Abstract
Loss of central inhibition has been hypothesized to underpin tinnitus and impact auditory acuity. Taurine, a partial agonist at inhibitory glycine and γ-amino butyric acid receptors, was added to the daily diet of rats to examine its effects on chronic tinnitus and normal auditory discrimination. Eight rats were unilaterally exposed once to a loud sound to induce tinnitus. The rats were trained and tested in an operant task shown to be sensitive to tinnitus. An equivalent unexposed control group was run in parallel. Months after exposure, 6 of the exposed rats showed significant evidence of chronic tinnitus. Two concentrations of taurine in drinking water were given over several weeks (attaining average daily doses of 67 mg/kg and 294 mg/kg). Water consumption was unaffected. Three main effects were obtained: (1) The high taurine dose significantly attenuated tinnitus, which returned to near pre-treatment levels following washout. (2) Auditory discrimination was significantly improved in unexposed control rats at both doses. (3) As indicated by lever pressing, taurine at both doses had a significant group-equivalent stimulant effect. These results are consistent with the hypothesis that taurine attenuates tinnitus and improves auditory discrimination by increasing inhibitory tone and decreasing noise in the auditory pathway.
Keywords: tinnitus, taurine, animal model, GABA, glycine, auditory discrimination
INTRODUCTION
Chronic tinnitus, the perception of sound without an external acoustic stimulus, affects a significant proportion of the adult population. For those who experience tinnitus, 3 to 5 percent find it to be bothersome and at times disabling (Cooper, 1994). The pathophysiology responsible for tinnitus is not completely understood. Animal models have been developed to understand the basic neuroscience of tinnitus and to screen potential therapeutics (Brozoski et al., 2008). Using these models, evidence from a number of laboratories points to decreased inhibition in the auditory pathway as an important component of tinnitus pathology (Bauer et al., 2008; Kaltenbach, 2007; Salvi et al., 2000; Wang et al., 2009). We recently reported that vigabatrin, a γ-amino butyric acid (GABA) agonist, effectively and reversibly eliminated the evidence of acoustic-trauma-induced chronic tinnitus in rats (Brozoski et al., 2007a). Using a similar procedure, the present study examined the effect of taurine on psychoacoustic performance and tinnitus.
Taurine is a sulfur-containing β-amino acid found at high concentrations in mammals. As a “non-coding” amino acid, it is derived directly from food sources and indirectly from the metabolism of other common sulfur-containing amino acids such as cysteine and methionine (Birdsall, 1998; Huxtable, 1992; Oja et al., 2007). Taurine is distributed widely in the mammalian body (approximately 1g/kg in humans) including blood plasma, heart, muscle, and brain tissue, and may beneficially participate in diverse physiological processes. Examples reported in the literature include, cell volume regulation (Hoffmann et al., 2009), weight reduction (Tsuboyama-Kasaoka et al., 2006), antioxidant action (Atmaca, 2004; Fang et al., 2002), facilitation of neural development (Aerts et al., 2002; Sturman, 1986; Sturman et al., 1986) and thermoregulation (Birdsall, 1998; Huxtable, 1992; Oja et al., 2007; Sgaragli et al., 1981; Sgaragli et al., 1996). Experimentally induced taurine depletion has been shown to exacerbate the retinopathy produced by the GABA-transaminase inhibitor, vigabatrin (Jammoul et al., 2009).
Relevant to the present study, taurine has been shown to act as an inhibitory neuromodulator, although its status as a neurotransmitter is unresolved. There is evidence of taurine specific receptors (Frosini et al., 2003; Wu et al., 1992; Wu et al., 1990). Taurine has been shown to inhibit neural activity by acting at glycine (GlyR), GABAA (GABAAR), and GABAB (GABABR) receptors (Albrecht et al., 2005), and is distributed throughout the central and peripheral auditory system (Contreras et al., 1979; Harding et al., 1993). In the central auditory system it has been shown to activate GlyRs in rat inferior colliculus (IC) (Xu et al., 2004; Xu et al., 2006), and may act similarly in the auditory midbrain and brainstem, including the cochlear nucleus, superior olivary complex, and nuclei of the lateral lemniscus (Friauf et al., 1997). Although the inhibitory role of taurine is widespread in the CNS, it may have an excitatory role in the periphery. Liu et al., (Liu et al., 2008; Liu et al., 2006) have shown that increased taurine elevates cochlear outer hair cell and spiral ganglion neuron Ca2+ influx.
In addition to its action at strychnine sensitive GlyRs, taurine has been shown to activate extrasynaptic GABAARs, producing a long lasting tonic Cl− current. This has been shown in mouse ventrobasal thalamus (Jia et al., 2008). Recently we have identified a dose-dependent tonic inhibition of neurons in the rat medial geniculate body (MGB), mediated by extrasynaptic δ-subunit containing GABAARs (Richardson et al., 2009). Extrasynaptic GABAARs have high ligand selectivity, low benzodiazepine sensitivity, and are slow to desensitize. These receptors are responsible for a long-lasting tonic Cl- influx that decreases cellular excitability. This stabilizing effect has been well documented in thalamic (Cope et al., 2005; Jia et al., 2005) neurons, the dentate gyrus of the hippocampus (Stell et al., 2002; Yeung et al., 2003), and cerebellar granule cells (Belelli et al., 2009; Brickley et al., 1996; Nusser et al., 2002). In thalamus, a number of electrophysiological and molecular studies have shown extrasynaptic GABAARs contain α4 and δ subunits (Chandra et al., 2006; Herd et al., 2009; Sur et al., 1999; Wisden et al., 1992). GABAARs containing α4 and δ subunits may be particularly important for the central inhibitory effect of taurine.
Not only can taurine in the brain be derived from sulfur-containing amino acids, but it can also enter by crossing the blood brain barrier via a Na+/Cl− dependent taurine transporter, TauT (Chung et al., 1994; Kang et al., 2002; Ohtsuki, 2004; Ramanathan et al., 1997; Tamai et al., 1995). It has been shown that performance on various behavioral tasks is significantly altered by supplemental dietary taurine as well as taurine administered by gastric lavage (El Idrissi, 2008; Vohra et al., 2000). Long-term continuous taurine administration is likely more effective than acute dosing since brain levels of taurine were not significantly altered before and after a single gastric lavage (Sved et al., 2007).
In the present experiment it was hypothesized that increasing systemic taurine levels through dietary supplement, would modulate neural activity in the central auditory pathway through either GlyR activation or increasing tonic inhibition mediated by extrasynaptic GABAARs. Enhanced inhibition, in the thalamus and elsewhere, would significantly modulate auditory sensation and may be capable of attenuating ascending activity that comprises the tinnitus signal.
METHOD
Subjects
Seventeen adult male Long–Evans rats, 4 months old at the start of the experiment, were individually housed and maintained at 25° C with a 12/12 hr reversed light/dark schedule. One subject was discarded at the beginning of the experiment because of failure to acquire basic operant skills necessary for auditory testing. The sixteen remaining subjects were trained, exposed, and tested in parallel throughout the study.
The methods used in the present study were similar to those reported in previous studies investigating the effect of drugs on tinnitus in rats (Bauer et al., 2001; Brozoski et al., 2007a). A summary of the experimental time line appears in Table 1.
Table 1.
