Abstract
Reactive oxygen species (ROS) play a role in a number of degenerative conditions including osteoporosis. Mice deficient in Cu,Zn-superoxide dismutase (Sod1) (Sod1−/− mice) have elevated oxidative stress and decreased muscle mass and strength compared to wild-type mice (WT) and appear to have an accelerated muscular aging phenotype. Thus, Sod1−/− mice may be a good model for evaluating the effects of free radical generation on diseases associated with aging. In this experiment, we tested the hypothesis that the structural integrity of bone as measured by bending stiffness (EI; N/mm2) and strength (MPa) is diminished in Sod1−/−compared to WT mice. Femurs were obtained from male and female WT and Sod1−/− mice at 8 mo. of age and three-point bending tests were used to determine bending stiffness and strength. Bones were also analyzed for bone mineral density (BMD; mg/cc) using micro-computed tomography. Femurs were approximately equal in length across all groups, and there were no significant differences in BMD or EI with respect to gender in either genotype. Although male and female mice demonstrated similar properties within each genotype, Sod1−/− mice exhibited lower BMD and EI of femurs from both males and females compared with gender matched WT mice. Strength of femurs was also lower in Sod1−/− mice compared to WT as well as between genders. These data indicate that increased oxidative stress, due to the deficiency of Sod1 is associated with decreased bone stiffness and strength and Sod1−/− mice may represent an appropriate model for studying disease processes in aging bone.
Keywords: oxidative stress, ROS, mineralization
INTRODUCTION
Osteoporosis is a leading cause of fractures, especially among the elderly [1]. As the population ages, morbidity, mortality and financial cost related to osteoporosis is expected to rise [2]. Osteoporosis is characterized by reduced bone mass and diminished bone integrity. Patients suffering from this degenerative disease experience a decrease in bone strength and consequently greater vulnerability to fractures [3].
Measures of BMD are the gold standard for assessing fracture risk due to reduced bone mass [3, 15]. Dual-energy X-ray Absorptiometry (DEXA) is commonly used in clinical practice to analyze BMD but is limited to measuring BMD based on area. In contrast, micro-computed tomography (micro-CT) provides information on the volumetric BMD distribution and three dimensional architecture of bone, making it a superior methodology to DEXA scanning [13]. In addition to the amount of mineral and its spatial distribution, the ability of a bone to resist fracture depends on the intrinsic material properties of the tissue [13, 16]. The bone remodeling process, specifically the balance between the formation and resorption of matrix mineralization, mediates changes that can influence bone strength [6]. The resultant mineral quantity and quality of bone matrix has been shown to correlate with bone strength [14]. Therefore, processes that impact bone remodeling can significantly influence bone’s resistance to fracture.
Reactive oxygen species (ROS) are considered to be a factor in the onset of a number of age-associated conditions [4, 7]. Approximately 3 to 10% of the oxygen utilized by tissues is converted to ROS intermediates, including superoxide radicals (O2-). Superoxide radicals impair cell and tissue function by oxidizing and degrading biologically important molecules, including proteins, lipids, and DNA [9]. Recently, it has been suggested that under normal physiological conditions, ROS produced by osteoclasts stimulates and facilitates resorption of bone tissue [17, 19]. When the production of ROS overwhelms the natural antioxidant defense mechanisms, the associated oxidant stress may lead to extensive bone loss and skeletal fragility, characteristic of osteoporosis [5, 7, 8].
Cu,Zn-superoxide dismutase (Sod1) binds copper and zinc ion cofactors and is one of three Sod1 isozymes located primarily in the cytosol. Sod1 is important in the defense against oxidative stress in tissue and acts by catalyzing the conversion of superoxide radicals (O2-) to hydrogen peroxide, which can then be further reduced to water [10, 11]. Mice deficient in Sod1 (Sod1−/− mice) display elevated oxidative stress, decreased body mass, decreased musculoskeletal mass, and decreased whole muscle strength compared to wild-type mice (WT) [9,20]. Decreases in body mass, muscle mass and muscle strength are also observed with aging, and as a result, Sod1−/− mice are considered by some to be a good model to study the role of oxidative stress in the aging of the musculoskeletal system. The purpose of the current study was to determine whether the deficiency of Sod1 leads to premature aging and osteoporotic bone fragility. We hypothesized that bending stiffness (EI; N/mm2) and BMD (mg/cc) for bones of the Sod1−/− mice would be diminished compared to the WT mice. This degenerative phenotype may lead to osteoporosis-related premature bone fragility in this animal model.
