Abstract
Cochlear implant (CI) users’ spectral resolution is limited by the number of implanted electrodes, interactions between the electrodes, and the underlying neural population. Current steering has been proposed to increase the number of spectral channels beyond the number of physical electrodes, however, electric field interactions may limit CI users’ access to current-steered virtual channels (VCs). Current focusing (e.g. tripolar stimulation) has been proposed to reduce current spread and thereby reduce interactions. In this study, current steering and current focusing were combined in a four-electrode stimulation pattern, i.e. quadrupolar virtual channels (QPVCs). The spread of excitation was measured and compared between QPVC and Monopolar VC (MPVC) stimuli using a forward masking task. Results showed a sharper peak in the excitation pattern and reduced spread of masking for QPVC stimuli. Results from the forward masking study were compared with a previous study measuring VC discrimination ability and showed a weak relationship between spread of excitation and VC discriminability. The results suggest that CI signal processing strategies that utilize both current steering and current focusing might increase CI users’ functional spectral resolution by transmitting more channels and reducing channel interactions.
Keywords: current steering, current focusing, cochlear implant, psychophysics
Introduction
Most cochlear implant (CI) users have excellent speech recognition in quiet, which requires only four spectral channels (Shannon et al., 1995; Loizou et al., 1999). Recognition of speech in noise and music perception are more difficult tasks with which many CI users struggle, and they require many more independent channels of information (e.g. Shannon et al., 2004). Modern CI devices typically transmit up to 22 spectral channels, but most CI users perform as if they are receiving only 4–8 independent channels of information (Friesen et al., 2001). The broad current spreads from the stimulated electrodes and the resulting overlapping populations of activated neurons are thought to limit CI users’ access to all of the spectral information transmitted by the device. These channel interactions ultimately limit CI performance in noise, particularly dynamic noise (Fu et al., 1998; Fu and Nogaki, 1998).
Current steering with virtual channels (VCs) has been proposed to increase the number of spectral channels beyond the number of physical electrodes. VCs are typically created by simultaneously stimulating two adjacent electrodes in-phase. The peak of excitation is “steered” between the component electrodes using a factor “α”, which denotes the proportion of current delivered to the basal electrode. When α = 0, all of the current is delivered to the apical electrode, and when α = 1, all of the current is delivered to the basal electrode. The peak of excitation is shifted between the component electrodes, which can elicit an intermediate pitch percept (Donaldson et al., 2005; Firszt et al., 2007). Donaldson et al. (2005) found that some subjects could reliably discriminate α steps as small as 0.11 for some electrode pairs, although the threshold for discrimination varied considerably. Only one subject (out of six) in that study could not discriminate the component electrodes, and therefore could not discriminate intermediate pitch percepts. In those studies, VCs were implemented using monopolar (MP) stimulation (see Figure 1). Throughout this manuscript, these dual-electrode MP stimuli will be referred to as monopolar virtual channels (MPVCs).
Figure 1.
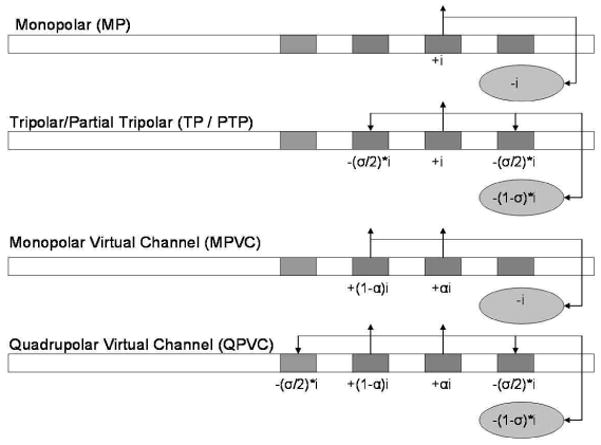
Illustration of different stimulation modes. The oval below each rectangular electrode array represents the extracochlear electrode. Note that this is the second phase of a cathodic-first bi-phasic pulse. “i” represents the current amplitude, α represents the fraction of current delivered to the basal electrode, and σ represents the fraction of current returned on the flanking electrodes within the array.
MPVCs have been implemented in Advanced Bionics’ Fidelity 120 speech processing strategy; 120 spectral channels are transmitted between 16 physical electrodes. While some CI users prefer the sound quality of Fidelity 120 (Brendel et al., 2008), no consistent or significant advantage in speech understanding has been observed with Fidelity 120 compared to the HiRes strategy (Brendel et al., 2008; Berenstein et al., 2008). If CI users can only access 8 of the 12–22 spectral channels provided by the physical electrodes, it is not surprising that they cannot access 120 VCs between the physical electrodes. The broad current spread associated with MP stimulation may activate greatly overlapping neural populations that limit sensitivity to spectral detail, especially in a multi-channel context. Busby et al. (2008) and Saoji et al. (2009) demonstrated that the current spread associated with MPVCs was similar to that of MP stimulation of single electrodes. This suggests that MPVC strategies (e.g. Fidelity 120) might perform similarly to MP strategies with only physical electrodes (e.g. Continuously Interleaved Sampling or CIS; Wilson et al., 1991) due to the similar amounts of current spread and resulting overlapping neural populations. Reducing current spread and neural interactions might allow CI users to access more of the spectral cues provided by the device (whether via virtual or physical channels).
Current focusing has been proposed to reduce current spread. In MP stimulation, current is delivered to an active electrode; an equal amount of current in opposite phase is delivered to an extra-cochlear electrode. In tripolar (TP) stimulation, current is delivered to an active electrode, and an equal amount of current in opposite phase is simultaneously delivered to the two adjacent flanking electrodes (see Figure 1). Because the current loop is entirely intra-cochlear with TP stimulation, current spread is reduced. However, the narrow current spread with TP stimulation requires higher current amplitude to achieve adequate loudness, necessitating long phase durations to achieve comfortable listening levels. For example, Litvak et al. (2007) needed to use pulse phase durations of 107 μsec or 205 μsec to achieve adequate loudness growth with TP stimulation. Even with these long phase durations, it is sometimes difficult to achieve sufficient loudness within the compliance limits of the device.
Partial tripolar (PTP) stimulation has been proposed to provide some degree of current focusing with a greater loudness at the same current amplitude as a TP stimulus (e.g. Mens and Berenstein, 2005; Litvak et al., 2007). In PTP stimulation, the portion of current returned to the flanking electrodes is σ (which ranges from 0 to 1), and the remainder (1 − σ) is returned via an extra-cochlear electrode, as in MP stimulation (see Figure 1). When σ = 0, 100% of the current is returned to the extra-cochlear electrode (i.e. true MP stimulation). When σ = 1, 100% of the current is returned to the adjacent electrodes (i.e. true TP stimulation). For intermediate σ values, the current spread is reduced compared to MP stimulation while providing better loudness growth than with TP stimulation (e.g. Litvak et al., 2007).
