Abstract
Purpose
To determine the effect of cryopreservation on acH4K12 in oocytes and their respective zygotes.
Methods
AcH4K12 in fresh or vitrified-warmed oocytes and their respective zygotes at 70 min–12 h post-fertilization were assessed using fluorescent staining.
Results
1. AcH4K12 levels increased significantly in vitrified oocytes compared to controls. 2. Respective zygotes derived from vitrified oocytes had abnormal chromatin distribution or acH4K12 patterns before and after pronuclear formation.
Conclusion
Cryopreservation alters AcH4K12 patterns in oocytes, which subsequently affect the chromatin distribution and acH4K12 in fertilized oocytes.
Keywords: Cryopreservation, Histone acetylation, Oocytes, Pronuclear, Zygotes, Mouse
Introduction
The cryopreservation of oocytes is very important for the preservation of genetic resources in farm and laboratory animals and in the human. Poor development after cryopreservation, however, has greatly limited its application in these fields [1, 2]. Still remaining are unique challenges that must be overcome before cryopreservation can be routinely used for the storage of oocytes and their subsequent embryos. A wide variety of approaches have been used to improve upon and optimize methods for cryopreservation [3].
Freezing the oocyte induces a number of unique anomalies such as altered distribution of cortical granules [4], disorganization of the spindle [5] and disruption of chromosomes [5, 6]. Some of these anomalies are transient for the spindle and chromosomal aberrations can mostly be restored upon warming and incubation [7]. A recent study showed that vitrification of oocytes also induces zona pellucida hardening [8], which can be overcome by zona-drilling in assisted fertilization [9, 10] or by using a calcium-free vitrification protocol [8]. Assuming that fertilization of vitrified-warmed oocytes can be improved to the level of fresh oocytes, there still remains the problem of reduced embryo development. It is clear that developmental competence in cryopreserved oocytes is decreased; what is not so clear are the events leading up to the decrease.
Histone acetylation is pivotal to many cellular functions such as chromosome condensation, DNA double-strand breakage repair and transcription [11–13]. During the early period of fertilization, sperm chromatin is decondensed and then recondensed, and histone acetylation patterns change regularly in the process [14]. For example, various lysine residues on histone 3 and histone 4 are unacetylated during meiosis of oocytes, whereas most of these lysine residues are acetylated in preimplantation embryos [15–17]. Previous studies demonstrated that acH3K14 or acH4K12 increases in oocytes could induce aberrant spindles and subsequent aneuploidy after fertilization [18, 19]. Other studies also indicated aberrant acH4K12 in male gametes after fertilization results in insufficient sperm chromatin compaction or inappropriate transfer of epigenetic information to the zygote, which might be responsible for in male infertility [20]. We hypothesized that changes in acH4K12 are also attributable to reduced development of cryopreserved oocytes.
In the present study we assessed the acetylation status of histone H4 at lysine K12 (acH4K12) in oocytes before and after freezing. We also examined acH4K12 patterns in zygotes derived from cryopreserved oocytes.
Materials and methods
All chemicals and media were purchased from Sigma chemical Co. (St. Louis, MO), unless otherwise indicated. Animals used in the study were Kunming white mice (Academy of Military Medical Sciences, Beijing, China) and were maintained at 20–22°C on a 14-h (6:00–20:00) light and 10-h (20:00–6:00) dark schedule. Experimental protocols for handing the mice were in accordance with the guidelines of the Institutional Animal Care and Use Committee of the China Agricultural University.
Oocytes collection
Six-eight week-old female mice were superovulated with 10 IU (i.p.) pregnant mare serum gonadotrophin (PMSG) (Ningbo Hormone Products CO., China) followed 48 h later by 10 IU of hCG (Ningbo Hormone Products CO., China). Fourteen hours post hCG injection, oocytes were obtained from the oviducts and put into M2 medium. The cumulus cells were removed with a hyaluronidase (300 IU/ml) treatment for 3–5 min in the same solution. Only normal oocytes with second polar body were used.
