Abstract
Purpose
The aim of the present study is to compare three previously described mouse embryonic stem cell derivation methods to evaluate the influence of culture conditions, number of isolated blastomeres and embryonic stage in the derivation process.
Methods
Three embryonic stem cell derivation methods: standard, pre-adhesion and defined culture medium method, were compared in the derivation from isolated blastomeres and whole embryos at 4- and 8-cell stages.
Results
A total of 200 embryonic stem cell lines were obtained with an efficiency ranging from 1.9% to 72%.
Conclusions
Using either isolated blastomeres or whole embryos, the highest rates of mouse embryonic stem cell establishment were achieved with the defined culture medium method and efficiencies increased as development progressed. Using isolated blastomeres, efficiencies increased in parallel to the proportion of the embryo volume used to start the derivation process.
Keywords: Embryo volume, ESC derivation, Isolated blastomeres, mESC
Introduction
Because of their high capacity to selfrenew and their regenerative potential, embryonic stem cells (ESCs) have generated a great interest as a developmental model in biology and as a new source of cells for tissue replacement treatments in regenerative medicine. ESCs have the ability to proliferate indefinitely without entering senescence and do not have specialized functions. Therefore, they are in an undifferentiated state and, after a differentiation process, they can produce different tissues of the three germinal layers, even extraembryonic tissue in the case of human ESCs [1].
In the 1980s, the first technique for pluripotent ESCs establishment from mouse blastocysts was developed [2, 3]. In this case, cells from the inner cell mass (ICM) were co-cultured with mouse STO embryo fibroblasts that had been previously inactivated by a mitomycin C treatment. Since then, many groups have achieved the establishment of mouse ESC lines [4] and human ESC lines [5] from blastocysts. However, ESC derivation from blastocysts has some drawbacks. First, it results in the destruction of the embryos from which the ESCs are derived, a controversial issue when dealing with human embryos, and second, it may not produce fully totipotent ESCs [1]. In fact, it is considered that mouse ESCs derived from blastocysts do not have the same developmental potential as those derived from embryonic stages previous to blastocyst. In experiments on chimera formation, it has been observed that ESCs from the ICM can not colonize trophectoderm (TE) when injected into blastocysts [1, 6]. This is due to the fact that, in the mouse, the formation of TE is simultaneous or even previous to the formation of the ICM. This is not the case in humans, as TE can develop from ESCs in vitro [5].
Concurrently, new experiments aimed to establish ESC lines from stages previous to blastocyst were attempted. In this sense, mouse ESC lines have been derived from morulae [7] using the traditional culture conditions mentioned before [2]. Tesar (2005) [8] also derived mouse ESC lines from several embryonic stages previous to blastocyst, but in this case introducing a variation in the traditional derivation method consisting in the attachment of the embryo to the culture dish prior to the derivation process. In humans, Strelchenko et al. (2004) [9] were the first who achieved the derivation of human ESCs from the morula stage.
On the other hand, different groups have tried to derive ESCs from isolated blastomeres [10–14]. The production of ESC lines from isolated blastomeres arose as an answer to the ethic and moral debate about the use of whole embryos for ESC derivation and it is based on the preimplantation genetic diagnosis (PGD) experience. In PGD studies one blastomere is removed from an embryo at the 6-8-cell stage for its analysis while the rest of the embryo is cultured in vitro until transfer [15]. It is well known that the human embryo remains viable and able to produce a normal pregnancy as long as at least a quarter of the embryo is intact [16].
