi: 10.1901/jaba.2005.114-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Benowitz NL, Auerback GM, Moscicki A. A randomized trial of nicotine nasal spray in adolescent smokers. Pediatrics. 2008;122:e595–e600. doi: 10.1542/peds.2008-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Duhig AM, Liss T, McFetridge A, Wu R, Cavallo DA, Dahl T, Jatlow P, Krishnan-Sarin S. Contingency management for smoking cessation: Enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine & Tobacco Research. 2008;10:1495–1501. doi: 10.1080/14622200802323209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies, NSDUH Series H-34, DHHS Publication No. SMA 08-4343. Rockville, MD: 2008. [Accessed January 18, 2009]. Results from the 2007 National Survey on Drug Use and Health: National Findings. Available online at: http://oas.samhsa.gov/NSDUH/2k7NSDUH/2k7results.cfm#Ch4. [Google Scholar]
- Sussman S. Effects of sixty-six adolescent tobacco use cessation trials and seventeen prospective studies of self-initiated quitting. Tobacco Induced Diseases. 2002;1:35–81. doi: 10.1186/1617-9625-1-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychology. 2006;25:549–557. doi: 10.1037/0278-6133.25.5.549. [DOI] [PubMed] [Google Scholar]
- Tevyaw TO, Colby SM, Tidey JW, Kahler CW, Rohsenow DJ, Barnett NP, Gwaltney CJ, Monti PM. Contingency management and motivational enhancement: A randomized clinical trial for college student smokers. Nicotine & Tobacco Research. 2009;11:739–749. doi: 10.1093/ntr/ntp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Reducing Tobacco Use: A Report of the Surgeon General. Atlanta, GA: United States Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2000. [Accessed January 18, 2010]. Available online at: http://www.cdc.gov/tobacco/data_statistics/sgr/sgr_2000/index.htm. [Google Scholar]
- Upadhyaya HP, Brady KT, Wang W. Bupropion SR in adolescents with comorbid ADHD and nicotine dependence: A pilot study. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:199–205. doi: 10.1097/00004583-200402000-00016. [DOI] [PubMed] [Google Scholar]