Cryopreservation of oocytes
Manufacture of the open pulled straws (OPS)
The OPS were made according to the method described by Vajta et al [21], with some modifications. The 0.25-ml plastic straws (I.V.M., 1’Aigle, France) were heat-softened over a self-made little alcohol burner and pulled manually to get a straw of approximately 0.10 mm in inner diameter and 0.05 mm in wall thickness, which could get about 20,000C/min in cooling rate.
Vitrification solutions
EDFS30: Ethylene glycol (EG) and Dimethyl Sulphoxide (DMSO) were diluted to 15% and 15% (v/v) in Ca2+-free M2 medium containing 30% (w/v) Ficoll (FW: 70000) and 0.5 M sucrose.
10% EG+10% DMSO: EG and DMSO were diluted to 10% and 10% (v/v) in Ca2+-free M2 medium.
Vitrification and warming of oocytes
Oocyte freezing was performed within 30 min after collection, and it was handled at ambient temperature (25 ± 0.5 C). Vitrification media and oocytes were maintained at 37 C on a warming plate (Wenesco, Inc. Chicago, USA). Oocytes were vitrified in EDFS30 using the OPS method. Oocytes were pretreated in 10% EG+10% DMSO for 30 s and then transferred to EDFS30 in the narrow end of the pulled straw and held for 25 s. The straws were then immediately plunged into liquid nitrogen (LN2). Fifteen oocytes were loaded into each OPS. After storage for at least 24 h in LN2, oocytes were removed for warming. The tip of OPS was put into 0.5 mol/L sucrose, and the oocytes were released and kept in 0.5 mol/L sucrose for 5 min. Afterwards the oocytes were placed into 100 μl of M2 droplets in a petri dish (35 mm × 10 mm, Corning Incorporated, Corning, NY 14831, USA) and incubated in a CO2 incubator for 30 minutes before immunocytochemical staining and fertilization procedure.
AcH4K12 analysis of oocytes by immunocytochemical staining
Fresh or frozen/thawed oocytes were fixed with 3.7% paraformaldehyde for 30 min, and permeabilized with 0.5% Triton X-100 for 30 min. The oocytes were then blocked in 0.1% BSA for 1 h at room temperature, and then incubated with an antibody against acetylated H4K12 (Upstate Biotechnology, Lake Placid, NY) (1:300) at 4°C overnight. The oocytes were washed extensively and labeled with FITC (Fluorescein isothiocyanate)-conjugated anti-rabbit IgG (1:100) for 30 min at room temperature. The DNA was counterstained with 10 μg/ml propidium iodide (PI) for 10 min, followed by an extensive washing. The oocytes were then mounted on slides and fluorescence was detected with a Nikon spectral confocal scanning microscope. System settings were kept constant for all the replicates and each experiment was repeated three times. A minimum of 78 oocytes were stained in each experiment. Oocytes were treated with 0.1% BSA instead of primary antibody for the negative control.
Fluorescence intensities were quantified using EZ-C1 Free Viewer software described previously [22] with some modification. The pixel value of fluorescence was measured within a constant area from five different regions of chromosomes and five different regions of cytoplasm, and the average cytoplasmic value was subtracted from the average chromosomal value. Oocytes were classified into three grades (“++”, “+”, and “–”) according to intensity of fluorescence as elsewhere described [18].
