Abstract
OBJECTIVE
Type 1 diabetes (T1D) is associated with a high risk for and mortality from premature coronary artery disease (CAD), including coronary artery calcification (CAC), a subclinical marker, and lower extremity arterial disease (LEAD). Pulse Wave Analysis (PWA) arterial stiffness indices have been associated with cardiovascular disease (CVD) risk factors and outcomes in various populations, but little is known regarding these relationships in T1D.
METHODS
PWA was performed using the Sphygmocor Px device on 144 participants in the Pittsburgh EDC Study of childhood onset T1D. The cross-sectional associations between arterial stiffness indices, augmentation index (AIx) and augmentation pressure (AP), and subendocardial viability ratio (SEVR), an estimate of myocardial perfusion, with prevalent CAD, electron beam computed tomography-measured CAC and low (<0.90) ankle-brachial index (ABI) were examined.
RESULTS
Higher AP (but not AIx) and lower SEVR were univariately associated with prevalent CAD, high CAC score, and low ABI. AP and SEVR’s association with CAD and CAC did not, however, remain significant after adjustment for age. In individuals not using nitrates, which profoundly affect PWA measures, AP was significantly higher in those with CAD events and explained more of the variance than either age or brachial blood pressure measures. SEVR was associated with low ABI in multivariable models.
CONCLUSIONS
Greater augmentation pressure is independently associated with prevalent CAD and estimated myocardial perfusion with low ABI in type 1 diabetes. These measures may thus help to better characterize CVD risk in type 1 diabetes and need to be examined prospectively.
Keywords: pulse wave analysis, type 1 diabetes, coronary artery disease, coronary artery calcification, lower extremity arterial disease, arterial stiffness
INTRODUCTION
Type 1 diabetes (T1D) is associated with high risk (10-fold or greater) of, and increased mortality from, premature coronary artery disease (CAD) and lower extremity arterial disease (LEAD).1–3 This increased risk is especially apparent in women with T1D, virtually eliminating the traditional female advantage seen in the absence of diabetes.4 In T1D, the atherogenic process appears to start earlier and occur more rapidly thereby leading to early morbidity and mortality.5 Traditional factors that increase the risk for both CAD and LEAD in T1D include dyslipidemia, hypertension and nephropathy.4–6 Coronary artery calcium (CAC), measured using electron beam tomography, is a marker of atherosclerotic burden, and in those with T1D, is closely related to clinical CAD.7
Pulse wave analysis measures vascular stiffness by determining the pulse waveform and the timing of reflected waves. Increased arterial stiffness increases the velocity of both forward arterial blood flow as well as reflected waves, causing central pressure augmentation. This augmentation pressure (AP) can be measured non-invasively using applanation tonometry and standardized as the augmentation index (AIx), which is the AP expressed as a percentage of the aortic pulse pressure (PP). AIx, which has been shown to be elevated in T1D,8 has also been found to correlate with traditional CAD risk factors, cardiovascular outcomes, and both coronary and extracoronary atherosclerosis.9–11 The subendocardial viability ratio (SEVR), an estimate of myocardial perfusion and a measure of the propensity to cardiac ischemia, can also be determined using applanation tonometry.
Arterial stiffness indices such as augmentation pressure, augmentation index and/or pulse wave velocity have been associated with CAD, CAC, and LEAD in the general population.12–14 Since these relationships have yet to be explored in T1D, the aim of this study is to cross-sectionally examine the relationships between indices of arterial stiffness and myocardial perfusion with prevalent CAD, CAC, and LEAD in a population with childhood-onset type 1 diabetes. We also assess the potential impact of medications which affect PWA measures, particularly nitrates, which have a major influence.15
METHODS
Participants in the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, a prospective investigation of patients with childhood-onset (age < 17 years) T1D were selected for study. EDC participants were either diagnosed or treated within 1 year of diagnosis at Children’s Hospital of Pittsburgh between 1950 and 1980 and were on insulin therapy at initial discharge. Initial evaluation in the EDC Study occurred in 1986–1988 with 18 years of follow-up. The 18 year examination (November 2004–November 2006) included pulse wave analysis. The study protocol was approved by the University of Pittsburgh Institutional Review Board.
Questionnaires concerning demographic, health care, self-care, and medical history information were sent to participants prior to clinic examinations. Smoking history (≥100 cigarettes in lifetime) and current smoking status were obtained. All medications were coded according to the World Health Organization’s Anatomical, Therapeutical, Chemical Classification/Defined Daily Doses (ATC/DDD) codes. Medications with potential effects on pulse wave reflection measures (angiotensin converting enzyme inhibitors (ACEIs), angiotensinogen receptor II blockers (ARBs), calcium channel blockers (CCBs), beta-blockers (BB) and nitrates) were of particular interest, and use of one or more of these medications was categorized as “pulse wave drug” (PWD) use. Antilipidemic agents were also of interest to this study.
