Abstract
Purpose
Create a 3-Dimensional artificial human ovary to mature human oocytes.
Methods
Theca and granulosa cells were isolated from antral follicles of reproductive-aged women, seeded into micro-molded gels and self-assembled into complex 3D microtissues. Immunohistochemistry and live-dead staining confirmed theca cell identity and cellular viability at one week respectively. Placement of granulosa cell spheroids or cumulus-oocyte complexes into theca cell honeycomb openings resulted in creation of an artificial human ovary. Oocytes from this construct were assessed for polar body extrusion.
Results
Theca and granulosa cells self-assembled into complex microtissues, remaining viable for one week. At 72 h after artificial human ovary construction, theca cells completely surrounded the granulosa spheroids or COCs without stromal invasion or disruption. Polar body extrusion occurred in one of three COCs assessed.
Conclusions
An artifical human ovary can be created with self-assembled human theca and granulosa cell microtissues, and used for IVM and future oocyte toxicology studies.
Keywords: Artificial ovary, In vitro maturation, Oocytes, Self-assembly, Theca cells, Tissue engineering
Introduction
Ovarian failure is the greatest obstacle facing reproductive-aged women who wish to conceive after chemotherapy or radiation treatment for cancer. Patients diagnosed with cancer have minimal time to pursue fertility preservation options. Patients are usually limited to one in vitro fertilization (IVF) treatment cycle with embryo or oocyte cryopreservation before the initiation of treatments toxic to ovarian folliculogenesis. Some centers cryopreserve surgically removed ovarian cortical tissue for future auto-transplantation or in vitro maturation of immature oocytes. Transplantation of autologous cryopreserved ovarian tissue [1] or whole ovary transplantion between identical twins [2, 3] has resulted in pregnancies but the transplants have limited functional longevity. Theoretically, in vitro maturation (IVM) of primordial oocytes promises to yield the greatest number of fertilizable oocytes for future reproduction. To date, however, this method of maturing fertilizable oocytes resulting in offspring is only successful in mice [4, 5].
Culture systems designed for mammalian and human in vitro oocyte maturation have met with limited success. Most 3D culture systems utilize serum-free media [6, 7], alginate gels [8–12] or oocyte encapsulation via collagen [13, 14]. These methods adequately provide physical support for follicular growth but fail to recreate endocrine and paracrine interactions between the granulosa and theca cells critical for in vivo follicular maturation.
Recently, our group developed a new method for the self-assembly of 3D microtissues from monodispersed cells [15, 16]. Cells are seeded into the recesses of micro-molded agarose gels, and allowed to self-assemble into 3D microtissues. These microtissues can be harvested from the molds, combined and co-cultured to form more complex microtissues [17]. This system was used to create an artificial human ovary composed of the three functional ovarian follicle cell types: theca, granulosa and oocytes. We hypothesize the artificial human ovary more closely recreates the 3D interaction between the three follicular cell types critical to follicular maturation, can be used to mature early antral oocytes, and serves as a model for testing follicular physiology and toxicology.
Materials & methods
Theca cell isolation
Human theca interna were isolated from women ages 25 to 46 undergoing oophorectomy for benign indications according to a Women & Infants’ Hospital IRB approved protocol. Ovarian tissue sections (2 × 1 cm) were placed in Dulbecco’s Modified Eagle’s Medium (DMEM) (InvitrogenTM) medium with 10% fetal bovine serum (FBS) (Hyclone Laboratories Inc, Logan, Utah), 1% penicillin/streptomycin (MP Biomedicals LLC, Solon, Ohio) and 2.8 μg/mL amphotericin B (Fischer-Scientific, Pittsburgh, PA) (Ovarian Culture Medium). Antral follicles were excised from the tissue and placed in a 35 mm Petri dish. The follicles were bisected and the internal surface scraped gently with a sterile spatula to remove the granulosa cells. The follicle wall was flushed with ovarian culture medium followed by phosphate buffered saline (PBS). It was cut into 1–2 mm segments, and digested at 37°C for 1 h in 15 mL of DMEM containing 120 mg of collagenase (0.5%) (InvitrogenTM). The cells and undigested tissue were centrifuged at 800 rpm for 5 min and resuspended in 25 mL Versene (1% EDTA in PBS) which was repeated twice. After the third centrifugation, the cells and tissue were resuspended in 25 mL of ovarian culture medium, and distributed into a 6 well plate in 2 mL aliquots. The cells were cultured overnight at 37 0C after which the undigested tissue was removed. After 48 h of culture, the cells were trypsinized with 1 mL of 0.25% trypsin (InvitrogenTM) per well for 5 min at 37 0C. Two mL of ovarian culture medium was added to each well and the cells in the suspension were counted with the hemocytometer. Two million cells were allocated to each T175 flask and cultured in 25 mL of ovarian culture medium, which was exchanged every 2 days.