TP and PTP stimulation have been studied from various perspectives. Physiological studies in animals (Bierer and Middlebrooks, 2002; Snyder et al., 2004) and computational models (e.g. Spelman et al., 1995; Briare and Frijns, 2000) have shown that for a fixed current amplitude and for σ values > 0.5, there is reduced current spread within the cochlea for TP/PTP stimulation relative to MP stimulation (for a review, see Bonham and Litvak, 2008). However, TP and PTP stimulation typically require much greater current to maintain equal loudness to MP stimulation (Litvak et al., 2007; Berenstein et al., 2008). In general, current spread increases with current amplitude (Chatterjee and Shannon, 1998). Although TP/PTP may produce less current spread than MP at the same amplitude, the two modes should be compared at equal loudness levels. Furthermore, stimulation modes should be compared at comfortably loud levels, as these will produce representative amounts of current spread with CI speech processing. While current spread has not been previously compared between MP and TP/PTP stimulation modes at equally-loud, comfortable listening levels, Berenstein et al. (2008) showed better spectral ripple resolution for multi-channel signal processing with PTP stimulation (σ = 0.75) than with MP stimulation. Previous work by Chatterjee et al. (2006) showed greater current spread for bipolar (BP) maskers whose component electrodes were widely spaced (BP+10) than when narrowly spaced (BP+1). In that study, narrow and wide BP maskers were compared at 50%, 70% and 80% dynamic range (DR), i.e., similar, but not necessarily equally loud.
Thus, the two approaches to improving spectral resolution (current steering and current focusing) each have limitations. Current steering via MPVCs may transmit more effective channels, but the functional spectral resolution is limited by interactions between overlapping neural populations (caused by broad electric fields). Current focusing via TP/PTP stimulation may reduce interactions, but provide two fewer spectral channels than the number of physical electrodes (the most apical and most basal electrodes can only be used as ground electrodes). To address these limitations, Landsberger and Srinivasan (2009) proposed using quadrupolar virtual channels (QPVCs) to combine current steering and current focusing. Theoretically, QPVCs could provide “the best of both worlds” – using current steering to transmit more spectral channels beyond the number of implanted electrodes and using current focusing to reduce channel interactions, thereby providing better functional spectral resolution. QPVC stimulation (defined in Landsberger and Srinivasan, 2009) consists of 4 simultaneously stimulated intracochlear electrodes (see Figure 1): two center “steering” electrodes and two outer “focusing” electrodes. The two steering electrodes are stimulated in phase, and the amount of current delivered to each electrode is determined by α. As with MPVCs, when α = 0, 100% of the current is delivered to the apical electrode; when α = 1, 100% of the current is delivered to the basal electrode. The two focusing electrodes are stimulated in opposite phase to the steering electrodes and are used as intra-cochlear grounds. A fraction of the current delivered to the steering electrodes is returned to the focusing electrodes according to σ. As with PTP stimulation, when σ = 0, 0% of the current is returned to the extra-cochlear electrode (i.e. MPVC stimulation); when σ = 1, 100% of the current is returned to the focusing electrodes. The value of σ can range from 0 to 1. [The term “Quadrupolar” has been used differently in the literature; some studies have referred to PTP stimulation as Quadrupolar (e.g. Jolly et al., 1996) because they count the extracochlear ground electrode as one of four stimulating electrodes. However, in referring to QPVCs, we are only counting the 4 intracochlear electrodes.] In the present study, σ was fixed at 0.75 (75% of the current was returned to the outer two electrodes).
Landsberger and Srinivasan (2009) compared VC discrimination between MPVC and QPVC (σ =0.75) stimulation modes in Advanced Bionics CI users. For each stimulation mode, VCs were created according to different α values (α = 0, 0.2, 0.4, 0.6, 0.8 and 1) at three stimulation sites (apical, middle and basal regions of the cochlea). VC discrimination was measured for each mode at loudness-balanced comfortable listening levels. Results showed significantly better VC discrimination with QPVC stimulation than with MPVC stimulation. For 20 out of 21 electrode pairs (across subjects and stimulation sites), cumulative d′ scores were better for QPVCs than for MPVCs. While the improved VC discrimination suggests better spatial selectivity (i.e. less current spread) with QP stimulation, this has not been directly measured.
In this study, psychophysical forward-masked excitation patterns were compared between MPVC and QPVC stimuli in 9 CI users, at equally loud comfortable listening levels. We hypothesized that the location of the peak in the excitation patterns would be similar between the two modes, but that the “skirts” of the excitation would be different, with QPVC stimulation providing a sharper excitation pattern. We hypothesized that the reduced spread of excitation with QPVC stimulation would persist even at the higher current levels needed to maintain equal loudness to the MPVC stimuli. The present forward masking data were compared to the VC discrimination data from Landsberger and Srinivasan (2009). We hypothesized that electrode pairs with narrower forward masking curves would also have better VC discrimination, because sharper excitation patterns might make VC peaks more perceptually salient.
2. Materials and Methods
Subjects
Nine users of the Advanced Bionics Clarion II or HiRes90K (all with the HiFocus electrode array) device participated in this experiment. All subjects were postlingually deafened and used the HiRes or Fidelity 120 speech processing strategy in their clinical speech processor. Table 1 shows relevant subject demographics. Subjects C1, C3, C4, C7, C8, and C9 participated in the previous Landsberger and Srinivasan (2009) study. All subjects provided informed consent in accordance with IRB regulations and all subjects were compensated for their participation.
Table 1.
Relevant demographic information for subjects participating in this study. Electrode pairs tested in the VC discrimination study are indicated. Electrode pairs shown in a bold font were also tested in the forward masking experiment.