During clinic visits, systolic and diastolic blood pressures (SBP and DBP) were measured twice after a 5-minute rest using a random zero sphygmomanometer, according to a standard protocol. Hypertension was defined as SBP≥130 mmHg, DBP≥80 mmHg, or the use of antihypertensive medication. Height and weight were measured to calculate BMI (kg/m2). Waist and hip circumferences were measured twice (thrice if first two measures differed by more than >0.5 cm). The two (or three) waist measures and hip measures were averaged, and then mean waist and mean hip measures were used to calculate waist-to-hip ratio.
Blood samples were analyzed for hemoglobin A1C using a DCA 2000 analyzer (Bayer Diagnostics, Tarrytown, NY). Total cholesterol and triglycerides were measured enzymatically. High-density lipoprotein (HDL) cholesterol level was determined using a precipitation technique (heparin and manganese chloride) with modification of the Lipid Research Clinics method.16 Non-HDL cholesterol was calculated as total cholesterol minus HDL cholesterol.
Coronary artery disease was defined as myocardial infarction confirmed by hospital records or Q waves on ECG (Minnesota codes 1.1 or 1.2), angiographic stenosis (≥50% blockage), coronary artery bypass surgery, or angioplasty. During the 10-, 16- and 18-year follow-up exams, coronary artery calcification (CAC) was assessed using electron beam tomography with a GE-Imatron ultrafast computed tomographic scanner (GE-Imatron, San Francisco, CA), as previously described.7 Only one CAC score was measured at year 10, whereas two CAC scores were obtained and averaged at years 16 and 18. The most recent CAC score (or mean of scores) was used in the present study. CAC scores were also categorized as either presence (≥100) or absence (0–99) of clinically significant CAC for analysis.
Resting ankle-brachial SBPs were taken in the supine position with a Doppler blood-flow detector. Ankle-to-brachial index (ABI) was calculated using the brachial pressure measurement taken closest in time to the ankle (right and left tibialis posterior and dorsalis pedis) pressures. LEAD comprised an ABI<0.9 on either side at rest, and/or a history of claudication as determined by questionnaire, and/or self-reported history of amputation for ischemia.
Of the 309 EDC study participants examined at the 18-year exam, pulse waveform analysis (PWA) was available only of 144 participants, as PWA testing began in January 2006, part way through the 18-year exam period. Aortic augmentation index (AIx), aortic augmentation pressure (AP) and subendocardial viability ratio (SEVR) were derived via waveforms measured at the radial artery using the SphygmoCor Px version 7.01 (AtCor Medical, Sydney, Australia), as previously described.17
The pressure wave created by left ventricular contraction propagates forward until meeting sites of resistance and reflecting backward. Stiffer artery walls result in earlier wave reflection, and when the reflected wave returns during systole rather than diastole, systolic pressure is augmented. Augmentation pressure (AP) is a measure of the contribution of the early reflected wave to central systolic pressure. Augmentation index (AIx) represents the level of augmentation and is expressed as a percentage of pulse pressure (AIx = AP/PP × 100). Heart rate is inversely associated with both AP and AIx.18 Subendocardial viability ratio (SEVR), which is the ratio of the diastolic area under the curve (AUC) of an arterial pulse wave to the systolic-AUC,19 is an estimate of myocardial perfusion (as coronary artery perfusion takes place primarily during diastole) to myocardial contraction. The SphygmoCor device provides a quality index (QI), which represents waveform reproducibility. If QI<80, the measure was repeated.
Distributional characteristics including normality were assessed. Student’s t-test, the Mann-Whitney U test for non-normally distributed variables (AP, triglycerides, albumin excretion rate, and serum creatinine), and the χ2 test were used as appropriate to compare cases and non-cases, adjusting for multiple comparisons using the Bonferroni correction. Comparison of AIx and SEVR values were assessed between clinically significant CAC groups using Student’s t-test, while AP comparison between CAC groups was performed using the Kruskal-Wallis test. When performing regression modeling, all continuous variables were standardized by subtracting the mean and dividing by their standard deviation. Thus, a unit change in the predictor variable was interpreted as a change of one standard deviation. Stepwise logistic regression was performed separately for prevalent CAD, low ABI and high CAC score. P-values < 0.05 were considered statistically significant. SPSS 15.0 for Windows (SPSS, Chicago, IL) was used for all analyses.