Theca cell cryobanking
Theca were tryspzinized and pelleted in a 50 mL conical tube. One million cells were concentrated in 900 μl ovarian culture media. One hundred microliters of dimethyl sulfoxide (DMSO) (ACROS, Morris Plains, NJ) was added to the cell suspension. One ml aliquots of the 10% DMSO solution were allotted to 1 mL cryovials, stored in isopropyl alcohol overnight at −80 0C, and moved to the cryotank at −80 0C. For use, cryovials were thawed at room temperature and cells immediately plated in ovarian culture medium.
Immunohistochemistry
Immunohistochemistry was performed according to the Autostainer Protocol for EnVision + ™ Dual Link HRP (DAB+) (www.dakousa.com) (Dako North America, Carpinteria, CA). Theca cells were plated onto glass slides at 25,000, 50,000 and 75,000 cells per slide. Slides were fixed with 10% neutral buffered formalin and air dried. Antigen retrieval was performed in Tris-Buffered Saline Solution with Tween (TBST, pH 7.6) at 95 0C for 20 min. Slides were cooled at room temperature and loaded into the programmed DakoCytomation Autostainer. After several rinses, slides were incubated with the mouse anti-human antibody to 1) vimentin (Ready to use, N1521) (Dako Denmark A/S, Glostrup, Denmark) or 2) calretinin (1:50) (Dako Denmark A/S, Glostrup, Denmark) for 30 min. Slides were incubated with the EnvisionTM HRP labeled polymer (Dako Denmark A/S, Glostrup, Denmark) for 30 min after a buffered rinse, then in Substrate-Chromogen Solution (DAB+) for 5–10 min, followed by hemotoxylin staining for 5–10 min.
Polydimethylsiloxane (PDMS) mold design
PDMS molds were designed using computer-assisted design (CAD) (Solid Works Corporation; Concord, MA) and a ThermoJet rapid prototyping machine (3D Systems Corporation—Valencia, CA) was used to make the wax molds. Wax molds were covered with Reprorubber (16116, Flexbar Machine Corporation; Islandia, NY), a synthetic casting material, that was removed after it solidified, creating a negative replica of the gel. This was sprayed with epoxy parfilm release agent (16136, Flexbar), filled with polydimethylsiloxane (PDMS; Dow Corning, Midland, MD) and cured at 950C for 2 h. The PDMS positive replicate was removed from the Reprorubber and cured for 1 h.
Micro-molded agarose gel formation
Three mL of a 2% autoclaved ultrapure electrophoresis grade agarose (Powder Ultrapure Agarose 15510-027, InvitrogenTM) solution was poured into the PDMS molds. The solidified agarose gel was removed from the PDMS mold after 15 min. The molds were equilibrated with 2 mL of ovarian culture medium in a 6-well plate at 37°C. The medium was changed twice, 4 h apart, prior to seeding.
Theca cell spheroid formation and characterization
Theca cells were rinsed with Versene prior to trypsinization (0.25% trypsin) for 5 min at 37°C. Addition of an equal volume of ovarian culture medium stopped trypsinization. The cell suspension was centrifuged at 800 rpm for 5 min. Approximately 800,000 cells were concentrated in 250 μl of ovarian culture medium and seeded into spheroid molds containing 822 wells. Cells were photographed at 1, 4, 24 and 48 h.
Live-dead viability assay
The medium in the agarose well was aspirated and the gel containing spheroids was immersed in 2.5 mL of PBS for 5 min. The PBS was aspirated and 300 μl of Live-Dead viability assay was added to each gel. The Live-Dead viability assay (InvitrogenTM) was made by mixing 2.5 μl Calcein-AM, 10 μl ethidium homodimer-1 and 5 mL of PBS. The gel was incubated in the dark for 30 min and rinsed in PBS for 5 min.
