Summary
The nonapeptide oxytocin and its receptor have been implicated in the regulation of mammalian social behavior and stress physiology. Evidence is accumulating that the quality of the parental environment is associated with oxytocin biology in children. The present study was designed to examine the interaction of the single nucleotide polymorphism (SNP) rs2254298 within the oxytocin receptor (OXTR) gene and quality of parental environment in predicting children's psychosocial functioning. More specifically, in a sample of 92 Caucasian adolescent girls (9- to 14-years old), we examined whether adverse parental environment, operationalized as mothers' history of recurrent major depressive disorder, interacts with the rs2254298 SNP on the OXTR gene to predict daughters' symptoms of depression and anxiety. Caucasian girls who both were heterozygous for the OXTR rs2254298 polymorphism and had high early adversity reported the highest levels of symptoms of depression, physical anxiety, and social anxiety. These findings highlight the potential importance of this OXTR gene polymorphism in the etiology of depression and anxiety disorders.
Keywords: oxytocin receptor gene, rs2254298, maternal depression, anxiety, adolescents
Introduction
A large literature has documented the importance of oxytocin in affiliation. This nonapeptide hormone has also been found to attenuate the stress response in both rats and humans (see Lee et al., 2009, for a review). Intracerebral oxytocin has been shown to inhibit the responsiveness of the hypothalamic-pituitary-adrenal axis in rats (Neumann, 2002), and intranasal administration of oxytocin has been associated with decreased amygdalar activation in response to threatening scenes in humans (Kirsch et al., 2005). Importantly, researchers have found that oxytocin biology is associated with the quality of parental environments. Adult female rats that received lower levels of maternal licking and grooming as pups subsequently exhibited decreased oxytocin receptor binding in several brain regions (Francis et al., 2000). Adverse early parental environments have been associated with lower oxytocin concentrations in cerebrospinal fluid (Winslow et al., 2003; Heim et al., 2008) and disruption of oxytocin biology in regulating stress-related cortisol responses in adult men (Meinlschmidt and Heim, 2007). These findings suggest that disruption of oxytocin biology is one mechanism through which early adverse environments lead to greater vulnerability to stress and stress-related psychosocial outcomes.
An unexplored alternative mechanism through which adverse environments may lead to poor psychosocial outcomes is the interaction of parental environments with genetic variability in oxytocin biology. One such candidate is the oxytocin receptor (OXTR) gene. For example, being an AG carrier for the single nucleotide polymorphism (SNP) rs2254298 on the OXTR gene has been found to be associated with loneliness in adolescents (Lucht et al., 2009). Whether this SNP, in interaction with an adverse parental environment, is associated with poor psychosocial functioning remains unclear.
The present study was designed to examine the interactive effects of an early adverse parental environment and the OXTR rs2254298 polymorphism in predicting poor psychosocial outcomes. Early adverse environment was operationalized based on mothers' history of Major Depressive Disorder (MDD). Compared to nondepressed mothers, depressed mothers report more negative and critical perceptions of their children and exhibit more hostility and intrusive behaviors towards their offspring (see Lovejoy et al., 2000, for a review). Maternal depression significantly increases the risk that offspring will develop psychiatric disorders (see Hammen, 2009, for a review). Thus, participants in the present study were adolescent girls whose mothers either had a history of recurrent depression during their daughters' lifetime (high early adversity) or had no current or past psychopathology (low early adversity). Psychosocial outcomes were operationalized as symptoms of depression and anxiety. Depression and anxiety have been found to be associated with the OXTR rs2254298 polymorphism in adults (Costa et al., 2009). We hypothesized that exposure to high early adversity would interact with the OXTR rs2254298 polymorphism to predict symptom levels of girls' depression and anxiety.
Methods
Participants were 129 girls between the ages of 9 and 14 years (M = 12.1, SD = 1.5 years). The racial/ethnic make-up of the sample was 71.3% Caucasian, 10.9% biracial, 10.1% Asian American, 4.7% Latina, and 3.1% African American. Approximately 49% (n=47) of participants who provided reports (n=97) had their first menses. Mothers and daughters provided written informed consent and assent, respectively; dyads were compensated $25 per hour. The study was approved by the university's Institutional Review Board.