RESULTS
Cross-sectional characteristics of the PWA population from the Pittsburgh EDC Study are detailed by CAD status, CAC score, and ABI category in Table 1. Although crude comparisons of AIx, AP and SEVR showed significant sex differences, height-adjusted comparisons did not (data not shown). Males, however, did have significantly higher SBP (119.1±16.9 vs. 112.2±15.5 mmHg, p=0.01) and DBP (69.2±8.4 vs. 63.9±9.3 mmHg, p<0.001), lower HDL-c (53.2±15.1 vs. 64.9±16.1 mg/dL, p<0.001), and greater waist-to-hip ratio (0.91±0.07 vs. 0.82±0.09, p<0.001). Overall, 12.5% (n=18) of the population had prevalent CAD, which was more common in males than in females (18.3% vs. 6.8%, p=0.04). CAC scores were available for 138 (68 males, 70 females) PWA participants. Fifty (36.2% of those with CAC scores) participants had a CAC score ≥100, with no sex difference (women=34.3% vs. men=38.2%, p=.63). Sixteen participants (11.1%) had a low ABI (<0.9) and 28 (20.1%) had a high ABI (>1.3), the latter being more prevalent in men (p=0.02).
Table 1.
Cross-sectional characteristics of the Pittsburgh EDC Pulse Wave Analysis study population by CAD, CAD and LEAD categories
| CAD Status | CAC Score | LEAD (ABI) Categorya | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Overall | No CAD | CAD | 0–99 | ≥ 100 | <0.9 | 0.9–1.3 | >1.3 |
| % (N) | 100.0 (144) | 87.5 (126) | 12.5 (18) | 63.8 (88) | 36.2 (50) | 11.1 (16) | 69.4 (100) | 19.4 (28) |
| Demographic | ||||||||
| Female Sex (% (n)) | 50.7 (73) | 54.0 (68) | 27.8 (5)* | 52.3 (46) | 48.0 (24) | 68.8 (11) | 54.0 (54) | 28.6 (8)*†† |
| Age (yrs) | 44.7±7.43 | 44.0±7.26 | 49.1±7.27** | 42.3±6.57 | 49.0±6.84*** | 49.7±7.61** | 43.5±7.41 | 46.0±6.00 |
| Diabetes Duration (yrs) | 36.4±6.74 | 35.9±6.74 | 39.9±5.71* | 34.4±6.10 | 40.1±6.25*** | 41.0±6.07** | 35.2±6.71 | 37.9±5.88* |
| Ever Smoker (% (n)) | 40.4 (57) | 38.2 (47) | 55.6 (10) | 36.0 (31) | 49.0 (24) | 56.3 (9) | 42.9 (42) | 22.2 (6)† |
| Key Variables | ||||||||
| AIx (%) | 23.0±11.0 | 22.7±10.2 | 25.1±15.5 | 22.4±10.6 | 24.4±11.7 | 27.3±12.0 | 23.1±10.7 | 20.4±11.1† |
| AP (mmHg) | 8.0 (5.0–12.0) | 8.00 (5.0–12.0) | 9.50 (5.0–15.0) | 7.00 (4.0–12.0) | 9.00 (6.0–13.3)* | 10.5 (6.0–16.5) | 8.00 (5.0–12.0) | 6.00 (4.3–10.8) |
| SEVR (%) | 142.2±31.1 | 142.1±32.6 | 143.0±18.7 | 147.3±33.0 | 133.3±26.9* | 126.2±26.1* | 143.8±32.1 | 145.5±28.1† |
| Prevalent CAD (% (n)) | 12.5 (18) | --- | --- | 3.4 (3) | 28.0 (14)*** | 37.5 (6)** | 8.0 (8) | 14.3 (4) |
| CAC Score ≥100 (% (n)) | 36.2 (50) | 29.8 (36) | 82.4 (14)*** | --- | --- | 62.5 (10)* | 31.9 (30) | 35.7 (10) |
| Low ABI (% (n)) | 11.1 (16) | 7.9 (10) | 33.3 (6)** | 6.8 (6) | 20.0 (10)* | --- | --- | --- |
| High ABI (% (n)) | 20.3 (28) | 19.0 (24) | 22.2 (4) | 20.5 (18) | 20.0 (10) | --- | --- | --- |
| PWD use (% (n)) | 62.5 (90) | 59.5 (75) | 83.3 (15) | 48.9 (43) | 88.0 (44)*** | 81.3 (13) | 58.0 (58) | 67.9 (19) |