The Experimental Time Line.
| Phase | Arrive | Sound exposure | Initial training | Tinnitus test | Taurine 0 mg | Taurine 1 mg | Taurine 4 mg | Washout1 | Washout 2 |
|---|---|---|---|---|---|---|---|---|---|
| Study Week | 1 | 15 | 22 | 39 | 42 | 44 | 50 | 57 | |
| Subject Age (mo) | 3.0 | 3.1 | 6.4 | 8.0 | 11.9 | 12.6 | 13.0 | 14.4 | 16.0 |
Acoustic exposure and calibration methods
Eight randomly selected subjects were exposed once to loud sound before operant training and pre-drug testing. These subjects will be referred to as “exposed.” The eight unexposed control subjects will be referred to as “unexposed.” All subjects were anesthetized to an areflexive state with an isoflurane/O2 mixture (Aerrane, Baxter Healthcare Corp., Deerfield, IL, USA) placed in a masked head holder, and had hearing thresholds determined using auditory brainstem-evoked potentials (ABR, described below). The exposed subjects were then exposed once unilaterally for 90 min to band-limited noise (similar to Bauer & Brozoski, 2001; Brozoski, et al., 2007). The exposure stimulus was produced using a noise generator (Grayson-Stadler 1724, Eden Prairie, MN 55344, USA), band-pass filter (KrohnHite 3384, 8 pole Butterworth filter, Brockton, MA, USA), audio amplifier (55ES, Sony, New York, NY, USA), delivered monaurally using a speaker driver (FT17H, Fostex, Tokyo, Japan) in a custom enclosure funneling to a 2 cm flexible tube that fit into the auditory canal. Peak stimulus level, centered at 16 kHz, was 116 dB sound pressure level (SPL), with an approximately linear decay to ambient levels at 6 and 24 kHz. Acoustic values were calibrated using a Brüel & Kjaer (Norcross, GA, USA) Pulse sound measurement system (Pulse 13 software), equipped with a 3560C high-frequency module, and a 4138 pressure-field microphone (Brüel & Kjaer) coupled to the transducer using rubber tubing with the internal dimensions of an adult rat external auditory canal. The sound measurement system permitted linear sound level measurements between 0 and 140 dB (re 20 μPa) and spectral analysis between 6.5 Hz and 100 kHz. Calibrations were carried out as unweighted linear SPLs. All sound levels reported in the present experiments are unweighted measures.
Sound levels were calibrated in the operant test chambers using the Brüel & Kjaer Pulse system described above, equipped with a Brüel & Kjaer 4191-L free-field microphone. This system permitted linear sound level measurements to be made between 0 and 140 dB (re 20 μPa) with spectral resolution between 3.15 Hz and 40 kHz. The microphone was positioned in each test chamber at a location 10 cm below the lid-mounted speaker, in the approximate location of a rat’s head during testing. A cloth bundle approximating the volume of a rat was placed in the test chamber along with the microphone to distort the sound field as it would be by a rat.
Hearing thresholds
ABR thresholds were obtained before loud-sound exposure, immediately after exposure (if exposed), and at the conclusion of behavioral testing. ABR measurements were obtained using a Tucker Davis Technologies System 3 Real Time Signal Processing System running BioSig32 and SigGen (Tucker Davis Technologies, Alachua, FL, USA). Stimuli were 5 msec tone bursts with a 2 msec cosine rise-decay and 1 msec plateau, stepped in 10 dB decrements from 90 to 10 dB (SPL). The bursts, at 50/sec, were presented to the entrance of each ear canal (separately) using the speakers and the audio system described above. Evoked responses were differentially recorded from a subcutaneous vertex needle electrode referenced to an electrode at the occiput. Evoked responses for 10 msec epochs following stimulus onset were amplified × 100,000, bandpass filtered (100–3,000 Hz), and averaged for 512 repetitions of each frequency-sound-level combination. Digitized records of the evoked responses (40 μsec resolution) were exported to Excel (Microsoft, Redmond, WA, USA) for analysis and threshold determination. Hearing thresholds for each ear were defined by the lowest stimulus level that produced a statistically distinct waveform at each frequency (8 to 32 kHz). Standardized criteria were derived from the absolute deviation and standard deviation of evoked potentials for each stimulus frequency at each level. Hearing threshold was indicated as the stimulus level at which the deviation functions reached a lower-bound asymptote, i.e., stimulus-evoked deflections no longer were evident. Visual inspection of the waveforms was used to corroborate and temper the statistical analysis.
Behavior: Initial training
Subjects were trained and tested using a procedure shown to detect tinnitus in animals (Bauer et al., 2001). All subjects were run in parallel throughout training and testing, i.e., they differed only in their initial exposure to loud sound. Training and testing occurred in individual commercial operant conditioning chambers (Lafayette Instruments, Mod. 80001, Lafayette, IN, USA). The rats were maintained on restricted food intake and trained to lever press for food pellets (45 mg, MLab Rodent, TestDiet, Richmond, IN, USA). Diet restriction was individually tailored to each subject, sufficient to produce a minimum of 200 lever presses per session (study average per subject, 802) with less than 10% within-session variation. Supplemental food was given at the conclusion of each session sufficient to maintain body weight at or above 80 percent of normative age–weight values. Five 60 min sessions were run per week. Operant training required 4 weeks to achieve criterion performance using a variable-interval reinforcement schedule. The variable interval schedule had a median value of 20 sec. (on average, a food pellet was available 20 sec after the previous pellet), with an upper limit of 30 sec and lower limit of 6 sec. The reinforcement schedule was in place throughout every session, including all stimulus presentations. Broad-band noise (BBN) at 60 dB SPL was presented to each operant chamber via a speaker (Optimus, 40-1219, Tandy), centermounted in each chamber lid (calibration procedure described above). The BBN was constant, except when acoustic test stimuli were presented, at which time it was turned off. Experimental control and data acquisition was accomplished using desktop computers running in-house programs and custom interfaces (Keithley/MetraByte, Cleveland, OH, USA).
Behavior: The suppression ratio, a running relative measure
In each 1-min period of each session, a suppression ratio (R) was calculated for each subject, using the formula R = B/(A+B), where A was the number of lever presses in the preceding period and B the number of lever presses in the current period. R can vary between 0 and 1: A value of 0 is attained when lever pressing in the current 1- min period is 0, a value of 0.5 when lever pressing in the current 1-min period is equal to that of the previous period, and a value of 1 when lever pressing in the previous period is zero. The runtime control program intervened in the few instances when subjects did not lever press for two or more successive minutes, in which case, R was defined as 0. R provided a running index of behavior and enabled a quantitative comparison between subjects as well as unbiased compilation of group data. With R as the performance measure, each subject contributed equally to group data irrespective of overall response rate. In the present type of experiment, R has additional utility in that it is very sensitive to short-term behavioral effects, such as those that might be produced by presentations of test stimuli, but is very insensitive to long-term behavioral effects, such as those that might be produced by shifts in motivational status, for example, satiation.
Behavior: acclimation to acoustic variation
Following initial training, acoustic test stimuli were introduced using a procedure designed to acclimate subjects to the presentation of acoustic events other than BBN. During stimulus introduction, which extended over six sessions, all behavioral contingencies remained the same as in training. Ten acoustic stimuli were digitally synthesized (Stanford Research Systems, DS-345, Palo Alto, CA, USA) and during the 60 min test session each was presented for 60 sec over the lid-mounted speakers. Stimulus presentations could not occur within 2 min of one another, or within 2 min of the beginning or end of the session. Two of the ten presentations were always speaker-off periods. The remaining eight presentations were either BBN, or 10, 16, or 20 kHz tones presented at four different sound levels, randomly ordered, with the sound levels extending across the subject’s sensitivity range. Each test stimulus level repeated once within the session. Test stimulus type, i.e., BBN, 10 kHz, etc., varied randomly between sessions, but remained constant within a session. Background sound was off during the test presentations.
