Abstract
Cochlear implants (CI) are commonly used to treat deafness in young children. While many factors influence the ability of a deaf child who is hearing through a CI to develop speech and language skills, an important factor is that the CI has to stimulate the auditory cortex. Obtaining behavioral measurements from young children with CIs can often be unreliable. While a variety of noninvasive techniques can be used for detecting cortical activity in response to auditory stimuli, many have critical limitations when applied to the pediatric CI population. We tested the ability of near-infrared spectroscopy (NIRS) to detect cortical responses to speech stimuli in pediatric CI users. Neuronal activity leads to changes in blood oxy- and de-oxyhemoglobin concentrations that can be detected by measuring the transmission of near-infrared light through the tissue. To verify the efficacy of NIRS, we first compared auditory cortex responses measured with NIRS and fMRI in normal-hearing adults. We then examined four different participant cohorts with NIRS alone. Speech-evoked cortical activity was observed in 100% of normal-hearing adults (11 of 11), 82% of normal-hearing children (9 of 11), 78% of deaf children who have used a CI >4 months (28 of 36), and 78% of deaf children who completed NIRS testing on the day of CI initial activation (7 of 9). Therefore, NIRS can measure cortical responses in pediatric CI users, and has the potential to be a powerful adjunct to current CI assessment tools.
Keywords: Cochlear implant, NIRS, fMRI, hearing, brain, auditory cortex, speech detection
Introduction
The development of communication and language skills during early childhood is greatly dependent upon hearing. Deafness is the fourth most common developmental disorder and the most common sensory disorder (Bhasin et al., 2006). Although deaf children can and do learn to use sign language to communicate, cochlear implantation (CI) has become the most common treatment. Many factors can impact long-term language outcomes after cochlear implantation (Miyamoto et al., 1994), but one critical issue is the ability of the CI to accurately convey the sound information within speech to the auditory nerve. If the appropriate information reaches auditory cortex, then the child has the best chance of learning normal speech and language. While many children can gradually catch up to their peers, many do not. Therefore, we sought to develop a brain-based measure as a supplement to existing techniques of measuring CI function.
A variety of noninvasive techniques can be used for detecting neural activity in response to auditory stimuli, but have critical limitations when applied to the pediatric CI population (Witte et al., 2003). While fMRI is the most common method for measuring human brain function (Friston, 2009), the ferromagnetic components of modern CIs are incompatible with the high magnetic fields generated by the MR scanner. Electroencephalography (EEG) can be used to identify cortically-generated event-related potentials. However, even though techniques have been developed to minimize artifacts in the EEG signal caused by CIs (Debener et al., 2008), when testing CI users the auditory stimuli are generally limited to short sounds, such as tone pips or clicks, to minimize artifacts due to the electrical current produced by the CI (Gilley et al., 2006). Thus, it is difficult to measure the cortical response to language, the stimulus category of greatest interest, with EEG. Positron emission tomography (PET) involves the use of ionizing radiation, which is not ideal for testing children or for repeated use. Finally, magnetoencephalography (MEG) techniques are limited by the magnetic fields associated with the CI device.
Near-infrared spectroscopy (NIRS) is a non-invasive neuroimaging technique used in both animal and human research that presents an alternative means of recording speech-evoked neural activity in CI users. NIRS assesses cerebral hemodynamics based on changes in the transmission of low power near-infrared light directed through the scalp and skull and into the surface of the brain (Abdelnour et al., 2009; Dehghani et al., 2003; Huppert et al., 2009a; Huppert et al., 2006). Given the differential absorption of specific wavelengths of near-infrared light by oxygenated hemoglobin (HbO), and deoxygenated hemoglobin (HbR), the changes in concentration of these chromophores can be determined by measuring changes in the amount of light transmitted across time. Changes in optical density are recorded and converted to relative concentrations of oxy-hemoglobin and deoxyhemoglobin. Due to the low absorbance of these wavelengths by biological tissue, the cerebral cortex can be imaged (Huppert et al., 2006; Okada et al., 2003). Additionally, NIRS can localize responses to within 1 to 2 cm of the area activated (Boas et al., 2004; Taga et al., 2003), providing sufficient spatial resolution to measure evoked responses within the cortical regions of interest. Because the NIRS equipment is quiet and the technique can be used in unsedated participants, it is ideal for auditory studies in awake, behaving pediatric CI users. There are no known risks of NIRS, and because it is not affected by CI-generated electrical signals it can be used with relatively long samples of speech. Therefore, NIRS has many appealing qualities for use in assessing cortical responses to speech in people of all ages who use cochlear implants.
Our recent work has shown that NIRS can detect significant hemodynamic responses to verbal stimuli in the receptive language center of the auditory cortex in normal-hearing, awake, and cooperative infants (Bortfeld et al., 2007; Bortfeld et al., 2009). In the present study, we sought to directly test the ability of NIRS to measure speech-evoked cortical responses within pediatric CI users. We first compared cortical measurements of speech-evoked activity obtained with fMRI and NIRS from normal-hearing adults in order to validate our experimental paradigm. We then used NIRS to measure speech-evoked activity in the auditory cortices of normal-hearing children and children hearing through CIs.