In vitro fertilization and acH4K12 analysis of the zygotes
Fresh or frozen/thawed oocytes were placed into 75 μl of human tubal fluid (HTF) medium supplemented with 4 mg/ml BSA. Ten micro liters of Kunming white mouse sperm, which had been incubated for 1–1.5 h in HTF medium supplemented with 4 mg/ml BSA at 37°C for capacitation, was added to the oocytes. The mixture was incubated at 37°C, in an atmosphere of 5% CO2 in air. Some of the fertilized oocytes were used to analyze the acetylation patterns of H4K12 pre- and post-pronuclear formation. The time points that we used during zygote development has been described previously [23]. Pre-pronuclear stage times were 70, 100, 150 and 280 min post insemination, and 6, 8, 10 and 12 h were used for the post-pronuclear stage formation. Each group was replicated three times and a minimum of 51 fertilized zygotes were assessed in each group. Immunocytochemical staining and acetylation assessment of pronuclear zygotes are as that as described above for oocytes, and the size of the sperm head 70 min post fertilization was also assessed using EZ-C1 Free Viewer software. For the assessment of the development potential of oocytes after fertilization, some of the embryos derived from fresh or vitrified/warmed oocytes were washed in HTF medium to remove the sperm after 4–6 h incubation, and then transferred into 75 μl drops of HTF medium. At this point, the oocytes were assessed for survival. Oocytes having dark or granular cytoplasm, or being non-spherical or shrunken were defined as survival. The normal oocytes were cultured in HTF for 24 and 96 h for counting of the cleavage and blastocyst formation separately.
Statistical analysis
Data were analyzed using the T-test with SPSS12.0 software. P values less than 0.05 were considered statistically significant.
Results
Cryopreservation effect on survival and subsequently oocyte developmental potential
The survival rate in Table 1 between the fresh and vitrified treatment groups was not significantly different. The cleavage and blastocyst rates, however, were significantly reduced when vitrified oocytes were fertilized and compared to the untreated control (55.9% and 66.1% vs. 76.1% and 76.8%, P < 0.05).
Table 1.
Effect of cryopreservation on survival and subsequent development of oocytes and corresponding embryos
| Groups | No. MII Oocytes | No. Survival (%) | No. 2-Cell (%) | No. Blastocyst (%)a |
|---|---|---|---|---|
| Fresh | 255 | – | 194 (76.1)a | 149 (76.8)a |
| Vitrified | 234 | 222 (94.9) | 124 (55.9)b | 82 (66.1)b |
Different letters within same column indicate statistically significant difference (P < 0.05)
aIn percentage of cleaved embryos
AcH4K12 in fresh or vitrified-warmed oocytes
Acetylation of H4K12 was significantly enhanced in the cryopreserved oocytes compared to that in fresh control (Fig. 1a). A proportion of oocytes in the cryopreserved group exhibited an intense increase in fluorescence signal as compared to the fresh control [(++) 55.3% vs. 32.1%] either weak [(+) 30.6% vs. 38.5%] or absent signal [(−) 14.1% vs. 29.5%] a decrease, respectively (Fig. 1b).
Fig. 1.
Acetylation patterns of H4K12 in fresh and vitrified-warmed oocytes. Acetylation patterns of H4K12 was significantly enhanced in the cryopreserved oocyte (bottom) compared to that of fresh control (top) (a). AcH4K12 and DNA were labeled as green and red, respectively. Scale bar was 20 μm. The proportion of oocytes with an intense increased acH4K12, a weak or absent or a decreased signal in the cryopreserved group compared to fresh control (b). Intense and weak fluorescence signals are denoted with “++” and “+,” respectively, and the absence of a signal is denoted with “–”
AcH4K12 patterns of gametes before pronuclear formation
Under natural conditions as shown in mode graph (Fig. 2a), there is a continuous morphological change in either paternal or maternal chromatin in fresh oocytes during fertilization. The acetylation level of H4K12 of paternal chromatin was undetectable in fresh oocytes 70 min post fertilization (Fig. 2b-a’). Thereafter and in conjunction with development, acetylation of H4K12 occurs and spread uniformly throughout paternal chromatin (Fig. 2b-b’, c’ and d’). In the maternal chromatin after fertilization the residue was continuously acetylated (Fig. 2c-a’, b’, c’ and d’). The acetylation patterns were different in fertilized vitrified-warmed oocytes. Unlike fresh oocytes, histone acetylation on H4K12 was detected on paternal chromatin in vitrified-warmed oocytes 70 min post fertilization, indicating that acetylation occurred earlier in the cryopreserved oocytes (Fig. 2b-e’). In order to explain whether this early appearance of acetylation of sperm was attribute to its faster decondense in vitrified oocytes, we have measured the size of sperm head at 70 min, and the result indicated that the sperm heads in vitrified oocytes larger than that in fresh oocytes (Fig. 3). Furthermore, we also observed that 37% of the vitrified oocytes had an abnormal chromatin distribution after fertilization.
