Abstract
Introduction
Embarrassment is commonly reported in essential tremor (ET) patients yet there is no formal tool to assess embarrassment in ET. Our aim was to develop such a tool and to assess its clinimetric properties. A quantitative measure of embarrassment could be used to assess response to treatment in clinical practice and clinical trials.
Methods
Based on surveys of international tremor experts and ET patients, we constructed the Essential Tremor Embarrassment Assessment (ETEA), a brief, easily administered, 14-item self-assessment scale. The ETEA was assessed for validity, reliability and other clinimetric properties in 75 ET patients.
Results
Forty-seven tremor experts from eight countries were surveyed. On average, they estimated that 75% of their patients experienced embarrassment, yet there was very little agreement (range = 10 – 95%). Among ET patients, three-quarters (77.3%) reported at least occasional embarrassment due to their tremor and one-third (36.4%) reported daily embarrassment. ETEA scores correlated with a tremor disability questionnaire score (p = 0.02 and p = 0.01) and Center for Epidemiologic Studies Depression Scale scores (p <0.001 and p <0.001). Test-retest reliability was high (p <0.001). Factor analysis identified four factors, explaining 62.4% of the variance. For the major factors (I and II), high internal consistency was found (Cronbach’s alpha = 0.85 and 0.74).
Conclusion
Embarrassment is commonly experienced by ET patients. The ETEA is a reliable and valid tool to measure embarrassment in patients with this disease.
Keywords: Essential tremor, clinical, embarrassment, assessment, epidemiology
Introduction
Essential tremor (ET) is the most common tremor disorder [1–4]. Prior studies of ET patients have demonstrated that tremor impacts on daily function and quality of life [5–7].
Embarrassment is a potentially important contributor to lower quality of life in patients with outwardly visible movement disorders, including ET. A recent study reported embarrassment to be very common among patients with ET, with 18.9% of a population sample and 58.2% of a clinical sample of ET patients reporting embarrassment from tremor [8]. In that study, embarrassment (i.e., independent of tremor severity) was responsible for a doubling of tremor medication usage [8]. What, in particular, makes ET patients embarrassed about their condition, however, has not been well defined.
Despite its high prevalence and potential importance, embarrassment is not routinely assessed as a modifiable quantifiable outcome in therapeutic trials in ET. A quantitative measure of embarrassment could be used to assess response to treatment and satisfaction with therapy in routine clinical settings. One problem is that there is no formal tool to measure embarrassment in patients with ET.
The main aim of this study was to develop and validate the Essential Tremor Embarrassment Assessment (ETEA). Additional aims were to further understand in what settings ET patients experience tremor-related embarrassment and to gauge the extent to which tremor experts viewed embarrassment to be an issue of importance in ET.
Methods
All study procedures were reviewed and approved by the Columbia University Medical Center (CUMC) Internal Review Board and written informed consent was obtained from participants.
Development of the ETEA
Written Survey of Tremor Experts
In September 2008, the senior author (EDL) identified individuals with expertise in ET using the following sources: (1) membership list of the Medical Advisory Board of the International Essential Tremor Foundation, (2) membership list of the Tremor Research Group, (3) Pubmed search using the term essential tremor to identify first- and senior-authors who had published two or more research papers on ET during the preceding five years. Using this approach, 52 individuals from eight countries were identified, nearly all of whom were neurologists specializing in movement disorders. In November 2008, each expert was sent by email a brief survey. The goals of this survey were to: (1) ascertain the extent to which tremor experts thought that embarrassment was a problem for their patients and (2) obtain their opinion about the sources of embarrassment in ET (esp. life situations that caused embarrassment). Thus, they were asked, “Approximately what percentage of your ET patients do you think feel embarrassment because of their tremor?” They were also asked to note the three most common reasons for embarrassment in ET; choices were provided and they were given the opportunity to write in additional reasons. These survey data were later used when constructing the ETEA.