Diagnostic interviews
Daughters and mothers were administered a structured clinical interview, Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL), about the daughters. Both informants needed to report an absence of current/past mental health disorders in the daughters to be eligible for participation. Mothers were also administered a clinical interview, the Structured Clinical Interview for the DSM–IV (SCID), to assess their Axis I disorders. Dyads were eligible to participate if the mothers had experienced either (1) no current/past disorders (low early adversity); or (2) at least two major depressive episodes during their daughter's lifetime but were currently in remission (high early adversity). Approximately 35% (n=45) of the mothers had recurrent depression; the remaining 65% (n=84) had no history of mental health disorders. The racial/ethnic make-up of the high adversity sample was 71.1% (n=32) Caucasian, 15.6% (n=7) biracial, 4.4% (n=2) Asian American, 6.7% (n=3) Latina, and 2.2% (n=1) African American.
Depressive symptoms
Girls completed the 10-item form of the Children's Depression Inventory (CDI-S; Kovacs, 1985). This widely-used self-report instrument has been found in several investigations to be a valid and reliable measure of depressive symptoms in 8- to 17-year-old children. Girls indicated which of a series of statements best described how they have been feeling recently. Internal consistency for the CDI-S was α = .76.
Anxiety symptoms
Girls' anxiety levels were assessed using the self-report Multidimensional Anxiety Scale for Children (MASC; March et al., 1997). The MASC consists of 39 items assessing four subscales: physical symptoms (α = .80), social anxiety (α = .86), separation anxiety (α = .77), and harm avoidance (α = .69). The MASC has been found in previous studies to have acceptable validity and reliability (March et al., 1997).
Genotyping
DNA was extracted from saliva using the Oragene DNA saliva kit (DNA Genotek, Ottawa, Ontario, Canada). OXTR SNP rs2254298 was amplified using the primers 5′-TGA AAG CAG AGG TTG TGT GGA CAG G-3′ and 5′-AAC GCC CAC CCC AGT TTC TTC-3′. The PCR reactions were carried out in a final volume of 15μl consisting of 50ng of genomic DNA, 50ng each of sense and antisense primers, 7.5ul of Taq PCR Master mix (Qiagen, Cat.#201445) and 10% DMSO. The PCR conditions included an initial denaturation step at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 30 s., annealing at 58°C for 45 s. and extension at 72°C for 1 min, with a final extension of 10 min at 72°C. 7.5 μl of the 307 bp PCR products was digested at 65°C for 3 hours with 5 U of the restriction enzyme BsrI (New England Biolabs, Cat#R0527L). The products were electrophoresed through 10% Polyacrylamide gel(Acrylamide/bis-Acrylamide ratio 19:1) at 150 V for 40 min. 10bp marker was used to measure the fragments size. The A allele yields 164bp, 136bp and 8bp. The G allele yields 164bp, 101bp, 34 and 8bp. All genotypes were scored by research assistants who had no knowledge of the phenotypic data. This genotyping yielded three groups of girls: 86 girls carrying two G alleles (GG), 41 girls carrying one A and one G allele (AG), and 2 girls carrying two A alleles (AA). Because OXTR rs2254298 SNP exhibits a different allele frequency in Asian and Caucasian populations (www.hapmap.org), and the sizes of the minority samples in this study do not allow for reliable comparison, we report findings only for the Caucasian girls (n=92), who comprised 72.1% of the overall sample of GGs (n=62), 70.7% of AGs (n=29), and 50% of AAs (n=1). The allelic frequencies for OXTR SNP rs2254298 were in the Hardy-Weinberg equilibrium, χ2= 1.44 (df=2, n=92), ns. The single AA Caucasian girl was excluded from the remaining analyses. Thus, we present results below for the Caucasian girls: 38 low-adversity GGs, 22 low-adversity AGs, 24 high-adversity GGs, and 7 high-adversity AGs.