Methods
Participants
We studied four different cohorts: normal-hearing adults, normal-hearing children (≤19 years of age), deaf children who had >4 months experience hearing through a cochlear implant prior to testing, and deaf children who were tested on the day of CI initial activation (Table 1). The inclusion criteria were that the participants and their parents agreed to attempt testing and that they had exposure to spoken English language on a daily basis (i.e. at home and/or in school). Exclusion criteria included a lack of daily exposure to spoken English language. This study was approved by the Baylor College of Medicine IRB and all participants or their parent/guardian were consented by one of the authors prior to participation. Prior to performing the experiments, normal-hearing participants (both adults and children) passed a hearing screen demonstrating that they had auditory thresholds better than 20 dB HL at 250, 500, 1000, 2000, and 4000 Hz. CIs from all three FDA-approved brands (Cochlear, Med-El, and Advanced Bionics) were represented in the sample of children tested.
Table 1. NIRS Speech-Evoked Responses.
| Normal-hearing adults (n=11/11) |
Normal-hearing children (n=11/12) |
Deaf children with > 4 mo. CI use (n=37/40) |
Deaf children at CI activation (n=9/13) |
|
|---|---|---|---|---|
| Significant HbO Cortical Response | 9 (82%) | 8 (73%) | 19 (51%) | 7 (78%) |
| Significant HbR Cortical Response | 11 (100%) | 8 (73%) | 26 (70%) | 5 (56%) |
| Significant HbO or HbR Cortical Response | 11 (100%) | 9 (82%) | 28 (76%) | 7 (78%) |
|
Age of Responders1 [Range] |
30.4 ± 8.3 [24 – 48] |
9.4 ± 3.4 [4 – 15] |
7.9 ± 3.8 [2 – 19] |
4.7 ± 1.6 [2 – 8] |
n=x/y: x is number of subjects who completed testing (allowing for data analysis); y is total number of subjects in which testing was attempted.
Values in parentheses represent the percentage of subjects who demonstrated a response out of those who completed testing.
Mean ± S.D. in years
Stimuli
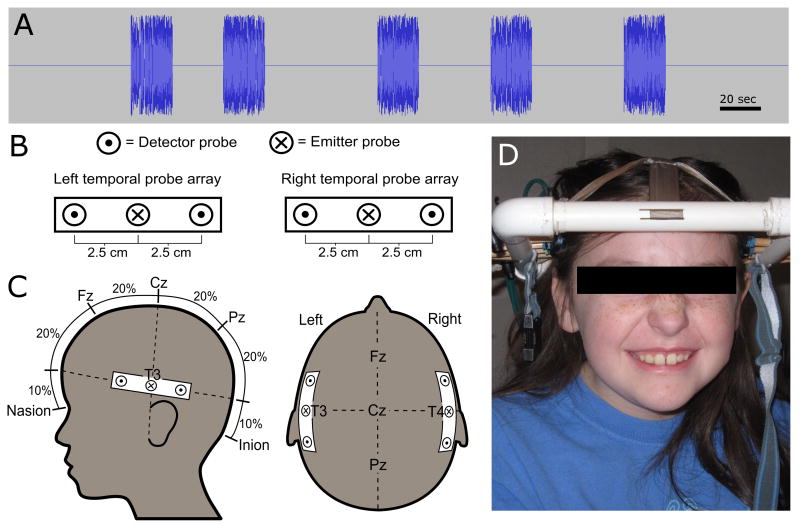
The acoustic speech stimuli consisted of digital recordings of a highly animated female voice reading from children's stories in English. The recordings were digitally edited into 20 second speech segments, each consisting of a single vignette from a story. The segments were interspersed with periods of silence that ranged from 25-55 seconds between them, in order to introduce periodic variations in cortical activity (Fig. 1A). The stimulus presentation protocols were similar for the NIRS and the fMRI measurement techniques.
Fig. 1. Experimental paradigm.
(A) The acoustic speech stimulus had five 20-second blocks of speech with periods of silence (ranging from 25-55) seconds between them. (B) Probe layout with emitter probe (X) and two detector probes (dot) 2.5 cm on either side. (C) Localization of the probes near T3 and T4 permits simultaneous measurements of the left and right auditory cortex. (D) A deaf child preparing to undergo NIRS measurement of cortical activity during a cochlear implant clinic visit.
The stimulus intensity was calibrated by measuring the peak amplitudes of the speech segments using a standard booth calibration microphone. For normal-hearing participants (adults and children), we used a peak stimulus intensity of 50 dB HL, which is 20 dB louder than a normal-hearing speech reception threshold (SRT) of ∼30 dB HL. For CI users, we used a peak stimulus intensity that was 20 dB above each participant's most recent SRT or speech awareness threshold (SAT) as established by their audiologist. Because the loudness settings for each CI user's program is gradually increased over a period of months as they get used to using the device, the stimulus intensities included in these experiments ranged from 30-80 dB.