| ACEI/ARB use (% (n)) | 55.9 (80) | 53.6 (67) | 72.2 (13) | 46.6 (41) | 72.0 (36)** | 68.8 (11) | 52.5 (52) | 60.7 (17) |
| Other Variables | ||||||||
| Body Mass Index (kg/m2) | 27.2±4.66 | 27.3±4.68 | 27.2±4.68 | 27.2±4.64 | 26.9±4.19 | 27.9±4.59 | 27.2±4.83 | 27.2±4.18 |
| Waist-to-Hip Ratio | 0.87±0.09 | 0.86±0.09 | 0.92±0.07* | 0.86±0.09 | 0.89±0.09* | 0.89±0.11 | 0.86±0.09 | 0.89±0.09* |
| HbA1c (%) | 7.44±1.37 | 7.42±1.40 | 7.57±1.14 | 7.51±1.47 | 7.31±1.20 | 6.99±1.27 | 7.40±1.32 | 7.83±1.52† |
| Systolic BP (mmHg) | 115.2±15.9 | 115.2±15.8 | 115.8±17.4 | 113.4±15.5 | 119.1±16.5* | 117.6±17.2 | 113.8±15.7 | 118.8±15.6 |
| Diastolic BP (mmHg) | 66.4±9.19 | 66.6±9.25 | 65.2±8.95 | 67.6±9.18 | 64.2±9.18* | 60.1±8.59* | 66.7±9.46 | 69.3±6.86†† |
| Hypertension (% (n)) | 20.8 (30) | 19.8 (25) | 27.8 (5) | 18.2 (16) | 26.0 (13) | 18.8 (3) | 21.0 (21) | 21.4 (6) |
| Heart Rate (bpm) | 75.9±12.3 | 76.3±12.5 | 72.9±10.9 | 75.4±12.8 | 76.9±10.9 | 73.6±13.2 | 75.7±12.6 | 77.9±11.0 |
| Non-HDL-c (mg/dl) | 114.3±32.4 | 115.6±33.3 | 104.1±23.8 | 116.6±33.0 | 107.3±28.2 | 114.5±27.2 | 115.6±32.2 | 109.6±36.5 |
| HDL-c (mg/dl) | 59.1±16.5 | 59.7±16.6 | 54.3±15.0 | 60.5±16.8 | 57.0±14.9 | 57.6±18.8 | 59.4±16.2 | 58.7±16.5 |
| Serum Creatinine (mg/dl) | 1.0 (0.9–1.1) | 1.00 (0.9–1.1) | 1.00 (0.9–1.3) | 0.90 (0.8–1.1) | 1.00 (0.9–1.2)* | 1.00 (0.9–1.2) | 1.00 (0.8–1.1) | 1.00 (0.9–1.2) |
| WBC Count (×10−9/L) | 6.44±2.01 | 6.31±2.04 | 7.38±1.47* | 6.20±2.07 | 6.84±1.93 | 7.52±2.80* | 6.26±1.82 | 6.43±1.97 |
Continuous variables presented as mean±SD or median (IQR), unless otherwise noted.
ABI categories compared to 0.9 ≤ ABI ≤ 1.3 category;
p<.05,
p<.01,
p<.001;
Linear trend:
p<.05;
p<.01
Abbreviations: EDC, Epidemiology of Diabetes Complications; CAD, coronary artery disease; CAC, coronary artery calcification; LEAD, lower extremity arterial disease; ABI, ankle-brachial index; AIx, Augmentation Index, AP, Augmentation Pressure; SEVR, Subendocardial Viability Ratio; BP, blood pressure; HDL-c, high density lipoprotein cholesterol; WBC, white blood cell; ACEI, Angiotensin converting enzyme inhibitor; ARB, Angiotensin II receptor blocker; PWD, pulse wave drug.
Those with prevalent CAD were more likely to be older with a longer diabetes duration, have a clinically significant CAC score (≥100) and a low ABI. HbA1c, blood pressure (BP), and cholesterol levels did not vary by CAD status, nor did taking a drug with potential effects on PWA measures, likely due to the overall prevalent use of ACEI/ARB medications (55.9%).
A high CAC score (≥100) was associated with higher age, diabetes duration, systolic BP, and serum creatinine level, but with lower diastolic BP. A high CAC score was also associated with low ABI and any PWD use, as well as ACEI/ARB use, specifically.