Telephone Interviews with ET Patients
To complement the expert-derived data, we also sought patient-derived data on the sources of embarrassment in ET. Our goal was to contact and interview 25 randomly-selected ET patients; that number, based on previous studies on embarrassment in ET [8], was expected to provide us with a broad range of responses. In February 2009, we queried the computerized billing database at the Neurological Institute of New York, CUMC for all tremor patients (International Classification Disease code 333.1) seen in the past twelve months at the Center for Parkinson’s Disease and Other Movement Disorders. This query generated 258 patients. Using medical record numbers and a random-digit table, 90 patients were selected from this list and their charts reviewed by the senior author (EDL) to confirm that the assigned diagnosis was ET and not another tremor disorder. Patients were excluded if they did not have ET or if they had both ET as well as another movement disorder. Using this method, 55 of 90 patients were excluded and 35 remained. A trained resident neurologist (RET) was able to successfully contact 25 of these 35 by telephone and 22 (88%) agreed to the telephone interview. The 9-question interview included both closed- and open-ended questions about their tremor and causes of embarrassment. They were asked to note reasons for embarrassment in ET; choices were provided and they were given the opportunity to write in additional responses.
Construction of the ETEA
The feedback obtained from the written surveys of ET experts and telephone interviews with ET patients was reviewed and used to develop the ETEA. The ETEA consists of 14-items endorsed as the most relevant by experts and patients. The ETEA items are structured as a series of 14 statements (Table 1) and the participant is asked to first provide a simple response (disagree or agree) and then to provide a more nuanced response (0 – 5 point Likert scale ranging from disagree [0] - agree strongly [5]). The ETEA is administered by a health care provider or researcher, yet it collects data on the patient’s self-assessment of embarrassment. The sum of the simple responses yields an initial score (Score A, range = 0 – 14) and the sum of the nuanced responses yields a second score (Score B, range = 0 – 70), with higher scores indicating greater embarrassment. The ETEA can be administered in 3 – 10 minutes.
Table 1.
Essential Tremor Embarrassment Assessment (ETEA)
For every question, indicate whether you agree or disagree with the statement. If you agree, then indicate, on a scale of 0 – 5 how much you agree (0 = disagree to 5 = agree strongly).
|
Patient Enrollment
To assess the properties of the ETEA, we enrolled a new group of ET patients. Our goal was to enroll 75 ET patients, which according to our sample size calculations would provide 87.3% power to detect correlations (r) of 0.35 or higher between the ETEA and other continuous measures (e.g., tremor disability questionnaire score) (assuming alpha = 0.05).
ET patients were recruited from the clinical practices of neurologists at the Center for Parkinson’s Disease and Other Movement Disorders, CUMC. Recruitment began in July 2009 and the rate of enrollment was dependent on the availability of the first author (RET), a resident neurologist, for approaching and enrolling cases. On days in which the first author was available, she reviewed the list of outpatients visiting the center who carried a diagnosis of ET. If the treating physician confirmed the diagnosis, then she obtained consent from the patient. The first 75 patients who were approached all agreed to participate.
Assessment Procedure
After obtaining written informed consent, the first author administered the following assessments: (1) collection of demographic data, medical history, medications, characteristics of tremor (duration, location); (2) drawing four (2 right, 2 left) Archimedes spirals that were blindly scored (EDL) using a validated 0 – 3 ordinal rating scale [9] resulting in a tremor severity score (range = 0 – 12); (3) functional disability from tremor assessed with a 10-item version of the Tremor Disability Questionnaire (TDQ) (range of scores = 0 – 20 [most disabled]) [10]; (4) depression assessed with the 10-item Center for Epidemiologic Studies Depression Scale (CES-D)(range of scores = 0 – 30 [maximal depressive symptoms]) [11] and (5) ETEA.