| Subject | Gender | Age | Device/Strategy/Electrode Type | VC Discrimination Study | Electrode Pairs |
|---|---|---|---|---|---|
| C1 | M | 77 | CII/Fidelity 120/HiFocus | Landsberger & Srinivasan (2009) | 3+4, 9+10, 14+15 |
| C3 | F | 53 | HR90K/Fidelity 120/HiFocus | Landsberger & Srinivasan (2009) | 2+3, 7+8, 13+14 |
| C4 | F | 62 | HR90K/HiRes/HiFocus | Landsberger & Srinivasan (2009) | 3+4, 7+8, 13+14 |
| C7 | F | 60 | HR90K/Fidelity 120/HiFocus | Landsberger & Srinivasan (2009) | 2+3, 7+8, 13+14 |
| C8 | F | 62 | HR90K/Fidelity 120/HiFocus | Landsberger & Srinivasan (2009) | 2+3, 7+8, 13+14 |
| C9 | M | 67 | CII/HiRes/HiFocus | Landsberger & Srinivasan (2009) | 2+3, 7+8, 13+14 |
| C14 | M | 44 | HR90K/Fidelity 120/HiFocus | This study | 2+3, 7+8, 13+14 |
| C15 | F | 56 | HR90K/HiRes/HiFocus | This study | 7+8 |
| C16 LE | F | 56 | HiRes90K/HiFocus | This study | 2+3, 7+8, 13+14 |
| C16 RE | F | 56 | HiRes90K/HiFocus | This study | 2+3, 7+8, 13+14 |
2.1 Forward Masking
Stimuli
Stimuli were generally similar to those in Landsberger and Srinivasan (2009), i.e., MPVCs and QPVCs presented at three cochlear locations (apical, middle and basal regions of the cochlea). Due to subject availability, we were unable to collect data from all regions for all subjects (see Table 1 for the electrode pairs tested for each subject). Maskers were either MPVCs or QPVCs (σ=0.75 in all cases) steered to the middle of the electrode pair (α = 0.5 in all cases). Probe stimuli were always QPVCs (σ = 0.75 in all cases). Only the stimulation mode of the masker was varied in order to maintain the probe stimuli as a constant “measuring stick”. Probes were steered to one of 7 locations between the component electrodes (α = 0, 0.2, 0.4, 0.5, 0.6, 0.8 and 1). MPVC and QPVC maskers were loudness balanced at the “most comfortable” listening level (according to the Advanced Bionics 10-point loudness scale used for clinical fitting). All stimuli were cathodic-first, biphasic pulse trains. The stimulation rate was 1000 pulses per second (pps) and the pulse phase duration was 226 μsec. The masker pulse train duration was 300 msec and the probe pulse train duration was 20 msec. The masker-probe interval was 5 msec.
Procedure
Loudness growth was estimated for each MPVC and QPVC masker. Starting with an initial stimulation level of 5 μA, the amplitude was gradually increased in 5 μA steps for MPVCs or 10 μA steps for QPVCs. The subject indicated the loudness according to the Advanced Bionics’ 10-point loudness scale. Current levels were recorded for loudness levels corresponding to “Barely Audible”, “Soft”, “Most Comfortable” and “Maximal Comfort”. The procedure was stopped when the subject indicated that “Maximal Comfort” loudness was obtained.
Maskers were presented at equal loudness (instead of equal amplitude or equal percent dynamic range). At each stimulation site, the QPVC masker was loudness-balanced to the MPVC masker at the “Most Comfortable” listening level. Loudness-balancing was performed using an adaptive, double-staircase, two-interval forced-choice (2IFC) procedure. The MPVC masker was the reference. The current amplitude of the QPVC masker was adjusted in 0.2 dB steps, with the upper staircase (3-down/1-up) converging on the level at which the reference was louder than the target 79.4% of the time and the lower staircase (1-up/3-down) converging on the level at which the reference was softer than the target 79.4% of the time (Levitt, 1971). Ten reversals were recorded for each staircase and the last six reversals of each staircase were averaged to estimate the point of subjective equality. The average of three runs was taken to be the loudness balanced level. Note that QPVC maskers were loudness-balanced to their MPVC counterparts at each stimulation site. MPVC maskers were not loudness-balanced across sites, although all stimulation levels corresponded to the “Most Comfortable” listening level. During testing, no trial-by-trial feedback was provided.
Before measuring forward-masked thresholds, detection thresholds for all QPVC probe stimuli were measured (with no masker) using an adaptive (3-down/1-up) 2IFC procedure (0.5 dB step size), converging on the 79.4% correct level (Levitt, 1971). Ten reversals were recorded and the last 6 reversals were averaged. The average of three runs was taken as the unmasked probe threshold.
Forward-masked thresholds were measured for all probes in the presence of the MPVC or QPVC masker using an adaptive (3-down/1-up) 2IFC procedure (0.2 dB step size), converging on the 79.4% correct level (Levitt, 1971). The maskers were fixed at the loudness-balanced “Most Comfortable” level. Ten reversals were recorded and the last six reversals were averaged. The average of three runs was taken as the masked threshold.
2.2 Virtual Channel Discrimination
Most of the subjects in this experiment also participated in a previous experiment (Landsberger and Srinivasan, 2009) that compared VC discrimination between two physical electrodes in MPVC or QPVC stimulation mode. In order to relate the forward masking data to the discrimination data of Landsberger and Srinivasan (2009), we repeated the VC discrimination experiment for subjects (C14, C15, and C16) who had not participated in the previous experiment. Subject C16 has bilateral implants, and we collected VC discrimination data for both ears; however, due to time constraints, we were unable to collect forward masking results for the right ear. The stimuli and procedure are briefly described here. For further details please refer to Landsberger and Srinivasan (2009).
Stimuli
Apical, middle and basal sets of electrode pairs were examined for subjects C14 and C16 (both ears); only the middle electrode pair was tested for subject C15. For most subjects, the MPVCs were created for electrode pairs 2+3, 7+8 and 13+14. See Table 1 for a listing of subjects and electrode pairs tested. The QPVCs were created by adding the simultaneous flanking electrodes to the MPVC pairs as described in Figure 1. At each stimulation site, 6 MPVCs and 6 QPVCs were created, steering through α values from 0 to 1, in 0.2 α steps. Note that these were the same α values used in the forward masking experiment, with the exception of α = 0.5. An α of 0.5 was not included for the 3 new subjects tested in this study in order to accurately replicate the procedure used in Landsberger and Srinivasan (2009). Similar to the forward masking stimuli, stimuli were 1000 pps pulse trains with a 226 μsec phase duration. Each stimulus was 300 msec in duration.
Procedure
All α values at each stimulation site for both MPVCs and QPVCs were loudness balanced to a MPVC with α = 0, at the “Most Comfortable” level. Loudness levels of the stimuli were balanced by repeatedly playing the standard stimulus (MPVC with α = 0) followed by the comparison stimulus with an interstimulus interval of 300 msec. The stimulus was adjusted in 1 μA steps using a knob (Griffin Powermate). The subject was asked to adjust the loudness of the comparison stimulus until the two sounds were the same loudness. The procedure was repeated at least 3 times per balance and the loudness balanced level was set as the average of all repetitions.
VC discrimination was measured independently for MPVCs and QPVCs using a 3 interval forced-choice (3IFC) procedure. Two intervals presented the same α value and the third presented a different α value. Subjects were instructed to pick the interval that was different in any way other than in loudness. The amplitude in each interval was roved by ± 0.6 dB to reduce any remaining loudness cues. All pairs of α values were compared 30 times within a block of testing, and 15 blocks were tested for each stimulation site and stimulation mode.