Results
Demographic characteristics
Logistic regression conducted on the girls' menarche status (0=no menses, 1=experienced first menses) did not yield significant effects for early adversity, χ2(1) = .36, ns, for OXTR, χ2(1) = 1.96, ns, or when both were included as predictors, χ2(2) = 2.70, ns. Moreover, menarche status did not predict symptoms of depression or anxiety, either as a main effect or in interaction with OXTR (Fs < 1.0; ps > .05); consequently, we do not consider menarche status further in this report.
Depressive and anxiety symptoms
Correlations among the measures are presented in Table 1. A two-way (early adversity × OXTR) multivariate analysis of variance (MANOVA) conducted on depression and the four anxiety scores yielded a significant interaction of early adversity and OXTR, F(5,76) = 4.42, p < .01. Separate univariate ANOVAs yielded significant interactions for depressive symptoms, F(1,84) = 8.50, p < .01, physical symptoms, F(1,82) = 9.89, p < .01, social anxiety, F(1,82) = 13.02, p < .01, and separation anxiety, F(1,82) = 5.80, p < .05, but not for harm avoidance, F(1,82) = 1.51, ns. As can be seen in Figure 1, heterozygous (AG) girls in the high early adversity group reported significantly higher symptom levels of depression, physical anxiety, and social anxiety than did participants in the other three groups, ts > 2.31, ps < .05, and higher symptom levels of separation anxiety than did the high-adversity GG group, t(27) = 3.00, p < .01.
Table 1.
Correlations among measures
| Physical Symptoms | Social Anxiety | Separation Anxiety | Harm Avoidance | |
|---|---|---|---|---|
| Depressive Symptoms | .33** | .24* | .07 | -.22* |
| Physical Symptoms | - | .58** | .49** | .30** |
| Social Anxiety | - | .40** | .27* | |
| Separation Anxiety | - | .54** | ||
| Harm Avoidance | - |
p < .05,
p < .01
Figure 1.
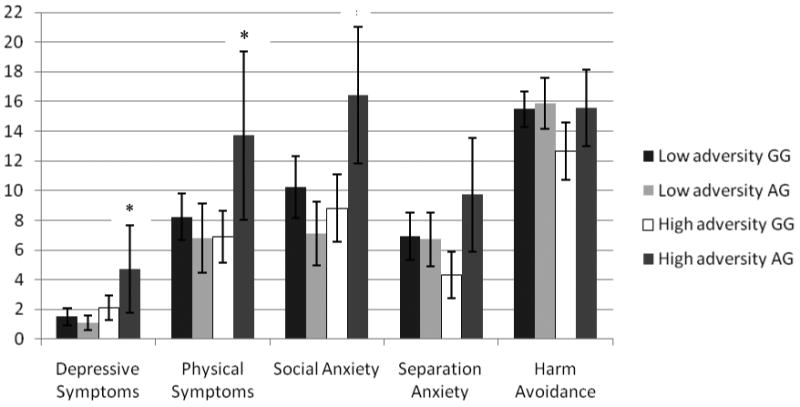
Mean symptom levels of depression and anxiety by early adversity and OXTR rs2254298. Error bars represent 95% confidence intervals (CI). Mean differences are statistically significant at p < .05 when the proportion overlap between CIs is half the average margin of error between two groups (Cumming & Finch, 2005). * = high-adversity AG group differs significantly from the other three groups. Note that the measure of depressive symptoms has a different scaling than do the four measures of anxiety symptoms.
Discussion
In this study we examined the interactive effects of OXTR rs2254298 polymorphism and exposure to early adversity on psychosocial functioning (depressive and anxious symptoms) in a group of adolescent girls. As we predicted, high early adversity, operationalized as having a mother with recurrent MDD, interacted with the OXTR rs2254298 polymorphism to predict girls' levels of anxiety and depressive symptoms. Girls who both were heterozygous for OXTR rs2254298 and had high early adversity reported the highest symptom levels of depression, physical anxiety, and social anxiety. In contrast, girls in the high early adversity group who were homozygous for the G allele were more similar to girls in the low early adversity group regardless of their genotype. Thus, having an allelic variation of AG for the OXTR rs2254298 polymorphism appears to function as a vulnerability factor for girls with a depressed mother even at a time when their mothers were not in a current depressive episode.