Assessing the Acceptability, Reliability and Convergent Validity of the ETEA
To assess the acceptability of the ETEA, we examined the difference between the mean and median scores, defining an acceptable difference as ≤10% of the maximum possible score; we also assessed the extent of floor and ceiling effects, setting the maximum acceptable limit at 15% [12].
To assess the reliability of the ETEA, the first author re-administered the ETEA to 50 participants 1 to 6 months after their initial assessment. Test-retest reliability was assessed for each ETEA A item (agree-disagree response) using Kappa statistics and for each ETEA B item (5-point scale) using intraclass correlation coefficients (i.e., one-way random effects analysis of variance model). For ETEA total scores (A and B), intraclass correlation coefficients coefficients (i.e., one-way random effects analysis of variance model) were used (SPSS version 17.0). Guidelines for levels of agreement were as follows: 0.20 – 0.39 (fair agreement), 0.40 – 0.59 (moderate agreement), 0.60 – 0.79 (good agreement), and ≥0.8 (very good agreement) [16].
Factor structure of the ETEA A score was assessed using principle component extraction with promax rotation. This rotation method was used because the independence of factors could not be assumed. Scree plots resulting from the principal components analysis were also examined. Assessment of reliability also included testing of internal consistency (Cronbach’s alpha) of individual items within factors identified by the factor analysis.
To assess the convergent validity of the ETEA, we examined its correlation (Pearson’s correlation coefficient) with several of other measures, hypothesizing that it would correlate with two self-reported psychosocial items (functional disability from tremor, depression) and possibly with tremor severity. As previously defined, r = 0 – 0.19 is consistent with a negligible association, r = 0.20 – 0.34 is consistent with a weak association, r = 0.35 – 0.50 is consistent with a moderate association, and r >0.50 is consistent with a close association [12].
Results
Written Survey of Tremor Experts
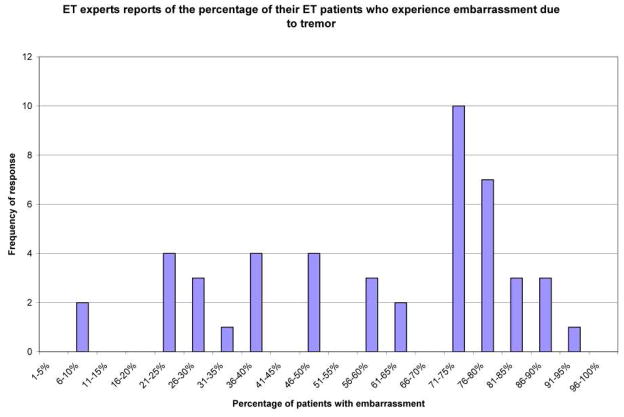
Forty-seven (90.4%) of 52 tremor experts completed the survey. These 47 experts were from eight countries and 46 were clinical neurologists (see acknowledgements). They estimated, on average, that 75% of their patients experienced embarrassment due to tremor, yet there was very little agreement (standard deviation = 22.7% and range = 10 – 95%, Figure 1). At the two extremes, 10 (21.3%) experts estimated that a third or fewer patients were embarrassed while 14 (29.8%) estimated that 75% or more were embarrassed by tremor.
Figure 1.
Telephone Interviews with ET Patients
Twenty-two patients (mean age = 72.4 ± 11.9 years, range = 39 – 93 years; mean tremor duration = 22.0 years, range 3 – 60 years) were interviewed by telephone. Seventeen (77.3%) reported at least occasional embarrassment from their tremor and 8 (36.4%) reported daily embarrassment from tremor. The three most commonly reported reasons for experiencing embarrassment were eating or drinking in public (16 [72.7%]), difficulty writing clearly in front of others (13 [59.1%]) and feeling that the tremor draws attention from strangers (12 [54.5%]). While patients and experts agreed on many of the common sources of embarrassment (e.g., eating and drinking in public) there were some disagreements (e.g., only 2 [4.1%] experts thought that “tremor draws attention from strangers” would be among the three most common reasons for experiencing embarrassment). Seven patients (31.8%) reported that they take ET medications because tremor embarrasses them.