MATERIALS AND METHODS
Animals
These studies were performed using WT and Sod1−/− mice. Male and female mice, 8 mo of age, were obtained from Dr. Holly Van Remmen at the University of Texas Health Science Center at San Antonio. All mice were housed in a specific pathogen free facility at University of Texas Health Science Center at San Antonio prior to their arrival at the University of Michigan. At the University of Michigan, the mice again were maintained under barrier conditions in a temperature-controlled environment and fed a commercial mouse chow (Teklad diet LM485) ad libitum. Mice were housed in our facility a minimum of two weeks prior to any procedures. Animal housing, operations, and subsequent animal care were carried out in accordance with the guidelines of the Unit for Laboratory Animal Medicine at the University of Michigan. Mice were anesthetized with pentobarbitone sodium with an initial dose of 65 mg/100 g of body mass via an intraperitoneal injection. Mice were euthanized by an overdose of the anesthetic and femurs were subsequently harvested from both male and female WT and Sod1−/− mice, by surgically dissecting away the surrounding muscle and tendon. The femurs were stored at 4°C in Dulbecco's Phosphate Buffered Saline (DPBS, Gibco BRL) until testing. All animal care and animal surgeries were in accordance with the Guide for Care and Use of Laboratory Animals (Public Health Service, 19965, NIH Publication No. 85-23).
Micro-Computed Tomography Testing
Samples were scanned for BMD (mg/cc) and bone mineral content (BMC; mg) using Micro Computed Tomography (GE Healthcare) and analyzed using commercially available software (MicroView 2.2 Advanced Bone Analysis). In addition to mineralization, total bone volume was measured from the three dimensional images of the scanned bones. Cortical bone was analyzed by standardizing a region of interest spanning 18% of the length of the bone, placed in the mid-diaphysis. A consistent threshold was used for each image to distinguish between cortical and trabecular bone in our samples. Choosing a standardized cortical region and threshold ensured a homogeneous representation of cortical bone from which the cross-sectional area could be determined for use in our mechanical analyses. BMD was also determined in this cortical section to compare regional differences between cortical bone and total bone densities. Statistical analysis was performed using a two-way anova test.
Three-Point Bending Testing
Three-point bending testing was performed using Dynamic Mechanical Analyzer (DMA) (RSA III, TA Instruments, New Castle, DE) to determine the bending stiffness (EI; N/mm2) and maximum elastic stress, defined as strength (MPa) of the bone samples. The bone was positioned horizontally with the anterior surface upward and centered between two supports 10 mm apart. A compression load at rate of 1 mm/sec was applied at mid-diaphysis until failure occurred.
The elastic portion of the load vs. displacement curve was collected. The displacement measured by the DMA is the midpoint deflection (δ) of the bone in bending. This deflection is related to the load in three-point bending of a long, slender beam via where P is the load response, L = 10 mm is the distance between the two supports, E is the Young’s modulus (MPa) of the material, I is the moment of inertia about the neutral bending axis (mm4) and EI is the bending stiffness [34]. The bones used in the three-point bending experiments had an insufficient aspect ratio (length to diameter) for this elementary beam theory to be strictly valid; application of the precise theory in this case requires independent measurement of the shear modulus of the bone, and neglecting this correction amounts to approximately a 5% overprediction of EI [35]. The bending stiffness of the bone specimens is the slope of a plot of (ordinate) vs. δ (abcissa). Statistical analysis was performed using a two-way anova test.