Presumptively, ESC derivation efficiency from isolated blastomeres may be lower than from whole embryos. For this reason, during many years several groups have attempted to determine the most favorable culture and technical conditions for the efficient derivation and maintenance of such ESC lines in an undifferentiated state in vitro. These approaches include changes in the composition of the culture medium and in the adhesion properties and communication of the cells. In this sense, Chung et al. (2006) [10] developed a new method consisting in the association of mouse isolated blastomeres with a clump of proven ESCs expressing the green fluorescent protein (ESCs-GFP). Later, Klimanskaya et al. (2006) [13] established human ESC lines from isolated blastomeres in a conditioned culture medium from proven ESCs-GFP but in this case without contact between both cell types. Wakayama et al. (2007) [14] produced mouse ESC lines introducing a variation in the culture medium in order to improve the derivation efficiency. Particularly, fetal calf serum (FCS) was replaced by Knockout Serum Replacement (KSR) because it is known that FCS may contain some factors that promote ESCs differentiation. Moreover, the adrenocorticotropic hormone (ACTH) was added to the culture because it favours the derivation from a single blastomere and the culture propagation from a single ESC without losing pluripotency. This method provided higher efficiencies of derivation than the method of aggregation with proven ESCs-GFP. Recently, Chung et al. (2008) [11] have found that the derivation efficiency of human ESC lines established from 8-cell stage blastomeres can be improved by culturing them in a medium containing laminin and fibronectin, a new approach that recreates the ICM niche. By contrast, Geens et al. (2009) [12] described the derivation of human ESC lines from isolated blastomeres of 4-cell stage embryos by simply culturing blastomeres onto a monolayer of feeder cells.
Although a bulk of information has been obtained from these studies, there are still some aspects that need to be addressed. In this study, three previously described methods for ESC derivation that combine variations in the technical and culture conditions were compared for the establishment of mouse ESC lines both from isolated blastomeres and whole embryos at different developmental stages. This allowed us to address the importance of different parameters such as the composition of the culture medium, adhesiveness, and the influence of the number of blastomeres and the embryonic stage on ESC derivation efficiency.
Material and methods
Feeder cells culture
STO mouse embryo fibroblasts (ECACC, Salisbury, UK) were inactivated by a mitomycin C (Invitrogen, Prat de Llobregat, Barcelona, Spain) treatment during 3 hours at a concentration of 10 μg/ml to produce feeder cells [17]. The culture medium used for feeder cells was Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Prat de Llobregat, Barcelona, Spain) supplemented with 15% FCS (Invitrogen, Prat de Llobregat, Barcelona, Spain).
Embryo collection
Embryos were collected from 129/Sv female mice mated with C57Bl/6 male mice [18]. Prior to mating, females were subjected to a superovulation process by intraperitoneal injection of 5 iu of pregnant mare serum gonadotrophin (PMSG) (Intervet, Alcobendas, Madrid, Spain) followed by 5 iu of human chorionic gonadotrophin (hCG) (Farma-Lepori, Barcelona, Spain) 48 h after the first injection. Embryos at the 2-cell stage were obtained by flushing the oviducts with KSOM-H [19] 48 h after the injection with hCG and were cultured at 37 C under 5% CO2 until the stages of 4-cell, 8-cell, morula and blastocyst were reached.
All this methodology has been carried out according to the Guiding Principles in the Care and Use of Animals and has been approved by the Comissió d’Ètica en Experimentació Animal i Humana of the Universitat Autònoma de Barcelona and of the Departament de Medi Ambient i Habitatge of the Generalitat de Catalunya.
Blastomere isolation
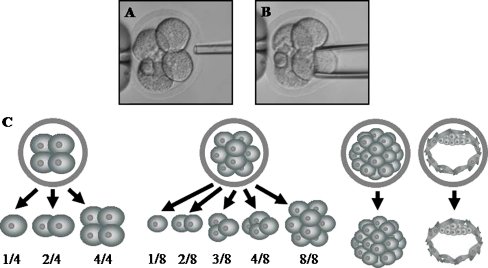
Blastomeres from 4- and 8-cell stage embryos were isolated by micromanipulation [15] in a calcium- and magnesium-free phosphate buffered solution (PBS). Briefly, the zona pellucida was disrupted with a 10 μm drilling micropipette containing Tyrode’s acid solution and individual blastomeres were aspirated with a 30 or 20 μm diameter micropipette for 4- and 8-cell stages, respectively (Fig. 1, a and b). Blastomeres were isolated constituting the groups of 1/4 and 2/4 from the isolation of 1 and 2 blastomeres at the 4-cell stage, respectively, and the groups of 1/8, 2/8, 3/8 and 4/8 from the isolation of 1, 2, 3 and 4 blastomeres at the 8-cell stage, respectively (Fig. 1c).