Acceptability, Reliability, and Convergent Validity of the ETEA
The 75 ET patients evaluated in person (37 [49.3%] men) represented a rich clinical range, including patients over a wide age range (28 – 91 years, mean = 71.9 ± 13.0 years) with broad representation of tremor durations (range = 3 – 69 years, mean = 29.1 ± 17.4 years). Tremor severity scores similarly ranged widely (range = 3 – 12, mean = 7.4 ± 2.3) as did CES-D scores (range = 0 – 27, mean = 8.0 ± 7.4). Approximately one-half took medication for tremor (43 [57.3%]).
ETEA scores are shown (Table 2). Items 1 (my tremor is embarrassing to me), 4 (I am embarrassed by tremor when I eat or drink in public), and 14 (I sometimes try to hide my tremor) were the most commonly endorsed, and seven of the items were endorsed by ≥50% of patients. Our sample was clinic-based rather than population-based. A major difference between the two is that population-dwelling cases tend to have milder tremor and hence, possibly less embarrassment. A subsample of 23 (30.7%) of our ET cases had tremor on Archimedes spiral that was mild in both their right and left arms and, in this respect, they more closely approximated a population-based sample of ET cases. A sub-analysis of these mild ET cases indicated that five items were endorsed by ≥50% of patients, with each of these five items also endorsed by the larger group of 75 patients.
Table 2.
ETEA scores in 75 ET patients
| Item | ETEA A | ETEA B | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| 1 | 54 (72%) Agree | 21 | 3 | 9 | 12 | 10 | 20 |
| 2 | 22 (29%) Agree | 31 | 1 | 3 | 3 | 5 | 10 |
| 3 | 41 (55%) Agree | 33 | 5 | 3 | 8 | 11 | 14 |
| 4 | 49 (65%) Agree | 26 | 5 | 3 | 11 | 4 | 26 |
| 5 | 43 (57%) Agree | 32 | 2 | 6 | 10 | 7 | 18 |
| 6 | 42 (56%) Agree | 33 | 3 | 9 | 11 | 4 | 15 |
| 7 | 30 (40%) Agree | 38 | 4 | 4 | 3 | 3 | 16 |
| 8 | 37 (49%) Agree | 35 | 5 | 6 | 13 | 2 | 11 |
| 9 | 42 (56%) Agree | 33 | 8 | 4 | 10 | 10 | 10 |
| 10 | 22 (29%) Agree | 53 | 3 | 2 | 2 | 5 | 10 |
| 11 | 10 (13%) Agree | 65 | 2 | 0 | 4 | 3 | 1 |
| 12 | 24 (32%) Agree | 51 | 7 | 3 | 5 | 5 | 4 |
| 13 | 31 (41%) Agree | 44 | 6 | 0 | 6 | 8 | 11 |
| 14 | 46 (61%) Agree | 27 | 6 | 9 | 7 | 6 | 18 |
Both the ETEA A score and B scores were normally distributed (respective Kolmogorov-Smirnov test z scores = 1.08 [p = 0.20] and 1.04 (p = 0.23]). The mean ETEA A score = 6.6 ± 4.7 (median = 7.0, range = 0 – 14) and the mean ETEA B score = 23.3 ± 19.9 (median = 19.0, range = 0 – 69). The ETEA A and ETEA B scores were correlated with one another (Pearson’s r = 0.91, p <0.001). Both ETEA A and B met criteria for acceptability: for ETEA A, the difference between the mean and median was 0.4 points (i.e., 2.9% of the maximum possible score of 14); for ETEA B, the difference (mean – median) was 4.3 points (i.e., 6.1% of the maximum possible score of 70). Floor effects were as follows: ETEA A = 20.0%, ETEA B = 20.0%. Ceiling effects were: ETEA A = 4.0%, ETEA B = 0.0%.