Behavior: Suppression training
Suppression training followed stimulus acclimation. In suppression training, the subjects received a 1-sec, 0.5-mA foot shock through the grid floor of the test chamber if they lever pressed above a criterion level in speaker-off periods. When scheduled, only one foot shock was given at the conclusion of the speaker-off period. The purpose of suppression training was twofold: It trained the subjects to listen carefully to their acoustic environment throughout the session because foot shocks could only be avoided if the unpredictable speaker-off periods were detected. Secondly, it trained subjects to discriminate between the speaker-on and speaker-off periods. A single foot shock was given at the end of a speaker-off period if R ≥ 0.1. When subjects decreased their lever pressing during speaker-off periods, so that R < 0.1, foot shock was avoided. It is important to note that whenever the speaker was on, irrespective of the acoustic signal, lever presses never led to foot shock. Although the majority of subjects achieved criterion-level suppression within two sessions, five suppression-training sessions were given to all subjects. The foot shock contingency during speaker-off periods remained in place throughout the experiment, including the acoustic test sessions described below. In that sense, suppression training was a constant feature of the experimental paradigm, once implemented. On average, subjects received less than one foot shock per month for the remainder of the study. The tandem constant parameters of food reinforcement for lever pressing, and foot shock for speaker-off lever pressing, maintained discrimination behavior in a steady state.
Behavior: Acoustic stimulus testing
The objective of acoustic stimulus testing was to determine the presence of tinnitus using a measure more sensitive than behavior during speaker-off periods. In the present experiment, the sensation associated with speaker-off periods determines behavior during test stimulus presentations: Subjects without tinnitus hear nothing with the speaker off, whereas those with tinnitus hear their tinnitus. In acoustic testing, when brief periods (60 sec) of noise or tones are presented, test stimuli with sensory features resembling tinnitus have a special meaning to exposed subjects but no special meaning for normal hearing subjects. For subjects with tinnitus, test stimuli that resembled the tinnitus served as a signal for response suppression. In other words, acoustic stimuli with sensory features resembling tinnitus should produce greater suppression in subjects with tinnitus. In testing, as in acclimation, the acoustic stimuli were presented in pseudorandomly scheduled, 60-sec test periods.
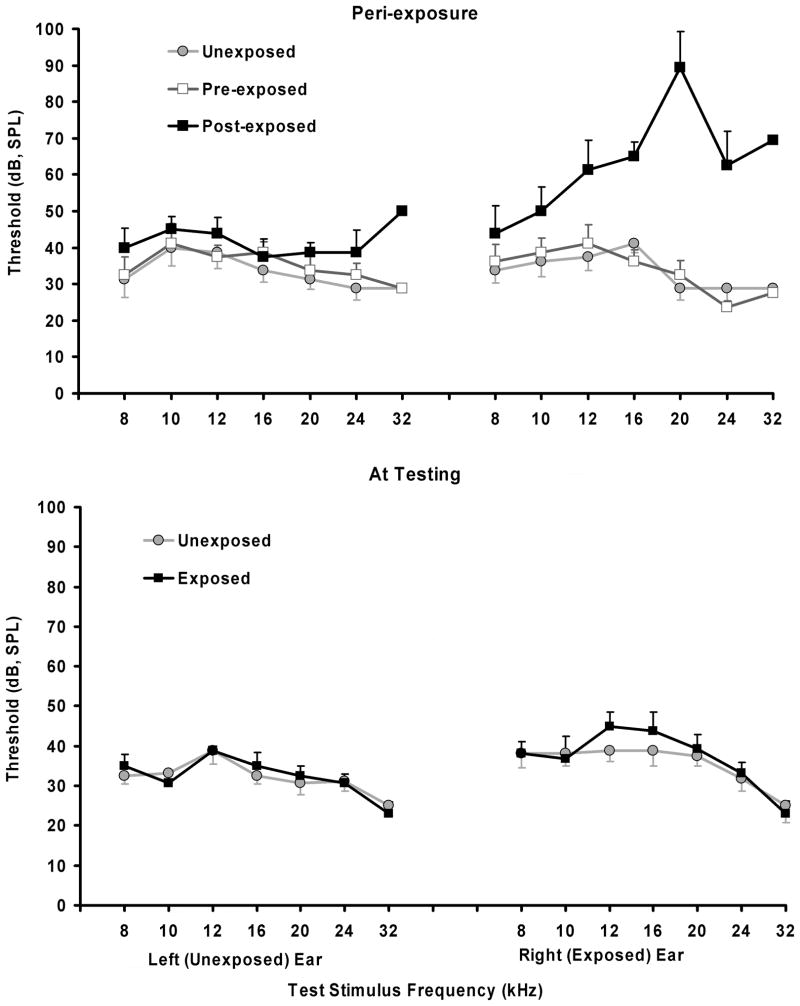
Previous research (Bauer et al., 2001) has shown that Long–Evans adult rats unilaterally exposed to loud band-limited noise show evidence of tinnitus in a range between 10 and 30 kHz. Four different test stimuli were used in the present experiment: BBN, and 10, 16, and 20 kHz tones. One of the four stimuli was presented eight times within a session, using four different sound levels, with each level repeated once, for a total of eight (4 × 2) presentations per session. Only one type of stimulus was tested in a given session, with sound-level order randomized. Every test series included two additional, randomly inserted, speaker-off periods. As in acclimation, test presentations and speaker-off periods were 60 sec in duration, separated from one another, and the session start and end, by a minimum of 2 min of background sound. The range of test sound levels depended upon the stimulus and always extended over a range broad enough to capture the psychometric hearing function for that stimulus. Sound levels were identical in all chambers. At the time of testing, hearing thresholds for exposed and unexposed animals were identical for unexposed ears and nearly identical for exposed ears (Fig. 1). Previous research has shown that unilateral threshold elevation does not affect performance in the present test procedure (Bauer & Brozoski, 2001).
Fig. 1.
Hearing thresholds as determined by acoustic brainstem evoked potentials (ABR), before and immediately after loud sound exposure (top panel), and at the time of psychophysical testing (lower panel). Immediately after exposure a large temporary threshold elevation was evident in the exposed ear. At the time of psychophysical testing (lower panel) there was no statistically significant difference between Exposed and Unexposed subjects.
Each stimulus type (i.e., BBN, 10, 16, and 20-kHz tones) was tested over a minimum of five sessions, with stimulus type randomized across sessions. The background sound was off when the test stimuli were present, as well as in speaker-off periods. The food reinforcement schedule was in place throughout, whereas the foot shock was criterion-dependent upon speaker-off lever pressing. Exposed and unexposed subjects were treated identically and tested in parallel, five sessions per week. Individual subject and group stimulus discrimination functions were derived from test-stimulus suppression-ratio data. Evidence of tinnitus was determined by the divergence of individual and group discrimination functions of exposed subjects from the group discrimination functions of unexposed subjects. In the present procedure, i.e., tinnitus induction before training, tinnitus was indicated by a function downshift (i.e., enhanced suppression).