In order to help maintain participant arousal and minimize head movement artifact, participants continuously viewed a silent video unrelated to the auditory stimulus. The video contained scenes of animals in their native habitats, without any speech or language cues.
fMRI measurements
Blood-oxygen level dependent functional magnetic resonance imaging (BOLD fMRI) was performed on three normal-hearing adults. NIRS testing was conducted on the same day in the same participants using the same stimuli as used for fMRI to allow comparison between the techniques. Anatomical MRI scans were obtained from each participant using a 3-Tesla whole-body MR scanner and 8-channel receiver head coil (Phillips Medical Systems, Bothell, WA) (Beauchamp et al., 2008). Images were collected using a magnetization-prepared 180 degree radio-frequency pulses and rapid gradient-echo (MP-RAGE) sequence optimized for gray-white matter contrast with 1 mm thick sagittal slices and an in-plane resolution of 0.938 × 0.938 mm. AFNI software (Cox, 1996) was used to analyze MRI data. 3D cortical surface models were created with FreeSurfer (Fischl et al., 1999) and visualized in SUMA (Argall et al., 2006). Functional images were collected using a gradient-recalled-echo echo-planar-imaging sequence sensitive to the BOLD signal. Thirty-three axial slices were collected with a repetition time (TR) of 2000 ms, an echo time (TE) of 30 ms and a flip angle of 90 degrees. Slice thickness was 3 mm and in-plane resolution was 2.75 mm × 2.75 mm. Each scan series contained 150 scans.
Following motion correction and slice timing correction, data were smoothed with a spatial Gaussian filter with root-mean-square deviation of 3 mm. Then, the time series data were analyzed with the general linear model using the periodic variation in the auditory stimulus as the regressor of interest; the motion correction estimates were used as regressors of no interest. A region of interest was created from all contiguous voxels showing significant (p < 10-6) activity in the superior temporal gyrus, the location of the primary auditory cortex and auditory association areas (Patterson et al., 2008; Upadhyay et al., 2008) (Fig. 2A-C).
Fig. 2. NIRS concept.
(A) Partially-inflated cortical surface models of the right and left hemisphere in a normal-hearing adult while listening to our speech stimulus showing significant BOLD fMRI activity in the superior temporal gyrus. (B) The NIRS probe locations (red arrows, emitters; green arrows, detectors) and a schematic of light transmission through the tissues are shown overlying an axial fMRI image of the same subject in (A). (C) fMRI measurements in a normal-hearing adult demonstrate a response within the left auditory cortex in response to our speech stimulus with NIRS probe layout overlay (X=laser in; O=laser out). (D) Model of NIRS sensitivity and coverage of the auditory cortex in this subject using our probe layout (X=laser in; O=laser out). The color scale shows the theoretical sensitivity (Log10 scale) of the optical measurement to hemoglobin changes at the cortical surface based on a finite element model of light propagation following the diffusion approximation in the head obtained from segmentation of the MRI anatomical images.
NIRS testing protocol
All NIRS testing was performed in a sound proof booth equipped with standard audiology equipment. Participants were seated in a chair or in a parent's lap in front of a television. The animal video was played silently throughout the session. The auditory speech stimuli were presented in a sound field through speakers centered directly in front of the participant. If a participant wished to take a break, cried, fell asleep for at least 30 seconds, or moved so much that the probes affected data collection, the session was terminated, and that participant's data were not included in the study. A typical NIRS testing session took ∼20 minutes to complete.
NIRS hardware
Testing was conducted using a four channel NIRS 2CE machine (TechEn, Inc., Milford, MA), which emits near-infrared light through the scalp and detects the amount returned. This machine contains two 690 nm and two 830 nm laser diodes. We used a power setting of ∼12 mW for the 690 nm light and ∼6 mW for the 830 nm light (measured at the scalp probe). Fiber optic cables coupled the light from one 690 nm and one 830 nm diode into a single emitter probe. Thus, there were two distinct emitter probes, each with both wavelengths of light. The transmitted light was returned to the NIRS machine via fiber-optic cables, filtered to separate the two different wavelengths, detected with photodiodes, and then digitized. Simultaneously, the voltage waveform of the auditory stimulus was recorded by the NIRS machine to synchronize the stimulus and the NIRS data.
In order to measure auditory responses, we developed a custom head frame to hold the emitters and detectors against the scalp while adjusting to accommodate the range of head sizes of the participants without disturbing the CI hardware external to the ear (Fig. 1B-D). One emitter was directed to each side of the head; each was located halfway between two detectors placed 2.5 cm anterior and posterior to each emitter. The two emitter probes were placed against the scalp at the T3 and T4 positions based on the International 10/20 system (Niedermeyer et al., 2004). A model of the coverage of our NIRS probes, based upon our earlier work (Dehghani et al., 2003; Huppert et al., 2006), predicted excellent coverage of a large swath of the temporal lobe and superior temporal gyrus (Fig. 2D).
NIRS data analysis
NIRS data were processed using HOMER software (Huppert et al., 2009b) and custom software written in MATLAB (version R2008b, The Mathworks, Natick, Massachusetts). This involved converting the transmission efficiency for each of the two wavelengths of light to oxygenated and deoxygenated hemoglobin levels (HbO and HbR, respectively) using the modified Beer-Lambert law (Cope et al., 1988; Kocsis L, 2006). Bandpass filtering of the data from 0.008 Hz to 0.1 Hz was then performed to reduce artifacts from participant motion, signal drift, pulse, respiration, and blood pressure changes. The full tracing was analyzed to determine the response from each participant to the speech stimulus. Wavelets were extracted to calculate average responses for individuals and cohort grand averages.