3. Results
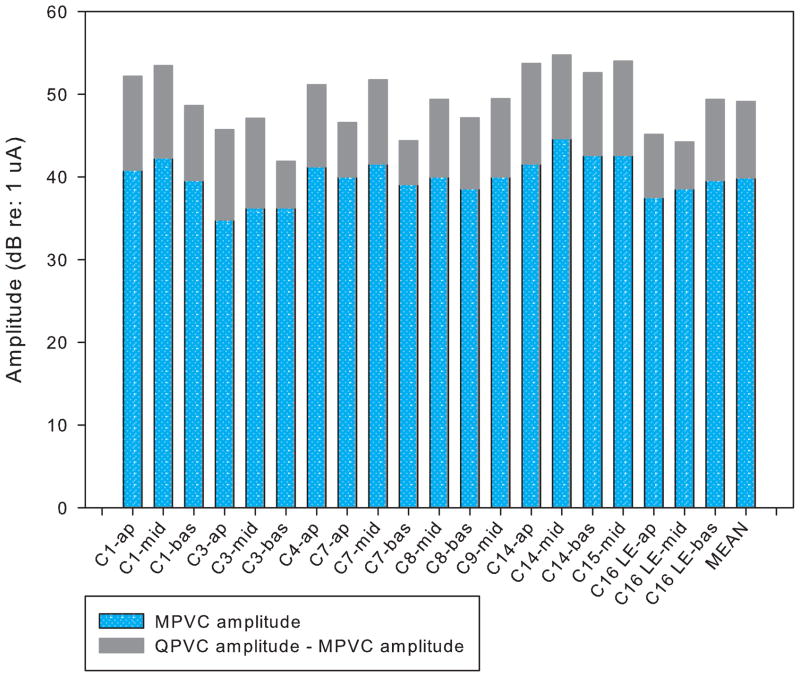
Figure 2 shows the current level (in dB) needed to maintain equal loudness between MPVC and QPVC stimuli as measured in the forward masking experiment. Results are shown for each subject and each stimulation site. On average, QPVCs required 9.3 dB more current to maintain equal loudness to the MPVC reference (range: 5.3 – 12.1 dB). A two-tailed paired t-test showed a significant difference in amplitude required to achieve equal loudness (t19 = 19.98, p<0.01).
Figure 2.
Loudness balanced levels for MPVC and QPVC stimuli (in dB) for individual subjects and electrode pairs (with α = 0.5 in all conditions) as measured in the forward masking task. Black bar indicates MPVC amplitude, gray bar indicates amplitude increase required for loudness balanced QPVC stimulus.
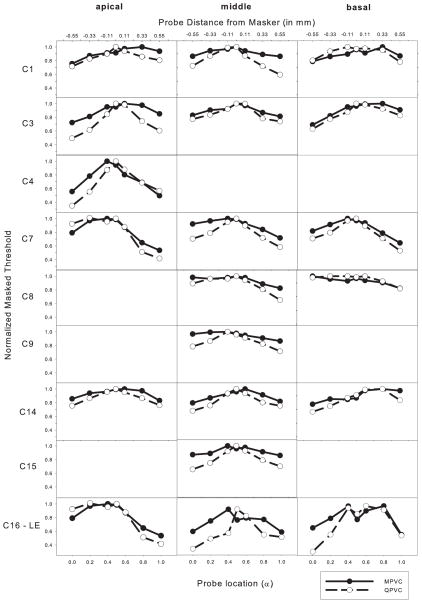
Figure 3 shows normalized masked threshold shifts for individual subjects and different stimulation sites with the MPVC and QPVC maskers. Masked threshold shifts were calculated by subtracting the unmasked threshold from the masked threshold (in μA). The masked threshold shifts were then normalized (in μA) to the peak shift with each stimulation mode, similar to Chatterjee and Shannon (1998) and Chatterjee et al. (2006). Note that masked threshold shifts are shown across a limited spatial extent (1.1 mm, i.e. the distance between adjacent electrodes in the Advanced Bionics HiFocus electrode array). In general, the threshold shift functions were steeper with the QPVC maskers. The magnitude of the difference varied considerably across subjects. Current focusing clearly affected the spread of excitation for some subjects at some locations (e.g., the middle VC pairs for subjects C1 and C7) and had little effect at others (e.g., the basal VC pairs for subjects C1 and C8). There was also great inter-subject variability in terms of the amount of masking, regardless of stimulation mode, e.g. relatively little masking for subject C4 and relatively large masking for subject C8.
Figure 3.
Normalized forward masking patterns with MPVC (filled symbols) and QPVC (open symbols) maskers, for individual subjects. Data is shown for the apical (left), middle (middle), and basal (right) electrode pairs; the specific VC component electrodes are listed in Table 1. The lower x-axis shows the α-value for the QPVC probe and the upper x-axis shows the distance (in mm) of the probe from the masker. The masker α value was always 0.5. The y-axis shows the normalized threshold (in μA), relative to the peak masking. Data were not collected at all locations for all subjects.
Although the masker was always steered to the middle of the electrode pair (α = 0.5), the peak of the masking pattern did not always correspond to that location. However, peak masking tended to occur within ± 0.11 mm of the masker location, with some exceptions (e.g., the basal MPVC maskers for subjects C1, C3 and C8). Additionally, there were several instances of a “tip shift” between the MPVC and QPVC masking patterns (e.g. subjects C4 and C16). In these tip-shift cases, the peak occurred at the α = 0.5 masker location for the QPVC maskers, but was shifted for the MPVC maskers.
In order to compare the masking curves of the MPVC and QPVC stimulation modes, the area under the normalized threshold shift curves shown in Figure 3 was calculated for the MPVC and QPVC maskers, similar to Chatterjee et al. (2006). The top panel of Figure 4 shows the difference in area under the masking curves for MPVC and QPVC maskers, for individual subjects and electrode pairs. On average, the area under the QPVC curves was 6.4% smaller than that under the MPVC curves, and the QPVC area was smaller than the MPVC area for 18/20 stimulation sites. A two-tailed paired t-test showed a significant difference in area across stimulation mode (t19 = 5.57, p<0.01).
Figure 4.
Top: Difference in the areas under the normalized masked threshold curves (MPVC area – QPVC area) shown in Figure 3. Subjects and stimulation sites are ordered according to the magnitude of difference. Bottom: Difference in the average percent masking at the endpoints of the normalized masked threshold curves (MPVC percent masking – QPVC percent masking) shown in Figure 3. Subjects and stimulation sites are shown in the same order as in the top panel.
Another measure of the difference between the MPVC and QPVC normalized threshold shifts shown in Figure 3 is the difference in masking response at the endpoints (α=0 and α=1) of the masking curves. This was calculated for the MPVC and QPVC maskers by calculating the average of the normalized percent masking at the apical and basal endpoints of each curve for each stimulation mode. The bottom panel of Figure 4 shows the difference in average percent masking at the endpoints for MPVC and QPVC masking functions, for individual subjects and electrode pairs. On average, the QPVC functions had 11.1% less masking at the endpoints compared to the MPVC functions, and the QPVC functions were steeper than the MPVC functions for 19 out of 20 stimulation sites. A two-tailed paired t-test showed a significant difference in average percent masking at the endpoints of the masking functions (t19 = 7.47, p<0.01).