Test-retest reliability was assessed in 50 patients over a broad age range (28 – 91 years, mean = 72.6 ± 12.6 years) whose tremor duration (range = 3 – 72 years, mean = 27.8 ± 17.1 years), tremor severity scores (range = 3 – 8, mean = 7.3 ± 2.4) and CES-D scores (range = 0 – 27, mean = 9.1 ± 7.8) were similar to the larger group of 75 patients. Test-retest reliability for the ETEA A and B scores was high (A = 0.84 [p < 0.001) and B = 0.83 [p < 0.001], Table 3). For the large majority of individual items (11 of 14 items on the ETEA A and 13 of 14 items on the ETEA B), reliability was in the good range (0.60 – 0.79) or very good range (≥0.80) (Table 3).
Table 3.
Test-retest reliability of the ETEA
| Item | ETEA A (kappa statistic) | ETEA B (intraclass correlation coefficient) |
|---|---|---|
| 1 | 0.88 (very good agreement) | 0.77 (good agreement) |
| 2 | 0.79 (good agreement) | 0.76 (good agreement) |
| 3 | 0.69 (good agreement) | 0.82 (very good agreement) |
| 4 | 0.59 (moderate agreement) | 0.74 (good agreement) |
| 5 | 0.86 (very good agreement) | 0.73 (good agreement) |
| 6 | 0.62 (good agreement) | 0.63 (good agreement) |
| 7 | 0.67 (good agreement) | 0.68 (good agreement) |
| 8 | 0.69 (good agreement) | 0.73 (good agreement) |
| 9 | 0.72 (good agreement) | 0.68 (good agreement) |
| 10 | 0.67 (good agreement) | 0.78 (good agreement) |
| 11 | 0.43 (moderate agreement) | 0.70 (good agreement) |
| 12 | 0.57 (moderate agreement) | 0.57 (moderate agreement) |
| 13 | 0.72 (good agreement) | 0.70 (good agreement) |
| 14 | 0.74 (good agreement) | 0.87 (very good agreement) |
Guidelines for levels of agreement are as follows: 0.20 –0.39 (fair agreement), 0.40 – 0.59 (moderate agreement), 0.60 – 0.79 (good agreement), and 0.8 (very good agreement) [16].
Factor analysis identified four factors, explaining 62.4% of the variance. The four factors were ETEA questions: (I) 1, 4, 5, 6, and 9, (II) 10, 11, 12, and 13 (III) 7 and 8, (IV) 2 and 3. Factor I explained far more variance than any other factor (32.4% compared to 11.3%, 10.7%, and 8.1% for each of the remaining factors). A number of the items in Factor I dealt with performance issues (eating, drinking, writing clearly) whereas all of the items in Factor II dealt with the issue of social isolation (e.g., people are hesitant to spend time with me, people treat me differently). For the 5 items that comprised factor I, high internal consistency was found (Cronbach’s alpha = 0.85). For factor II, comprised of 4 items, Cronbach’s alpha was 0.74. The remaining factors were only composed of two items.
In terms of convergent validity, the ETEA scores correlated with the CES-D scores (correlation coefficients = 0.45 – 0.53, which are in the moderate to strong range) and with the TDQ score (correlation coefficients = 0.26 and 0.28, which are both in the weak range) (Table 4). Younger age was associated with greater embarrassment, with correlation coefficients (r = −0.39) in the moderate range (Table 4). The correlation with tremor severity was in the appropriate direction (i.e., greater embarrassment with greater tremor severity) but not significant and within the negligible range (Table 4). Among the 60 ET cases with an ETEA A score >0 (i.e., among the patients who endorsed embarrassment), both the ETEA A score and the ETEA B score correlated more robustly with the TDQ score (for ETEA A score, correlation coefficient = 0.40, which is in the moderate range; for ETEA B score, correlation coefficient = 0.41, which is in the moderate range). Among our sub-sample of 23 milder ET cases, results were similar (e.g., correlations between ETEA A score and CES-D score = 0.67 [p < 0.001], ETEA A score and TDQ score = 0.53 [p = 0.009], and ETEA A score and tremor severity score = 0.11 [p = 0.62]).