We analyzed both the HbO and HBR responses obtained from the two light wavelengths by comparing the measured responses to a predicted responses consisting of a gamma-variate function convolved with the stimulus timing (see Figure 3A for a sample predicted response). This procedure is similar to analyses we have previously used for fMRI (Beauchamp, 2005; Beauchamp et al., 2007; Beauchamp et al., 2004). Linear regression was used to find the best-fit between the predicted and measured HbO and HbR responses. The significance and beta-weight, β, returned by the regression was used to classify responses as statistically-significant activity if they exceeded a significance threshold of p < 10-5 and a magnitude β >0.1 μM. All figures show both HbO and HbR responses.
Fig. 3. Neuroimaging of a representative normal-hearing adult.
(A) Predicted fMRI BOLD response for the 20-second stimulus. (B) Average of the BOLD responses within the superior temporal gyrus across five trials in the same representative adult participant from Fig. 2A-C. The shaded box indicates the stimulus duration. (C) Normalized BOLD responses measured with fMRI during the five trials. (D) Predicted HbO response for the 20-second stimulus. (E) Average of the five normalized HbO responses measured with NIRS from the left and right hemispheres. (F) Raw HbO data tracings. (G) Predicted HbR response for the 20-second stimulus. (H) Average of the five normalized HbR responses measured with NIRS. (I) Raw HbR data tracings.
Results
Speech-evoked cortical responses in adults
Three normal-hearing adult participants underwent fMRI and NIRS testing using the same speech stimuli on the same day. All three showed significant bilateral BOLD fMRI responses within the superior temporal gyrus, a cortical region identified in previous neuroimaging studies as relevant to processing fluent speech (Belin et al., 2000; Coez et al., 2008; Giraud et al., 2000; Mortensen et al., 2006; Wong et al., 1999; Wong et al., 2002). Spatial localization data (Fig. 2A-C) and time series data (Fig. 3A-C) of the BOLD responses from one representative participant are presented. For all three participants, the cortical responses measured using NIRS were similar. The NIRS measurements from the same representative participant are presented in Fig. 3D-I. Signal drift, often related to subject movement, was sometimes noted (for example, see the large variations within the blue tracing in the first 20 seconds of recording in Fig. 3I).
Further NIRS data (without fMRI) was collected in an additional eight normal-hearing adults. Significant cortical responses in either HbO or HbR were detected in all of the 11 adults (Table 1). In general, the HbO response was larger than the HbR response (Fig. 4B&G), consistent with previous research (Lloyd-Fox et al., 2010).
Fig. 4. Cortical responses of representative participants measured with NIRS.
(A&F) Predicted NIRS responses. (B-E) Average of the five HbO, and (G-J) HbR speech-evoked cortical responses measured using NIRS from representative individuals in four cohorts. Significance (p-values) are given at the bottom of each figure. (B&G) A normal-hearing adult, (C&H) a normal-hearing child, (D&I) a deaf child who has used a cochlear implant for 5 months, and (E&J) a deaf child hearing through a cochlear implant on the day of initial activation. Note that the left side (blue tracings) was not significant for this child in either HbO or HbR.
Speech-evoked cortical responses in children
We then attempted to use NIRS to detect cortical responses to speech stimuli in normal-hearing children, deaf children who had been using a CI for >4 months, and deaf children on the day of initial activation of the CI. In general, most children tolerated the testing procedures (Table 1). Common reasons for not completing the testing were patient movement, inconsolable crying, and drowsiness. Nevertheless, even in the most difficult category of children to test, those that just had CI surgery 4 weeks previously and were being activated that day, 9/13 (69%) permitted completion of the entire test. This indicates that our experimental testing procedures were generally appropriate for this subject population.
We then analyzed data from participants who completed testing to determine what proportion of them demonstrated measurable cortical responses with NIRS. We found that significant cortical responses to the speech stimuli were detectable with NIRS in all groups of children (Fig. 4 and Table 1). The percentages of children in which a significant response was detected in either HbO or HbR were similar among the cohorts of children but lower than adults (76-82% for children vs. 100% for adults). While the most commonly-detected cortical response pattern was bilateral (Table 2), some subjects only demonstrated statistically significant responses on one side. An example is shown in Fig 4E&J, in which there was a significant response on the right side (red trace) but not on the left side (blue trace). Most of the children that were CI users >4 months only had a single side implanted. The most commonly-detectable response pattern for children hearing through a CI was bilateral HbO and HbR responses. This pattern was generally similar for deaf children on the day of their CI activation although there were equal numbers who demonstrated only an ipsilateral response. Together, these data indicate that NIRS can be used to measure cortical responses in children hearing through a CI.
Table 2. NIRS Responder Laterality.
| Normal-hearing adults | Normal-hearing children | Deaf children with > 4 mo. CI | Deaf children at CI activation | |
|---|---|---|---|---|
| Side of CI1 | - | - | L:8 (22%) | L:1 (14%) |
| R:22 (59%) | R:6 (86%) | |||
| B:7 (19%) | B:0 (0%) | |||
| HbO Response Laterality | ||||
| Right Only | 1 (11%) | 3 (38%) | 7 (37%) | 3 (43%) |
| Left Only | 3 (33%) | 2 (25%) | 4 (21%) | 1 (14%) |
| Bilateral | 5 (56%) | 3 (38%) | 8 (42%) | 3 (43%) |
| Contralateral Only | - | - | 6 (32%) | 1 (14%) |
| Ipsilateral Only | - | - | 5 (26%) | 3 (43%) |
| HbR Response Laterality | ||||
| Right Only | 3 (27%) | 2 (25%) | 7 (27%) | 2 (40%) |
| Left Only | 3 (27%) | 2 (25%) | 7 (27%) | 1 (20%) |
| Bilateral | 5 (45%) | 4 (50%) | 12 (46%) | 2 (40%) |
| Contralateral Only | - | - | 10 (38%) | 1 (20%) |
| Ipsilateral Only | - | - | 4 (15%) | 2 (40%) |
Values in parentheses are percentages of subjects who completed testing.
