Abstract
Auditory-evoked-potential (AEP) data from two studies originally designed for other purposes were reanalyzed. The auditory brainstem response (ABR), middle-latency response (MLR), and long-latency response (LLR) were measured. The latencies to each of several peaks were measured for each subject for each ear of click presentation, and the time intervals between successive peaks were calculated. Of interest were differences in interpeak intervals between the sexes, between people of differing sexual orientations, and between the two ears of stimulation. Most of the differences obtained were small. The largest sex differences were for interval I → V in the ABR and interval N1 → N2 of the LLR (effect sizes > 0.6). The largest differences between heterosexuals and nonheterosexuals were for the latency to Wave I in both sexes, for the interval Na→ Nb in females, and for intervals V → Na and Nb → N1 in males (effect sizes > 0.3). The largest difference for ear stimulated was for interval N1 → N2 in heterosexual females (effect size ~0.5). No substantial differences were found in the AEP intervals between women using, and not using, oral contraceptives. Left/right correlations for the interpeak intervals were mostly between about 0.4 and 0.6. Correlations between the ipsilateral intervals were small; i.e., interval length early in the AEP series was not highly predictive of interval length later in the series. Interpeak intervals appear generally less informative than raw latencies about differences by sex and by sexual orientation.
Keywords: auditory evoked potentials (AEPs), interpeak intervals, sex differences, sexual orientation, oral contraceptives, ear differences
1. Introduction
Following the presentation of a brief acoustic stimulus such as a click, a succession of peaks is evoked in the brain waves that are detected using simple electrode arrays on the scalp (e.g., Hall, 2007). This succession of peaks is known as the auditory evoked potentials (AEPs). The most common way to analyze and report AEP data is to measure either the time elapsed between stimulus presentation and the maximum magnitude of the peak of interest (the latency), or the amplitude of that peak measured from its maximum magnitude to the following minimum magnitude (e.g., Davis, 1976). By convention, the succession of peaks seen is divided into three temporal groups: the auditory brainstem responses (ABRs) having peak latencies between 0 and about 10 ms, the middle-latency responses (MLRs) having peak latencies between about 10 and 50 ms, and the long-latency responses (LLRs) having peak latencies between about 50 and 300 ms (Picton et al., 1974).
A number of factors affect the latencies of the peaks in the AEP (for a review, see Burkard and Don, 2007). However, because the peaks are loosely associated with anatomical regions within the central auditory nervous system (e.g., Møller, 1998), the time intervals between the latencies of successive peaks do provide a rough measure of the transmission times between regions of the brain, and these intervals have proved to be useful for neuro-diagnostic purposes (Hall, 2007). The presence of neurological pathology typically leads to a prolongation of the interpeak intervals (Møller, 2007). The transmission times of the ABR have been studied most extensively (e.g., Fabiani et al., 1979; Griffiths et al., 1989).
Calculating interpeak intervals has been most common for early peaks such as the interval between wave I and wave III (I → III), between wave III and wave V (III → V), or between wave I and wave V (I → V) of the ABR (e.g., Fabiani et al., 1979; Griffiths et al., 1989; Grillon et al., 1989; Skoff et al., 1980; Rosenhall et al., 2003). Less commonly, investigators sometimes have reported interpeak intervals between later waves such as Na, Pa, and Nb of the MLR (e.g., Davis & Zerlin, 1966; Grillon et al., 1989; Ozdamar & Kraus, 1983). The interval I → V has been labeled “central conduction time” (Gorga et al., 1988) and is used clinically to assess the presence of retrocochlear pathology between the auditory nerve and the brainstem.
AEPs long have been known to exhibit sex differences in latency or amplitude of certain peaks (e.g., Cowell et al., 1994; Stockard et al., 1978). The most commonly studied peak is Wave V of the ABR, and both its latency and the interpeak interval I → V are shorter (faster) in females than in males (Beagley & Sheldrake, 1978; Stockard et al., 1978; Jerger & Hall, 1980). Although sex differences are well established in adults, the results are less clear-cut in infants and young children where sex differences sometimes are not observed (e.g., Sininger et al., 1998; Stockard et al., 1979). In a previous paper, we presented evidence that certain AEP peaks also are different in latency or amplitude in people of different sexual orientations (McFadden and Champlin, 2000). Specifically, we showed that the mean values for homosexual and bisexual females can be shifted in the direction of males (called “masculinized”), and the mean values for homosexual and bisexual males can be shifted so far away from the female means that they exceed the means for the heterosexual males (called “hyper-masculinized”). A masculinization also has been observed in the otoacoustic emissions (OAEs) of nonheterosexual females, but the OAEs of heterosexual and nonheterosexual males did not differ (McFadden and Pasanen, 1998, 1999; reviewed by McFadden, 2008, 2009).
We wished to know if the elapsed times between successive peaks in the AEPs also can differ between heterosexual and nonheterosexual males or females, and if sex (or ear) differences exist in intervals other than interval I → V of the ABR. To maximize the sample size, the data from a previously published study (McFadden, 2000; McFadden and Champlin, 2000) were pooled with later-collected data using substantially the same recruitment and measurement procedures.
2. Methods
2.1. General
The analyses reported here were performed on data collected in two AEP studies designed for purposes other than measuring the time intervals between successive peaks in the AEP waveforms. The first data set was the basis for two previous reports (McFadden, 2000; McFadden and Champlin, 2000). The second data set was collected over a period of several years beginning soon after the publication of the first study. The AEP procedures, equipment, and analyses were essentially identical for the two studies. The data-collection and data-analysis procedures are described briefly below; additional details can be found in McFadden (2000) and McFadden and Champlin (2000). The research protocols for both studies were approved in advance by the Institutional Review Board of The University of Texas.
2.2. Subjects
For the first study, data were collected from 49 heterosexual females, 57 nonheterosexual females, 50 heterosexual males, 53 nonheterosexual males (plus 35 additional heterosexual females who were using oral contraceptives--see below). For the second study, data were collected from 75 heterosexual females, 25 nonheterosexual females, 64 heterosexual males, and 27 nonheterosexual males; all these females were non-users of oral contraceptives. Because usable data occasionally were missing for an interval or an ear, the Ns varied slightly across conditions and comparisons. The Ns contributing to the various comparisons, and the breakdown for heterosexuals and nonheterosexuals are provided as part of the results.
The means (and standard deviations) for years of age in study 1 were: 21.2 (3.6), 26.1 (6.6), 24.1 (4.5), and 25.3 (5.8) for the heterosexual females, nonheterosexual females, heterosexual males, and nonheterosexual males, respectively; the average age of the heterosexual females using oral contraceptives was 21.3 (2.4). The means (and standard deviations) of the years of age in study 2 were: 20.2 (2.0), 21.8 (2.4), 20.3 (2.5), 22.0 (3.3) for the heterosexual females, nonheterosexual females, heterosexual males, and nonheterosexual males, respectively. Some subjects in study 2 were twins, but for same-sex twins, only one member of each pair was included (pseudorandomly) in the data analysis here.
2.3. Procedure
For both studies, subjects were recruited using advertisements on campus and in the campus newspaper and by word of mouth. Notices about the studies also were distributed to on-campus organizations for nonheterosexuals. Prospective subjects were screened for recent exposure to intense sounds and recent use of various drugs. Because ABRs are known to fluctuate with the menstrual cycle (Elkind-Hirsch et al. 1992a, 1992b, 1994), test sessions for females in both studies were scheduled during the midluteal phase of the cycle (days 16 – 26). Because oral contraceptives can masculinize AEPs (McFadden, 2000), the data from users and non-users of oral contraceptives are treated separately here.