Taurine testing
The effect of dietary taurine on the psychophysical performance of exposed and unexposed subjects was tested using an ascending-dose series followed by two washout series obtained after supplemental taurine discontinuation. All subjects received the same doses in parallel. Purified taurine (C2H7NO3S, 99%, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in tap water at either 0, 1, or 4 mg/ml, depending on the dose condition. Fresh solutions were made daily and given to subjects in 100-ml graduated cylinders with no-leak drinking spouts. The drug solutions were substituted for the subjects’ normal drinking water and made continuously available, except in the operant test chambers. Average daily water intake for all subjects at 0 mg concentration was 28 ± 8.4 ml, at 1 mg concentration was 31 ± 8.7 ml yielding an average taurine dose of 66.6 ± 20.3 mg/kg/da, and at 4 mg concentration was 33 ± 7.8 ml yielding an average taurine dose of 293.6 ± 75.8 mg/kg/da. Each dose series required 12 test days to complete. Dose testing was followed by two washout periods without supplemental taurine. The first followed taurine treatment by 27– 42 days, and the second followed taurine treatment by 69 – 91 days. Therefore the effect of supplemental taurine on auditory performance was tested in five successive series, of 0, 67, 294, 0 and 0 mg/kg/da. The oral route of taurine administration via drinking water has been used in other studies of taurine’s effect on behavior (Jammoul et al., 2009). While drinking-water administration of taurine resulted in intersubject dose variation, several important benefits accrued: (a) Drug administration was benign, eliminating the potential disruption of behavior in daily test sessions; (b) rats on a restricted diet drink a reasonably consistent volume from day to day; therefore, the subjects maintained a consistent chronic systemic dose; and (c) chronic administration was maintained without the attendant risks of systemic infection, often associated with repeated parenteral injections, or gastric/pulmonary distress, associated with oral gavage.
Data analysis
All data were entered into spreadsheets. For inclusion in statistical analysis, individual-subject psychophysical data sets had to pass two quality filters: (a) There had to be a minimum of 200 total lever presses in the session, and (b) the mean session R for background BBN had to be at least 0.4. The first criterion insured that a sufficient number of responses were present for meaningful suppression ratios to be determined for 1-min periods. The second criterion insured that responses were distributed evenly across the session so that discrimination of the randomly scheduled test stimuli was equitably measured regardless of when they occurred in the session. The quality filters were automatically applied to all data by formulas residing in each spreadsheet data record. Descriptive and inferential statistical analyses and graphic depictions were done using Excel (Professional Edition, 2007, Microsoft, Redmond, WA, USA). Two-factor mixed ANOVAs were used to compare exposed–unexposed performance at each dose level within each test stimulus condition: Noise-exposed vs unexposed was the independent group comparison and stimulus level was the repeated-measures comparison.
The experimental protocol was approved by the Laboratory Animal Care and Use Committee of Southern Illinois University School of Medicine.
RESULTS
Main effects
Three main effects were obtained with supplemental dietary taurine: (1) A significant, and reversible, tinnitus therapeutic effect was evident at the high dose level (4 mg/ml, or 294 mg/kg/da), but not the low dose level (1 mg/ml, or 67 mg/kg/da). (2) At the low dose, taurine may have exacerbated high-frequency components of the tinnitus. (3) Auditory discrimination performance was enhanced in normal-hearing rats in a dose-dependent manner.
Sound exposure, hearing thresholds, and tinnitus
The 1 hr loud sound exposure produced a maximum 60 dB ABR threshold elevation at 20 kHz in the exposed ear immediately after exposure, and a 0 to 11 dB threshold elevation in the unexposed ear (Fig. 1, top panel). At the time of psychophysical testing, ABR thresholds in the unexposed ear of Exposed subjects were identical to those of Unexposed subjects, while exposed ear thresholds were slightly but not significantly elevated at 12 and 16 kHz (F1,28 = 2.20; p = 0.149) (Fig. 1 lower panel). This profile of temporary hearing loss in rats so exposed has been previously reported (Brozoski et al., 2007a; Brozoski et al., 2007b; Wang et al., 2009).
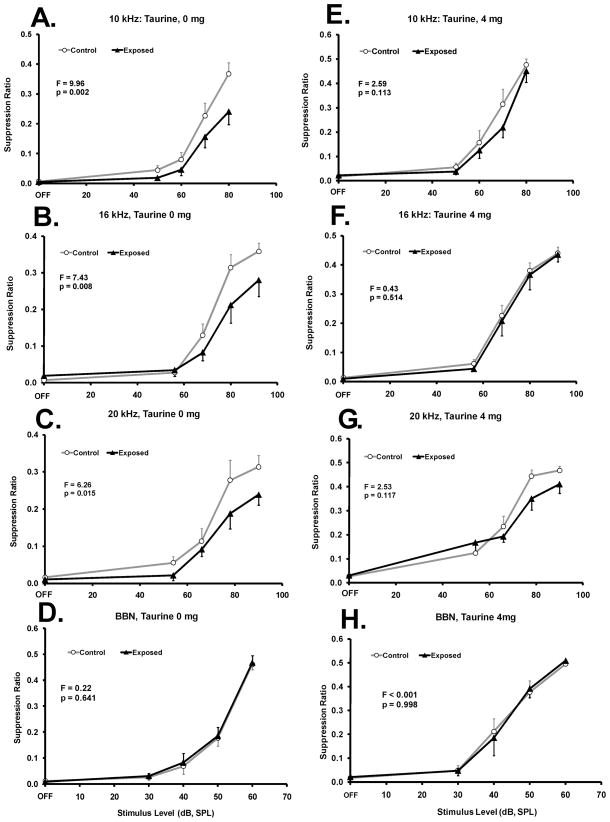
Six of the eight exposed rats showed evidence of chronic acoustically-induced tinnitus as indicated by a visible down shift in their individual psychophysical functions. Data from the two non-tinnitus rats (i.e., post-exposure functions not down shifted) were not included in the present analysis. In the six rats displaying tinnitus, it was reflected by a broad downshift in their discrimination functions between 10 and 20 kHz (Fig. 2A-C, F statistics indicated in each panel). This “broad-band” effect has been previously reported with extended acoustic exposure (Bauer et al., 1999). However the tinnitus was not equivalent to BBN. When tested with BBN there was no evidence of a tinnitus-like shift (Fig. 2D). There was no interaction between sound exposure (i.e., Exposed vs Unexposed) and test stimulus level (range: F1,63 = 0.012; p = 0.998; to F1,63 = 1.34; p = 0.274).
Fig. 2.
Taurine at an average daily dose of 294 mg/kg (4 mg/ml drinking-water concentration is indicated) significantly decreased the evidence of chronic tinnitus in rats. Control n = 8, Exposed n = 6. Performance as a suppression ratio (see text for defininition) is shown as a function of stimulus sound level. Panels A - D show pre-taurine performance (0 mg/ml concentration). Panels E – H show performance on the highest taurine concentration (4 mg/ml). Statistical analysis, between group F tests (df = 1,56), are summarized in each panel; error bars indicate the standard error of the mean.
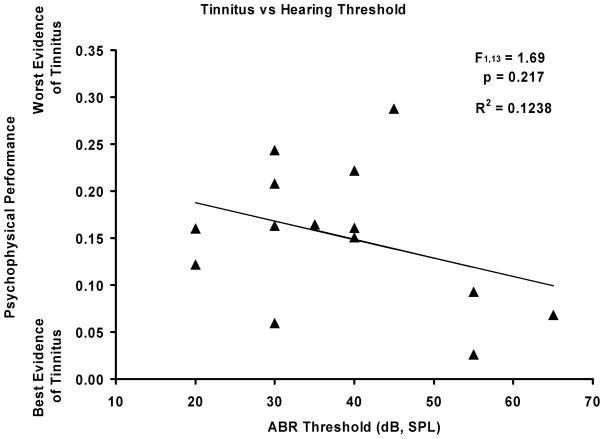
To examine the potential relationship between hearing loss and tinnitus, a regression analysis was done on the psychophysical data that most consistently reflected tinnitus (10 kHz), and immediate post-exposure ipsilateral ABR thresholds (also at 10 kHz). Fig. 3 shows the scatterplot for all subjects in the study, as well as the regression coefficient and statistical analysis. There was a non-significant trend of higher threshold subjects showing greater tinnitus, with a cluster of three subjects primarily responsible for the trend.