R: Right, L: Left, B: Bilateral; Subjects who had bilateral cochlear implants were only allowed to use one of them during these testing procedures. This was the one that had been in the longest.
Grand Averages
We extracted wavelets from all of the statistically-significant subjects, normalized them, and then averaged them to calculate cohort grand averages (Fig. 5). All cohorts demonstrated similar response shapes. There was a change in the signal after the stimulus started (increase for HbO and decrease for HbR) followed by a recovery of the signal back to baseline. While the predicted waveform and the fMRI data indicate that there should be a relatively steady level of the signal during the time of the stimulus, we rarely identified this feature. This could be due to the filtering algorithms applied to the NIRS data which removed constant signals. Nevertheless, the similarity in the shapes of the responses among the cohorts indicates similarities in the cortical hemodynamic responses in children hearing through a CI to normal-hearing adults and children, even at the time of CI activation.
Fig. 5. Cohort grand averages of cortical responses measured with NIRS.
These data were normalized and only wavelets from participants who demonstrated statistically-significant responses were included. (A-D) HbO and (E-H) HbR. (A&E) normal-hearing adults (n=11), (B&F) normal-hearing children (n=10), (C&G) deaf children who have used a cochlear implant for ≥4 months (n=31), and (D&H) deaf children hearing through a cochlear implant on the day of initial activation (n=10).
Discussion
This study describes a preliminary attempt to determine if the novel neuroimaging modality of NIRS could be useful in assessing auditory function in deaf children using CIs. Encouragingly, these data indicate that the measurement technique is feasible in this subject population and demonstrate that cortical responses can be measured. While this study did not address what features of the acoustic stimulus are the most effective drivers of the NIRS response (e.g. by comparing the NIRS response to speech vs. non-speech sounds), this study suggests that NIRS may be useful as a clinical tool in determining an individual child's cortical responses to sounds. Since a major goal for most CI candidates is to improve speech and language skills, it is the ability of NIRS to measure cortical responses to speech that may make it an important modality for this patient population.
An interesting observation that should be investigated in future studies is our finding that many responses in deaf children with a new CI appeared to be localized to the hemisphere ipsilateral to the CI, which was most often the right hemisphere (because most CIs were implanted in the right ear). This finding of right-sided activation is in contrast to the normal adult finding of left-hemisphere dominant language and suggests that auditory brain development in deaf children may be altered by the lack of normal sensory input (Neville et al., 2002). A caveat is that the low numbers of patients we have studied to date limit our ability to make any firm conclusions regarding this concept. As well, the laterality of the cortical response to language stimulation has been shown to be dependent upon the age (for review see (Holland et al., 2007)) and the listening task (Sharp et al., 2004).
To ensure the proper development of language function, it is critical that children receive the appropriate auditory input at a young age. Even though the CI can be placed in very young children (Lin et al., 2010; Oghalai et al., 2009), it can be difficult to perform the necessary behavioral testing to program the CI, especially if there are co-existent developmental delays which are often found in this patient population (Beauchamp et al.; Cristobal et al., 2008; Katzenstein et al., 2009; Kushalnagar et al., 2007; Pierson et al., 2007). Therefore, an objective method to measure brain activity would be give valuable additional information. NIRS is a useful technique in this regard and has some potential benefits compared with other imaging modalities. As well our data suggests that NIRS may be a useful complement to existing techniques such as fMRI, PET, ERP, and transcranial doppler sonography to study brain development in children with CIs (Gilley et al., 2008; Knecht et al., 1998; Kral et al., 2009; Ponton et al., 1996; Schmithorst et al., 2005; Sharma et al., 2009; Strelnikov et al., 2009; Thai-Van et al.).
The lower spatial resolution of NIRS means that is unlikely to replace fMRI as a research tool for understanding cortical function. Nevertheless, it has a large advantage over fMRI in that it can be easily used in subjects with CIs (Deneuve et al., 2008). Even in CIs designed to be “MRI-compatible”, functional imaging of the cortex ipsilateral to the CI is inhibited by artifact (Vincent et al., 2008; Witte et al., 2003). It should be noted, though, that fMRI has been used in subjects implanted with the Ineraid device, a discontinued model that does not require radiofrequency signal transmission or signal processing in proximity of the subject. These data with the Ineraid device have demonstrated activity in the primary auditory cortex, specifically near Heschl's gyrus (Berthezene et al., 1997; Lazeyras et al., 2002; Seghier et al., 2005), an area of the cortex that our modeling suggests is partially measured by our NIRS protocol. It is also possible to use fMRI to measure cortical function in deaf patients before the cochlear implant is placed, a method that may provide prognostic outcome information (Patel et al., 2007).