After informed consent was obtained, subjects were screened audiometrically. This included otoscopy, audiometric screening at the frequencies between 250 and 6000 Hz in both ears, and tympanometry. To pass the hearing screening, a subject’s hearing had to be 15 dB Hearing Level or better at octave frequencies from 250 – 4000 Hz and 20 dB or better at 6000 Hz. Each subject completed a questionnaire containing items about history of exposure to intense sounds and drugs, and items about various aspects of sexual behavior. In study 2, otoacoustic emissions (OAEs) were collected as well as AEPs. The single test session lasted approximately 2 hours in study 1 and approximately 2 – 2.5 hours in study 2.
For both studies, sexual orientation was assessed using versions of the two traditional Kinsey items about fantasy and experience, plus an additional multiple-choice item asking for a self-assessment of orientation (heterosexual, homosexual, bisexual). When answers were inconsistent or incomplete (human-subjects regulations required allowing subjects not to answer individual questions), the data for that subject were excluded from the analyses. For the analyses reported here, the homosexual and bisexual subjects were pooled to create one nonheterosexual group per sex.
The two studies contributing data to this report differed in one important regard: namely, no LLR conditions were tested in study 2, only ABRs and MLRs. Accordingly, the Ns here for the LLR intervals are considerably smaller than those for the other interpeak intervals. One reason for omitting LLRs from study 2 was that the first study revealed no differences by sexual orientation in either latency or amplitude for any of the LLR peaks (McFadden and Champlin, 2000); also, LLRs require considerable time to collect. A second difference between the two studies that proved relevant during data analysis (see below) was bioamplifiers from different manufacturers.
For the AEP measurements, the subjects were fitted with four gold-plated surface electrodes, in accord with standard AEP procedures (Hall, 2007). They were seated in a reclining chair inside an electrically shielded, sound-treated room, and were informed about the importance of wakefulness. State of arousal was monitored using the ongoing electroencephalic waveform, and brief rest breaks were used as necessary to maintain and restore wakefulness. ABRs, MLRs, and LLRs were obtained in different experimental runs within the test session. Experienced experimenters were responsible for fitting the electrodes and collecting the AEP data.
Electrical pulses of 100-microsecond duration and negative polarity were presented to a shielded insert microphone (Etymotic, model ER-3A) in order to produce the click stimuli. The level of the clicks was about 70 dB above mean behavioral threshold, as measured in 20 separate listeners with normal hearing. The clicks were presented at a continuous repetition rate which was 18.1, 7.1, and 1.1 clicks per second for the ABR, MLR, and LLR measurements, respectively. The order of data collection was ABR, MLR, and LLR for all subjects. The scalp-recorded potentials were differentially amplified (gain = 200,000) and band-pass filtered (filter rejection rate = −6 dB/octave). The pass band of the filter was 0.1 – 3.0 kHz for the ABR, 10 – 300 Hz for the MLR, and 1 – 30 Hz for the LLR measurements. During and following each click presentation, the voltage waveform in each channel was digitized using a 16-bit digital-to-analog converter having a sampling rate of 10 kHz (MLR and LLR) or 50 kHz (ABR). Any sweep having a peak voltage exceeding a predetermined criterion was discarded. The number of click presentations ultimately averaged to obtain a waveform was 4,000 for ABR, 2,000 for MLR, and 400 for LLR.
The four surface electrodes permitted the collection of two channels of data simultaneously, one from the electrode on the earlobe ipsilateral to the ear containing the earphone and one from the electrode on the contralateral earlobe. For the analyses reported here, the contralateral data were discarded, and interpeak intervals are reported only between successive peaks in the ipsilateral left and ipsilateral right waveforms. We acknowledge that this procedure ignores the fact that the neural output of each cochlear nucleus (where all ipsilateral primary auditory fibers synapse) flows to both sides of the head.
2.4. Analyses
Audiology graduate students having past experience with AEP waveforms measured the latencies for six peaks from each of the six averaged waveforms for each subject (ipsilateral left and ipsilateral right for ABR, MLR, and, in study 1 only, LLR). The work of each experimenter was spot-checked by a second experimenter who independently analyzed approximately 5% of the waveforms in each latency category. Differences between measurements made by the two experimenters were resolved through reanalysis of the peak in question. For each subject, for each side of the head, the latency was measured for the six peaks traditionally labeled waves I and V of the ABR, peaks Na and Nb of the MLR, and peaks N1 and N2 of the LLR. The time differences between successive pairs of ipsilateral peaks were calculated to yield five interpeak intervals: I → V, V →Na, Na → Nb, Nb → N1, and N1 → N2. One additional value also was calculated and called Click → I; this is simply the latency from the presentation of the click stimulus to wave I. (Obtaining these latter values required making a correction that is described below.) Thus, each subject in study 1 contributed six interval measurements for each side of the head for a total of 12 intervals per subject (fewer for study 2, where LLRs were not measured). For study 1, all waveform measurements were made in ignorance of the sex and sexual orientation of the subject under consideration, but not in study 2. The decision to calculate interpeak intervals was made long after the judges had finished measuring the latencies for the various peaks.
Some waveforms were excluded because they were judged to be too noisy for accurate determination of the relevant peaks. Also, various checks were performed on the data: outlying values and marked lateral asymmetries for particular intervals were identified and either verified as accurate or excluded from subsequent analyses. A few instances of especially short-latency Na peaks were noted. These were attributed to post-auricular muscle response (PAMR; O’Beirne and Patuzzi, 1999), and the latencies for these peaks were omitted from the calculations of intervals. Whenever there was uncertainty about an individual peak or interval, it was excluded from the final analyses. Accordingly, the Ns varied across measures and comparisons.
Of interest were several questions, including: Were there sex differences or differences by sexual orientation in the lengths of any of the interpeak intervals? Because of the large number of pair-wise comparisons necessary to answer these questions for this data set, the various comparisons will be summarized using effect sizes rather than statistical tests. Effect sizes were calculated here as the difference between the means of the two groups of interest divided by the square root of the weighted mean of the variances of those two groups. By tradition, effect sizes of 0.2, 0.5, and 0.8 are regarded as small, medium, and large, respectively (Cohen, 1992). In order to estimate how likely that each obtained difference was simply a chance outcome, a resampling procedure was used (details are below).
In order to obtain comparable values for the interval Click → I, the measured latencies to wave I had to be corrected for the subjects in the study 2. The reason is that the bioamplifier used in study 2 (TDT HS4/DB4) introduced an electronic time delay that was longer than the time delay introduced by the bioamplifier used in study 1 (Grass 512P). According to the manufacturer’s specifications, that difference was nominally 1 ms. However, examination of the data from the two studies revealed that the actual difference in time delays was smaller than the nominal difference. For that reason, the difference in mean latency to wave I for the two studies was calculated for the left and right side for heterosexual females and heterosexual males, those four differences were averaged, and that average value (0.84 ms) was subtracted from the wave-I latencies for all subjects in study 2. This correction eliminated the difference between studies and allowed the differences between the sexes and sexual orientations to be revealed.
2.5. Resampling
When a large number of pairwise comparisons are made using measures that are not independent, it is difficult to know how best to control for the experiment-wise error rate. Resampling techniques using effect sizes can identify which outcomes are rare and unlikely to be attributable to chance.
To implement our resampling procedure, the data for the subjects in the two conditions of interest were pooled, that pool was partitioned at random into two groups of the same size as the original groups, and all of the relevant effect sizes were calculated. This process was repeated many thousands of times, and a record was kept of how often the effect sizes calculated were equal to, or larger than, the effect sizes actually obtained in the study. For example, when the comparison of interest was sex difference, the heterosexual females were pooled with the heterosexual males, the pool was randomly partitioned into “females” and “males” in accord with the relative numbers of actual females and males in the study, the effect sizes for all six designated intervals on the left side of the head were calculated for that resample, the effect sizes for all six designated intervals on the right side of the head were calculated for that resample, and the absolute values of those 12 individual effect sizes were stored for later comparison with the absolute values of the 12 effect sizes actually obtained for those conditions. That process constituted one resample, and 20,000 such random resamples were implemented. The use of absolute values for these comparisons is conservative; the rationale behind that decision was that in some cases we had no strong expectations about the direction of effect even though the literature does suggest that the interpeak intervals for ABRs are smaller for females than for males (e.g., Elberling and Parbo, 1987; Watson, 1996).