Fig. 3.
Scatterplot of the relationship between ABR hearing thresholds and psychophysical performance indicative of tinnitus. The analysis was done on 10 kHz data where the evidence for tinnitus was most pronounced, and included all subjects in the study. The relationship was not statistically significant (statistics presented in figure).
Tinnitus modulation
The higher dose of taurine tested (4 mg/ml concentration), produced a significant therapeutic effect that was evident across all test frequencies (Fig. 2E-G). The therapeutic effect was indicated by the exposed pure tone discrimination functions converging on those of the unexposed group, and the loss of statistically significant separation between the functions (F statistics are indicated in Fig. 2 panels). Taurine did not significantly affect BBN discrimination (Fig. 2D vs. 2H). Some frequency components of the tinnitus (e.g., 16 kHz, Fig. 2B vs 2F) appeared to have been more effectively attenuated by taurine than others (e.g., 20 kHz, Fig. 2C vs 2G). While a significant therapeutic effect of taurine was evident at the higher dose, none was obtained with the low dose (1 mg/ml concentration), i.e., significant evidence of tinnitus remained at 10 kHz (F1,56 = 8.44; p = 0.005), 16 kHz (F1,56 = 25.05; p < 0.0001), and 20 kHz (F1,56 = 6.97; p = 0.010). At neither dose of taurine was there a significant interaction between sound exposure (i.e., Exposed vs Unexposed) and test stimulus level (range: F1,63 = 0.0006; p = 0.979; to F1,63 = 1.37; p = 0.260).
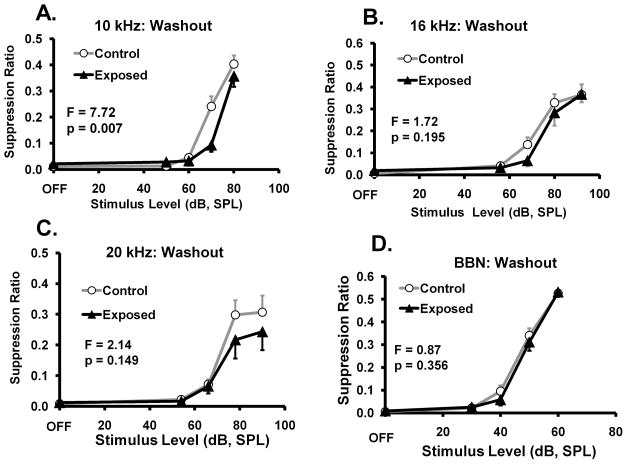
When supplemental taurine was discontinued, there was a gradual return to pretreatment performance indicative of tinnitus. Washout end points are shown in Fig. 4. Evidence of tinnitus was again significant at 10 kHz (Fig. 4A), but failed to reach significance at other test frequencies (Fig. 4B – D). Based on studies of acute taurine kinetics (Lee et al., 2004) this lingering therapeutic was surprising. However studies of tissue compartment levels after chronic administration of taurine, suggest that elevated tissue levels may persist for 6 to 11 weeks (Pacioretty, et al., 2001).
Fig. 4.
Washout return to pre-treatment performance showing significant evidence of tinnitus returning at 10 kHz (A). Tinnitus was evident at other frequencies, but failed to reach statistical significance (panels B, C, D). Data were collected 30 to 90 da after supplemental taurine was discontinued. Statistical analyses, between group F tests (df = 1,56), are summarized in each panel; error bars indicate the standard error of the mean.
Improved auditory discrimination
The procedure used to assess tinnitus derives from a determination of auditory discrimination. In the present context, auditory discrimination is characterized by functions that slope upward, when plotting performance against stimulus level. The functions assume this shape because the animals have been trained to respond when the sound is on, and not respond when the sound is off (e.g., Figs. 2, 4 and 5). For unexposed control animals, the discrimination functions therefore depict the rats’ distinction between sound and silence.
Fig. 5.
Improved auditory discrimination in normal control rats (n = 8) tested as described in the text. Two doses of supplemental taurine were tested, shown as taurine concentration (mg/ml) in drinking water. For each test stimulus, A – D, high-dose performance was significantly improved compared to 0 dose performance (statistics summarized in Table 2).
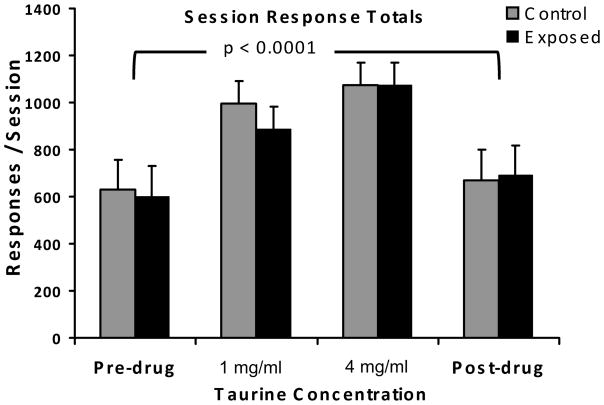
At both the low and high concentrations, taurine improved auditory discrimination, in unexposed rats, as indicated by a left shift of the discrimination functions (Fig. 5; statistics summarized in Table 2). This effect was most significant at the highest concentration (4 mg/ml) and highest frequency tested (20 kHz; F 1,56 = 26.72, p < 0.0001; Fig. 5D) but also evident at lower frequencies (Fig. 5B). After discontinuation of supplemental taurine, performance returned to pre-treatment levels (circular data points, gray function, Fig. 5A and B). It is also notable that supplemental taurine at both dose levels had a general energizing effect on performance, as reflected by total lever presses/session (F 3,248 = 50.6, p < 0.0001), and that this effect, depicted in Fig. 6, was the same in both exposed and unexposed subjects (F1,248 = 1.01, p = 0.315).
Table 2.
ANOVA Summary.
The effect of taurine on Unexposed subject discrimination at two dose levels, compared to 0 mg/ml performance.
| BBN | 10 kHz | 16 kHz | 20 kHz | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | F | df | p | F | df | p | F | df | p | F | df | p |
| 1 mg/ml | 15.64 | 1, 56 | 0.0002 | 5.49 | 1, 56 | 0.0227 | 11.33 | 1, 56 | 0.0014 | 0.12 | 1, 56 | 0.645 |
| 4 mg/ml | 19.44 | 1, 56 | <0.0001 | 7.13 | 1, 56 | 0.0099 | 14.34 | 1, 56 | 0.0004 | 26.72 | 1, 56 | <0.0001 |
Fig. 6.
The stimulant property of supplemental taurine as reflected by session total lever presses. The stimulant effect was equivalent in both treatment groups (F1,248 = 1.013, p = 0.315), but significantly different between both drug doses and non-drug levels (p value shown, F 3,248 = 50.6).