Granulosa cell isolation & spheroid formation
Cumulus granulosa cells, stripped from the oocytes of IVF patients were rinsed with PBS in a 35 mm Petri dish. Extraneous blood and tissue was removed with a glass pipette. In Cell-TrackerTM (InvitrogenTM) experiments, the granulosa cells were incubated in DMEM with high glucose (InvitrogenTM) and Cell-TrackerTM Green (1 vial mixed with 7.1 μl DMSO & 21.6 mL serum-free media) for 45 min prior to separation. The cumulus cells were separated by pipetting and centrifuged at 800 rpm for 10 min. Approximately 900,000 granulosa cells were resuspended in 250 μl of ovarian culture medium and seeded into agarose micro-molds for 42 h prior to placement in the theca cell honeycombs.
Artificial human ovary construction with granulosa cell spheroids
Approximately 2.7 million theca cells were concentrated into 400 μl of ovarian culture medium and seeded into an agarose mold containing 18 honeycomb-shaped wells. After 18 h the formed honeycomb microtissues, which had “popped off” the pegs, were transferred with a sterile spatula to a 35 mm Petri dish layered with 2 ml of sterile solidified agarose. Ovarian culture medium was added to barely cover the surface of the honeycombs. Granulosa cell spheroids, cultured for 42 h, were harvested by flipping the molds and centrifuging them at 500 rpm for 5 min. The granulosa cell spheroids were retrieved with a pipette, placed in the openings of the theca cell honeycomb and cultured for another 48 h. In Cell Tracker experiments, theca cell honeycombs were cocultured with granulosa cell spheroids in pegless wells for 1 week.
Artificial human ovary construction with cumulus granulosa-ooycte complexes
Cumulus-oocyte complexes were removed from early antral follicles (<10 mm) of reproductive age women undergoing oophorectomy via follicular puncture with a 27 gauge needle followed by flushing with DMEM. The follicles were opened and further rinsed with DMEM in a Petri dish. Oocytes were retrieved and transferred to 60 μl drops of HEPES medium (CO2 buffer for pH maintenance) (Sage-Cooper Surgical, Pasadena, CA) in a separate Petri dish using a Drummond 3 μl pipette (300 micron tips) (The Drummond Scientific Co, Broomall, PA). A total of 550,000 theca cells, rinsed in Versene and trypsinized, were concentrated into 60 μl of SAGE® IVM medium (Sage-Cooper Surgical) containing 10% albumin (Sage-Cooper Surgical), follicle stimulating hormone (FSH) (75 mIU/mL of Repronex®) (Ferring, Parsippany, NJ) and human chorionic gonadotropin (hCG) (100 mIU/mL Novarel®) (Ferring). The theca cells were seeded in an agarose mold containing two honeycomb shaped wells. Oocytes were transferred into the mold between the two honeycomb wells to allow co-culture with the theca cells overnight during honeycomb self-assembly. The patients from which the cumulus oocyte complexes were derived were not age-matched with patients who provided the theca cells. The well surrounding the agarose mold was filled with one milliliter of cleavage medium, a low glucose medium for pronuclear to 8-cell embryos (Sage-Cooper Surgical). The following day, theca cell honeycombs were transferred using a pipette to another 18 well agarose mold containing hexagon-shaped, pegless wells. Oocytes were transferred from the seeding mold into the openings of the honeycombs using a Drummond Microdispenser Pipette (300 micron tips). At 45 h after initiation of coculture, the oocyte was mechanically removed from the theca cell construct, stripped of cumulus granulosa cells and assessed for polar body extrusion.
Results
Theca cell isolation and immunohistochemistry
Several million theca cells, isolated from a single antral follicle, were cryopreserved, thawed and cultured in simple growth medium consisting of DMEM with high glucose, 10% FBS, penicillin/streptomycin and amphotericin B. Immunohistochemistry staining with mouse anti-human vimentin (Fig. 1a) and anti-calretinin antibodies confirmed isolation of a pure population of theca cells.