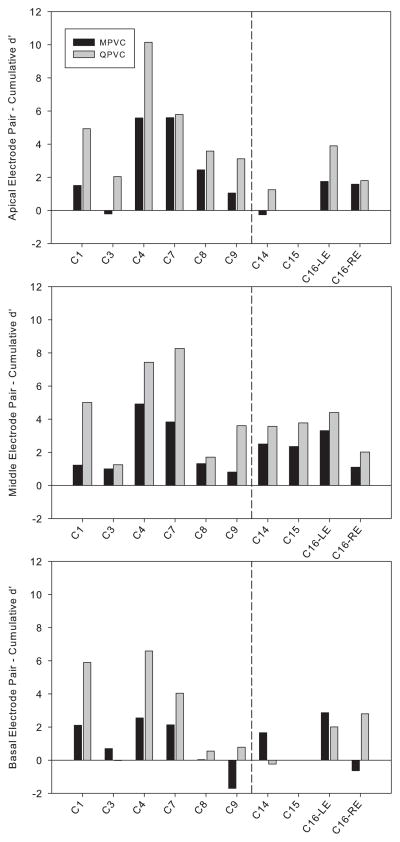
The forward masking curves were compared with VC perceptual discrimination data collected for subjects C1, C3, C4, C7, C8 and C9 in Landsberger and Srinivasan (2009) and for subjects C14, C15, and C16 (both ears) collected in this study (see Table 1 for electrode pairs tested for each subject). The cumulative d′ was calculated across all α values at each stimulation site, using the tables of Hacker and Ratcliff (1979). In general, cumulative d′ values were larger for QPVCs than for MPVCs, suggesting that QPVCs provide better spectral resolution between adjacent electrodes. Figure 5 shows the cumulative d′ values for MPVC and QPVC stimulation for all subjects and all stimulation sites in both the previous study (Landsberger & Srinivasan, 2009) and this study. Out of 28 electrode pairs, three showed a smaller QPVC cumulative d′ compared to the MPVC cumulative d′ (the basal electrode pairs of subjects C3, C14, and C16 – left ear). Results for the three newly tested subjects are similar to those in Landsberger and Srinivasan (2009). In that paper, we found one electrode pair out of 21 with a lower QPVC cumulative d′ than MPVC cumulative d′ (basal electrode pair for subject C3). In this study, we used the same protocol and found two electrode pairs having a smaller QPVC cumulative d′ than MPVC cumulative d′ (basal electrode pairs for subjects C14 and C16 – left ear). The three electrode pairs with lower QPVC cumulative d′ scores than MPVC cumulative d′ scores all had narrower QPVC forward masking curves than MPVC (assessed by both area and percent masking at the endpoints), although the magnitude of difference for C3 was quite small. The VC discrimination scores from the previous study (Landsberger and Srinivasan, 2009) were combined with the data collected in this study and the complete set was analyzed (omitting the data for C15, since we were unable to collect data from all three cochlear regions). A two-way repeated measures analysis of variance (RM ANOVA) revealed a main effect of stimulation mode [F(1, 9) = 18.84, p<0.005] and a main effect of cochlear region [F(2, 18) = 4.18, p<0.05]. Multiple pairwise t-tests with Bonferroni corrections showed a significant difference between the basal and middle regions (p<0.05) when cumulative d′ scores were collapsed across stimulation mode; there were no significant differences between any other locations. The average cumulative d′ scores for the apical, middle and basal regions were 3.05, 3.21 and 2.02, respectively (collapsed across stimulation mode). There was no significant interaction between stimulation mode and cochlear region [F(2, 18) = 0.32, p=0.73].
Figure 5.
A summary of VC discrimination results for all electrode pairs measured. Bars show the cumulative d′ score for each electrode pair in a stimulation mode. A larger cumulative d′ indicates greater perceptual resolution. Data to the left of the dashed line indicates data described in a previous paper (Landsberger & Srinivasan, 2009) and data to the right was collected specifically for this study.
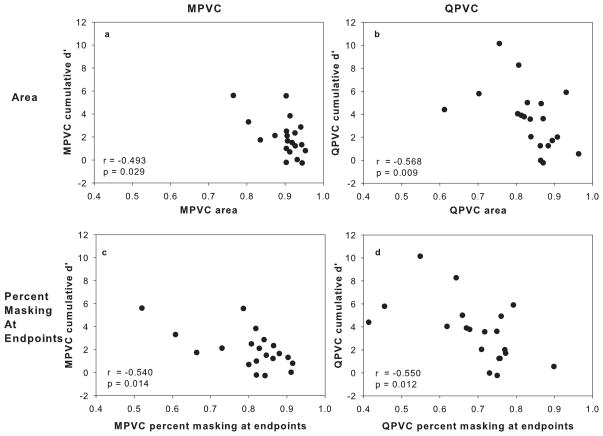
The VC discrimination data from Landsberger and Srinivasan (2009) showed that VC discrimination varied widely across subjects and electrode pairs. We hypothesized that the degree of spread of excitation is related to VC spectral resolution. In order to determine if spread of excitation patterns are predictive of VC discrimination ability, the correlation between cumulative d′ and masking curve area and width were calculated. Figures 6a and 6b show cumulative d′ data as a function of the area under the normalized masking functions for MPVC and QPVC maskers (respectively). Similarly, Figures 6c and 6d show cumulative d′ data as a function of the average percent masking at the endpoints of the masking function for MPVC and QPVC maskers (respectively). All electrode pairs tested are plotted for each subject. In general, the forward masked excitation patterns were not strong predictors of cumulative d′ data, although sharper masking patterns seem more likely to produce larger cumulative d′ values. All correlations were significant (p<0.05) using the Spearman’s rank correlation coefficient.
Figure 6.
Panel A: MPVC cumulative d′ as a function of the area under the normalized MPVC masking curve. Panel B: QPVC cumulative d′ as a function of the area under the normalized QPVC masking curve. Panel C: MPVC cumulative d′ as a function of the average percent masking at the endpoints of the MPVC masking curve. Panel D: QPVC cumulative d′ as a function of the average percent masking at the endpoints of the QPVC masking curve. The correlation coefficient r and the p-value calculated from the Spearman’s rank correlation are shown.