DISCUSSION
Summary of results
(1) Supplemental taurine at approximately 300 mg/kg/da effectively eliminated evidence of chronic acoustic-exposure-induced tinnitus in rats, thus confirming the primary experimental hypothesis that enhanced inhibition could significantly modulate auditory sensation and attenuate neural activity that comprises the tinnitus signal. (2) The tinnitus therapeutic effect, although reversible, was slow to washout, with partial effects lingering to at least 90 days post treatment (Fig. 4). The persistence of the therapeutic effect exceeded initial expectation, extrapolating from studies of single-treatment taurine efflux from rat brain (Lee et al., 2004). However studies of tissue compartment concentrations after chronic administration, suggest that much more protracted systemic elevations may persist (Pacioretty, et al., 2001). (3) Supplemental dietary taurine at both concentrations (1 mg/ml and 4 mg/ml) improved auditory discrimination in normal intact rats as well as in rats with psychophysical evidence of tinnitus. This improvement was more evident at the higher concentration and higher frequencies than lower, e.g., Fig. 5B vs Fig. 5D. Although somewhat unexpected, this general improvement in auditory discrimination is consistent with the hypothesized action of taurine at glycine and GABAA receptors, inhibitory amino acid receptors prevalent in central auditory circuits (Albrecht et al., 2005). Increased inhibition could improve signal-to-noise processing and frequency discrimination by reducing temporal jitter, especially at lower levels of the ascending auditory pathway. (4) Supplemental taurine at both the low (1 mg/ml) and high (4 mg/ml) dose levels had a general energizing effect, reflected as an increase in lever pressing (Fig. 6). This effect was equally evident in both the experimental and control group. Excitatory effects of taurine on peripheral systems have been reported (Liu et al., 2006), and extended oral dosing of taurine has been reported to have stimulant properties reflected in locomotor behavior (El Idrissi et al., 2009).
The Age Parameter
The rats in the present study were trained as young adults and the effects of supplemental taurine were tested between 12 and 16 months of age (Table 1). In this time frame Long-Evans rats typically show robust psychophysical performance and well-preserved hearing as indicated by ABR. That taurine was effective in alleviating tinnitus and generally improving auditory performance in this age range indicates that normal auditory performance may nevertheless be improved with an appropriately chosen agonist.
Anesthesia during loud sound exposure
In the present and related studies, the subjects were under general anesthesia during traumatic sound exposure. This insured a constant and known sound level at the tympanum, as well as only unilateral exposure. Preserving completely normal hearing in one ear is important, because a bilateral hearing loss will affect psychophysical performance, albeit rather differently than tinnitus (Brozoski et al., 2008). Nevertheless, a question may be raised about the modulatory effect of anesthesia on the physiological impact of the sound exposure. Gas anesthetics such as isoflurane have complex central effects, inhibiting both excitatory glutamatergic and inhibitory GABAergic systems (Larsen, et al, 1998; Franks, 2008; Jokosovic, et al., 2009). Therefore, their net influence on the physiological impact of intense sound exposure is difficult to predict, and may not bias the impact in any particular direction. Although it has been shown that some anesthetics, including isoflurane, confer a peripheral oto-protective effect (e.g., Duan et al., 2000; Kim et al., 2005), we have shown elsewhere that cochlear hair cell loss is not a good predictor of tinnitus level (Bauer et al., 2007).
Mechanism of action
The enhancement of auditory discrimination and therapeutic action of taurine on tinnitus could result from its relatively high affinity for inhibitory strychnine-sensitive glycine receptors prevalent in the dorsal cochlear nucleus (DCN) and other brainstem auditory structures (Wang et al., 2009; Xu et al., 2006). The DCN has been implicated in the establishment of tinnitus (Kaltenbach, 2007), analysis of spectrally complex signals (Bandyopadhyay et al., 2007), and sound localization (Oertel et al., 2004). Enhanced inhibition within the auditory brainstem would likely suppress the increased spontaneous and bursting activity observed in DCN output neurons in animal models of tinnitus (Bauer et al., 2008; Brozoski et al., 2002; Chang et al., 2002; Finlayson et al., 2009). Taurine has intermediate-to-low affinity for wild-type GABAAR constructs (Bureau et al., 1993) found throughout central auditory system, but has a higher affinity for extrasynaptic GABAAR constructs containing α4 and δ subunits found in thalamus (Chandra et al., 2006; Herd et al., 2009; Jia et al., 2008; Sur et al., 1999; Wisden et al., 1992). Extrasynaptic GABAARs are present in high concentration in the rat medial geniculate body (Caspary et al., 2007; Richardson et al., 2009). Evidence suggests that physiologic concentrations of taurine could inhibit thalamic neurons and regulate excitability acting at extrasynaptic GABAARs (Jia et al., 2008).
Assuming that taurine functions as an effective agonist at inhibitory amino acid neurotransmitter receptors in the auditory pathway, it may have the capacity to reduce background neural activity through enhanced inhibition, and thereby improving signal-to-noise separation. In the present study normal-hearing intact rats, while on a moderately-high level of supplemental taurine, improved their discrimination performance across a broad frequency range. Loss of coding fidelity is observed in aged animals in conjunction with loss of GABA and glycine function in the auditory neuraxis (Caspary et al., 2008; Hughes et al., 2009; Schatteman et al., 2008; Wang et al., 2009). Consistent with these findings, it would be expected that enhanced inhibitory amino acid neurotransmitter function would facilitate frequency detection (Gleich et al., 2003). In the present study, normal-hearing intact rats on a moderately-high level of supplemental taurine improved their discrimination performance across a broad frequency range. The same mechanism, that is, increased agonistic activity at inhibitory amino acids receptors in the auditory neuraxis, could explain the attenuation of tinnitus. This would be particularly true if tinnitus emerges from brainstem areas and derives from bursting activity with high peak discharge rates (Bauer et al., 2008). Beyond specific improvements in auditory processing, taurine and other extra-synaptic GABA agonists, including GABA itself, may play an important role generally stabilizing brain systems.
Lingering therapeutic effect
In the present study the tinnitus therapeutic effect of taurine was slow to wash out. Seventy to ninety days after the supplement was discontinued, evidence of tinnitus returned only at 10 kHz (Fig. 4A) but not at other frequencies (Fig 4B – D). Clearance of acute exogenous taurine from rat brain is estimated to be quite rapid at about 57 min (Lee et al., 2004). However when cats were given supplemental taurine (0.15 mg/kg) for five months, the half life of taurine in skeletal muscle was 11.2 weeks, while in whole blood, it was 6.2 weeks (Pacioretty, et al., 2001). According to Pacioretty et al. (2001) tissue biopsy and analysis most accurately reflects potential taurine availability. Considering that the rats in the present study were given supplemental taurine for 27 days (14 days at 1 mg/ml; 13 days at 4 mg/ml), it is possible that elevated brain taurine levels persisted well into the washout period. Future research should address this issue.
Focus of therapeutic effect
Some frequency aspects of the tinnitus (e.g., 16 kHz, Fig. 2B vs 2F) were more effectively attenuated by taurine than others (e.g., 20 kHz, Fig. 2C vs 2G). Reasons for this therapeutic profile were not clear, although the traumatizing stimulus had its maximum level, and maximum long-term threshold effect at 16 kHz. If, as hypothesized, taurine stabilizes auditory processing, its therapeutic impact may have been greatest in tonotopic areas were the impact of acoustic trauma was also greatest.