Table 4.
Correlation of ETEA to TDQ, CES-D, tremor severity score, and age
| ETEA A score | ETEA B score | |||
|---|---|---|---|---|
| Correlation Coefficient | Significance | Correlation Coefficient | Significance | |
| TDQ | 0.26 | 0.02 | 0.28 | 0.01 |
| CES-D | 0.45 | <0.001 | 0.53 | <0.001 |
| Tremor severity score | 0.16 | 0.19 | 0.18 | 0.13 |
| Age | −0.39 | 0.001 | −0.39 | 0.001 |
Discussion
Embarrassment is a common problem in patients with ET, a motivator for health-care seeking behavior [8] and a potentially useful outcome measure in clinical practice and therapeutic trials. Because there was no formal tool to assess embarrassment in ET, we set out to develop such a tool and to assess its clinimetric properties.
Prior studies have measured internalized stigma in other medical and neurological illnesses [13–15], but, to our knowledge, no prior scale has been developed to assess embarrassment either in ET or in another disease.
To develop the scale, initial surveys were completed with movement disorders specialists and ET patients. Although, on average, tremor specialists reported that three-quarters (75%) of their patients experienced embarrassment, there was very little agreement, with answers ranging widely from nearly no patients (10%) to nearly all patients (95%). Of course, the mix of patients sampled by these practitioners could have been different, but most were specialists at tertiary referral centers, so this is unlikely. A more likely explanation is that embarrassment is not routinely assessed using a systematic instrument.
Interviews with patients indicated that embarrassment was indeed common and was a daily experience among one-in-three cases. Despite many commonalities, the experts and the patients did not demonstrate complete overlap in their responses and, at least for some items, there was a sizable difference (e.g., tremor draws attention from strangers). Obtaining a routine and structured assessment of embarrassment in ET patients will likely enhance the clinical dialogue and the transfer of relevant information from patient to physician.
ETEA scores correlated most robustly with depressive symptoms, which may reflect the psychosocial nature of both embarrassment and depression in ET patients. It also correlated with tremor disability scores, but to a lesser extent. The correlation with tremor severity was in the correct direction but negligible in terms of magnitude. These analyses also indicated that there is an element of embarrassment that is independent of tremor severity and more reflective of the patient’s psychosocial state.
In terms of its clinimetric properties, the ETEA demonstrated reasonable acceptability (i.e., small differences between mean and median values [i.e., ≤10% of the maximum possible score] [12], small ceiling effects [well under the maximum acceptable limit of 15%] [12], and modest floor effects [slightly higher than the 15% limit][12]). For the total score, test-retest reliability was high with correlation coefficients in excess of 0.8. Cronbach’s alpha within the two major domains (Factors I and II) was similarly high. The correlations with other scales indicate variable convergent validity with pre-specified correlations of interest [12], with the strongest correlation being between the ETEA and depressive symptoms.
A strength of this study was our survey both of ET experts and ET patients to develop the ETEA. A limitation is that the scale was validated in ET patients receiving treatment in a movement disorders center, which increases the likelihood that patients had severe tremor and, perhaps, more marked embarrassment. This being said, the subsample of ET patients who were the subject of the validation study did include a broad range of tremor severities and durations. We performed a sub-sample analysis of the 23 (30.7%) ET cases who had mild tremor, and the results of these analyses were similar to the results for the entire sample of 75 cases. Yet it is important to emphasize that this initial study of the ETEA is an early one and it would be useful to assess the utility of this instrument in population-based settings as well its application to other types of tremor aside from ET.