Fig. 2.
H4K12 acetylation patterns of paternal or maternal chromatin in fresh or vitrified-warmed oocytes during the early fertilization stage. Paternal and maternal chromatin changes during the early fertilization stage were shown by schematic representation. Parental and maternal chromatin is represented by blue and red colors, respectively (a). H4K12 acetylation patterns of paternal chromatin in fresh (top) and vitrified-warmed (bottom) oocytes during the early fertilization stage are shown in Fig. 2 (b). H4K12 acetylation patterns of maternal chromatin in fresh (top) and vitrified-warmed (bottom) oocytes are shown in Fig. 2 (c). Picture letters without superscript, DNA; letters with (’), AcH4K12; and letters with (”), pictures were produced by Nikon EZ-C1 Free Viewer software and different colors were employed to represent the different intensity of the fluorescence of H4K12 acetylation
Fig. 3.
Effect of oocyte vitrification on the size of sperm head 70 min post fertilization. Different letter indicate statistically significant difference (P < 0.05)
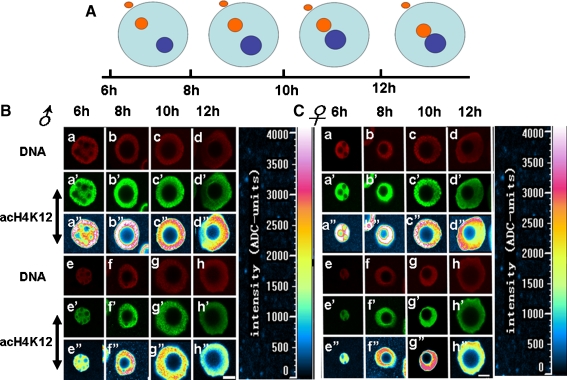
Pronuclear AcH4K12 patterns in zygotes
As shown in mode graph (Fig. 4a), under natural conditions, the male and female pronuclei expanded gradually, approached each other and then merging at about 12 h post-fertilization. In untreated control oocytes, the acetylation of H4K12 for male and female pronuclei distributed uniformly in the central region 6 hours post-fertilization (Fig. 4b-a” and Fig. 4c-a”). The acetylation signal decreased in the central region and increased in peripheral regions along with embryo development, which was very pronounced at 10 or 12 h post-fertilization (Fig. 4b-c”,d” and Fig. 4c-c”,d”). The acH4K12 patterns of male and female pronuclei in zygotes derived from vitrified-warmed oocytes gradually took on a normal composition 6–12 h post-fertilization, with very similar distribution patterns as that in controls at 12 h post-fertilization. The intensity of the acetylation signal in the vitrified-warmed group (Fig. 4b-e”,f”, g”, h” and Fig. 4c-e”, f”, g”, h”) was lower than that in the untreated control (Fig. 4b-a”,b”, c”, d” and Fig. 4c-a”, b”, c”, d”) at corresponding phases, especially at 6 and 8 h after fertilization.
Fig. 4.
AcH4K12 patterns in male and female pronuclei in zygotes from fresh or vitrified-warmed oocytes. Schematic representation of fertilized zygotes 6, 8, 10 and 12 h post fertilization (a). The acH4K12 patterns of male and female pronuclei in zygotes derived from both vitrified-warmed oocytes (bottom) and fresh control (top) at different time points are shown in (b) and (c)
Discussion
In this study, we first investigated putative cryopreservation-induced alterations of acetylation patterns on H4K12 in oocytes and their subsequent zygotes. The research focused on providing a better understanding of how cryopreservation affects the oocyte at the molecular level and how this information might lead to an improvement of gamete cryopreservation. Our research showed that acetylation levels on H4K12 in vitrified-warmed oocytes increased significantly when compared to that observed in fresh controls. Enhanced acH4K12 has been previously reported in oocytes from aged mice and in trichostatin A (a histone deacetylase inhibitor)-treated oocytes from young mice, which is both cases resulted in aneuploidy and embryo death [19, 24]. Our results, and in a previous study [19], showed that the acetylation signal of H4K12 is very low, even undetectable, in fresh oocytes from young mice, The enhanced histone acetylation that we observed in oocytes might account for the decreased developmental capacity of cryopreserved oocytes in earlier reports [10, 25]. We also observed that the rate of cleavage and development to the blastocyst stage was significantly decreased when vitrified oocytes were fertilized. Whether histone acetylation during fertilization can be changed by the cryopreservation process is worth further investigation.