When the comparison of interest was sexual orientation, the heterosexual females were pooled with the nonheterosexual females or the heterosexual males were pooled with the nonheterosexual males, the pool was randomly partitioned in accord with the Ns of the two contributing groups, and so on until 20,000 resamples had been obtained for each sex.
By counting how often an experimentally obtained effect size was exceeded in magnitude in random resamples of the same data, one can gain insight into how (un)likely an experimentally obtained outcome was. One desirable characteristic of the resampling procedure described is that whatever dependencies might exist among the multiple dependent measures, they were preserved in the resamples.
The actual process of pooling the data was done in a staged manner in order to guarantee that subjects from the study 1 (who did contribute measures for the higher intervals) were proportionately randomly partitioned before adding in the proportionately randomly partitioned subjects from study 2. That way the Ns for the resamples were appropriate for all the designated intervals.
2.6. Comment
Before presenting the data, it is necessary to make two important points. First, it is tempting to think about the times between successive peaks in the AEP as representing the speed of neural propagation in the central nervous system. Thus, shorter interpeak intervals exist either when the speed of propagation across a fixed distance is greater or when the distance between two locations is shorter. Møller (2007) has discussed several ways that greater speed of propagation could be implemented, including larger diameters of the individual neural fibers and more efficient processing at the relevant synapses and within the neurons themselves, but whatever the underlying mechanism, one is tempted to think of greater speed of propagation as somehow being associated with a faster, and thus, a better brain. As intuitive as it might be, however, reasoning of this sort is almost surely an oversimplification in the current context, especially for the later interpeak intervals. It is correct to think of AEP peaks as representing more-or-less synchronous firings of collections of neurons; the more neurons contributing, or the more simultaneous their firings, the larger the peak that emerges from the background neural noise. However, for the peaks occurring later than wave I of the ABR, it is undoubtedly not correct to think of all those synchronously firing neurons as being located in the same place in the brain. Collections of neurons located at multiple places in the brain are contributing to each peak, some of which are even sending information from higher to lower neural centers. Because the collections of individual generators for the various later peaks are widely distributed, interpeak intervals of the sort reported below do not translate simply into the speed of the flow of neural information from one discrete location in the auditory brain to the next on the path from periphery to sensorium. Furthermore, our gross, far-field technique prohibited any attempt to identify the multiple brain locations contributing to a given peak or interval (Møller, 2007). Accordingly, the interpeak intervals discussed here are perhaps best thought of as representing global or overall synchrony in the auditory system.
Second, even though interpeak intervals in the AEP do not relate simply to speed of flow of the afferent information, the size difference in female and male heads still might play a major role in any sex differences in interpeak intervals observed. Because the studies in which the current subjects participated were not designed initially with interpeak intervals in mind, no measures of head size were obtained for these subjects. However, past research has indicated that the sex differences in absolute latencies, at least for the ABR peaks, do continue to exist after head size is taken into account (e.g., Edwards et al., 1983; Dempsey et al., 1986; Trune et al., 1988; Don et al., 1993). Also, we have reported a substantial sex difference in the latency to wave V in the absence of a sex difference in the latency to wave I, and that sex difference in latency for wave V was not present in every subsequent peak (McFadden and Champlin, 2000). Neither of these outcomes would be expected if the sex differences were attributable solely to head size. Accordingly, we believe that head size was not a crucial factor in the results presented here.
Of interest here were differences in the interpeak intervals by sex, by sexual orientation, by side of head, and by use of oral contraceptives. As noted, the intervals were calculated using only the data collected from the electrode array on the same side of the head as the clicks were presented (ipsilateral left and ipsilateral right). Results are reported for both ears separately, in part because two-ear averages could obscure laterality effects in the data that ultimately may prove important. In addition, correlations were calculated among the multiple measures obtained from the individual subjects to examine laterality and other questions. Again, ABR, MLR, and LLR measures all were obtained in study 1, but only ABR and MLR were obtained in study 2.
3. Results
3.1. Sex Differences
Only the heterosexual subjects were included in the analyses for differences between the sexes. The magnitudes and variability of the various intervals are compared for females and males in Figure 1, where the succession of six temporal intervals is illustrated as a succession of stacked arrows. Most of the sex differences proved to be quite small. In accord with past research (Rosenhamer et al., 1980; Dempsey et al., 1986; Trune et al., 1988), moderately large sex differences were found for the interval I → V, for both the left and right sides of the head; in addition, the LLR interval N1 → N2 on the left side also exhibited a substantial sex difference.
Figure 1.
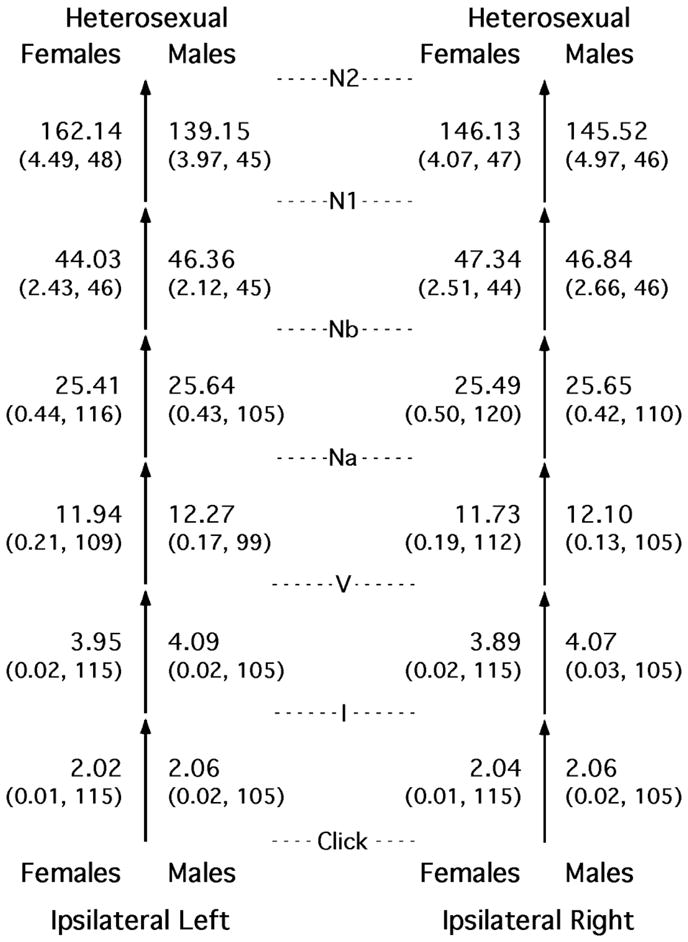
Means (standard errors, Ns) for the time intervals between successive peaks in the auditory evoked potential, shown separately for heterosexual females, heterosexual males, and the two sides of the head. The succession of six temporal intervals is illustrated as two stacks of six arrows, one for the left and one for the right side of the head. All entries shown were obtained from the side of the head ipsilateral to the ear being stimulated acoustically. The means and SEs are in milliseconds. The data necessary for the calculation of the two latest intervals were collected only in study 1.