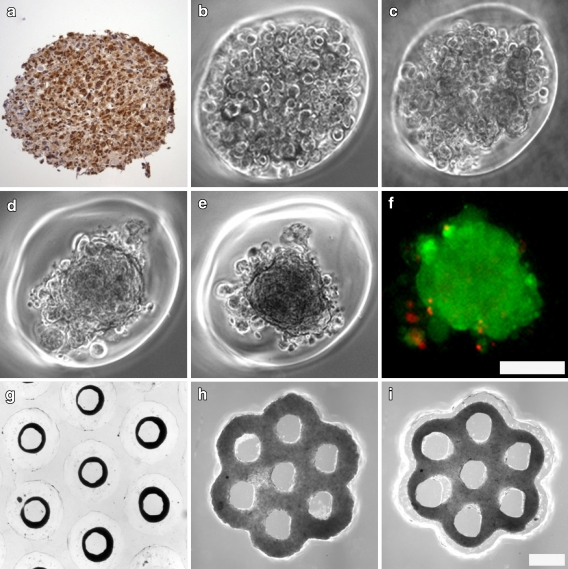
Fig. 1.
Theca cells can self-assemble into complex 3-dimensional microtissues. Isolation of theca cells from antral follicles was confirmed immunohistochemically with calretinin staining (a). Theca cells were seeded into spheroid shaped wells and self assembly into microtissues was documented starting at 1 h post seeding (b). Theca cells began to aggregate by 4 h (c) and compacted into spheroids by 24 h (d). Compaction into spheroids was complete at 48 h (e) and live-dead staining confirmed the viability (green) of theca cells in complex microtissues (f). Theca cells self-assembled into luminal structures such as toroids (g) seen at 24 h after seeding. Theca cells also formed complex microtissues containing multiple openings (lumina) including the honeycomb at 18 h (i) after initial seeding (h). Scale bars (100 microns) are the same for panels b, c, d, e, f, and for panels g, h,i
Theca cell microtissue formation and characterization
Theca cells self-assembled into complex three dimensional microtissues including the spheroid, toroid and honeycomb (Fig. 1b, c, d, e, f, g, h, i). Theca cells formed visible intercellular interactions at 4 h with significant structural compaction into spheroids occurring between 24–48 h after seeding (Fig. 1d, e). Granulosa cell spheroid compaction occurred in a very similar manner (Fig. 2a). Compaction was maintained 48 h to 7 days. A live-dead assay (Fig. 1f) demonstrated that theca cells within spheroids were alive after 7 days of culture (data not shown), while cells detached from the spheroid were predominantly dead.
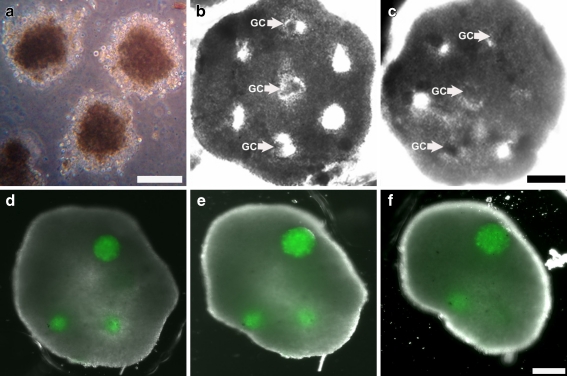
Fig. 2.
A model Artificial Human Ovary can be constructed with the theca cell honeycomb and granulosa spheroids while maintaining microtissue integrity. Granulosa cell spheroids (GC) self-assembled into spheroids and compacted within 48 h (a) in a comparable manner to theca cells. Granulosa cell spheroids could be placed into theca cell honeycombs seeded 22 h prior to form a model artificial human ovary (b). The theca cell honeycomb and granulosa cell spheroids maintained their respective shape after 24 h of coculture (c). Labeling of granulosa cell spheroids with Cell-Tracker Green confirms that microtissues placed 24 h prior within the theca cell honeycomb maintain their integrity (d) during envelopment by the theca, just as in panel C. Continued coculture and envelopment of granulosa cell spheroids within the model artificial ovary shows maintenance of microtissue integrity at 48 h (e) and 5 days (f) after initial placement of spheroids. Scale bars (100 microns) are the same for panels b, c, and for panels d, e, f
Artificial human ovary construction with granulosa cell spheroids
Theca cells formed stable honeycomb microtissues (Fig. 1h, i) and “popped off” of their pegs at 12–18 h after seeding. Granulosa cells spheroids, cultured for 42 h prior and compacted in a manner analogous to theca cell spheroids (Fig. 2a), could be placed in the honeycomb openings (Fig. 2b). The theca cell honeycomb and granulosa cell spheroids of the Model Artificial Human Ovary maintained their respective structural integrity for 48 h of co-culture on flat agar (Fig. 2c). Granulosa cell spheroids labeled with Cell Tracker Green and placed in the honeycomb openings maintained their integrity for 5 days (Fig. 2d, e, f) without stromal invasion by the surrounding theca.