RESULTS
Micro-Computed Tomography
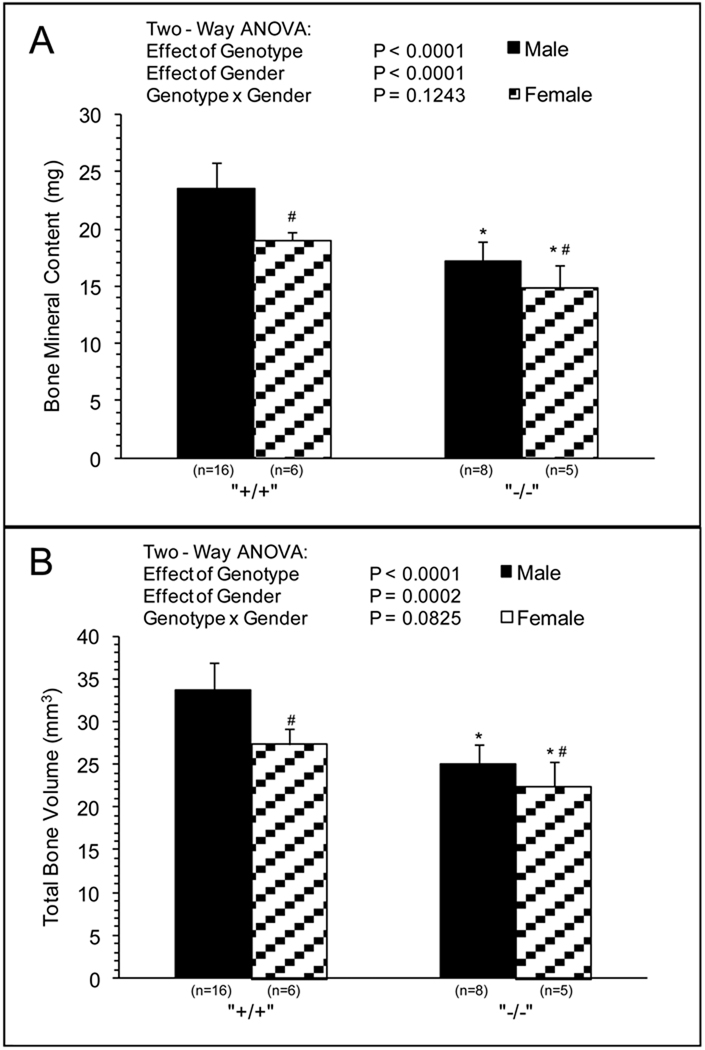
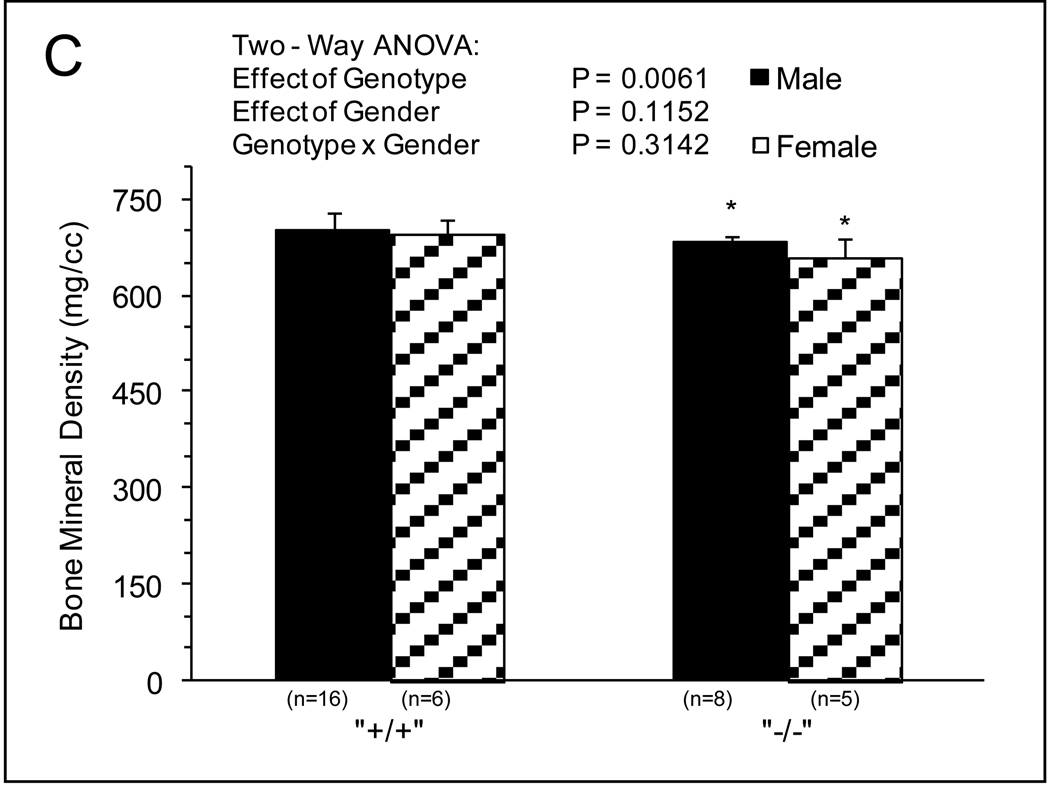
MicroCT and mechanical analysis was preformed on WT and Sod1−/− mice. Negligible differences in femur length were observed between Sod1−/− mice and WT. Significant differences in BMC and total bone volume were observed between genotype and gender (Fig.1A and 1B). BMC was 10%–20% lower for bones of female compared with male mice, and ~25% lower for bones of Sod1−/− compared with WT mice with no effect of gender on the impact of the Sod1 deficiency. The effects of genotype and gender on total bone volume were similar to those observed for BMC, with bones of female mice displaying 10%–20% smaller volumes than bones of male mice and the deficiency of Sod1 resulting in 20%–30% reductions in volume. Normalizing the mineral content to bone volume resulted in a small but significant decrease in BMD with respect to genotype (Fig.1C). Femurs of Sod1−/− mice showed approximately 4 percent lower BMD than those of WT mice, and negligible gender differences were observed. BMD in the cortical region was more severely affected by the Sod1 deficiency with values for femurs of Sod1−/− mice approximately 14 percent less than those of WT mice (Fig.2A). The cortical cross sectional area was also diminished in femurs of Sod1−/− mice femurs, consistent with a reduction in total bone size (Fig.2B).
Fig. 1.
Total Bone MicroCT. (A) BMC (mg), (B) Total Bone Volume (mm3), (C) BMD (mg/cc) of femurs extracted from male and female wild-type and Sod1−/− C57BI6 mice. Values are means ± SD. * Significant difference in wild-type vs. Sod1−/− bones P < 0.05. # Significant difference in male vs. female mice, P < 0.05.
Fig. 2.
Cortical MicroCT. (A) Cortical BMD (mg/cc), (B) Cross Sectional Area (mm3) of femurs extracted from male and female wild-type and Sod1−/− C57BI6 mice. Values are means ± SD. * Significant difference in wild-type vs. Sod1−/− bones P < 0.05.