Future research should examine the ETEA in a larger cohort of ET patients, consider whether this scale could be useful in tracking response to medications or surgery in ET, or whether it might be adapted and applicable to other movement disorders or other outwardly visible neurological diseases.
Acknowledgments
Financial disclosures: Dr. Louis has received research support from the NIH [NINDS #R01 NS42859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R56 NS042859 (principal investigator), NINDS #T32 NS07153-24 (principal investigator), NIA #2P01 AG0027232-16 (principal investigator), and NINDS #R01 NS36630 (co-Investigator)], the Parkinson’s Disease Foundation (principal investigator), the Arlene Bronstein Essential Tremor Research Fund (Columbia University) and the Claire O’Neil Essential Tremor Research Fund (Columbia University). Rebecca Traub, Marina Gerbin and Mary Mullaney have not received any support.
The following people participated as neurologists who are experts in ET, through completion of email surveys: Peter Bain, Imperial College London; Julian Benito-Leon, Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED); Felix Bermejo-Pareja, Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED); Kailash Bhatia, University College London, Institute of Neurology; Susan Bressman, Mirken Department of Neurology, Beth Israel Medical Center; John Caviness, Parkinson’s Disease and Movement Disorders Center, Mayo Clinic; Guenther Deuschl, Department of Neurology, Christian-Albrechts-University Kiel; Okan Dogu, Department of Neurology, Mersin University; Rodger Elble, Southern Illinois University School of Medicine; Samuel Ellias, Department of Neurology, Boston University Medical Center; Stanley Fahn, Neurological Institute, Columbia University; Leslie Findley, The Essex Neurosciences Unit, Queens Hospital; Steven Frucht, Neurological Institute, Columbia University; Alexandre Gironell, Department of Neurology, Sant Pau Hospital, Autonomous University of Barcelona; Deborah Hall, Department of Neurology, School of Medicine, University of Colorado Denver; Mark Hallett, Human Motor Control Section, NINDS NIH; Adrian Handforth, Department of Neurology, Veterans Affairs Greater Los Angeles Healthcare System; Peter Hedera, Department of Neurology, Vanderbilt University; Jean Hubble, Boehringer Ingelheim; Rivka Inzelberg, Department of Neurology, The Sagol Neuroscience Center, Sheba Medical Center; Joseph Jankovic, Parkinson’s Disease Centre and Movement Disorders Clinic, Department of Neurology, Baylor College of Medicine; Felix Jimenez-Jimenez, Medicine-Neurology, Hospital “Príncipe de Asturias”, Universidad de Alcalá; Amos Korczyn, Sackler School of Medicine, Tel Aviv University; Peter LeWitt, Department of Neurology, Henry Ford Hospital; Delia Lorenz, Klinik für Neurologie, Universitätsklinikum Schleswig-Holstein, Campus Kiel, Christian-Albrechts-Universität Kiel; Elan Louis, Neurological Institute, Columbia University; Kelly Lyons, Departments of Neurology, Kansas City, University of Kansas School of Medicine; Joseph Matsumoto, Department of Neurology, Mayo Clinic College of Medicine; William Ondo, Parkinson’s Disease Center and Movement Disorders Clinic, Department of Neurology, Baylor College of Medicine; Rajesh Pahwa, University of Kansas Medical Center; Seth Pullman, Neurological Institute, Columbia University; Alex Rajput, Division of Neurology, University of Saskatchewan and Saskatoon Health Region; Ali Rajput, Department of Medicine, Saskatoon Health Region, University of Saskatchewan; Annette Schrag, Department of Clinical Neurosciences, University College; Kapil Sethi, The Movement Disorder Clinic, Medical College of Georgia; Holly Shill, Sun Health Research Institute; Mark Stacy, Division of Neurology, Duke University; Matthew Stern, Department of Neurology, University of Pennsylvania; E-K