Stimulant properties
Despite the potential role of taurine as a global neuromodulator, it seems unlikely that its net effect on brain function is entirely inhibitory. In the present study both the low and high taurine doses produced significant increases in lever pressing in both groups of rats. The maximum lever-press rate obtained in the present study, however, was well below the empirical maximum of 3500/hr attainable by rats tested using this procedure. The stimulant effect of taurine suggests a spectrum of action that includes inhibition of other inhibitory systems. An alternative interpretation would be that improved signal-to-noise processing in the thalamus and other structures, facilitated focus on the requirements of the behavioral task. The net effect of improved attention to task requirements might well elevate lever pressing, thereby maximizing reinforcement.
Taurine is a prominent ingredient in many energy drinks, with concentrations as high as 17 mg/ml. The highest concentration of taurine used in the present study was 4 mg/ml. Whether the taurine available in energy drinks improves auditory discrimination, or attenuates tinnitus is unknown. However the typical energy drink also contains substantial concentrations of caffeine and sodium, neither of which is likely to contribute to tinnitus alleviation. On the other hand, the energizing effect of taurine on lever pressing obtained in the present study suggests that taurine additives may contribute to the stimulant properties of energy drinks.
Acknowledgments
Supported by NIH grant 1RO1DC009669-01
Abbreviations
- ABR
acoustic brainstem evoked response
- BBN
broad-band noise
- DCN
dorsal cochlear nucleus
- GABA
γ-amino butyric acid
- GlyR
glycine receptors
- GABAAR
GABAA receptors
- GABABR
GABAB receptors
- IC
inferior colliculus
- MGB
medial geniculate body
- R
suppression ratio
- SPL
sound pressure level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Donald M. Caspary, Email: dcaspary@siumed.edu.
Carol A. Bauer, Email: cbauer@siumed.edu.
Benjamin D. Richardson, Email: brichardson@siumed.edu.
References
- Aerts L, Van Assche FA. Taurine and taurine-deficiency in the perinatal period. J Perinat Med. 2002;30:281–6. doi: 10.1515/JPM.2002.040. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res. 2005;30:1615–21. doi: 10.1007/s11064-005-8986-6. [DOI] [PubMed] [Google Scholar]
- Atmaca G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med J. 2004;45:776–88. doi: 10.3349/ymj.2004.45.5.776. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Reiss LA, Young ED. Receptive field for dorsal cochlear nucleus neurons at multiple sound levels. J Neurophysiol. 2007;98:3505–15. doi: 10.1152/jn.00539.2007. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J of the Assoc for Res in Otolaryngol. 2001;2:54–64. doi: 10.1007/s101620010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Myers K. Primary afferent dendrite degeneration as a cause of tinnitus. J Neurosci Res. 2007;85:1489–98. doi: 10.1002/jnr.21259. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M. Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg. 1999;121:457–62. doi: 10.1016/S0194-5998(99)70237-8. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–78. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–63. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall TC. Therapeutic applications of taurine. Altern Med Rev. 1998;3:128–36. [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497 (Pt 3):753–9. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. Learning about tinnitus from an animal model. Seminars in Hearing. 2008;29:242–258. [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–90. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Spires TJ, Bauer CA. Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J Assoc Res Otolaryngol. 2007a;8:105–18. doi: 10.1007/s10162-006-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Ciobanu L, Bauer CA. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI) Hear Res. 2007b;228:168–79. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Bureau MH, Olsen RW. GABAA receptor subtypes: ligand binding heterogeneity demonstrated by photoaffinity labeling and autoradiography. J Neurochem. 1993;61:1479–91. doi: 10.1111/j.1471-4159.1993.tb13643.x. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–91. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Hutson P, Wang HN, Turner JG, Hughes LF. Altered α4δ GABAA receptors in the medial geniculate body of young and aged rats in a noise-exposure model of tinnitus. Society for Neuroscience Abstract. 2007;37:504.4. [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–5. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Chen K, Kaltenbach JA, Zhang J, Godfrey DA. Effects of acoustic trauma on dorsal cochlear nucleus neuron activity in slices. Hear Res. 2002;164:59–68. doi: 10.1016/s0378-5955(01)00410-5. [DOI] [PubMed] [Google Scholar]
- Chung SJ, Ramanathan V, Giacomini KM, Brett CM. Characterization of a sodium-dependent taurine transporter in rabbit choroid plexus. Biochim Biophys Acta. 1994;1193:10–6. doi: 10.1016/0005-2736(94)90326-3. [DOI] [PubMed] [Google Scholar]
- Contreras NEIR, Bachelard HS. Some Neurochemical Studies on Auditory Regions of Mouse-Brain. Exp Brain Res. 1979;36:573–584. doi: 10.1007/BF00238524. [DOI] [PubMed] [Google Scholar]
- Cooper JC., Jr Health and Nutrition Examination Survey of 1971–75: Part II. Tinnitus, subjective hearing loss, and well-being. J Am Acad Audiol. 1994;5:37–43. [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–63. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan M, Agerman K, Ernfors P, Canlon B. Complementary roles of neurotrophin 3 and a N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc Natl Acad Sci U S A. 2000;97:7597–602. doi: 10.1073/pnas.97.13.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Idrissi A. Taurine improves learning and retention in aged mice. Neurosci Lett. 2008;436:19–22. doi: 10.1016/j.neulet.2008.02.070. [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Boukarrou L, Heany W, Malliaros G, Sangdee C, Neuwirth L. Effects of Taurine on Anxiety-Like and Locomotor Behavior of Mice. In: JAe, et al., editors. Taurine 7, Advances in Experimental Medicine and Biology. Springer Science+Business Media, LLC; New York: 2009. pp. 207–215. [DOI] [PubMed] [Google Scholar]
- Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–9. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Kaltenbach JA. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear Res. 2009;256:104–17. doi: 10.1016/j.heares.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Friauf E, Hammerschmidt B, Kirsch J. Development of adult-type inhibitory glycine receptors in the central auditory system of rats. J Comp Neurol. 1997;385:117–34. doi: 10.1002/(sici)1096-9861(19970818)385:1<117::aid-cne7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Frosini M, Sesti C, Saponara S, Ricci L, Valoti M, Palmi M, Machetti F, Sgaragli G. A specific taurine recognition site in the rabbit brain is responsible for taurine effects on thermoregulation. Brit J Pharmacol. 2003;139:487–494. doi: 10.1038/sj.bjp.0705274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich O, Hamann I, Klump GM, Kittel M, Strutz J. Boosting GABA improves impaired auditory temporal resolution in the gerbil. Neuroreport. 2003;14:1877–80. doi: 10.1097/00001756-200310060-00024. [DOI] [PubMed] [Google Scholar]
- Harding NJ, Davies WE. Cellular localisation of taurine in the organ of Corti. Hear Res. 1993;65:211–5. doi: 10.1016/0378-5955(93)90214-l. [DOI] [PubMed] [Google Scholar]