Beginning at the bottom left of Figure 1, it can be seen that, for the first five intervals, the times between successive peaks in the AEP from the left side of the head were slightly shorter for the females than for the males. Then, for the final interval the directionality of this sex difference changed and the magnitude increased dramatically so that interval N1 → N2 on the left side of the head was substantially longer for females than for males. On the right side, the directionality of the sex difference also changed, but after the fourth interval in this case, and the magnitudes of the sex differences on the right side remained small throughout. That is, in females, the interval N1 → N2 was highly asymmetric, with the longer interval on the left side, but in males the asymmetry was much smaller and the longer interval was on the right side. This pattern of differences across intervals and across the head further suggests that head size does not play a major role in the sex differences in interpeak intervals (also see Edwards et al., 1983; Dempsey et al., 1986; Trune et al., 1988; Don et al., 1993). Note that any comparison of “long” intervals such as Click → N2 could produce a misleading picture of the differences between the sexes because some of the constituent intervals are shorter for females and others shorter for males.
Table 1 provides some perspective for the sex differences shown in Figure 1. This table contains effect sizes for the sex differences for each of the interpeak intervals and for both sides of the head. Also shown are the Ns upon which the individual effect sizes were based. Of the 12 effect sizes shown, only 5 were larger than 0.2 (defined as a small effect—Cohen, 1992). The three largest effect sizes were for interval I → V for both sides of the head and for interval N1 → N2 for the left side of the head. Resampling revealed that those three experimental outcomes were extremely unlikely to be attributable to chance.
Table 1.
Effect sizes (female minus male) and Ns for sex differences using heterosexual subjects only.
| Interval | Effect Sizes | Ns (Female/Male) | ||
|---|---|---|---|---|
| Side of Head | Side of Head | |||
| Left | Right | Left | Right | |
| Click → I | − 0.23 | − 0.18 | 115/105 | 115/105 |
| I → V | − 0.64 | − 0.74 | 115/105 | 115/105 |
| V → Na | − 0.16 | − 0.22 | 109/99 | 112/105 |
| Na → Nb | − 0.05 | − 0.03 | 116/105 | 120/110 |
| Nb → N1 * | − 0.15 | + 0.03 | 46/45 | 44/46 |
| N1 → N2 * | + 0.79 | + 0.02 | 48/45 | 47/46 |
Bold font indicates that fewer than 5% of 20,000 resamples had an effect size whose absolute value was equal to or greater than the obtained effect size shown.
A plus sign indicates a longer interpeak interval in the females.
Measurements obtained in study 1 only
Of the 20,000 random resamplings done for the interval I → V for each side of the head, none yielded an effect size equal to or larger than the two effect sizes obtained experimentally for the basic sex difference. In fact, only 4 and 9 resamples exceeded an effect size of 0.5 for the left and right sides of the head, respectively; both of those results correspond to a proportion of less than 0.0004. Thus, the data obtained for interval I→ V were highly unlikely to be attributable to chance. Others also have reported a smaller I → V interval for females (Dempsey et al., 1986; Trune et al., 1988). For the interval N1 → N2 on the left side of the head, only 3 of the 20,000 random resamples exceeded the effect size obtained experimentally, which corresponds to a proportion of about 0.0002. To our knowledge, this outcome has not been reported previously. To complete the set of effect sizes that exceeded 0.2 in Table 1: the proportion of resamples that exceeded the experimentally obtained effect size for the interval Click → I on the left side of the head was 0.11, and the proportion for the interval V → Na on the right side of the head was about 0.12. Accordingly, those outcomes may be attributable to chance.
By comparison with the small number of sex differences in these interpeak intervals, substantial sex differences were evident for 8 of the 19 absolute latencies and amplitudes measured in study 1 (McFadden and Champlin, 2000). Reite et al. (1995b) reported that an LLR-like peak had a slightly different site of origin in the brain depending upon the sex of the subject; that difference was larger on the right side of the head, unlike the laterality difference for LLR interval N1 → N2 in Table 1.
3.2. Sexual Orientation
Most of the differences between heterosexual and nonheterosexual subjects of the same sex were small. The basic comparisons are shown in Figures 2 and 3, for females and males, respectively.
Figure 2.
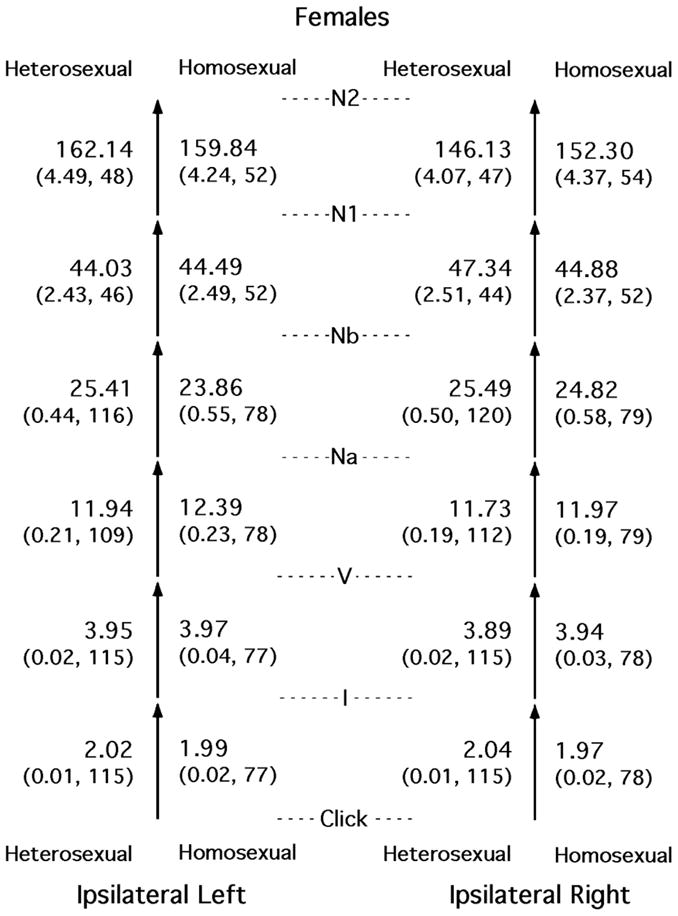
Means (standard errors and Ns) for the time intervals between successive peaks in the auditory evoked potential, shown separately for heterosexual females, homosexual females, and the two sides of the head. All entries shown were obtained from the side of the head ipsilateral to the ear being stimulated acoustically. The means and SEs are in milliseconds. The data necessary for the calculation of the two latest intervals were collected only in study 1.
Figure 3.
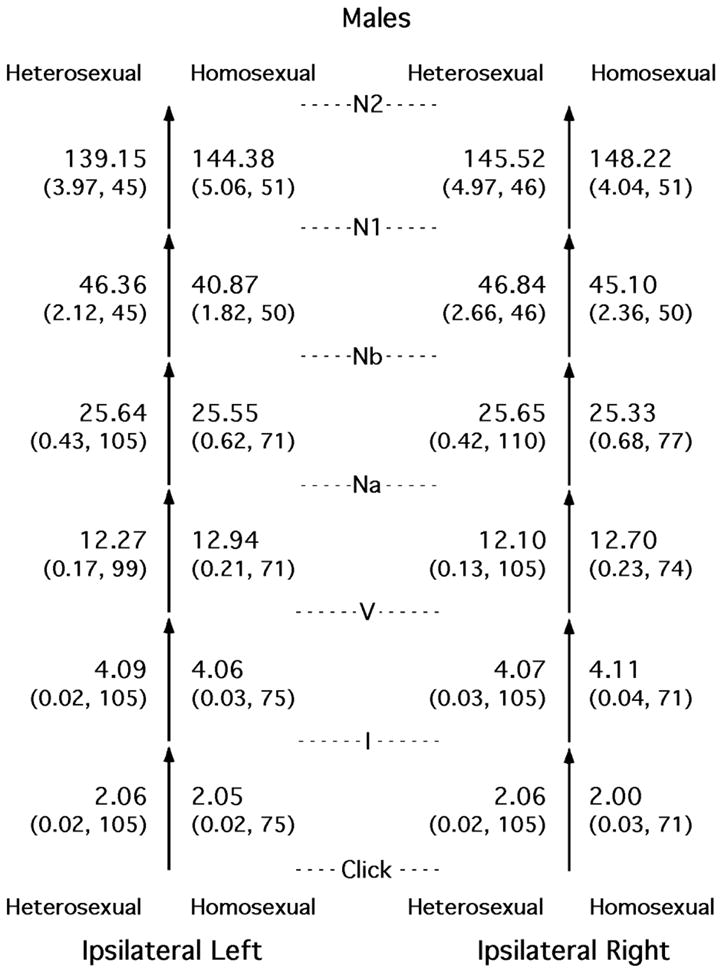
Means (standard errors and Ns) for the time intervals between successive peaks in the auditory evoked potential, shown separately for heterosexual males, homosexual males, and the two sides of the head. All entries shown were obtained from the side of the head ipsilateral to the ear being stimulated acoustically. The means and SEs are in milliseconds. The data necessary for the calculation of the two latest intervals were collected only in study 1.