Pulse Wave Analysis Measures and Coronary Artery Disease
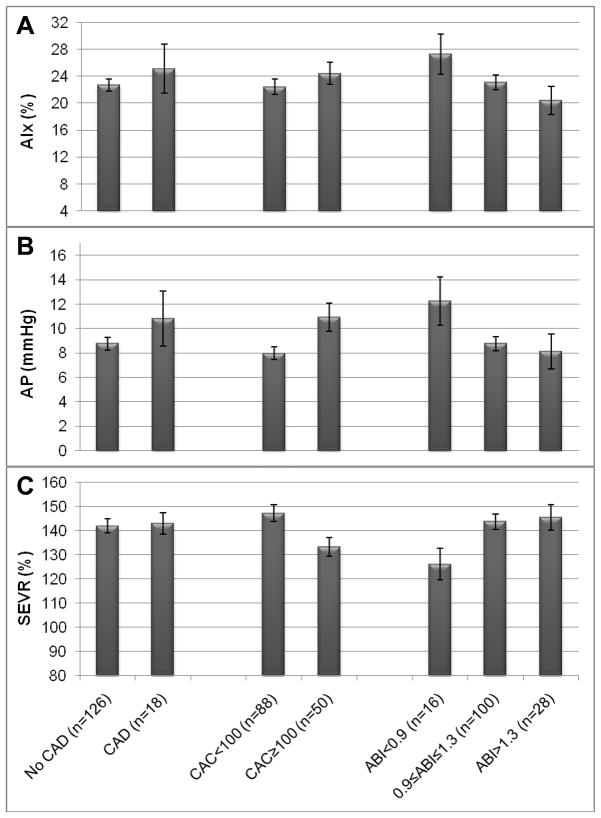
Augmentation index showed no significant univariate associations with any cardiovascular outcome (Table 1 and Figure 1a) and will not be considered further. Univariately, lower SEVR was not associated with prevalent CAD (Table 1 and Figure 1c). Augmentation pressure, although higher in CAD cases (Figure 1b), was not significantly different by CAD status (Table 1).
Figure 1.
Augmentation Index (mean and SEM) (Panel A), Augmentation Pressure (mean and SEM) (Panel B), and Subendocardial Viability Ratio (mean and SEM) (Panel C) by prevalent coronary artery disease (CAD) status, coronary artery calcification (CAC) score and ankle-brachial index (ABI) categories in the Pittsburgh EDC Pulse Wave Analysis study population.
In multivariable logistic regression for CAD, a standardized unit increase in AP was associated with an 87% increased chance (OR=1.87; 95%CI: 1.17–3.00; p=.009) of CAD in models adjusted for potential confounders (height, heart rate, PWD use). When allowing for other variables associated with CAD to enter models, age enters and eliminates the significant association between AP and prevalent CAD. Multivariable models with SEVR also showed no significant association with CAD (p=.77). Likewise, the addition of age to multivariable models further diminished the statistical significance of the SEVR-CAD relationship. Similar results were found if diabetes duration was modeled instead of age.
Due to the potential confounding effect of PWD use, multivariable models were performed after excluding each PWD use group, except for ACEI/ARB use (as most participants (56%) reported ACEI/ARB use). ACE/ARB use did not significantly differ between CAD cases (72.2%) and non-cases (53.6%). All of the models produced results similar to those discussed above, except for models that excluded nitrate use. Higher AP (OR=2.34; 95%CI: 1.04–5.28) was significantly related to CAD multivariately after excluding those taking nitrate medication (n=5) (Table 2), and AP entered the multivariable model preferentially over age or both systolic and diastolic BP. Results were similar when diabetes duration rather than age was made available (data not shown). A univariate comparison of AP by nitrate use (n=5) shows lower AP in those using nitrates (8.80 ± 10.2 vs. 9.05 ± 6.42, respectively).
Table 2.
Multivariable logistic regression models for CAD, high CAC score (≥100), and low ABI (<0.9)a in the Pittsburgh EDC Pulse Wave Analysis study population
| CADb | High CACb | Low ABI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Augmentation Pressurec | 2.34 | 1.04–5.28 | 0.04 | --- | --- | --- | 1.27 | 0.67–2.42 | 0.47 |
| Subendocardial Viability Ratiod | --- | --- | --- | 0.56 | 0.30–1.04 | 0.07 | 0.35 | 0.15–0.83 | 0.02 |
Odds ratios are per standardized unit.
Excludes those with High ABI (>1.3) (n=28).
Excludes those reporting nitrate use (n=5).
Model adjusted for potential confounders: heart rate and height
Model adjusted for potential confounder: heart rate
Other variables available for model selection: age, sex, SBP, DBP, WHR, HDL-c, Non-HDL-c, HbA1c, anti-lipidemic agent use, pulse wave drug use (use of at least one: ACEI/ARB, calcium channel blocker, beta-blocker), smoking history, albumin excretion rate.
Pulse Wave Analysis Measures and Coronary Artery Calcification
Univariately, augmentation pressure was significantly higher in individuals with high CAC (Table 1 and Figure 1b). Similarly, lower SEVR was associated with high CAC score (Table 1 and Figure 1c).
Multivariable models for high (≥100) CAC score were similar to those for prevalent CAD. AP was borderline significant (OR=1.55; 95%CI: 0.96–2.49; p=.07) in confounder adjusted models with entrance of age extinguishing the AP-CAC relationship. AP models adjusted for prevalent CAD showed no significant association between AP and high CAC. SEVR was significantly associated with having a high CAC score in confounder adjusted models (OR=0.46; 95%CI: 0.26–0.81, p=0.02) with age reducing the statistical significance (OR=0.61; 95%CI: 0.33–1.13; p=.12). CAD adjustment did not significantly alter SEVR models for high CAC. After excluding nitrate use, SEVR was significantly associated with a high CAC score (Table 2).