Lucht et al. (2009) found different patterns of association between the OXTR rs2254298 genotypes and loneliness in adolescents than in adults, suggesting that that the relation between loneliness and this polymorphism may change over the life course. The present results add to previous findings that being an AG carrier for OXTR rs2254298 is associated with negative outcomes in adolescents. These possible developmental differences represent one explanation for the discrepancies between our results and Costa et al.'s (2009) finding of high levels of depression and anxiety among the OXTR rs2254298 GG adults. Further, it is important to note that whereas the adults in Costa et al.'s study had current MDD, none of the girls in the present investigation had experienced an Axis-I disorder.
We operationalized early adverse environment as having a mother with a history of recurrent MDD. Because the offspring of mothers with MDD have higher rates of psychopathology than do children of nondepressed mothers (Hammen, 2009), this operationalization may include an increased likelihood of inheriting depressive and anxiety disorders through a variety of pathways. Nevertheless, within the high early adverse environment group, AG girls reported significantly higher symptom levels of depression, physical anxiety, social anxiety and separation anxiety than did GG girls. Our results suggest that assessing the allelic variations of the OXTR rs2254298 polymorphism provides additional explanatory power over simply examining and comparing the girls' level of early adversity. Indeed, Costa et al. (2009) posit that OXTR rs2254298 polymorphism is in a linkage disequilibrium block that could bring important allelic variants and, consequently, may be relevant for increasing our understanding of MDD.
Investigators examining the biology of oxytocin in the context of psychopathology have measured levels of plasma and CSF oxytocin at baseline or after experimental manipulation, have assessed the effects of intranasal oxytocin administration on a range of behavioral or biological variables, and have examined relations between oxytocin peptide gene and OXTR polymorphisms and severity of psychiatric symptoms (see Heinrichs et al., 2009; Lee et al., 2009, for reviews). Because the nature of the relations among plasma and CSF oxytocin and oxytocin peptide and receptor genes is not well understood, it is not yet clear how the present findings can best be integrated with the results of these studies. Clearly integrating findings from these different approaches is critical to being able to elucidate the role of oxytocin in psychopathology and is a vital direction for future research.
In closing, the present findings are important in demonstrating that both early childhood experiences and OXTR SNPs can profitably be considered in understanding the relation between maternal depression and symptomatology in offspring. Future research assessing oxytocin biology should examine more explicitly, and in larger samples, quality of maternal care and levels of adversity associated with having a depressed mother. The results of this study underscore the promise of continuing to elucidate the links among maternal depression, oxytocin biology, and children's internalizing symptoms.
Acknowledgments
The authors thank Hannah Burley, Yamanda Wright, and Chung Ping Liao for their help with this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Costa B, Pini S, Gabelloni P, Abelli M, Lari L, Cardini A, Muti M, Gesi C, Landi S, Galderisi S, Mucci A, Lucacchini A, Cassano GB, Martini C. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34:1506–1514. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Cumming G, Finch S. Inference by eye: Confidence intervals and how to read pictures of data. Am Psychol. 2005;60:170–180. doi: 10.1037/0003-066X.60.2.170. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behavior are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Hammen C. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. 2nd. Guilford Press; New York, NY: 2009. pp. 275–297. [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatr. 2008;14:954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression Inventory (CDI) Psychoph Bull. 1985;21:995–1124. [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS. Oxytocin: The great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O'Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clin Psychol Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, Rosenberger A, Grabe HJ, Schroeder W, Völzke H, Freyberger HJ, Herrmann FH. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness, and intelligence in normal subjects. Prog Neuro-Psychoph. 2009;33:860–866. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- March JS, Parker JDA, Sullivan D, Stallings P, Conners K. The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. J Am Acad Child Psy. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol Psychiat. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: Interactions with the hypothalamic-pituitary-adrenal axis. In: Poulain D, editor. Vasopressin and Oxytocin, Progress in Brain Research. Vol. 139. Elsevier Science; Amsterdam: 2002. pp. 147–162. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Moble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacol. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]