Three-Point Bending
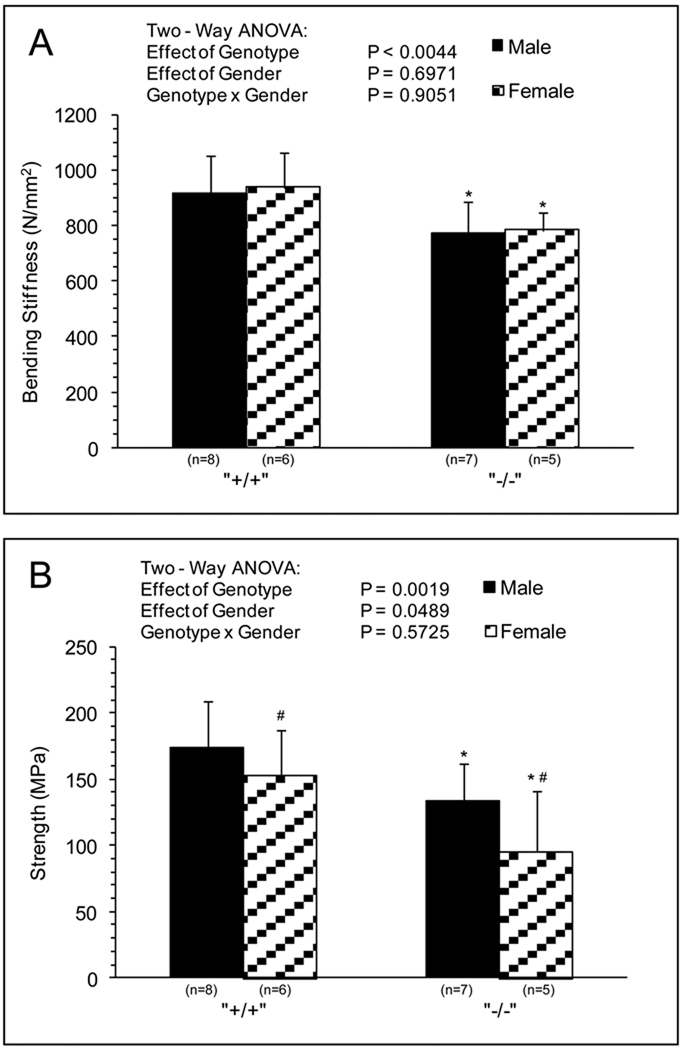
Three point bending analysis showed ~20% lower bone stiffness for Sod1−/− compared with WT mice with no effect of gender (Fig.3A). Strength of the bone was also diminished by Sod1 deficiency by ~30% (Fig.3B). Although strength of bones from female mice showed lower values than bones of male mice, the effect of the Sod1 deficiency on bone strength was similar for male and female mice.
Fig. 3.
Bone Mechanics. (A) Bending Stiffness (N/mm2), (B) Peak Strength (MPa) of femurs extracted from male and female wild-type and Sod1−/− C57BI6 mice. Values are means ± SD. * Significant difference in wild-type vs. Sod1−/− bones P < 0.05. # Significant difference in male vs. female mice, P < 0.05.
DISCUSSION
In this study, we investigated the effect that decreasing total body anti-oxidant defenses, through the knockout of Sod1, has on bone fragility. For mice in which the endogenous antioxidant systems were diminished, we observed decreased total and cortical BMD, BMC and bone volume [26, 36]. These results are consistent with previous reports of decreased BMD of Sod1−/− mice [10]. While BMD is the current clinical gold standard for analyzing reductions in bone mass and diagnosing osteoporosis, mechanical parameters are required to accurately investigate fragility [27]. Our finding of lower values for strength and bending stiffness of Sod1−/− mice femurs, compared to those of gender matched WT mice, are consistent with the detrimental effects observed for the morphological and structural properties of the Sod1 deficient bones.
Decreases in bone strength are reported to be associated with alterations in the amount of calcified matrix and its composition [18]. Our observation of genotypic differences in cortical BMD where the load is applied, correlate with decreases in overall strength. Bending stiffness has been reported to be dependent on both the mineral distribution within the bone as well as the area over which the load is applied during the test [32, 24]. Decreases in mineral density and cross-sectional area observed in Sod1−/− versus WT mice correlate to decreases in stiffness.
Evidence for increased levels of oxidative stress and damage in aging organisms are a result of increased generation of ROS coupled with decreased ability to detoxify ROS [21]. ROS have been proposed to be causative in aging overall and more recently, involved in the incidence and severity of osteoporosis [21–23]. Recent evidence suggests that ROS are involved in bone resorption, with a direct contribution of osteoclast-generated superoxide to bone degradation [12, 28, 31]. In addition, superoxide has been reported to specifically stimulate osteoclast formation within bones of rodents [33]. While the exact mechanism by which ROS accelerates bone resorption is still unclear, ineffective neutralization of ROS leading to oxidative stress in bone can increase bone loss and bone weakness, typical of osteoporosis [25, 29, 30].