Pulse Wave Analysis Measures and Ankle-Brachial Index
Cross-sectional characteristics of the PWA population by ABI category are described in Table 1. AIx was not significantly different across ABI categories, but did show an inverse linear trend across increasing ABI categories (Table 1 and Figure 1a). Univariately, AP was not significantly different by ABI category, but SEVR was lower in the low ABI (<0.9) category (p=0.03) and increased linearly across increasing ABI categories (Table 1 and Figures 1b and 1c). Participants with low ABI (<0.9) were older with a longer duration of diabetes, had lower diastolic BP and a higher WBC count. Participants with high ABI (>1.3) were also older (but not significantly) with a longer diabetes duration. Significant linear trends across ABI categories were seen for female gender (inverse), HbA1c (direct), diastolic BP (direct), and smoking history (inverse).
Multivariable models for low ABI, excluding those with high ABI, showed a 66% increased risk for low ABI per standardized unit increase in AP (OR=1.66; 95%CI: 0.95–2.90; p=.08). However, adjusting for age, the association between AP and low ABI was not significant (Table 2). SEVR, however, was significantly associated with low ABI and preferentially entered heart rate-adjusted multivariable models over systolic and diastolic BP (Table 2). PWD use was not significantly associated with low ABI, and adjusting for PWD use did not significantly alter models. When a broader definition of lower extremity arterial disease was used (including claudication and/or amputation), PWA measures were not significantly associated with presence of LEAD multivariately.
DISCUSSION
This study found that augmentation pressure (AP), measured non-invasively using radial applanation tonometry, is significantly and independently associated with prevalent coronary artery disease in T1D, defined as myocardial infarction, coronary blockage ≥ 50%, or history of revascularization (coronary artery bypass graft or angioplasty), when those using nitrate medication (n=5) were excluded. We also found that lower estimated myocardial perfusion, SEVR, is associated with presence of high CAC (≥100), again after excluding those reporting nitrate use. This was the case even when adjusting for age and prevalent CAD. Also, AP was higher in those with low ABI and remained statistically significant until the model was adjusted for prevalent CAD. However, reduced SEVR remained significantly associated with presence of low ABI multivariately even after adjusting for prevalent CAD.
In some of our multivariable models with CAD and high CAC as outcomes, adding age eliminated the significant associations between pulse wave analysis measures and disease. Age (and diabetes duration) is a major risk factor for CAD in T1D. As diabetes duration and age are highly correlated (r=.83), it is difficult to separate out independent contributions as both are likely to influence CAC and its progression in T1D.20, 21 Both increased age and longer diabetes duration have been significantly associated with higher arterial stiffness measures in the past,22, 23 while in our study, age appears a somewhat stronger predictor.
Use of medications known to influence PWA measures was quite common in this T1D population (62.5%), particularly in those with a history of CAD events (83.3%). Approximately 90% of PWD use was ACEI or ARB, both of which have been shown to be very effective in reducing arterial stiffness indices.24 Use of other medication types (e.g., calcium channel blockers, beta-blockers and nitrates) was more common among those with CAD. Therefore, we chose to examine the relationship between PWA measures and CAD in the absence of each PWD drug. Exclusion of calcium channel blockers and beta-blockers had little effect. However, by excluding those on nitrates (only 4 participants with CAD), significant changes occurred to multivariable models. Administration of exogenous nitrate has been shown to increase vasodilatation, arterial compliance and decrease systolic BP.25 In a double-blind randomized cross-over study in elderly participants with hypertension, Stokes et al found that administration of isosorbide mononitrate (ISMN) decreased not only brachial systolic and diastolic ambulatory BPs, but also aortic SBP and augmentation pressure.26 Another study by Stokes et al later confirmed that ISMN alters PWA measures, decreasing SBP by 16 mmHg, pulse pressure by 13 mmHg and AIx by 4% in older hypertensive patients.27
Presence of a low ABI is associated with both increased cardiovascular risk and increased cardiovascular and all-cause mortality risk in T1D.28 Previous studies have shown significant association between AIx and ABI in multivariable analyses.14 While we found no significant relationship between low ABI and AIx in our T1D population, we did find a relationship of low ABI with both augmentation pressure and SEVR. AIx is augmentation pressure as a percentage of pulse pressure, and its effectiveness in representing increased pulse wave reflection and arterial stiffness in older populations has been questioned.29 As T1D is thought to accelerate vascular aging,30 and our study population had a mean diabetes duration of 36 years, AIx may not be as accurate as augmentation pressure alone in estimating arterial stiffness in this population.