Artificial human ovary construction with cumulus granulosa-ooycte complexes
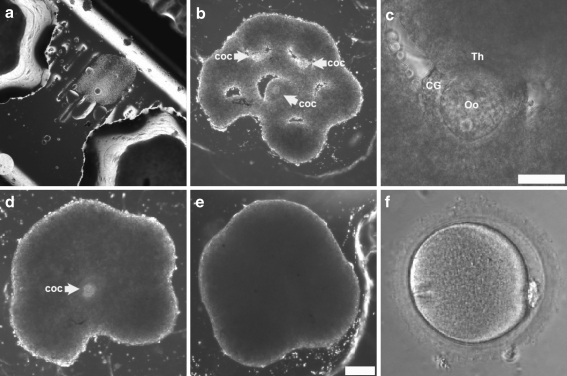
Cumulus granulosa-oocyte complexes (COCs) demonstrated cumulus expansion during overnight coculture with the theca (Fig. 3a) in SAGE® IVM medium containing FSH and hCG. At 10 h after placement of COCs in the honeycomb, the COCs became immobilized (Fig. 3b, c) due to contraction of the theca cells surrounding the opening. Over the next 48 h (Fig. 3d) theca cell envelopment of the COCs led to polar body extrusion (Fig. 3f) in 1 of 3 oocytes cultured in the theca cell honeycomb. At 72 h of coculture (Fig. 3e), the theca completely surrounded the COCs, eventually forming a large spheroid composed of all three cell types.
Fig. 3.
An Artificial Human Ovary can be formed from all three ovarian follicular cell types leading to polar body extrusion. Cumulus-oocyte complexes (COC) harvested from antral follicles were cocultured for 12–18 h with theca cell honeycombs during self-assembly. Three cumulus-oocyte complexes were placed into the openings of a theca cell honeycomb formed 21 h prior (b) to form an artificial human ovary from the 3 requisite cell types in ovarian folliculogenesis. A closeup (c) of panel b shows the theca (Th) enveloping a cumulus-oocyte complex without disruption of the cumulus granulosa(CG)-oocyte(Oo) interactions. At 24 h after placement of cumulus oocyte complexes within the human artificial ovary (d), two of the three cumulus oocyte complexes are completely enveloped. At 48 h after placement, the cumulus-oocyte complexes are completely enveloped by the theca cells which are beginning to assume a spheroidal shape (e). One of three oocytes removed from the artificial human ovary at 45 h, as in panel d, demonstrated maturation by extrusion of a polar body (f). Scale bars (100 microns) are the same for panels b, d and e
Discussion
This is the first demonstration of a 3-dimensional IVM culture system composed of 3 cell types with a proven physiologic, clinically applicable endpoint. This novel and promising approach differs from other 3-dimensional models by maximizing theca-granulosa cell interactions required for in vivo follicular maturation. Furthermore, the combination and coculture of microtissues can be used to create artificial organs such as the artificial ovary that mimic in vivo physiology. Maturation of early antral follicle (<10 mm) oocytes into metaphase II oocytes represents the first success in using 3D tissue engineering principles for in vitro oocyte maturation.
Valuable insight from the methods developed by Eppig and colleagues [5] serve as a template for addressing the challenge of in vitro maturation in humans. As suggested by Picton [18], the hypothesized four stage plan for in vitro maturation of human primordial to fertilizable metaphase II oocytes includes: 1) culture of primordial to secondary follicles in ovarian cortical tissue strips; 2) isolation and maturation of secondary follicles into antral follicles; 3) somatic cell (theca & granulosa cell) maturation and steroidogenesis; 4) nuclear and cytoplasmic maturation of germinal vesicle stage oocytes to fertilizable metaphase II oocytes. In vivo, the process of maturation of primordial to secondary follicles takes approximately 270 days [19]. In 1997, human primordial follicles in ovarian cortical strips were cultured into secondary follicles within 5 days [20]. By 2008, in vitro maturation of cortical ovarian strips in serum-free medium with activin A yielded late secondary and early antral follicles within 10 days [7]. Maturation of primordial oocytes beyond the late preantral stage has not been reported; it may be limited by the constraints of diffusion related to the high volume:surface area ratio in larger mammal and primate follicles.