NIRS is not without its limitations. Not all children in our study permitted NIRS testing. As in all neuroimaging modalities involving awake subjects, motion artifacts are present. Unfortunately, motion artifacts are generally greater with younger children, a cohort of particular interest for CI studies. Nevertheless, about 70% of our children completed the testing procedures, a number consistent with previous studies (Lloyd-Fox et al., 2010). We found that among those who completed testing, neither the side of CI placement nor movement artifact appeared to be a limiting factor in the detection of cortical activity.
In the past decade, NIRS has become an important tool in research on the linguistic and cognitive capabilities of neonates and young infants. Research with preverbal infants has demonstrated the utility of NIRS for studying early speech perception (Bortfeld et al., 2007), visual processing sensitivities (Wilcox et al., 2008; Wilcox et al., 2009), and the emergence of language laterality (Bortfeld et al., 2009). Our initial investigation shows that NIRS may allow for the accurate assessment of the ability of a CI to successfully stimulate the auditory cortex. This supports the notion that NIRS neuroimaging could help guide post-implant programming and therapy in the service of improving deaf children's speech and language outcomes.
Acknowledgments
This research was funded by The Dana Foundation (to JSO and HB), NIH-NIDCD R01 DC010075 and R56 DC010164 (to JSO), NSF Cognitive Neuroscience Initiative Research Grant 0642532 and NIH R01 NS065395 (to MSB). AS was supported by NIH-NIDCD T32 DC007367.
Abbreviations
- CI
cochlear implant
- IT
impedance testing
- eCAP
electrically evoked compound action potential
- BA
behavioral audiometry
- EEG
electroencephalography
- MEG
magnetoencephalography
- BOLD
Blood-oxygen level dependent
- fMRI
functional magnetic resonance imaging
- PET
positron emission tomography
- SPECT
single positron emission computed tomography
- HbO
oxygenated hemoglobin
- HbR
deoxygenated hemoglobin
- HL
hearing level
- SRT
speech reception threshold
- SAT
speech awareness threshold
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelnour AF, Huppert T. Real-time imaging of human brain function by near-infrared spectroscopy using an adaptive general linear model. Neuroimage. 2009;46:133–43. doi: 10.1016/j.neuroimage.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argall BD, Saad ZS, Beauchamp MS. Simplified intersubject averaging on the cortical surface using SUMA. Hum Brain Mapp. 2006;27:14–27. doi: 10.1002/hbm.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS. Statistical criteria in FMRI studies of multisensory integration. Neuroinformatics. 2005;3:93–113. doi: 10.1385/NI:3:2:093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Pasalar S, Ro T. Neural substrates of reliability-weighted visual-tactile multisensory integration. Front Syst Neurosci. 2010;4:25. doi: 10.3389/fnsys.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Kishan N, Ro T. Human MST but not MT responds to tactile stimulation. J Neurosci. 2007;27:8261–7. doi: 10.1523/JNEUROSCI.0754-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Frye RE, Ro T. Touch, sound and vision in human superior temporal sulcus. Neuroimage. 2008;41:1011–20. doi: 10.1016/j.neuroimage.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Argall BD, Bodurka J, Duyn JH, Martin A. Unraveling multisensory integration: patchy organization within human STS multisensory cortex. Nature neuroscience. 2004;7:1190–2. doi: 10.1038/nn1333. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–12. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Berthezene Y, Truy E, Morgon A, Giard MH, Hermier M, Franconi JM, Froment JC. Auditory cortex activation in deaf subjects during cochlear electrical stimulation. Evaluation by functional magnetic resonance imaging. Invest Radiol. 1997;32:297–301. doi: 10.1097/00004424-199705000-00007. [DOI] [PubMed] [Google Scholar]
- Bhasin TK, Brocksen S, Avchen RN, Van Naarden Braun K. Prevalence of four developmental disabilities among children aged 8 years--Metropolitan Atlanta Developmental Disabilities Surveillance Program, 1996 and 2000. MMWR Surveill Summ. 2006;55:1–9. [PubMed] [Google Scholar]
- Boas DA, Dale AM, Franceschini MA. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage. 2004;23 1:S275–88. doi: 10.1016/j.neuroimage.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Bortfeld H, Wruck E, Boas DA. Assessing infants' cortical response to speech using near-infrared spectroscopy. Neuroimage. 2007;34:407–415. doi: 10.1016/j.neuroimage.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortfeld H, Fava E, Boas DA. Identifying cortical lateralization of speech processing in infants using near-infrared spectroscopy. Dev Neuropsychol. 2009;34:52–65. doi: 10.1080/87565640802564481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coez A, Zilbovicius M, Ferrary E, Bouccara D, Mosnier I, Ambert-Dahan E, Bizaguet E, Syrota A, Samson Y, Sterkers O. Cochlear implant benefits in deafness rehabilitation: PET study of temporal voice activations. J Nucl Med. 2008;49:60–7. doi: 10.2967/jnumed.107.044545. [DOI] [PubMed] [Google Scholar]