Our study also showed that acetylation of H4K12 was undetectable in paternal chromatin in the fresh oocytes 70 min post fertilization. This observation confirms a previous report that H4K12 was under acetylated when the sperm was injected into an oocyte [26]. The sperm chromatin is compacted and combined with protamines but not histone under natural conditions. After gamete fusion, sperm-specific chromatin decondenses and recondenses and then protamines are replaced by histones [14], which could very well postpone acetylation processes in paternal chromatin. Following the development of a fertilized zygote, acetylation at this residue occurs and distributed uniformly on chromosomes after that. However, H4K12 remains acetylated during the entire process of fertilization in maternal chromatin.
Unlike in the fresh oocyte group, acH4K12 and bigger sperm head were detected on paternal chromatin 70 min post fertilization, thus indicating earlier acetylation and faster decondense of paternal chromatin occurred in the cryopreserved oocytes. The abnormal phenomena might be associated with the enhanced histone acetylation in cryopreserved oocytes. Because previous study suggested that acetylated histone might be a binding chromatin remodeler [27], which becomes pivotal for the remodeling of chromatin and subsequently affects chromosome structure when the sperm penetrates oocyte.
We observed in our study that the acH4K12 patterns of both male and female pronuclei in zygotes derived from vitrified-warmed oocytes can be partially restore from 6 to 12 h post-fertilization, with a similar distribution patterns compared to that in controls at 12 h post-fertilization. The intensity of acetylation signal in vitrified-warmed group, however, was lower than that in the untreated controls at the same time points after fertilization. Abnormal acetylation levels at these time points could hamper nucleosome disassembly and thereby delay DNA replication [28], which might lead to the degradation of vitrified oocytes. Yamanaka et al (2009) also indicated that the level of histone acetylation at the pseudo-pronuclear stage is pivotal for subsequent development in SCNT embryos [29]. The same mechanisms seem to apply in this study.
Conclusion
The acH4K12 patterns were first characterized in mouse oocytes and in their corresponding zygotes. The characteristic patterns could be altered by cryopreservation, which more than likely compromise chromatin distribution and development of the cryopreserved oocytes after fertilization.
Acknowledgements
This research was supported by National Key Technology R&D Program (No.2006BAD14B08) and the Research Fund for the Doctoral Program of Higher Education (No. 20060019031) from the China Ministry of Education.
Appendix

Table 2.
Different vitrification solution
| Solution | Ethylene glycol(E) | Dimethyl sulfoxide(D) | FS | mPBS/M2 medium |
|---|---|---|---|---|
| 10%E | 10 | – | – | 90 |
| 10%E+10%D | 10 | 10 | – | 80 |
| EFS30 | 30 | – | 70 | – |
| EDFS30 | 15 | 15 | 70 | – |
| EFS40 | 40 | – | 60 | – |
| EDFS40 | 20 | 20 | 60 | – |
Footnotes
Capsule
Oocyte cryopreservation can alter acetylation patterns on lysine 12 of histone H4 in oocytes and zygotes in mouse
Lun Suo and QingGang Meng contributed equally to this work.