The artificial human ovary addresses the limitations created by diffusion and size. Several million theca cells can be isolated from a single antral follicle, and cryopreserved, thawed and cultured further in media available in most laboratories. Immunohistochemistry confirmed that mechanical isolation is sufficient to achieve a pure population of theca cells that can self-assemble into complex microtissues. Theca cell contractility proved advantageous for artificial ovarian formation as granulosa spheroids and COCs were completely enveloped without disruption of their respective integrities. The ability of three cell types to aggregate into a 3D artificial ovary indicated that the follicular cells were aggregating and interacting on a macrocelulular level. Although the gap junctions were intact between the oocytes and granulosa cells, it was not determined if there were gap junctions formed between theca cells.
Sufficient theca cells may be retrieved from a patient’s single antral follicle for autologous artificial ovary construction, and in vitro maturation of oocytes retrieved from the ovarian cortex. The artificial human ovary which enveloped granulosa spheroids or COCs could not be cryosectioned for immunohistochemistry due to intolerance of shear forces produced by the blade. Microtissues appear to lack the intercellular cohesive properties which maintain whole ovarian tissue integrity in vivo. This attribute, in conjunction with the small construct size, may allow for enhanced diffusion of media, paracrine factors and byproducts compared to whole ovarian tissue culture, and could be advantageous for IVM culture systems. In this system only 1 in 3 of the oocytes appeared to reach the M2 phase which may be attributed to the presence of SAGE® IVM media containing FSH and hCG, or spontaneous oocyte maturation. The limitation of the study was the ability to collect enough human oocytes outside of an IVF cycle to give a definitive estimate of the benefit of the articial human ovary over the use of SAGE® IVM media containing FSH and hCG on oocyte maturation, or spontaneous oocyte maturation alone. High concentrations of exogenous gonadotropins were added to the culture as they are physiologically required for follicle maturation. While hormone production was not evaluated, the benefits of the paracrine and intracellular interacations cannot be underestimated and will be evaluated in future studies.
In the future, the approach to human IVM may involve isolation of primordial oocytes or ovarian cortical strips containing primordial oocytes and maturation within the artificial human ovary construct. Primordial oocytes exist in significantly greater quantities than secondary or antral follicle oocytes, and therefore may provide a better source of oocytes for in vitro maturation. Mechanical isolation of primordial follicles in rodents is easily accomplished, but significantly more difficult in humans [21–24] due to a tougher cortex. Follicles containing enzymatically isolated primordial follicles, freely cultured or grown in collagen gels, are burdened by collapse, migration of cumulus granulosa away from the follicle, or oocyte degeneration [22, 25, 26]. Enzymatic digestion also disrupts the theca cell layer and basement membrane [27], despite efforts to limit exposure to enzymes [28]. The development of methods to isolate intact primordial follicles will allow utilization of the significant primordial follicular pool for human IVM and fertility preservation. Current models (Telfer 2008) that mature human primordial oocytes to the early secondary stage culture the oocytes in whole ovarian tissue and then mechanically isolate them. This is disruptive to the three-layer stratification of oocyte-granulosa–theca cells. The human artificial ovary would allow mechanically isolated primordial oocytes or ovarian cortical strips, which have not undergone granulosa cell expansion or theca cell recruitment, to continue maturation in the same construct throughout development. Since theca cells are used in the construct, they could continue to produce hormones throughout the development of the oocytes unlike alginate or collagen scaffolding. Thus, we hypothesize the artificial human ovary will therefore be more advantageous to maturing the primordial oocytes into fertilizable metaphase II oocytes, and allow for more detailed study of human folliculogenesis.
Acknowledgements
This research was supported by Research Funds from the Division of Reproductive Endocrinology & Infertility at the Women & Infants’ Hospital of Rhode Island, and by a grant from the Rhode Island Science and Technology Council.