LEAD occurs due to atherosclerosis in the arteries of leading to or within the legs. Atherosclerotic changes in lower peripheral vessels may logically contribute to earlier reflection of the pulse wave and to an increase in central systolic pressure augmentation. Earlier wave reflection results in higher AP and, therefore, higher central systolic pressure. Over time, this increased pressure reduces both DBP and time in diastole, when coronary perfusion occurs. Our findings are consistent with this physiologic process, in that reduced diastolic pressure leads to impaired coronary perfusion (i.e., lower SEVR), even though this, in turn, did not translate into increased CAD events in our population.
The cross-sectional design of this study is one of its limitations and might explain why PWA measures were not significantly associated with all CAD outcomes. A prospective design using pulse wave analysis measurements to predict CAD events or CAC progression within a T1D population would more effectively elucidate the relationship between arterial stiffness indices and these outcomes. Another limitation of the study is that most participants with either CAD or high CAC scores also reported PWD use, which is to be expected, and may explain the lack of association between PWA measures and some of the CVD outcomes. The limited sample size of our study population is another limitation, as a larger sample size would have also allowed for stratification by medication use, age or diabetes duration, and/or sex. However, this study is the largest to date to assess PWA measures (AIx, AP, and SEVR) in type 1 diabetes.
In summary, augmentation pressure, an index of arterial stiffness, and subendocardial viability ratio, an estimate of myocardial perfusion, are associated with CAD and low ABI, respectively, in our T1D population. To our knowledge, this is the first study to examine the association between pulse wave reflection measures and prevalent cardiovascular disease in a type 1 diabetes population. Pulse waveform analysis is a simple, non-invasive measure of arterial stiffness indices, and further research into the association between these indices and T1D complications is needed.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01-DK034818). AMS was supported by a training grant from the National Institute of Diabetes and Digestive and Kidney Diseases (F30-DK082137).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deckert T. The prognosis of insulin-dependent diabetes mellitus. Z Gesamte Inn Med. 1981;36:4–8. [PubMed] [Google Scholar]
- 2.Maser RE, Wolfson SK, Jr, Ellis D, Stein EA, Drash AL, Becker DJ, Dorman JS, Orchard TJ. Cardiovascular disease and arterial calcification in insulin-dependent diabetes mellitus: interrelations and risk factor profiles. Pittsburgh Epidemiology of Diabetes Complications Study-V. Arterioscler Thromb. 1991;11:958–965. doi: 10.1161/01.atv.11.4.958. [DOI] [PubMed] [Google Scholar]
- 3.Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, Gatling W, Bingley PJ, Patterson CC. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46:760–765. doi: 10.1007/s00125-003-1116-6. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd CE, Kuller LH, Ellis D, Becker DJ, Wing RR, Orchard TJ. Coronary artery disease in IDDM. Gender differences in risk factors but not risk. Arterioscler Thromb Vasc Biol. 1996;16:720–726. doi: 10.1161/01.atv.16.6.720. [DOI] [PubMed] [Google Scholar]
- 5.Krolewski AS, Kosinski EJ, Warram JH, Leland OS, Busick EJ, Asmal AC, Rand LI, Christlieb AR, Bradley RF, Kahn CR. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–755. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 6.Forrest KY, Becker DJ, Kuller LH, Wolfson SK, Orchard TJ. Are predictors of coronary heart disease and lower-extremity arterial disease in type 1 diabetes the same? A prospective study. Atherosclerosis. 2000;148:159–169. doi: 10.1016/s0021-9150(99)00217-8. [DOI] [PubMed] [Google Scholar]
- 7.Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ. Coronary calcium in adults with type 1 diabetes: a stronger correlate of clinical coronary artery disease in men than in women. Diabetes. 2000;49:1571–1578. doi: 10.2337/diabetes.49.9.1571. [DOI] [PubMed] [Google Scholar]
- 8.Brooks B, Molyneaux L, Yue DK. Augmentation of central arterial pressure in type 1 diabetes. Diabetes Care. 1999;22:1722–1727. doi: 10.2337/diacare.22.10.1722. [DOI] [PubMed] [Google Scholar]