Examination of Figure 2 reveals that sometimes the nonheterosexual females had longer interpeak intervals than the heterosexual females (a masculinized pattern), but sometimes the reverse occurred. For the subset of these subjects who were in study 1, the absolute AEP latencies and amplitudes generally were masculinized in the nonheterosexual females (McFadden and Champlin, 2000).
Examination of Figure 3 also reveals no evident, consistent pattern of differences between the heterosexual and nonheterosexual males. The absolute AEP latencies and amplitudes for the subset of these subjects who were in study 1 generally were hyper-masculinized for the nonheterosexual males (McFadden and Champlin, 2000).
Table 2 provides effect sizes for the differences by sexual orientation shown in Figures 2 and 3. The inconsistencies in the signs of these effect sizes highlight the lack of regular patterns in the differences between heterosexuals and nonheterosexuals. Of the 24 comparisons shown in Table 2, nine had effect sizes exceeding 0.2 and six had effect sizes exceeding 0.3. Of the latter, resampling suggested that five were unlikely to be attributable to chance.
Table 2.
Effect sizes for sexual orientation (heterosexual minus nonheterosexual females or heterosexual minus nonheterosexual males).
| Interval | Females | Males | ||
|---|---|---|---|---|
| Side of Head | Side of Head | |||
| Left | Right | Left | Right | |
| Click -> I | + 0.19 | + 0.36 † | + 0.06 | + 0.32 ‡ |
| I -> V | − 0.09 | − 0.20 | + 0.10 | − 0.12 |
| V -> Na | − 0.20 | − 0.13 | − 0.38 § | − 0.37 § |
| Na -> Nb | + 0.32 | + 0.12 | + 0.02 | + 0.06 |
| Nb -> N1 * | − 0.03 | + 0.15 | + 0.41 ‡ | + 0.10 |
| N1 -> N2 * | + 0.07 | − 0.20 | − 0.16 | − 0.09 |
Bold font indicates that fewer than 5% of 20,000 resamples had an effect size whose absolute value was equal to or greater than the obtained effect size shown.
Measurements obtained in study 1 only.
Hyper-feminized
Hypo-masculinized
Hyper-masculinized
We begin with the two largest effect sizes for sexual orientation in females (left half of Table 2): Only 208 of the 20,000 random resamples for the interval Click → I on the right side of the head yielded effect sizes having absolute values equal to or larger than the effect size obtained experimentally, which corresponds to a proportion of about 0.01. So that comparison seems unlikely to be attributable to chance. Not so for the interval Na → Nb on the left side of the head in females, however; 2736 of those 20,000 resamples equaled or exceeded the effect size obtained experimentally, corresponding to a proportion of about 0.14. Also, none of the three comparisons for sexual orientation in females that had effect sizes of −0.2 were rare during resampling; the proportions of resamples equaling or exceeding those obtained effect sizes were between 0.2 and 0.3. Note that the interval showing the largest effect size (Click → I, right side) was shorter for the nonheterosexual females than for the heterosexual females, which is a shift away from the corresponding value for the heterosexual males. So, descriptively, the shift was a hyper-feminization.
For sexual orientation in the males (right half of Table 2), all four comparisons having an effect size exceeding 0.3 appear to be rather unlikely to be attributable to chance. For the interval Click → I on the right side of the head, 908 of the 20,000 resamples had effect sizes equaling or exceeding the effect size obtained experimentally, which corresponds to a proportion of about 0.045. The resampling for the interval V → Na on the left and right sides of the head yielded 392 and 414 instances, respectively, in which the effect sizes equaled or exceeded the effect sizes obtained experimentally, and those each correspond to proportions of about 0.02. For the interval Nb → N1 on the left side of male heads, the relevant number and proportion were 1027 and 0.051. Note that the signs of these four effect sizes were not the same. For the interval V → Na on both sides of the head (negative effect sizes), the interval lengths for the nonheterosexual males were longer than those for the heterosexual males, both of which were shifts away from the values for the heterosexual females; so descriptively these two shifts were hyper-masculinizations. The signs for the other two large effect sizes (Click → 1, right side, and Nb → N1, left side) were positive and correspond, descriptively, to hypo-masculinizations. Indeed, in both of these latter cases, the interval lengths for the nonheterosexual males were even shorter than those for the heterosexual females.
Certain explanations about the origins of nonheterosexuality lead one to expect that those interpeak intervals showing effects for sexual orientation would be intervals that also showed differences between the sexes. A comparison of Tables 1 and 2 reveals that this was not true here. We acknowledge, but cannot explain, this curious outcome. Note, however, that some of the absolute AEP latencies and amplitudes that showed effects for sexual orientation also did not show a basic sex difference (McFadden and Champlin, 2000).
3.3. Lateral Asymmetry
Examination of the data in Figures 1, 2, and 3 reveals no consistent pattern of differences for the side (ear) of stimulation for either sex, and the calculation of effect sizes (see Table 3) revealed that the side differences that did exist generally were quite small. The primary exception was the side difference already noted for interval N1 → N2 in the heterosexual females (effect size = +0.54; calculated as left minus right, see Figure 2 for Ns). That same interval showed a similar direction of effect for the nonheterosexual females (effect size = +0.24) but an opposite direction of effect for the heterosexual males (effect size = − 0.21). Thus, the nonheterosexual females appear to be shifted in the male direction for interval N1 → N2. A similar, but weaker, masculinization appears to exist for the interval I → V; heterosexual females exhibited a small positive effect size, heterosexual males were basically symmetric for that interval, and nonheterosexual females were more like the males than the heterosexual females.
Table 3.
Effect sizes for differences in side of head stimulated (left minus right).
| Interval | Females | Males | ||
|---|---|---|---|---|
| Heterosexual | Non-Ht | Heterosexual | Non-Ht | |
| Click -> I | − 0.10 | + 0.09 | − 0.04 | + 0.23 |
| I -> V | + 0.26 | + 0.09 | + 0.05 | − 0.15 |
| V -> Na | + 0.10 | + 0.22 | + 0.11 | + 0.13 |
| Na -> Nb | − 0.01 | − 0.19 | − 0.00 | + 0.04 |
| Nb -> N1 * | − 0.20 | − 0.02 | − 0.03 | − 0.28 |
| N1 -> N2 * | + 0.54 | + 0.24 | − 0.21 | − 0.12 |
Non-Ht = Nonheterosexual
Bold font indicates that fewer than 5% of 20,000 resamples had an effect size whose absolute value was equal to or greater than the obtained effect size shown.
A plus sign indicates a longer interpeak interval on the left side of the head.
Measurements obtained in study 1 only.