- Cope M, Delpy DT, Reynolds EO, Wray S, Wyatt J, van der Zee P. Methods of quantitating cerebral near infrared spectroscopy data. Advances in experimental medicine and biology. 1988;222:183–9. doi: 10.1007/978-1-4615-9510-6_21. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cristobal R, Oghalai JS. Hearing loss in children with very low birth weight: current review of epidemiology and pathophysiology. Arch Dis Child Fetal Neonatal Ed. 2008;93:F462–8. doi: 10.1136/adc.2007.124214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Hine J, Bleeck S, Eyles J. Source localization of auditory evoked potentials after cochlear implantation. Psychophysiology. 2008;45:20–4. doi: 10.1111/j.1469-8986.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- Dehghani H, Brooksby B, Vishwanath K, Pogue BW, Paulsen KD. The effects of internal refractive index variation in near-infrared optical tomography: a finite element modelling approach. Physics in medicine and biology. 2003;48:2713–27. doi: 10.1088/0031-9155/48/16/310. [DOI] [PubMed] [Google Scholar]
- Deneuve S, Loundon N, Leboulanger N, Rouillon I, Garabedian EN. Cochlear implant magnet displacement during magnetic resonance imaging. Otol Neurotol. 2008;29:789–90. doi: 10.1097/MAO.0b013e3181825695. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Modalities, modes, and models in functional neuroimaging. Science. 2009;326:399–403. doi: 10.1126/science.1174521. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman MF. Cortical reorganization in children with cochlear implants. Brain Res. 2008;1239:56–65. doi: 10.1016/j.brainres.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman M, Finley CC, Panch AS, Martin K. Minimization of cochlear implant stimulus artifact in cortical auditory evoked potentials. Clin Neurophysiol. 2006;117:1772–82. doi: 10.1016/j.clinph.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Truy E, Frackowiak RS, Gregoire MC, Pujol JF, Collet L. Differential recruitment of the speech processing system in healthy subjects and rehabilitated cochlear implant patients. Brain. 2000;123(Pt 7):1391–402. doi: 10.1093/brain/123.7.1391. [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema JM, Karunanayaka PR, Schmithorst VJ, Yuan W, Plante E, Byars AW. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46:533–51. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Allen MS, Diamond SG, Boas DA. Estimating cerebral oxygen metabolism from fMRI with a dynamic multicompartment Windkessel model. Hum Brain Mapp. 2009a;30:1548–67. doi: 10.1002/hbm.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt. 2009b;48:D280–98. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Hoge RD, Dale AM, Franceschini MA, Boas DA. Quantitative spatial comparison of diffuse optical imaging with blood oxygen level-dependent and arterial spin labeling-based functional magnetic resonance imaging. J Biomed Opt. 2006;11:064018. doi: 10.1117/1.2400910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenstein JM, Oghalai JS, Tonini R, Baker D, Haymond J, Caudle SE. Neurocognitive functioning of a child with partial trisomy 6 and monosomy 21. Neurocase. 2009;15:97–100. doi: 10.1080/13554790802631910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Ringelstein EB, Wirtz M, Lohmann H, Drager B, Huber T, Henningsen H. Reproducibility of functional transcranial Doppler sonography in determining hemispheric language lateralization. Stroke. 1998;29:1155–9. doi: 10.1161/01.str.29.6.1155. [DOI] [PubMed] [Google Scholar]
- Kocsis L, H P, Eke A. The modified Beer-Lambert law revisited. Phys Med Biol. 2006:N91–8. doi: 10.1088/0031-9155/51/5/N02. Epub. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Hubka P, Schiemann D, Heid S, Hartmann R, Engel AK. Spatiotemporal patterns of cortical activity with bilateral cochlear implants in congenital deafness. J Neurosci. 2009;29:811–27. doi: 10.1523/JNEUROSCI.2424-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushalnagar P, Krull K, Hannay J, Mehta P, Caudle S, Oghalai J. Intelligence, parental depression, and behavior adaptability in deaf children being considered for cochlear implantation. J Deaf Stud Deaf Educ. 2007;12:335–49. doi: 10.1093/deafed/enm006. [DOI] [PubMed] [Google Scholar]
- Lazeyras F, Boex C, Sigrist A, Seghier ML, Cosendai G, Terrier F, Pelizzone M. Functional MRI of auditory cortex activated by multisite electrical stimulation of the cochlea. Neuroimage. 2002;17:1010–7. [PubMed] [Google Scholar]
- Lin JW, Mody A, Tonini R, Emery C, Haymond J, Vrabec JT, Oghalai JS. Characteristics of malfunctioning channels in pediatric cochlear implants. The Laryngoscope. 2010;120:399–404. doi: 10.1002/lary.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci Biobehav Rev. 2010;34:269–84. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Miyamoto RT, Osberger MJ, Todd SL, Robbins AM, Stroer BS, Zimmerman-Phillips S, Carney AE. Variables affecting implant performance in children. The Laryngoscope. 1994;104:1120–4. doi: 10.1288/00005537-199409000-00012. [DOI] [PubMed] [Google Scholar]
- Mortensen MV, Mirz F, Gjedde A. Restored speech comprehension linked to activity in left inferior prefrontal and right temporal cortices in postlingual deafness. Neuroimage. 2006;31:842–52. doi: 10.1016/j.neuroimage.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Neville H, Bavelier D. Human brain plasticity: evidence from sensory deprivation and altered language experience. Progress in Brain Research. 2002;138:177–88. doi: 10.1016/S0079-6123(02)38078-6. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E, Lopes da Silva F. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Lippincott Williams & Wilkins; 2004. [Google Scholar]