The influence of ROS in this animal model suggests that this degenerative bone phenotype may be associated with age related skeletal fragility, and early onset of osteoporosis related bone weakness. Further work investigating micro-architecture, porosity, as well as matrix composition may provide a better understanding and assess the mechanism ROS plays in bone remodeling.
ACKNOWLEDGMENTS
The authors would like to acknowledge the technical assistance of Amit Kaushik
GRANTS
This project was supported from NIH, NIA Grant PO1 AG20591.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lim LS, Hoeksema LJ, Sherin K. Screening for osteoporosis in the adult U.S. population: ACPM position statement on preventive practice. Am J Prev Med. 2009;36:366–375. doi: 10.1016/j.amepre.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Davison KS, Kendler DL, Ammann P, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, McClung MR, Olszynski WP, Yuen CK. Assessing fracture risk and effects of osteoporosis drugs: bone mineral density and beyond. Am J Med. 2009;122:992–997. doi: 10.1016/j.amjmed.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, Johansson H, Oden A, McCloskey EV. Assessment of fracture risk. Eur J Radiol. 2009;71:392–397. doi: 10.1016/j.ejrad.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Banfi G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med. 2008;46:1550–1555. doi: 10.1515/CCLM.2008.302. [DOI] [PubMed] [Google Scholar]
- 5.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;15:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 6.Basu S, Michaëlsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001;288:275–279. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- 7.Finkel T, Holbrook NJ. Oxidants, oxidative stress, and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Rodríguez MA, Ruiz-Ramos M, Correa-Muñoz E, Mendoza-Núñez VM. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet Disord. 2007;19:124. doi: 10.1186/1471-2474-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasilaki A, Mansouri A, Remmen H, Van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell. 2006;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 10.Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, 2nd, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Noor R, Mittal S, Iqbal J. Superoxide dismutase--applications and relevance to human diseases. Med Sci Monit. 2002;8:210–215. [PubMed] [Google Scholar]
- 12.Yang S, Madyastha P, Bingel S, Ries W, Key L. A new superoxide-generating oxidase in murine osteoclasts. J Biol Chem. 2001;276:5452–5458. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- 13.Kreider JM, Goldstein SA. Trabecular bone mechanical properties in patients with fragility fractures. Clin Orthop Relat Res. 2009;467:1955–1963. doi: 10.1007/s11999-009-0751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currey JD. The effect of porosity and mineral content on the Young's modulus of elasticity of compact bone. J Biomech. 1988;21:131–139. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 15.Kaptoge S, Benevolenskaya LI, Bhalla AK, Cannata JB, Boonen S, Falch JA, Felsenberg D, Finn JD, Nuti R, Hoszowski K, Lorenc R, Miazgowski T, Jajic I, Lyritis G, Masaryk P, Naves-Diaz M, Poor G, Reid DM, Scheidt-Nave C, Stepan JJ, Todd CJ, Weber K, Woolf AD, Roy DK, Lunt M, Pye SR, O'neill TW, Silman AJ, Reeve J. Low BMD is less predictive than reported falls for future limb fractures in women across Europe: results from the European Prospective Osteoporosis Study. Bone. 2005;36:387–398. doi: 10.1016/j.bone.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Hans D, Fuerst T, Lang T, Majumdar S, Lu Y, Genant HK, Glüer C. How can we measure bone quality? Baillieres Clin Rheumatol. 1997;11:495–515. doi: 10.1016/s0950-3579(97)80017-9. [DOI] [PubMed] [Google Scholar]