Resampling revealed that only two of the observed differences for side of head were rare outcomes, and both of those were for the heterosexual females. Specifically, the interval N1 → N2 for heterosexual females had 187 resamples in which the absolute value of the effect size equaled or exceeded the effect size obtained experimentally, which corresponds to a proportion of about 0.009, and the interval I → V for heterosexual females yielded 1087 resamples equal to or larger than the obtained value, which corresponds to a proportion of 0.054. None of the other comparisons in Table 3 were likely to be attributable to anything other than chance. Reite et al. (1995a) reported that an LLR-like peak had sites of origin in the brain that were more bilaterally symmetric in homosexual than in heterosexual males; a similar, but weak, effect exists for the LLR interval N1 → N2 in males in Table 3.
3.4. Correlations
An unexpected outcome in these data was that correlations between the ABR, MLR, and LLR intervals were generally weak for all subject groups. That is, the early interpeak intervals in individual subjects were not good predictors of the later interpeak intervals on the same side of the head. The intervals I → V, Na → Nb, and N1 → N2 were chosen to represent the ABR, MLR, and LLR time periods, respectively, and the correlations between all pairs of those intervals are shown in the top half of Table 4. The interval I → V was poorly related to interval Na → Nb in all subject groups, and it was generally poorly related to interval N1 → N2 as well. The correlations between the intervals Na → Nb and N1 → N2 were greater than 0.2 only for the nonheterosexual subjects. These calculations reveal that interpeak intervals are not routinely short in some brains and routinely long in others. Although not shown here, the correlations with the intervals that cut across ABR/MLR and MLR/LLR boundaries (e.g., V → Na) similarly were quite small.
Table 4.
Correlations between interpeak intervals (top) and between sides of the head for the same interval (bottom).
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Heterosexual | Non-Ht | Heterosexual | Non-Ht | |||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| I -> V vs. Na -> Nb | + 0.02 | − 0.01 | −0.06 | −0.14 | −0.23 | −0.02 | −0.06 | − 0.05 |
| I -> V vs. N1 -> N2 | − 0.04 | − 0.32 | +0.18 | −0.00 | −0.17 | +0.18 | +0.23 | + 0.41 |
| Na -> Nb vs. N1 -> N2 | − 0.08 | + 0.12 | +0.06 | −0.23 | +0.01 | −0.01 | +0.25 | + 0.11 |
| Heterosexual | Non-Ht | Heterosexual | Non-Ht | |||||
| Lt vs. Rt | Lt vs. Rt | Lt vs. Rt | Lt vs. Rt | |||||
| I -> V | 0.58 | 0.41 | 0.48 | 0.51 | ||||
| Na -> Nb | 0.62 | 0.42 | 0.39 | 0.45 | ||||
| N1 -> N2 | 0.49 | 0.22 | 0.38 | 0.30 | ||||
| Means (Lt vs. Rt) | 0.56 | 0.35 | 0.42 | 0.42 | ||||
Non-Ht = Nonheterosexual
Bold font indicates that fewer than 5% of 20,000 resamples had an effect size whose absolute value was equal to or greater than the obtained effect size shown.
Shown in the bottom half of Table 4 are the correlations across the head for the three representative intervals I → V, Na → Nb, and N1 → N2. Those correlations were universally positive, and all were moderately strong for all groups, which is better in accord with intuition and expectations than the correlations in the top half of the table. (Note that interaural correlations for interpeak intervals should be lower than interaural correlations for the individual peaks in the AEP because estimates of intervals contain error variance associated with both the onset and the termination of each interval.) In all subject groups, the left/right correlations generally were high for the ABR interval I → V and grew gradually weaker for the representative MLR and LLR intervals. These left/right correlations also were somewhat stronger for the heterosexual females than for the nonheterosexual females, but this trend was not evident for the males. Note that the sharp reversal in the size and direction of the N1 → N2 interval mentioned above for the heterosexual females did not produce an unusual left/right correlation for that interval. These left/right correlations reveal that the two sides of the auditory brain are more similar than are the different levels of the auditory brain (correlations in the top half of Table 4).
In order to put into perspective the correlation values shown in Table 4, resampling was conducted as follows: The interval values actually obtained for each measure (i.e., interval I → V left, interval I → V right, interval Na → Nb left, etc.) were assigned correctly to each individual member of the group of interest (e.g., heterosexual females). Then, each individual subject also was assigned, at random (and without replacement), all the interval values for one of the other subjects. All of the relevant correlations were calculated, and the values noted. This process was repeated 20,000 times and, for each correlation of interest, the number of instances in which the absolute value of the resampled correlation equaled or exceeded the correlation value obtained experimentally were tallied. In Table 4, those correlations whose absolute values were exceeded by fewer than 5% of the resamples are printed in bold font. Clearly, the experimentally obtained correlations between the two sides of the head (bottom half of Table 4) were far more likely to be rare events than the correlations among the ABR, MLR, and LLR intervals (top half of Table 4).
3.5. Oral Contraceptives
Certain AEPs can shift in the male direction in women using oral contraceptives (OC; see McFadden, 2000). Knowledge of that fact raised the question of whether some AEP interpeak intervals might be different in subjects using and not using OC. So far in this report, all the data presented for females have been from non-users of OC, but in study 1, data were collected from both users and non-users so a comparison was possible. (Only normal-cycling females were hired for study 2.) The relevant latencies from the users of OC in study 1 were analyzed as above, and the results for heterosexual users and non-users were compared. Table 5 contains the effect sizes and Ns for the various intervals calculated for this report. Again, all of the differences were small.
Table 5.
Effect sizes (Non-Users minus Users) and Ns for use of oral contraception (heterosexuals from study 1 only).
| Interval | Effect Sizes | Ns (Non-users/Users) | ||
|---|---|---|---|---|
| Side of Head | Side of Head | |||
| Left | Right | Left | Right | |
| Click → I | − 0.06 | + 0.01 | 48/34 | 48/34 |
| I → V | − 0.21 | − 0.15 | 48/34 | 48/34 |
| V → Na | + 0.20 | − 0.33 | 47/34 | 46/35 |
| Na → Nb | − 0.32 | − 0.21 | 46/32 | 45/34 |
| Nb → N1 | − 0.45 | − 0.21 | 46/26 | 44/30 |
| N1 → N2 | + 0.10 | − 0.31 | 48/34 | 47/34 |
Resampling revealed that none of these effect sizes was large enough to occur less often than 5% of the time.
A plus sign indicates a longer interpeak interval in the non-users of OC.
Resampling revealed that the largest effect size in Table 5 (−0.45 for interval Nb → N1 on the left side of the head) was equaled or exceeded 1355 times, for a proportion of 0.07. The other, smaller effect sizes obviously were associated with correspondingly larger proportions. Thus, the effects of oral contraception on AEP intervals were small.
4. Discussion
The present results suggest that some of the time intervals between successive peaks in the AEPs differ by sex (Table 1), by sexual orientation (Table 2), and by side of head being tested (Table 3). Most of the effects were small, although a few of the sex differences were substantial. Also, the heterosexual females were markedly asymmetrical for the interval N1 → N2 (longer on the left side of the head), a finding that, to our knowledge, has not been reported previously. The use of oral contraception had very little effect on interpeak intervals (Table 5). Interpreting these differences is complicated by the facts discussed in the Comments section; namely, with the likely exception of wave I, AEP peaks almost surely represent neural activity originating from multiple generators and sites. Thus, group differences in a particular interpeak interval seemingly are best interpreted as differences in synchronized behavior for collections of cells relatively widely distributed through the auditory brain.
Females can have a slightly higher body temperature than males, and this factor has been considered in the past as a possible basis for the sex difference in AEPs (Don et al., 1993). However, a global difference in body temperature seemingly is inadequate as an explanation for the fluctuating pattern of sex differences seen in Figure 1. Don et al. (1993) also discussed other possible factors affecting sex differences in AEP latency.