References
- 1.Katayama KP, Stehlik J, Kuwayama M, Kato O, Stehlik E. High survival rate of vitrified human oocytes results in clinical pregnancies. Fertil Steril. 2003;80:223–224. doi: 10.1016/S0015-0282(03)00551-X. [DOI] [PubMed] [Google Scholar]
- 2.Paynter SJ, Fuller BJ. Cryopreservation of mammalian oocytes. Methods Mol Biol. 2007;368:313–324. doi: 10.1007/978-1-59745-362-2_22. [DOI] [PubMed] [Google Scholar]
- 3.Leibo SP. Cryopreservation of oocytes and embryos: optimization by theoretical versus empirical analysis. Theriogenology. 2008;69:37–47. doi: 10.1016/j.theriogenology.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Ghetler Y, Skutelsky E, Ben Nun I, Ben Dor L, Amihai D, Shalgi R. Human oocyte cryopreservation and the fate of cortical granules. Fertil Steril. 2006;86:210–216. doi: 10.1016/j.fertnstert.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 5.Zenzes MT, Bielecki R, Casper RF, Leibo SP. Effects of chilling to 0 degrees C on the morphology of meiotic spindles in human metaphase II oocytes. Fertil Steril. 2001;75:769–777. doi: 10.1016/S0015-0282(00)01800-8. [DOI] [PubMed] [Google Scholar]
- 6.Boiso I, Marti M, Santalo J, Ponsa M, Barri PN, Veiga A. A confocal microscopy analysis of the spindle and chromosome configurations of human oocytes cryopreserved at the germinal vesicle and metaphase II stage. Hum Reprod. 2002;17:1885–1891. doi: 10.1093/humrep/17.7.1885. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Lien Y, Chen S, Chao K, Ho H, Yang Y. Open pulled straws for vitrification of mouse mature oocytes preserves patterns of meiotic spindles and chromosomes better than conventional straws. Hum Reprod. 2000;15:2598–2603. doi: 10.1093/humrep/15.12.2598. [DOI] [PubMed] [Google Scholar]
- 8.Larman M, Sheehan C, Gardner D. Calcium-free vitrification reduces cryoprotectant-induced zona pellucida hardening and increases fertilization rates in mouse oocytes. Reproduction. 2006;131:53–61. doi: 10.1530/rep.1.00878. [DOI] [PubMed] [Google Scholar]
- 9.Lane M, Gardner DK. Vitrification of mouse oocytes using a nylon loop. Mol Reprod Dev. 2001;58:342–347. doi: 10.1002/1098-2795(200103)58:3<342::AID-MRD13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Meng Q, Li X, Wu T, Dinnyes A, Zhu S. Piezo-actuated zona-drilling improves the fertilisation of OPS vitrified mouse oocytes. Acta Vet Hung. 2007;55:369–378. doi: 10.1556/AVet.55.2007.3.11. [DOI] [PubMed] [Google Scholar]
- 11.Bird AW, Yu DY, Pray-Grant MG, Qiu Q, Harmon KE, Megee PC, Grant PA, Smith MM, Christman MF. Acetylation of histoneH4 by Esa1 is required for DNA double-strand break repair. Nature. 2002;419:411–415. doi: 10.1038/nature01035. [DOI] [PubMed] [Google Scholar]
- 12.Masumoto H, Hawke D, Kobayashi R, Verreault A. A role for cell cycle- regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature. 2005;436:294–298. doi: 10.1038/nature03714. [DOI] [PubMed] [Google Scholar]
- 13.Racedo SE, Wrenzycki C, Lepikhov K, Salamone D, Walter J, Niemann H. Epigenetic modifications and related mRNA expression during bovine oocyte in vitro maturation. Reprod Fertil Dev. 2009;21:738–748. doi: 10.1071/RD09039. [DOI] [PubMed] [Google Scholar]
- 14.Rousseaux S, Reynoird N, Escoffier E, Thevenon J, Caron C, Khochbin S. Epigenetic reprogramming of the male genome during gametogenesis and in the zygote. Reprod Biomed Online. 2008;16:492–503. doi: 10.1016/S1472-6483(10)60456-7. [DOI] [PubMed] [Google Scholar]