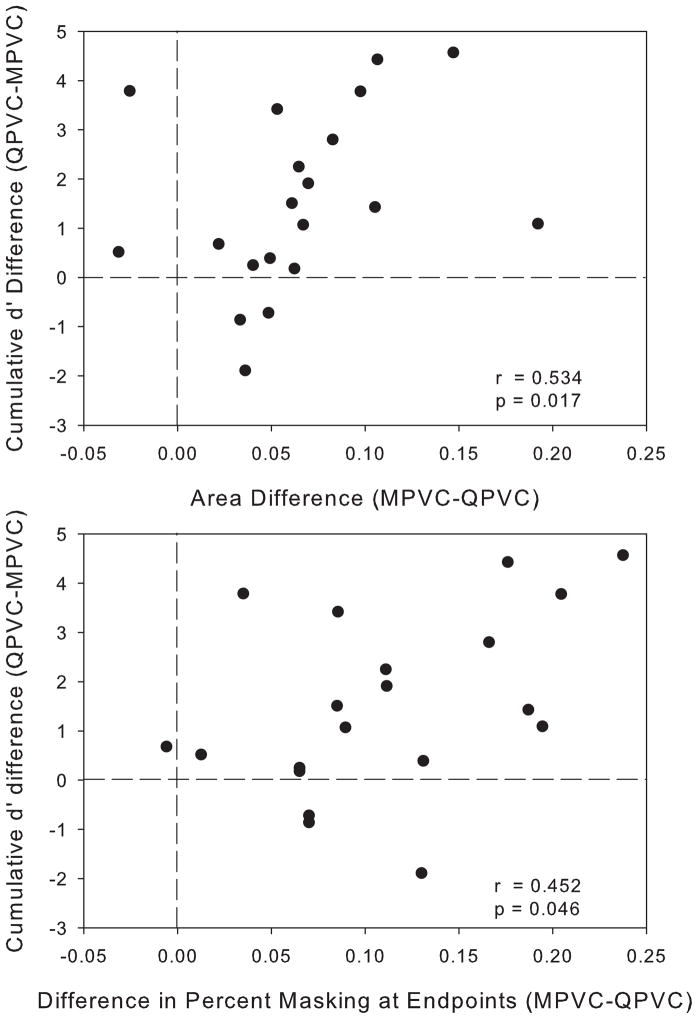
QPVC stimulation yielded a significant improvement in VC resolution compared to MPVC stimulation, although the amount of improvement varied across electrode pairs. In order to determine if the difference in masking curves between stimulation modes was predictive of the amount of improvement in VC discrimination with QPVC stimulation, the correlation between difference in cumulative d′ scores and difference in masking curves (area and width) was calculated using the Spearman’s rank correlation coefficient. Figure 7 shows the difference in cumulative d′ values between MPVCs and QPVCs as a function of the difference in area under the masking curves (Figure 7a) and the difference in average percent masking at the endpoints of the masking curve (Figure 7b). A cumulative d′ difference greater than 0 indicates that QPVC stimulation provided better VC resolution than did MPVC stimulation. Larger values for the difference in area under the masking curves or difference in percent masking at the endpoints indicate that the QPVC stimulation provided a smaller spread of excitation than did the MPVC stimulation. Both correlations were significant (p<0.05), although neither showed a very strong relationship.
Figure 7.
Top: difference in cumulative d′ (see Figure 5) between stimulation modes as a function of the difference in the area under the normalized forward masking functions (see Figure 3). Bottom: difference in cumulative d′ between stimulation modes as a function of the difference in percent masking response at the endpoints of the normalized forward masking functions. The dashed lines show no difference between stimulation modes. The correlation coefficient r and the p-value calculated from the Spearman’s rank correlation are shown.
4. Discussion
The present forward-masked excitation patterns showed significantly smaller spread of excitation with current focusing, compared to equally loud MP stimulation. Although focusing requires greater absolute current levels to achieve adequate loudness, the relative spread of excitation was smaller for QPVCs than for MPVCs. The present results are in agreement with previous physiological studies (e.g. Boham and Litvak, 2008) and models (Litvak et al., 2007) that show reduced current spread with TP/PTP stimulation, although equal loudness was not maintained across stimuli in those studies. The spread of excitation in this study was measured across a limited spatial extent (1.1 mm), and it is not certain that the results would hold if measured across a larger spatial extent.
This study measured a reduced spread of excitation for QPVCs with σ = 0.75 compared to MP stimuli. Animal models of TP/PTP stimulation imply that using larger focusing coefficients would lead to even greater reductions in current spread (Bierer and Middlebrooks, 2002). However, it is difficult to achieve a comfortable loudness with focusing coefficients greater than 0.75. It was impossible to achieve a useable DR with a fully focused configuration for any of the subjects tested in this study. In all likelihood, if QPVC stimulation were implemented in a speech processing strategy, a partially focused configuration (such as σ = 0.75 used here) would be necessary. Therefore, the study presented here is clinically relevant to the strategy that would most likely be implemented.
The effect of current focusing on spread of excitation has been studied previously with both TP/PTP and BP stimulation, and the present study generally agrees with previous results. Bierer and Faulkner (2010) showed that psychophysical tuning curves of MP maskers measured with PTP probes have significantly narrower widths than those measured with MP probes. However, the tuning curves were measured with probes fixed at low levels (1 dB or 3 dB above threshold), which may not give an accurate representation of excitation at current levels used in CI speech processing strategies. The difference in masking curves with BP maskers compared to MP maskers has been studied with somewhat mixed results. Kwon and van den Honert (2006) measured forward masking patterns for equally-loud BP and MP stimuli in 4 CI subjects and found no significant difference between the normalized masking functions with either mode. However, it is possible that the number of subjects in the study was too small to detect a significant difference. Nelson et al. (2008) found that the slopes of the tuning curves with BP stimuli were steeper than with MP stimuli, although that was not a within-subjects comparison.
The magnitude of the difference in masking curves between the two stimulation modes varied widely across electrode pairs and subjects. In subject C7, there was a relatively large difference between MPVC and QPVC masking patterns for the middle electrode pair, but a smaller difference at the apical site. At some locations and in some subjects (e.g. the basal pair for subjects C1 and C8), the masking patterns were equally broad for MPVC and QPVC stimulation. The variability in masking patterns might be related to the quality of the electrode-neuron interface, which would be affected by the distance of the electrode from the neurons, ossification, and the health of the spiral ganglion population. Bierer and Faulkner (2010) hypothesized that the broadness of psychophysical tuning curves was related to the quality of the electrode-neuron interface; they showed that channels with higher PTP thresholds (indicating electrodes far from a healthy neural population) also had tuning curves with shallower slopes. Conversely, computer modeling data suggests that there is little decrease in current spread for TP/PTP configurations compared to MP configurations with close electrode to nerve distances, and there might in fact be an increase in current spread for σ ≥ 0.75 for higher stimulation levels (Litvak et al., 2007). Alternatively, similar masking patterns between modes may have been due to the spatially limited region (1.1 mm) across the cochlea in which probe stimuli were presented in the present study. A larger spatial range of the probe (as is commonly used in forward masking studies) may have revealed larger differences in current spread between modes. Note that significant differences were observed between modes over the limited 1.1 mm extent; one would expect these differences to be magnified with a larger spatial probe extent, particularly when considering the reduction in current spread farther from the masker when using PTP or BP stimulation (Bierer and Faulkner, 2010; Nelson et al., 2008).