Tan, Department of Neurology, Singapore General Hospital; Louis Tan, Parkinson’s Disease and Movement Disorders Centre, Department of Neurology, National Neuroscience Institute, Singapore; Caroline Tanner, Department of Clinical Research, The Parkinson’s Institute; Daniel Tarsy, Department of Neurology, Beth Israel Deaconess Medical Center; Claudia Testa, Emory Department of Neurology and Center for Neurodegenerative Diseases; Ron Tintner, Parkinson’s Disease Center and Movement Disorders Clinic, Department of Neurology, Baylor College of Medicine; Eduardo Tolosa, Neurology Service, Institut Clínic de Neurociències, Hospital Clínic Universitari; Ryan Uitti, Department of Neurology, Mayo Clinic, Jacksonville; Theresa Zesiewicz, Department of Neurology, Parkinson’s Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13:5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- 2.Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18:389–94. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- 3.Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, et al. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey. Neurology. 2003;61:1804–6. doi: 10.1212/01.wnl.0000099075.19951.8c. [DOI] [PubMed] [Google Scholar]
- 4.Tan LC, Venketasubramanian N, Ramasamy V, Gao W, Saw SM. Prevalence of essential tremor in Singapore: a study on three races in an Asian country. Parkinsonism Relat Disord. 2005;11:233–9. doi: 10.1016/j.parkreldis.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz D, Schwieger D, Moises H, Deuschl G. Quality of life and personality in essential tremor patients. Mov Disord. 2006;21:1114–8. doi: 10.1002/mds.20884. [DOI] [PubMed] [Google Scholar]
- 6.Louis ED, Barnes L, Albert SM, Cote L, Schneier FR, Pullman SL, et al. Correlates of functional disability in essential tremor. Mov Disord. 2001;16:914–20. doi: 10.1002/mds.1184. [DOI] [PubMed] [Google Scholar]
- 7.Bain PG, Mally J, Gresty M, Findley LJ. Assessing the impact of essential tremor on upper limb function. J Neurol. 1993;241:54–61. doi: 10.1007/BF00870673. [DOI] [PubMed] [Google Scholar]
- 8.Louis ED, Rios E. Embarrassment in essential tremor: Prevalence, clinical correlates and therapeutic implications. Parkinsonism Relat Disord. 2008;15:535–8. doi: 10.1016/j.parkreldis.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis ED, Barnes L, Wendt KJ, et al. A teaching videotape for the assessment of essential tremor. Mov Disord. 2001;16:89–93. doi: 10.1002/1531-8257(200101)16:1<89::aid-mds1001>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED, Barnes LF, Wendt KJ, Albert SM, Pullman SL, Yu Q, et al. Validity and test-retest reliability of a disability questionnaire for essential tremor. Mov Disord. 2000;15:516–23. doi: 10.1002/1531-8257(200005)15:3<516::AID-MDS1015>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 12.Martinez-Martin P, Jimenez-Jimenez FJ, Carroza Garcia E, Alonso-Navarro H, Rubio L, Calleja P, et al. Most of the Quality of Life in Essential Tremor Questionnaire (QUEST) psychometric properties resulted in satisfactory values. J Clin Epidemiol. 2010;63:767–73. doi: 10.1016/j.jclinepi.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Lee SA, Yoo HJ, Lee BI. Factors contributing to the stigma of epilepsy. Seizure. 2005;14:157–63. doi: 10.1016/j.seizure.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin DP, Pachana NA, McFarland K. Stigma, seizure frequency and quality of life: the impact of epilepsy in late adulthood. Seizure. 2008;17:281–287. doi: 10.1016/j.seizure.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Van Brakel WH. Measuring health-related stigma--a literature review. Psychol Health Med. 2006;11:307–34. doi: 10.1080/13548500600595160. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG. Practical statistics for medical research. London, UK: Chapman & Hall; 1991. [Google Scholar]