Fig. 1.
a–b Embryo biopsy at the 4-cell stage. a Drilling process. b Blastomere removal. c Derivation groups from which embryonic stem cells were established
Establishment of embryonic stem cell lines
ESC lines were derived either from isolated blastomeres or whole embryos, using three already described derivation methods. When working with whole embryos, the zona pellucida was digested with Tyrode’s acid solution prior to the culture.
In the standard method [20], isolated blastomeres and whole embryos were seeded onto a feeder cell monolayer and kept in culture.
In the pre-adhesion method, adapted from Tesar (2005) [8], isolated blastomeres and whole embryos were first attached to the bottom of the culture dish by incubating them in a DMEM solution without proteins during 15 min. Then, a feeder cell suspension was gently poured on.
In both methods, the culture was performed in 4-well dishes with a 1.6 cm diameter per well (Nunc, Langenselbold, Germany) containing 1 ml per well of DMEM medium supplemented with 100 μM 2-ß-mercaptoethanol (Invitrogen, Prat de Llobregat, Barcelona, Spain), 200 mM L-glutamine (Invitrogen, Prat de Llobregat, Barcelona, Spain), 100X non-essential aminoacids (Invitrogen, Prat de Llobregat, Barcelona, Spain), 103 units/ml of leukemia inhibitory factor (LIF) (Millipore, Madrid, Spain) and 15% FCS at 37 C and in a 5% CO2 atmosphere. The medium was changed every 2 days.
Finally, in the defined culture medium method [14] isolated blastomeres and whole embryos were seeded onto a feeder cell monolayer cultured in 50 μl drops contained in a 60 mm Petri dish (Nunc, Langenselbold, Germany) with DMEM medium supplemented with 100 μM 2-ß-mercaptoethanol, 1mM L-glutamine, 1X non-essential aminoacids, 103 units/ml of LIF, 20% KSR (Invitrogen, Prat de Llobregat, Barcelona, Spain) and 0.1 mg/ml ACTH (Sigma, Madrid, Spain). As in the previous methods, culture was performed at 37 C in a 5% CO2 atmosphere and the medium was changed every 2 days.
In the three methods, isolated blastomeres or whole embryos were kept in culture until outgrowths of embryonic cells were observed. ESC outgrowths were passaged using a mechanical method 7 or more days after the blastomeres or embryos were plated. In brief, cells of the ESC-like clump were disaggregated by a trypsin-EDTA (LabClinics, Barcelona, Spain) treatment using a mouth-controlled Pasteur pipette to draw the clump in and out of the end of the pipette in order to reduce the ESC-like clump into smaller cell fragments and a few single cells [21]. The subculture procedure was performed approximately 7 times until ESC stable lines were considered to be definitively established.
Embryonic stem cells characterization
ESC lines were first selected based on the morphology of their colonies. An ESC colony had to present a defined morphology at its edge, without differentiated cells. Later, the pluripotency of the selected ESC-like lines was determined by immunofluorescence, using specific markers: Oct4, Nanog, Sox2, Cdx2 and SSEA3 (Table 1). Briefly, ESC lines were washed 3 times in a PBS solution, fixed in 4% paraformaldehyde (Sigma, Madrid, Spain) during 15 min and washed again 3 times in PBS. Blocking and permeabilization was performed in a PBS solution containing 0.5% Triton X-100 (Sigma, Madrid, Spain), 3% goat serum (Sigma, Madrid, Spain) and 0.2% sodium azide (Sigma, Madrid, Spain) during 30 min. Incubation with primary antibodies was done overnight at 4 C. Then, they were washed 3 times in PBS and incubated with the corresponding secondary antibody (Table 1) during 2 h at room temperature. Finally, samples were washed again in PBS and stained with Hoechst 33258 (Invitrogen, Prat de Llobregat, Barcelona, Spain) at 10 μg/ml as a nuclear counterstain. Samples were examined with an epifluorescence microscope Olympus Bx60 (Olympus, Barcelona, Spain) and an image capture and analyzing system (Software Genus, 3.0 version, Cupertino, California, USA).