- 15.Kim JM, Liu H, Tazaki M, Nagata M, Aoki F. Changes in histone acetylation during mouse oocyte meiosis. J Cell Biol. 2003;162:37–46. doi: 10.1083/jcb.200303047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Yin S, Ai JS, Liang CG, Hou Y, Chen DY, Schatten H, Sun QY. Histone deacetylation is required for orderly meiosis. Cell Cycle. 2006;5:766–774. doi: 10.4161/cc.5.7.2627. [DOI] [PubMed] [Google Scholar]
- 17.Kageyama S, Liu H, Nagata M, Aoki F. Stage specific expression of histone deacetylase 4 (HDAC4) during oogenesis and early preimplantation development in mice. J Reprod Dev. 2006;52:99–106. doi: 10.1262/jrd.17044. [DOI] [PubMed] [Google Scholar]
- 18.Huang JC, Yan LY, Lei ZL, Miao YL, Shi LH, Yang JW, Wang Q, Ouyang YC, Sun QY, Chen DY. Changes in histone acetylation during postovulatory aging of mouse oocyte. Biol Reprod. 2007;77:666–670. doi: 10.1095/biolreprod.107.062703. [DOI] [PubMed] [Google Scholar]
- 19.Akiyama T, Nagata M, Aoki F. Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. PNAS. 2006;103:7339–7344. doi: 10.1073/pnas.0510946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steger K, Paradowska A, Schumacher S, Schuppe HC, Bartkuhn M, Weidner W. Acetylated histone H4K12 exhibits diferent binding to sperm DNA between fertile men and infertile patients. Eur Urol Suppl. 2009;8:147. doi: 10.1016/S1569-9056(09)60112-3. [DOI] [Google Scholar]
- 21.Vajta G, Booth PJ, Holm P, Greve T, Callesen H. Successful vitrification of early stage bovine in vitro produced embryos with open pulled straw (OPS) method. Cryo Lett. 1997;18:191–195. [Google Scholar]
- 22.Aoki F, Worrad DM, Schultz RM. Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Dev Biol. 1997;181:296–307. doi: 10.1006/dbio.1996.8466. [DOI] [PubMed] [Google Scholar]
- 23.Heijden GW, Dieker JW, Derijck AA, Muller S, Berden JH, Braat DD, Vlag J, Boer P. Asymmetry in Histone H3 variants and lysine methylation between paternal and maternal chromatin of the early mouse zygote. Mech Dev. 2005;122:1008–1022. doi: 10.1016/j.mod.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Suo L, Meng QG, Pei Y, Yan CL, Fu XW, Bunch TD, Zhu SE. Changes in acetylation on lysine 12 of histone H4 (acH4K12) of murine oocytes during maternal aging may affect fertilization and subsequent embryo development. Fertil Steril. 2010;93:945–951. doi: 10.1016/j.fertnstert.2008.12.128. [DOI] [PubMed] [Google Scholar]
- 25.George M, Johnson M, Howlett S. Assessment of the developmental potential of frozen-thawed mouse oocytes. Hum Reprod. 1994;9:130–136. doi: 10.1093/oxfordjournals.humrep.a138302. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida N, Brahmajosyula M, Shoji S, Amanai M, Perry ACF. Epigenetic discrimination by mouse metaphase II oocytes mediates asymmetric chromatin remodeling independently of meiotic exit. Dev Biol. 2007;301:464–477. doi: 10.1016/j.ydbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Yang XJ. Lysine acetylation and the bromodomain: a new partnership for signaling. Bio Essays. 2004;26:1076–1087. doi: 10.1002/bies.20104. [DOI] [PubMed] [Google Scholar]
- 28.Kemp MG, Ghosh M, Liu G, Leffak M. The histone deacetylase inhibitor trichostatin A alters the pattern of DNA replication origin activity in human cells. Nucleic Acids Res. 2005;33:325–336. doi: 10.1093/nar/gki177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamanaka K, Sugimura S, Wakai T, Kawahara M, Sato E. Acetylation level of histone H3 in early embryonic stages affects subsequent development of miniature pig somatic cell nuclear transfer embryos. J Reprod Dev. 2009;55:638–644. doi: 10.1262/jrd.20245. [DOI] [PubMed] [Google Scholar]