- Oghalai JS, Tonini R, Rasmus J, Emery C, Manolidis S, Vrabec JT, Haymond J. Intraoperative monitoring of cochlear function during cochlear implantation. Cochlear implants international. 2009;10:1–18. doi: 10.1002/cii.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada E, Delpy DT. Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Appl Opt. 2003;42:2915–22. doi: 10.1364/ao.42.002915. [DOI] [PubMed] [Google Scholar]
- Patel AM, Cahill LD, Ret J, Schmithorst V, Choo D, Holland S. Functional magnetic resonance imaging of hearing-impaired children under sedation before cochlear implantation. Arch Otolaryngol Head Neck Surg. 2007;133:677–83. doi: 10.1001/archotol.133.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RD, Johnsrude IS. Functional imaging of the auditory processing applied to speech sounds. Philos Trans R Soc Lond B Biol Sci. 2008;363:1023–35. doi: 10.1098/rstb.2007.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson SK, Caudle SE, Krull KR, Haymond J, Tonini R, Oghalai JS. Cognition in children with sensorineural hearing loss: etiologic considerations. The Laryngoscope. 2007;117:1661–5. doi: 10.1097/MLG.0b013e3180ca7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton CW, Don M, Eggermont JJ, Waring MD, Masuda A. Maturation of human cortical auditory function: differences between normal-hearing children and children with cochlear implants. Ear and hearing. 1996;17:430–7. doi: 10.1097/00003446-199610000-00009. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Ret J, Duggins A, Arjmand E, Greinwald J. Cortical reorganization in children with unilateral sensorineural hearing loss. Neuroreport. 2005;16:463–7. doi: 10.1097/00001756-200504040-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Boex C, Lazeyras F, Sigrist A, Pelizzone M. FMRI evidence for activation of multiple cortical regions in the primary auditory cortex of deaf subjects users of multichannel cochlear implants. Cereb Cortex. 2005;15:40–8. doi: 10.1093/cercor/bhh106. [DOI] [PubMed] [Google Scholar]
- Sharma A, Nash AA, Dorman M. Cortical development, plasticity and re-organization in children with cochlear implants. J Commun Disord. 2009;42:272–9. doi: 10.1016/j.jcomdis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Wise RJ. Monitoring and the controlled processing of meaning: distinct prefrontal systems. Cereb Cortex. 2004;14:1–10. doi: 10.1093/cercor/bhg086. [DOI] [PubMed] [Google Scholar]
- Strelnikov K, Rouger J, Demonet JF, Lagleyre S, Fraysse B, Deguine O, Barone P. Does Brain Activity at Rest Reflect Adaptive Strategies? Evidence from Speech Processing after Cochlear Implantation. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp183. [DOI] [PubMed] [Google Scholar]
- Taga G, Asakawa K, Maki A, Konishi Y, Koizumi H. Brain imaging in awake infants by near-infrared optical topography. P Natl Acad Sci USA. 2003;100:10722–10727. doi: 10.1073/pnas.1932552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai-Van H, Veuillet E, Norena A, Guiraud J, Collet L. Plasticity of tonotopic maps in humans: influence of hearing loss, hearing aids and cochlear implants. Acta oto-laryngologica. 2010;130:333–7. doi: 10.3109/00016480903258024. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Silver A, Knaus TA, Lindgren KA, Ducros M, Kim DS, Tager-Flusberg H. Effective and structural connectivity in the human auditory cortex. J Neurosci. 2008;28:3341–9. doi: 10.1523/JNEUROSCI.4434-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C, Ruzza I, Vaneecloo FM, Dubrulle F. Magnetic resonance imaging with the Digisonic SP Neurelec cochlear implant. Eur Arch Otorhinolaryngol. 2008;265:1043–6. doi: 10.1007/s00405-007-0576-6. [DOI] [PubMed] [Google Scholar]
- Wilcox T, Bortfeld H, Woods R, Wruck E, Boas DA. Hemodynamic response to featural changes in the occipital and inferior temporal cortex in infants: a preliminary methodological exploration. Dev Sci. 2008;11:361–70. doi: 10.1111/j.1467-7687.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Bortfeld H, Woods R, Wruck E, Armstrong J, Boas D. Hemodynamic changes in the infant cortex during the processing of featural and spatiotemporal information. Neuropsychologia. 2009;47:657–662. doi: 10.1016/j.neuropsychologia.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte RJ, Lane JI, Driscoll CL, Lundy LB, Bernstein MA, Kotsenas AL, Kocharian A. Pediatric and adult cochlear implantation. Radiographics. 2003;23:1185–200. doi: 10.1148/rg.235025046. [DOI] [PubMed] [Google Scholar]
- Wong D, Miyamoto RT, Pisoni DB, Sehgal M, Hutchins GD. PET imaging of cochlear-implant and normal-hearing subjects listening to speech and nonspeech. Hearing research. 1999;132:34–42. doi: 10.1016/s0378-5955(99)00028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Pisoni DB, Learn J, Gandour JT, Miyamoto RT, Hutchins GD. PET imaging of differential cortical activation by monaural speech and nonspeech stimuli. Hearing research. 2002;166:9–23. doi: 10.1016/s0378-5955(02)00311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]