Footnotes
Capsule
An artificial human ovary can be created using self-assembled 3-dimensional theca & granulosa cell constructs to mature human oocytes in vitro
References
- 1.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–21. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 2.Silber SJ, Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med. 2007;356(13):1382–4. doi: 10.1056/NEJMc066574. [DOI] [PubMed] [Google Scholar]
- 3.Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril 2010 Feb 18. [Epub ahead of print] [DOI] [PubMed]
- 4.Eppig JJ, O’Brien MJ. Development In Vitro of Mouse Oocytes from Primordial Follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien MJ, Pendola JK, Eppig JJ. A Revised Protocol for In Vitro Development of Mouse Oocytes from Primordial Follicles Dramatically Improves Their Developmental Competence. Biol Reprod. 2003;68:1682–6. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 6.Picton HM, Mkandla A, Salha O, Wynn P, Gosden RG. Initiation of human primordial follicle growth in vitro in ultra-thin slices of ovarian cortex. Human Reprod. 1999;14:11. doi: 10.1093/humrep/14.1.11. [DOI] [Google Scholar]
- 7.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–8. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 8.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–23. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 9.West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–48. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-Engineered Follicles Produce Live, Fertile Offspring. Tissue Eng. 2006;12:2739–46. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated Three-Dimensional Culture Supports Development of Nonhuman Primate Secondary Follicles. Biol Reprod. 2009;81:587–94. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–40. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll J, Whittingham DG, Wood MJ. Effect of gonadotrophin environment on growth and development of isolated mouse primary ovarian follicles. J Reprod Fertil. 1991;93:71–9. doi: 10.1530/jrf.0.0930071. [DOI] [PubMed] [Google Scholar]
- 14.Hirao Y, Nagai T, Kubo M, Miyano T, Miyake M, Kato S. In vitro growth and maturation of pig oocytes. J Reprod Fertil. 1994;100:333–9. doi: 10.1530/jrf.0.1000333. [DOI] [PubMed] [Google Scholar]
- 15.Napolitano AP, Dean DM, Man AJ, et al. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 2007;13:2087–94. doi: 10.1089/ten.2006.0190. [DOI] [PubMed] [Google Scholar]
- 16.Dean DM, Napolitano AP, Youssef J, Morgan JR. Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB J. 2007;21:4005–12. doi: 10.1096/fj.07-8710com. [DOI] [PubMed] [Google Scholar]
- 17.Rago AP, Dean DM, Morgan JR. Controlling cell position in complex heterotypic 3D microtissues by tissue fusion. Cell Motil Cytoskeleton. 2009;66:129–41. doi: 10.1002/cm.20335. [DOI] [PubMed] [Google Scholar]
- 18.Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction. 2008;136:703–15. doi: 10.1530/REP-08-0290. [DOI] [PubMed] [Google Scholar]
- 19.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Human Reprod. 1986;1:81–7. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- 20.Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Human Reprod. 1997;12:1032–6. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- 21.Oktay K, Briggs D, Gosden RG. Ontogeny of follicle stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab. 1997;82:3748–51. doi: 10.1210/jc.82.11.3748. [DOI] [PubMed] [Google Scholar]
- 22.Abir R, Franks S, Mobberley MA, Moore PA, Margara RA, Winston RML. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril. 1997;68:682–8. doi: 10.1016/S0015-0282(97)00264-1. [DOI] [PubMed] [Google Scholar]
- 23.Huntriss J, Gosden R, Hinkins M, et al. Isolation, characterisation, and expression of the human factor in the germline alpha (FIGLA) in ovarian follicles and oocytes. Mol Hum Reprod. 2002;8:1087–95. doi: 10.1093/molehr/8.12.1087. [DOI] [PubMed] [Google Scholar]
- 24.Rice S, Ojha K, Mason H. Human ovarian biopsies as a viable source of pre-antral follicles. Human Reprod. 2008;23:600–5. doi: 10.1093/humrep/dem390. [DOI] [PubMed] [Google Scholar]
- 25.Spears N, Boland NI, Murray AA, Gosden RG. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Human Reprod. 1994;9:527–32. doi: 10.1093/oxfordjournals.humrep.a138539. [DOI] [PubMed] [Google Scholar]
- 26.Cortvrindt R, Smitz J, Steirteghem AC. In vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepubertal mice in a simplified culture system. Human Reprod. 1996;11:2656–6. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 27.Nayudu PL, Fehrenbach A, Kiesel P, Vitt UA, Pancharatna K, Osborn SM. Progress towards understanding follicle development in vitro: appearances are not deceiving. Arch Med Res. 2001;32:587–94. doi: 10.1016/S0188-4409(01)00339-3. [DOI] [PubMed] [Google Scholar]
- 28.Shuttleworth G, Broughton Pipkin F, Hunter MG. In vitro development of pig preantral follicles cultured in a serum-free medium and the effect of angiotensin II. Reproduction. 2002;123:807–18. doi: 10.1530/rep.0.1230807. [DOI] [PubMed] [Google Scholar]