- 17.Altindag O, Erel O, Soran N, Celik H, Selek S. Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol Int. 2008;28:317–321. doi: 10.1007/s00296-007-0452-0. [DOI] [PubMed] [Google Scholar]
- 18.Comelekoglu U, Bagis S, Yalin S, Ogenler O, Yildiz A, Sahin NO, Oguz I, Hatungil R. Biomechanical evaluation in osteoporosis: ovariectomized rat model. Clin Rheumatol. 2007;26:380–384. doi: 10.1007/s10067-006-0367-2. [DOI] [PubMed] [Google Scholar]
- 19.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang TT, Carlson EJ, Raineri I, Gillespie AM, Kozy H, Epstein CJ. The use of transgenic and mutant mice to study oxygen free radical metabolism. Ann NY Acad Sci. 1999;893:95–112. doi: 10.1111/j.1749-6632.1999.tb07820.x. [DOI] [PubMed] [Google Scholar]
- 21.Schoneich C. Reactive oxygen species and biological aging: a mechanistic approach. Exp Gerontol. 1999;34:19–34. doi: 10.1016/s0531-5565(98)00066-7. [DOI] [PubMed] [Google Scholar]
- 22.Sheweita SA, Khoshhal KI. Calcium metabolism and oxidative stress in bone fractures: role of antioxidants. Curr Drug Metab. 2007;8:519–525. doi: 10.2174/138920007780866852. [DOI] [PubMed] [Google Scholar]
- 23.Beckman K, Ames B. The free radical theory of aging. Physiological Reviews. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 24.Jämsä T, Jalovaara P, Peng Z, Väänänen HK, Tuukkanen J. Comparison of three-point bending test and peripheral quantitative computed tomography analysis in the evaluation of the strength of mouse femur and tibia. Bone. 1998;23:155–161. doi: 10.1016/s8756-3282(98)00076-3. [DOI] [PubMed] [Google Scholar]
- 25.Ozgocmen S, Kaya H, Fadillioglu E, Yilmaz Z. Effects of Calcitonin, Risedronate, and Raloxifene on Erythrocyte Antioxidant Enzyme Activity, Lipid Peroxidation, and Nitric Oxide in Postmenopausal Osteoporosis. Archives of Medical Research. 2007;38:196–205. doi: 10.1016/j.arcmed.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 27.Breen SA, Loveday BE, Sharpe PM, Millest AJ, Waterton JC. Measurement of bone mineral density in vivo in a murine model of osteoporosis: A longitudinal study. J Bone Miner Res. 1996;11:S471. [Google Scholar]
- 28.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 29.Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in co-cultures with mouse spleen cells. Endocrinology. 1989;125:1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- 30.Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, Meghji S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol. 2003;196:2–8. doi: 10.1002/jcp.10321. [DOI] [PubMed] [Google Scholar]
- 31.Galli F, Piroddi M, Annetti C, Aisa C, Floridi E, Floridi A. Oxidative stress and reactive oxygen species. Contrib Nephrol. 2005;149:240–260. doi: 10.1159/000085686. [DOI] [PubMed] [Google Scholar]
- 32.Crenshaw TD, Peo ER, Jr, Lewis AJ, Moser BD. Bone Strength as a Trait for Assessing Mineralization in Swine: a Critical Review of Techniques Involved. J Anim Sci. 1981;53:827–835. [Google Scholar]
- 33.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. 1990;85:632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beer Ferdinand P, Johnston E Russel, Jr, DeWolfe John T. Mechanics of Materials. 3rd Edition. Boston: McGraw Hill; 2002. [Google Scholar]
- 35.Timoshenko S. In: Strength of Materials Part I Elementary Theory and Problems. 3rd Edition. Robert E, editor. Huntington, NY: Krieger Publishing Company, Inc.; 1976. [Google Scholar]
- 36.Jepsen KJ, Hu B, Tommasini SM, Courtland HW, Price C, Terranova CJ, Nadeau JH. Genetic randomization reveals functional relationships among morphologic and tissue quality traits that contribute to bone strength and fragility. Mamm Genome. 2007;18:492–507. doi: 10.1007/s00335-007-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]