- 9.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Nurnberger J, Keflioglu-Scheiber A, Opazo Saez AM, Wenzel RR, Philipp T, Schafers RF. Augmentation index is associated with cardiovascular risk. J Hypertens. 2002;20:2407–2414. doi: 10.1097/00004872-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 12.Altunkan S, Oztas K, Seref B. Arterial stiffness index as a screening test for cardiovascular risk: a comparative study between coronary artery calcification determined by electron beam tomography and arterial stiffness index determined by a VitalVision device in asymptomatic subjects. Eur J Intern Med. 2005;16:580–584. doi: 10.1016/j.ejim.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Covic A, Haydar AA, Bhamra-Ariza P, Gusbeth-Tatomir P, Goldsmith DJ. Aortic pulse wave velocity and arterial wave reflections predict the extent and severity of coronary artery disease in chronic kidney disease patients. J Nephrol. 2005;18:388–396. [PubMed] [Google Scholar]
- 14.Khaleghi M, Kullo IJ. Aortic augmentation index is associated with the ankle-brachial index: a community-based study. Atherosclerosis. 2007;195:248–253. doi: 10.1016/j.atherosclerosis.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Protogerou AD, Stergiou GS, Vlachopoulos C, Blacher J, Achimastos A. The effect of antihypertensive drugs on central blood pressure beyond peripheral blood pressure. Part II: Evidence for specific class-effects of antihypertensive drugs on pressure amplification. Curr Pharm Des. 2009;15:272–289. doi: 10.2174/138161209787354186. [DOI] [PubMed] [Google Scholar]
- 16.Warnick GR, Albers JJ. Heparin--Mn2+ quantitation of high-density-lipoprotein cholesterol: an ultrafiltration procedure for lipemic samples. Clin Chem. 1978;24:900–904. [PubMed] [Google Scholar]
- 17.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. The Journal of physiology. 2000;525(Pt 1):263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckberg GD, Archie JP, Fixler DE, Hoffman JI. Experimental subendocardial ischemia during left ventricular hypertension. Surg Forum. 1971;22:124–125. [PubMed] [Google Scholar]
- 20.Hata N, Kunimi T, Kishida H, Miyagawa H, Ikema Y, Hayakawa H. Clinical significance of coronary artery calcification. Int Angiol. 1994;13:281–285. [PubMed] [Google Scholar]
- 21.Costacou T, Edmundowicz D, Prince C, Conway B, Orchard TJ. Progression of coronary artery calcium in type 1 diabetes mellitus. Am J Cardiol. 2007;100:1543–1547. doi: 10.1016/j.amjcard.2007.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahlgren AR, Sundkvist G, Wollmer P, Sonesson B, Lanne T. Increased aortic stiffness in women with type 1 diabetes mellitus is associated with diabetes duration and autonomic nerve function. Diabet Med. 1999;16:291–297. doi: 10.1046/j.1464-5491.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 23.Lambert J, Smulders RA, Aarsen M, Donker AJ, Stehouwer CD. Carotid artery stiffness is increased in microalbuminuric IDDM patients. Diabetes care. 1998;21:99–103. doi: 10.2337/diacare.21.1.99. [DOI] [PubMed] [Google Scholar]
- 24.Pannier BM, Guerin AP, Marchais SJ, London GM. Different aortic reflection wave responses following long-term angiotensin-converting enzyme inhibition and beta-blocker in essential hypertension. Clin Exp Pharmacol Physiol. 2001;28:1074–1077. doi: 10.1046/j.1440-1681.2001.03570.x. [DOI] [PubMed] [Google Scholar]
- 25.Simon AC, Levenson JA, Levy BY, Bouthier JE, Peronneau PP, Safar ME. Effect of nitroglycerin on peripheral large arteries in hypertension. British journal of clinical pharmacology. 1982;14:241–246. doi: 10.1111/j.1365-2125.1982.tb01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stokes GS, Ryan M, Brnabic A, Nyberg G. A controlled study of the effects of isosorbide mononitrate on arterial blood pressure and pulse wave form in systolic hypertension. Journal of hypertension. 1999;17:1767–1773. doi: 10.1097/00004872-199917120-00015. [DOI] [PubMed] [Google Scholar]
- 27.Stokes GS, Bune AJ, Huon N, Barin ES. Long-term effectiveness of extended-release nitrate for the treatment of systolic hypertension. Hypertension. 2005;45:380–384. doi: 10.1161/01.HYP.0000156746.25300.1c. [DOI] [PubMed] [Google Scholar]
- 28.Olson JC, Erbey JR, Williams KV, Becker DJ, Edmundowicz D, Kelsey SF, Tyrrell KS, Orchard TJ. Subclinical atherosclerosis and estimated glucose disposal rate as predictors of mortality in type 1 diabetes. Ann Epidemiol. 2002;12:331–337. doi: 10.1016/s1047-2797(01)00269-1. [DOI] [PubMed] [Google Scholar]
- 29.Fantin F, Mattocks A, Bulpitt CJ, Banya W, Rajkumar C. Is augmentation index a good measure of vascular stiffness in the elderly? Age Ageing. 2007;36:43–48. doi: 10.1093/ageing/afl115. [DOI] [PubMed] [Google Scholar]
- 30.Ronnback M, Fagerudd J, Forsblom C, Pettersson-Fernholm K, Reunanen A, Groop PH. Altered age-related blood pressure pattern in type 1 diabetes. Circulation. 2004;110:1076–1082. doi: 10.1161/01.CIR.0000139903.29522.8D. [DOI] [PubMed] [Google Scholar]