The durations of interpeak intervals were moderately, positively correlated across the head, as would be expected (Table 4, bottom). We are not aware of any other study in which interpeak intervals from the left and right ears were compared directly; however, Durrant et al. (1990) reported measurements that are somewhat related to ours. They stimulated only one ear, measured the latencies for certain ABR peaks in both the ipsilateral and contralateral records, and calculated interaural correlations for those absolute latencies. The correlations were 0.6 and 0.9 for wave III and V, respectively, and no analysis for sex differences was attempted. Also, the correlations here between representative ABR, MLR, and LLR intervals generally were small (Table 4, top), revealing that auditory brains are not universally “slow” or “fast.”
The one interpeak interval showing a difference with sexual orientation for females was hyper-feminized in the nonheterosexuals. Two of the four intervals showing a difference with sexual orientation for males involved a hypo-masculinization in the nonheterosexuals and two involved a hyper-masculinization. Finding different directions of effect for different measures is not uncommon in studies of sexual orientation; one possible explanation is what has been called “localized effects” (see McFadden, 2002). As noted above, the absolute AEP latencies and amplitudes showing effects for nonheterosexuals (McFadden and Champlin, 2000) were generally masculinized in females and generally hyper-masculinized in males.
Beginning with the neurons leaving the cochlear nucleus but continuing at multiple locations beyond the hindbrain, some afferent auditory neurons cross the midline of the brain and synapse contralaterally. Thus, there are multiple paths from periphery to cortex, some more direct than others. One might argue, then, that any investigation of successive interpeak intervals, like this one, should take this complex pattern of crossing into account when calculating intervals. The simple pattern of electrodes used here precluded an elaborate analysis of that sort. Further, too much still is unknown about the various pathways involved to do such detailed analyses (and, as noted in the Comment above, the later peaks in the AEP surely do not originate from single neural locations anyway). Rosenhall et al. (2003) argued that the primary flow of afferent information in the auditory system crosses the midline of the brain between the cochlear nucleus and the inferior colliculus. Accordingly, one might argue that here we should have calculated our intervals ipsilaterally only through, say, interval I → V, then calculated the interval ipsilateral-V → contralateral-Na, then continued with the contralateral intervals. We wish to point out that an analysis of that sort on the data available here was not likely to reveal additional differences by sex, by sexual orientation, or by side of head from those we obtained. Were this study ever repeated using multiple electrodes, and thus having better localization of the origins of the various AEP peaks, then consideration might be given to the issue of crossed pathways when calculating interpeak intervals.
AEP interpeak intervals have been reported to be longer than normal in people with retrocochlear disorders such as space-occupying lesions and degenerative pathologies, and following traumatic incidents. While prolonged interpeak intervals do not permit the identification of specific etiologies, they do suggest the general presence of neurological anomalies (e.g., Hall, 2007). Prolonged interpeak intervals also have been observed in individuals having developmental disorders such as autism and central auditory processing disorder (e.g., Skoff et al., 1980; Rosenhall et al., 2003; Jirsa and Clontz, 1990). The prolonged ABR intervals in autism may be caused by some of the anomalies in the normal developmental processes that also are responsible for the primary symptoms of the disorder. Because autism is far more prevalent in males than females, and the initial symptoms appear relatively early in life, both the symptoms and the accompanying prolonged AEP intervals may be attributable in part to the degree of exposure to androgens during prenatal development (see McFadden 2008, 2009). Specifically, if exposure to prenatal androgens somehow lengthened some AEP intervals, that would explain the basic sex differences summarized in Table 1, and it would provide an hypothesis about the interval differences seen in people with autism, nonheterosexuals, and possibly other special populations having inherent sex differences. Evidence of hormonal influences on AEP intervals has been noted in both animals and humans; estrogen replacement treatment can decrease ABR intervals and certain latencies of the MLR (e.g., Coleman et al., 1994; Caruso et al., 2000).
A final comment: McFadden and Champlin (2000) reported that a weaker click level often yielded larger differences between groups than a stronger click level. Here we have reported interpeak intervals obtained only from the data collected with the stronger click level (70 dB above the mean behavioral threshold of 20 separate listeners). The primary reason is that it was generally far more difficult to identify some of the various AEP peaks in the averaged waveforms collected with the weaker click. Not only did this diminish the Ns, it led to worries about the representativeness of the remaining data. If future investigators use more sophisticated electrode arrays or superior averaging procedures, they are encouraged to consider collecting data with weaker click levels than were employed here.
Acknowledgments
This work was supported by a research grant awarded to DM by the National Institute on Deafness and other Communication Disorders (NIDCD; RO1 DC000153). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health. E.G. Pasanen helped with the resampling, M.M. Maloney helped with the figures, and Diana Simmons and Lars Strother helped with early data analysis.
List of Abbreviations
- ABR
auditory brainstem response
- AEP
auditory evoked potential
- dB
decibel
- Ht
heterosexual
- Hz
Hertz
- LLR
long-latency response
- MLR
middle-latency response
- ms
millisecond
- N
number of subjects
- OAE
otoacoustic emission
- OC
oral contraceptive
- PAMR
post-auricular muscle response
- SEs
standard errors of the mean
- SPL
sound-pressure level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle D. Hsieh, Email: michellehsieh@mail.utexas.edu.
Craig A. Champlin, Email: champlin@austin.utexas.edu.
References
- Beagley HA, Sheldrake JB. Differences in brainstem response latency with age and sex. Br J Audiol. 1978;12:69–77. doi: 10.3109/03005367809078858. [DOI] [PubMed] [Google Scholar]
- Burkard RF, Don M. The auditory brainstem response. In: Burkard R, Don M, Eggermont J, editors. Auditory Evoked Potentials: Basic Principles and Clinical Application. Lippincott, Williams & Wilkins; New York: 2007. pp. 229–253. [Google Scholar]
- Caruso S, Cianci A, Grasso D, Agnello C, Galvani F, Maiolino L, et al. Auditory brainstem response in postmenopausal women treated with hormone replacement therapy: A pilot study. Menopause. 2000;7:178–183. doi: 10.1097/00042192-200007030-00008. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Coleman J, Campbell D, Cooper W, Welsh M, Moyer J. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res. 1994;80:209–215. doi: 10.1016/0378-5955(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes. J Neurosci. 1994;14:4748–4755. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H. Principles of electric response audiometry. Ann Otol Rhinol Laryngol. 1976;85:1–96. [PubMed] [Google Scholar]
- Davis H, Zerlin S. Acoustic relations of the human vertex potential. J Acoust Soc Am. 1966;39:109–116. doi: 10.1121/1.1909858. [DOI] [PubMed] [Google Scholar]