Significant relationships were found between the spread of excitation and the discriminability of VCs, for both MPVC and QPVC stimulation. Furthermore, there was a significant relationship between the difference in forward masking patterns and the VC discrimination improvement with QPVC stimulation. These relationships indicate that narrower spreads of excitation yield improved VC discriminability, and the amount of narrowing with focused stimulation compared to MP stimulation is related to the degree of improvement in perceptual discrimination tasks. This was not surprising, as both VC discrimination and forward masking tasks are at least somewhat dependent on similar factors (e.g. specifics of the electrode to nerve interface). However, the relationships found were not particularly strong, indicating that there are other factors beyond spread of excitation that limit VC discrimination.
We had hoped to find a stronger relationship between the forward masked excitation patterns and VC discrimination. Psychophysical forward masking and physiological forward masking using electrically evoked compound action potentials (ECAPs) have been shown to be correlated (Hughes and Stille, 2008). Had we found a strong relationship between forward masking patterns and VC discrimination, quick objective measures such as ECAPs (e.g. Abbas et al., 2004; Cohen et al., 2003) may have been strong predictors of discriminability for different stimulation modes. However, we found only weak relationships between the forward masked excitation patterns and VC discrimination across stimulation modes and between the difference in masking curves and the difference in VC discrimination between stimulation modes.
It is not surprising that we did not find a strong correlation between forward masking patterns and VC discrimination as other studies have had widely varying results. Throckmorton and Collins (1999) measured electrode discrimination (BP+1 and BP+2 stimulation modes) using a 2IFC procedure in which subjects indicated whether the two intervals were the same or different; results were significantly correlated to a psychophysical forward masking task. However, Hughes and Abbas (2006) measured electrode pitch ranking (MP stimulation mode) using a 2IFC procedure in which subjects indicated which stimulus was higher in pitch; no correlation was found between these data and ECAP forward masking patterns. There are several possibilities that may explain discrepancies between forward masking and VC discrimination data.
First, the forward masked threshold detection and VC discrimination tasks may have been too different, targeting different auditory processes. Forward-masked excitation patterns most likely reflect more peripheral processes, while VC discrimination may reflect both peripheral and central processes (i.e. pitch ranking). Also, subjects may have had greater difficulty with the VC discrimination task: subject C15 in particular had difficulty with the 3IFC discrimination task and performed better with a 2IFC task in which she indicated which interval was higher in pitch. This might indicate why Throckmorton and Collins (1999) found a significant relationship between forward masking and a 2IFC electrode discrimination task; the greater cognitive load of the 3IFC task may have depressed VC discrimination scores. Finally, the amplitude roving used for VC discrimination may have resulted in greater changes across stimuli, thereby increasing the difficulty of the task.
A stronger possibility is that the limited 1.1 mm spatial extent of the probe used for the present forward masking data may have been too small to allow for meaningful comparisons. In Throckmorton and Collins (1999), forward masking was measured across a larger portion of the electrode array. Other forward masking studies have used a “Q-value” (typically, the width in mm -1 dB or -3 dB from the peak) to characterize the masking patterns (e.g. Nelson et al., 2008). The limited spatial extent in the present study did not allow for such a measure, as masking patterns for some electrode pairs did not decrease 1 dB from the peak even at the farthest extents from the masker. It is possible that, given the spatially limited range of the probe, neither the area under the masking curves nor the percent masking at the endpoints are appropriate metrics for correlating to VC discrimination.
The present data show that QPVC stimulation produces less current spread than MPVC stimulation. The present VC discrimination data, along with Landsberger and Srinivasan (2009) show that QPVC stimulation can improve discriminability. However, it is unclear whether these single-channel measures will predict performance in a multi-channel context, as in a CI speech processing strategy. Previous studies have shown increased spectral resolution via current-steering in a single-channel context (Firszt et al., 2007; Donaldson et al., 2005), but little benefit when MPVCs were implemented in a CI speech processing strategy (e.g. Brendel et al., 2008). Simultaneous stimulation of two electrodes in MP mode (in essence, MPVCs) has been shown to have a similar current spread as MP stimulation of one electrode (Saoji et al., 2009). If so, MPVCs may activate broad populations of neurons, negating any gains in spectral resolution from the additionally transmitted spectral channels. QPVC stimulation may reduce channel interaction while preserving the additional spectral channels the VCs provide. Collecting data beyond the 1.1 mm spatial extent measured in this study (with either psychophysical forward masking or with ECAPs) may give clues regarding how QPVCs would perform in a multi-channel context.
One concern with implementing QPVCs in a CI speech processing strategy is the large amount of current needed to achieve adequate loudness. With the short pulse phase durations used in clinical processors to maintain high stimulation rates, QPVC stimulation may require current amplitudes beyond the device compliance voltage. Long phase durations may be used to offset these high current requirements, but at the expense of stimulation rate, which may reduce the temporal resolution. Methods of increasing the overall stimulation rate are discussed in detail in Landsberger and Srinivasan (2009). Additionally, the importance of fine temporal cues may be reduced if enough spectral channels are available (Xu et al., 2005; Fu et al., 2005). For example, Fu et al. (2005) in a voice gender discrimination study with NH subjects listening to acoustic CI simulations found near perfect performance with 32 channels, whether the temporal cutoff frequency was 40 Hz or 160 Hz; with only 4 channels, performance greatly improved when the envelope cutoff frequency was increased from 40 Hz to 160 Hz. Similarly, Rosen (1992) found that low-frequency envelope cues (<50 Hz) were most useful for speech recognition. Thus, long phase durations and the associated reduction in stimulation rate may not limit CI performance, especially if the functional spectral resolution is dramatically increased with QPVCs.
Acknowledgments
This work was supported by NIDCD Grants and Fellowship Numbers:. R01-DC- 001526, R03-DC-010064, and F31 DC011205-01. We gratefully acknowledge the CI subjects who participated in this study. We also thank John J. Galvin III for editorial help.