- Herd MB, Foister N, Chandra D, Peden DR, Homanics GE, Brown VJ, Balfour DJ, Lambert JJ, Belelli D. Inhibition of thalamic excitability by 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3-ol: a selective role for delta-GABA(A) receptors. Eur J Neurosci. 2009;29:1177–87. doi: 10.1111/j.1460-9568.2009.06680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- Hughes LF, Turner JG, Parrish JL, Caspary DM. Processing of broadband stimuli across A1 layers in young and aged rats. Hear Res. 2010;264:79–85. doi: 10.1016/j.heares.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–63. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Jammoul F, Wang QP, Nabbout R, Coriat C, Duboc A, Simonutti M, Dubus E, Craft CM, Ye W, Collins SD, Dulac O, Chiron C, Sahel JA, Picaud S. Taurine Deficiency Is a Cause of Vigabatrin-Induced Retinal Phototoxicity. Ann Neurol. 2009;65:98–107. doi: 10.1002/ana.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL. Taurine is a potent activator of extrasynaptic GABA(A) receptors in the thalamus. J Neurosci. 2008;28:106–15. doi: 10.1523/JNEUROSCI.3996-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksovic PM, Weiergräber M, Lee W, Struck H, Schneider T, Todorovic SM. Isoflurane-sensitive presynaptic R-type calcium channels contribute to inhibitory synaptic transmission in the rat thalamus. J Neurosci. 2009;29:1434–45. doi: 10.1523/JNEUROSCI.5574-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA. The dorsal cochlear nucleus as a contributor to tinnitus: mechanisms underlying the induction of hyperactivity. Progress in Brain Research. 2007;166:89–106. doi: 10.1016/S0079-6123(07)66009-9. [DOI] [PubMed] [Google Scholar]
- Kang YS, Ohtsuki S, Takanaga H, Tomi M, Hosoya K, Terasaki T. Regulation of taurine transport at the blood-brain barrier by tumor necrosis factor-alpha taurine and hypertonicity. J Neurochem. 2002;83:1188–1195. doi: 10.1046/j.1471-4159.2002.01223.x. [DOI] [PubMed] [Google Scholar]
- Kim JK, Lee HJ, Kang HH, Shin JW, Ku SW, Ahn JH, Kim YJ, Chung JW. Protective effect of isoflurane anesthesia on noise-induced hearing loss in mice. Laryngoscope. 2005;115:1996–1999. doi: 10.1097/01.mlg.0000180173.81034.4d. [DOI] [PubMed] [Google Scholar]
- Larsen M, Haugstad TS, Berg-Johnsen J, Langmoen IA. The effect of isoflurane on brain amino acid release and tissue content induced by energy deprivation. J Neurosurg Anesthesiol. 1998;10:166–70. doi: 10.1097/00008506-199807000-00007. [DOI] [PubMed] [Google Scholar]
- Lee NY, Kang YS. The brain-to-blood efflux transport of taurine and changes in the blood-brain barrier transport system by tumor necrosis factor-alpha. Brain Res. 2004;1023:141–7. doi: 10.1016/j.brainres.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Liu HY, Chi FL, Gao WY. Taurine modulates calcium influx under normal and ototoxic conditions in isolated cochlear spiral ganglion neurons. Pharmacol Rep. 2008;60:508–13. [PubMed] [Google Scholar]
- Liu HY, Gao WY, Wen W, Zhang YM. Taurine modulates calcium influx through L-type voltage-gated calcium channels in isolated cochlear outer hair cells in guinea pigs. Neurosci Lett. 2006;399:23–26. doi: 10.1016/j.neulet.2006.01.070. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. Journal of Neurophysiology. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Oertel D, Young ED. What’s a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004;27:104–10. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S. New aspects of the blood-brain barrier transporters; Its physiological roles in the central nervous system. Biol Pharm Bull. 2004;27:1489–1496. doi: 10.1248/bpb.27.1489. [DOI] [PubMed] [Google Scholar]
- Oja SS, Saransaari P. Pharmacology of taurine. Proc West Pharmacol Soc. 2007;50:8–15. [PubMed] [Google Scholar]
- Pacioretty L, Hickman MA, Morris JG, Rogers QR. Kinetics of taurine depletion and repletion in plasma, serum, whole blood and skeletal muscle in cats. Amino Acids. 2001;21:417–427. doi: 10.1007/s007260170006. [DOI] [PubMed] [Google Scholar]
- Ramanathan VK, Chung SJ, Giacomini KM, Brett CM. Taurine transport in cultured choroid plexus. Pharm Res. 1997;14:406–9. doi: 10.1023/a:1012074827388. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM. Presence and function of extrasynaptic GABAA receptors in the medial geniculate body. Society for Neuroscience Abstract. 2009;39:258.23. [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–74. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Schatteman TA, Hughes LF, Caspary DM. Aged-related loss of temporal processing: altered responses to amplitude modulated tones in rat dorsal cochlear nucleus. Neuroscience. 2008;154:329–37. doi: 10.1016/j.neuroscience.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgaragli G, Carla V, Magnani M, Galli A. Hypothermia induced in rabbits by intracerebroventricular taurine: specificity and relationships with central serotonin (5-HT) systems. J Pharmacol Exp Ther. 1981;219:778–85. [PubMed] [Google Scholar]
- Sgaragli G, Frosini M, Palmi M, Dixon HB, Desmond-Smith N, Bianchi L, Della Corte L. Role of taurine in thermoregulation and motor control. Behavioural and cellular studies. Adv Exp Med Biol. 1996;403:527–35. doi: 10.1007/978-1-4899-0182-8_57. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman JA. Nutritional taurine and central nervous system development. Ann N Y Acad Sci. 1986;477:196–213. doi: 10.1111/j.1749-6632.1986.tb40337.x. [DOI] [PubMed] [Google Scholar]
- Sturman JA, Gargano AD, Messing JM, Imaki H. Feline maternal taurine deficiency: effect on mother and offspring. J Nutr. 1986;116:655–67. doi: 10.1093/jn/116.4.655. [DOI] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha4 and delta subunits of the gamma-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–5. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Sved DW, Godsey JL, Ledyard SL, Mahoney AP, Stetson PL, Ho S, Myers NR, Resnis P, Renwick AG. Absorption, tissue distribution, metabolism and elimination of taurine given orally to rats. Amino Acids. 2007;32:459–66. doi: 10.1007/s00726-007-0494-3. [DOI] [PubMed] [Google Scholar]
- Tamai I, Senmaru M, Terasaki T, Tsuji A. Na(+)- and Cl(−)-dependent transport of taurine at the blood-brain barrier. Biochem Pharmacol. 1995;50:1783–93. doi: 10.1016/0006-2952(95)02046-2. [DOI] [PubMed] [Google Scholar]
- Tsuboyama-Kasaoka N, Shozawa C, Sano K, Kamei Y, Kasaoka S, Hosokawa Y, Ezaki O. Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology. 2006;147:3276–3284. doi: 10.1210/en.2005-1007. [DOI] [PubMed] [Google Scholar]
- Vohra BP, Hui X. Improvement of impaired memory in mice by taurine. Neural Plast. 2000;7:245–59. doi: 10.1155/NP.2000.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–59. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–62. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Tang XW, Tsai WH. Taurine receptor: kinetic analysis and pharmacological studies. Adv Exp Med Biol. 1992;315:263–8. doi: 10.1007/978-1-4615-3436-5_31. [DOI] [PubMed] [Google Scholar]
- Wu JY, Liao CC, Lin CJ, Lee YH, Ho JY, Tsai WH. Taurine receptor in the mammalian brain. Prog Clin Biol Res. 1990;351:147–56. [PubMed] [Google Scholar]
- Xu H, Zhou KQ, Huang YN, Chen L, Xu TL. Taurine activates strychnine-sensitive glycine receptors in neurons of the rat inferior colliculus. Brain Res. 2004;1021:232–40. doi: 10.1016/j.brainres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Xu H, Wang W, Tang ZQ, Xu TL, Chen L. Taurine acts as a glycine receptor agonist in slices of rat inferior colliculus. Hearing Res. 2006;220:95–105. doi: 10.1016/j.heares.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Yeung JYT, Canning KJ, Zhu GY, Pennefather P, Macdonald JF, Orser BA. Tonically activated GABA(A) receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Molecular Pharmacology. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]