- Dempsey JJ, Censoprano E, Mazor M. Relationship between head size and latency of the auditory brainstem response. Audiology. 1986;25:258–262. [PubMed] [Google Scholar]
- Don M, Ponton CW, Eggermont JJ, Masuda A. Gender differences in cochlear response time: An explanation for gender amplitude differences in the unmasked auditory brain-stem response. J Acoust Soc Am. 1993;94:2135–2148. doi: 10.1121/1.407485. [DOI] [PubMed] [Google Scholar]
- Durrant JD, Boston JR, Martin WH. Correlation study of two-channel recordings of the brain stem auditory evoked potential. Ear Hear. 1990;11:215–221. doi: 10.1097/00003446-199006000-00009. [DOI] [PubMed] [Google Scholar]
- Edwards RM, Squires NK, Buchwald JS, Tanguay PE. Central transmission time differences in the auditory brainstem response as a function of sex, age, and ear of stimulation. Internat J Neurosci. 1983;18:59–66. doi: 10.3109/00207458308985878. [DOI] [PubMed] [Google Scholar]
- Elberling C, Parbo J. Reference data for ABRs in retrocochlear diagnosis. Scand Audiol. 1987;16:49–55. doi: 10.3109/01050398709042155. [DOI] [PubMed] [Google Scholar]
- Elkind-Hirsch KE, Stoner WR, Stach BA, Jerger JF. Estrogen influences auditory brainstem responses during the normal menstrual cycle. Hear Res. 1992a;60:143–148. doi: 10.1016/0378-5955(92)90016-g. [DOI] [PubMed] [Google Scholar]
- Elkind-Hirsch KE, Wallace E, Stach BA, Jerger JF. Cyclic steroid replacement alters auditory brainstem responses in young women with premature ovarian failure. Hear Res. 1992b;64:93–98. doi: 10.1016/0378-5955(92)90171-i. [DOI] [PubMed] [Google Scholar]
- Elkind-Hirsch KE, Wallace E, Malinak LR, Jerger JJ. Sex hormones regulate ABR latency. Otolaryngol Head Neck Surg. 1994;110:46–52. doi: 10.1177/019459989411000105. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Sohmer H, Tait C, Gafni M, Kinart R. A functional measure of brain activity. Electroencephalogr Clin Neurophysiol. 1979;47:483–491. doi: 10.1016/0013-4694(79)90164-0. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Kaminski JR, Beauchaine KA, Jesteadt W. Auditory brainstem responses to tone bursts in normally hearing subjects. J Speech Lang Hear Res. 1988;31:87–97. doi: 10.1044/jshr.3101.87. [DOI] [PubMed] [Google Scholar]
- Griffiths SK, Chambers RD, Bilger RC. Statistical dependence among the wave V latencies of the auditory brain stem response. Ear Hear. 1989;10:299–303. doi: 10.1097/00003446-198910000-00005. [DOI] [PubMed] [Google Scholar]
- Grillon C, Courchesne E, Akshoomoff N. Brainstem and middle latency auditory evoked potentials in autism and developmental language disorder. J Autism Dev Disord. 1989;19:255–269. doi: 10.1007/BF02211845. [DOI] [PubMed] [Google Scholar]
- Hall JW., III . New Handbook of Auditory Evoked Responses. Pearson Education; Boston: 2007. [Google Scholar]
- Jerger J, Hall JW., III Interactions of age and sex on the auditory brainstem response (ABR) Arch Otolaryngol. 1980;106:387–391. doi: 10.1001/archotol.1980.00790310011003. [DOI] [PubMed] [Google Scholar]
- Jirsa R, Clontz K. Long latency event-related potentials from children with auditory processing disorders. Ear Hear. 1990;11:222–232. doi: 10.1097/00003446-199006000-00010. [DOI] [PubMed] [Google Scholar]
- McFadden D. Masculinizing effects on otoacoustic emissions and auditory evoked potentials in women using oral contraceptives. Hear Res. 2000;142:23–33. doi: 10.1016/s0378-5955(00)00002-2. [DOI] [PubMed] [Google Scholar]
- McFadden D. Masculinization effects in the auditory system. Arch Sex Behav. 2002;31:93–105. doi: 10.1023/a:1014087319682. [DOI] [PubMed] [Google Scholar]
- McFadden D. What do sex, twins, spotted hyenas, ADHD, and sexual orientation have in common? Perspect Psychol Sci. 2008;3:309–323. doi: 10.1111/j.1745-6924.2008.00082.x. [DOI] [PubMed] [Google Scholar]
- McFadden D. Masculinization of the mammalian cochlea. Hear Res. 2009;252:37–48. doi: 10.1016/j.heares.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Champlin CA. Comparison of auditory evoked potentials in heterosexual, homosexual, and bisexual males and females. J Assoc Res Otolaryngol. 2000;1:89–99. doi: 10.1007/s101620010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG. Comparison of the auditory systems of heterosexuals and homosexuals: Click-evoked otoacoustic emissions. Proc Natl Acad Sci USA. 1998;95:2709–2713. doi: 10.1073/pnas.95.5.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG. Spontaneous otoacoustic emissions in heterosexuals, homosexuals, and bisexuals. J Acoust Soc Am. 1999;105:2403–2413. doi: 10.1121/1.426845. [DOI] [PubMed] [Google Scholar]
- Møller AR. Twenty-five years of ABR: Historical perspective, current issues, and future directions. Semin Hear. 1998;19:11–27. [Google Scholar]
- Møller AR. Neural generators for auditory brainstem evoked potentials. In: Burkard R, Don M, Eggermont J, editors. Auditory Evoked Potentials: Basic Principles and Clinical Application. Lippincott, Williams and Wilkins; New York: 2007. pp. 336–354. [Google Scholar]
- O’Beirne GA, Patuzzi RB. Basic properties of the sound-evoked post-auricular muscle response (PAMR) Hear Res. 1999;138:115–132. doi: 10.1016/s0378-5955(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Ozdamar O, Kraus N. Auditory middle-latency responses in humans. Audiology. 1983;22:34–49. doi: 10.3109/00206098309072768. [DOI] [PubMed] [Google Scholar]
- Picton TW, Hillyard SA, Krausz HI, Galambos R. Human auditory evoked potentials: I. Evaluation of components. Electroencephalogr Clin Neurophysiol. 1974;36:179–190. doi: 10.1016/0013-4694(74)90155-2. [DOI] [PubMed] [Google Scholar]
- Reite M, Sheeder J, Richardson D, Teale P. Cerebral laterality in homosexual males: Preliminary communication using magnetoencephalography. Arch Sex Behav. 1995a;24:585–593. doi: 10.1007/BF01542181. [DOI] [PubMed] [Google Scholar]
- Reite M, Sheeder J, Teale P, Richardson D, Adams M, Simon J. MEG based brain laterality: Sex differences in normal adults. Neuropsychologia. 1995b;33:1607–1616. doi: 10.1016/0028-3932(95)00112-3. [DOI] [PubMed] [Google Scholar]
- Rosenhall U, Nordin V, Brantberg K, Gillberg C. Autism and auditory brain stem responses. Ear Hear. 2003;24:206–214. doi: 10.1097/01.AUD.0000069326.11466.7E. [DOI] [PubMed] [Google Scholar]
- Rosenhamer HJ, Lindstrom B, Lundborg T. On the use of click- evoked electric brainstem responses in audiological diagnosis: II. The influence of sex and age upon the normal response. Scand Audiol. 1980;9:93–100. doi: 10.3109/01050398009076342. [DOI] [PubMed] [Google Scholar]
- Sininger YS, Cone-Wesson B, Abdala C. Gender distinctions and lateral asymmetry in the low-level auditory brainstem response of the human neonate. Hear Res. 1998;126:58–66. doi: 10.1016/s0378-5955(98)00152-x. [DOI] [PubMed] [Google Scholar]
- Skoff BF, Mirsky AF, Turner D. Prolonged brainstem transmission time in autism. Psychiatry Res. 1980;2:157–166. doi: 10.1016/0165-1781(80)90072-4. [DOI] [PubMed] [Google Scholar]
- Stockard JJ, Stockard JE, Sharbrough FW. Nonpathologic factors influencing brainstem auditory evoked potentials. Am J EEG Technol. 1978;18:177–209. [Google Scholar]
- Stockard JE, Stockard JJ, Westmoreland BF, Corfits JL. Brainstem auditory-evoked responses: Normal variation as a function of stimulus and subject characteristics. Arch Neurol. 1979;36:823–831. doi: 10.1001/archneur.1979.00500490037006. [DOI] [PubMed] [Google Scholar]
- Trune DR, Mitchell C, Phillips DS. The relative importance of head size, gender and age on the auditory brainstem response. Hear Res. 1988;32:165–174. doi: 10.1016/0378-5955(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Watson DR. The effects of cochlear hearing loss, age and sex on the auditory brainstem response. Audiology. 1996;35:246–258. doi: 10.3109/00206099609071945. [DOI] [PubMed] [Google Scholar]