Abbreviations
- CI
Cochlear Implant
- VCs
Virtual channels
- QPVCs
Quadrupolar virtual channels
- MPVCs
Monopolar virtual channels
- MP
Monopolar
- CIS
Continuously Interleaved Sampling
- TP
Tripolar
- PTP
Partial Tripolar
- BP
Bipolar
- DR
Dynamic range
- 2IFC
Two-interval forced choice
- 3IFC
Three-interval forced choice
- RM ANOVA
Repeated Measures Analysis of Variance
- ECAP
Evoked Compound Action Potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas PJ, Hughes ML, Brown CJ, Miller CA, South H. Channel interaction in cochlear implant users evaluated using the electrically evoked compound action potential. Audiol Neurootol. 2004;9:203–13. doi: 10.1159/000078390. Cohen 2003. [DOI] [PubMed] [Google Scholar]
- Berenstein CK, Mens LHM, Mulder JJS, Vanpouke FJ. Current steering and current focusing in cochlear implants: comparison of monopolar, tripolar, and virtual channel electrode configurations. Ear Hear. 2008;29:250–260. doi: 10.1097/aud.0b013e3181645336. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Faulkner KF. Identifying cochlear implant channels with poor electrode-neuron interface: partial tripolar, single-channel thresholds and psychophysical tuning curves. Ear Hear. 2010;31:247–58. doi: 10.1097/AUD.0b013e3181c7daf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer JA, Middlebrooks JC. Auditory cortical images of cochlear-implant stimuli: dependence on electrode configuration. J Neurophysiol. 2002;87:478–92. doi: 10.1152/jn.00212.2001. [DOI] [PubMed] [Google Scholar]
- Bonham BH, Litvak LM. Current focusing and steering: modeling, physiology, and psychophysics. Hear Res. 2008;242:141–53. doi: 10.1016/j.heares.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel M, Buechnear A, Krueger B, Frohne-Buechner C, Lenarz T. Evaluation of the Harmony soundprocessor in combination with the speech coding strategy HiRes 120. Otol Neurotol. 2008;29:199–202. doi: 10.1097/mao.0b013e31816335c6. [DOI] [PubMed] [Google Scholar]
- Briare JJ, Frijns JH. Field patterns in a 3D tapered spiral model of the electrically stimulated cochlea. Hear Res. 2000;148:18–30. doi: 10.1016/s0378-5955(00)00104-0. [DOI] [PubMed] [Google Scholar]
- Busby PA, Battmer RD, Pesch J. Electrophysiological spread of excitation and pitch perception for dual and single electrodes using the Nucleus Freedom cochlear implant. Ear Hear. 2008;29:853–64. doi: 10.1097/AUD.0b013e318181a878. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Shannon RV. Forward masked excitation patterns in multielectrode electrical stimulation. J Acoust Soc Am. 1998;103:2565–72. doi: 10.1121/1.422777. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Galvin JJ, 3rd, Fu QJ, Shannon RV. Effects of stimulation mode, level and location on forward-masked excitation patterns in cochlear implant patients. J Assoc Res Otolaryngol. 2006;7:15–25. doi: 10.1007/s10162-005-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GS, Kreft HA, Litvak L. Place-pitch discrimination of single versus dual-electrode stimuli by cochlear implant users. J Acoust Soc Am. 2005;128:623–626. doi: 10.1121/1.1937362. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Koch DB, Downing M, Litvak L. Current steering creates additional pitch percepts in adult cochlear implant recipients. Otol Neurotol. 2007;28:629–36. doi: 10.1097/01.mao.0000281803.36574.bc. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110:1150–63. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Nogaki G. Noise susceptibility of cochlear implant users: the role of spectral resolution and smearing. J Asssoc Res Otolaryngol. 2005;6:19–27. doi: 10.1007/s10162-004-5024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Shannon RV, Wang X. Effects of noise and spectral resolution on vowel and consonant recognition: acoustic and electric hearing. J Acoust Soc Am. 1998;104:3586–96. doi: 10.1121/1.423941. [DOI] [PubMed] [Google Scholar]
- Hacker MJ, Ratcliff R. A revised table of d′ for M-alternative forced choice. Percept Psychophys. 1979;26:168–70. [Google Scholar]
- Hughes ML, Abbas PJ. The relation between electrophysiologic channel interaction and electrode pitch ranking in cochlear implant recipients. J Acoust Soc Am. 2006;119:1527–37. doi: 10.1121/1.2163273. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Stille LJ. Psychophysical versus physiological spatial forward masking and the relation to speech perception in cochlear implants. Ear Hear. 2008;29:435–52. doi: 10.1097/AUD.0b013e31816a0d3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly CN, Spelman FA, Clopton BM. Quadrupolar stimulation for Cochlear prostheses: modeling and experimental data. IEEE Trans Biomed Eng. 1996;43:857–65. doi: 10.1109/10.508549. [DOI] [PubMed] [Google Scholar]
- Kwon BJ, van den Honert C. Effect of electrode configuration on psychophysical forward masking in cochlear implant listeners. J Acoust Soc Am. 2006;119:2994–3002. doi: 10.1121/1.2184128. [DOI] [PubMed] [Google Scholar]
- Landsberger DM, Srinivasan AG. Virtual channel discrimination is improved by current focusing in cochlear implant recipients. Hear Res. 2009;254:34–41. doi: 10.1016/j.heares.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(Suppl 2):467. [PubMed] [Google Scholar]
- Litvak LM, Spahr AJ, Emadi G. Loudness growth observed under partially tripolar stimulation: model and data from cochlear implant listeners. J Acoust Soc Am. 2007;122:967–81. doi: 10.1121/1.2749414. [DOI] [PubMed] [Google Scholar]
- Loizou PC, Dorman M, Tu Z. On the number of channels needed to understand speech. J Acoust Soc Am. 1999;106:2097–103. doi: 10.1121/1.427954. [DOI] [PubMed] [Google Scholar]
- Mens LH, Berenstein CK. Speech perception with mono- and quadrupolar electrode configurations: a crossover study. Otol Neurotol. 2005;26:957–64. doi: 10.1097/01.mao.0000185060.74339.9d. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Donaldson GS, Kreft H. Forward-masked spatial tuning curves in cochlear implant users. J Acoust Soc Am. 2008;123:1522–43. doi: 10.1121/1.2836786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philos Trans R Soc Lond B Biol Sci. 1992;336:367–73. doi: 10.1098/rstb.1992.0070. [DOI] [PubMed] [Google Scholar]
- Saoji AA, Litvak LM, Hughes ML. Excitation patterns of simultaneous and sequential dual-electrode stimulation in cochlear implant recipients. Ear Hear. 2009;30:559–67. doi: 10.1097/AUD.0b013e3181ab2b6f. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–4. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Fu QJ, Galvin J., 3rd The number of spectral channels required for speech recognition depends on the difficulty of the listening situation. Acta Otolaryngol Suppl. 2004:50–4. doi: 10.1080/03655230410017562. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Bierer JA, Middlebrooks JC. Topographic spread of inferior colliculus activation in response to acoustic and intracochlear electric stimulation. J Assoc Res Otolaryngol. 2004;5:305–22. doi: 10.1007/s10162-004-4026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelman FA, Pfingst BE, Clopton BM, Jolly CN, Rodenhiser KL. Effects of electrical current configuration on potential fields in the electrically stimulated cochlea: field models and measurements. Ann Otol Rhinol Laryngol Suppl. 1995;166:131–6. [PubMed] [Google Scholar]
- Throckmorton CS, Collins LM. Investigation of the effects of temporal and spatial interactions on speech-recognition skills in cochlear-implant subjects. J Acoust Soc Am. 1999;105:861–73. doi: 10.1121/1.426275. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991;352:236–8. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]
- Xu L, Thompson CS, Pfingst BE. Relative contributions of spectral and temporal cues for phoneme recognition. J Acoust Soc Am. 2005;117:3255–67. doi: 10.1121/1.1886405. [DOI] [PMC free article] [PubMed] [Google Scholar]