Table 1.
Primary and secondary antibodies used for embryonic stem cell characterization
| Primary antibody | Localization | Dilution | Reference | Secondary antibody | Concentration | Reference |
|---|---|---|---|---|---|---|
| Mouse monoclonal anti-Oct4 | Nuclear | 1:50 | Santa Cruz sc-5279 | Chicken anti-mouse IgG Alexa Fluor 488 | 6 μg/ml | Molecular Probes A-21200 |
| Rabbit polyclonal anti-Nanog | Nuclear | 1:200 | Abcam ab21603 | Goat anti-rabbit IgG Alexa Fluor 594 | 6 μg/ml | Molecular Probes A-11037 |
| Rabbit polyclonal anti-Sox2 | Nuclear | 1:200 | Chemicon AB5603 | Goat anti-rabbit IgG Alexa Fluor 594 | 6 μg/ml | Molecular Probes A-11037 |
| Goat polyclonal anti-Cdx2 (negative control) | Nuclear | 1:50 | Santa Cruz sc-19478 | Rabbit anti-goat IgG Alexa Fluor 488 | 6 μg/ml | Molecular Probes A-11078 |
| Rat monoclonal anti-SSEA3 (negative control) | Membrane | 1:20 | Chemicon MAB4303 | Goat anti-rat IgM Alexa Fluor 488 | 6 μg/ml | Molecular Probes A-21212 |
| Rabbit polyclonal anti-Nestin | Cytosolic | 1:250 | Abcam ab5968 | Goat anti-rabbit IgG Alexa Fluor 594 | 6 μg/ml | Molecular Probes A-11037 |
| Rabbit polyclonal anti-AFP | Cytosolic | 1:400 | Dako A0008 | Goat anti-rabbit IgG Alexa Fluor 594 | 6 μg/ml | Molecular Probes A-11037 |
| Mouse monoclonal anti-α-SMA | Cytosolic | 1:400 | Sigma A5228 | Chicken anti-mouse IgG Alexa Fluor 488 | 6 μg/ml | Molecular Probes A-21200 |
In vitro differentiation of embryonic stem cell lines
To confirm the pluripotency of the established ESC lines, some of them were subjected to differentiating culture conditions to produce cells of the three germ layers: ectoderm, endoderm and mesoderm. The in vitro differentiation procedure was performed in ESC lines produced with the standard method and the defined culture medium method as these two methods are the most different in terms of culture conditions used to derive the lines. ESC lines were subculture to dishes pre-coated with gelatin (Sigma, Madrid, Spain) but in the absence of feeder cells and LIF. Moreover, in the defined culture medium method the KSR was replaced with FCS to favor differentiation. Culture medium was replaced every 2 days and after a period of approximately 7 days, the lines were fixed for immunofluorescence analysis.
Characterization of embryonic stem cell lines differentiated in vitro
The characterization of the in vitro differentiated ESC lines was performed by immunofluorescence for the detection of specific markers as the neuroepithelial stem cell protein (Nestin), characteristic of the ectodermal layer; alpha-fetoprotein (AFP), characteristic of endoderm; and α-smooth muscle actin (α-SMA), characteristic of mesoderm. Primary and secondary antibodies used are detailed in Table 1. All ESC lines were also immunostained with Oct4 in addition to each marker of differentiation to detect not fully differentiated cells and, finally, stained with Hoechst 33258 as a nuclear counterstain. The immunofluorescence procedure was the same used for the characterization of the undifferentiated ESC lines.
Experimental design
Experiments to determine ESC derivation efficiency were carried out in two series. In the first one, the influence of the number of isolated blastomeres used to start the derivation process was analysed while, in the second the influence of the embryonic stage on this parameter was assessed. Three ESC derivation methods aforementioned (the standard method, the pre-adhesion method and the defined culture medium method) were used for each set of experiments in order to determine which of them provided the highest derivation efficiency.
In the first set of the experiments, a minimum of 25 blastomeres for each test group (1/4 and 2/4 and 1/8, 2/8, 3/8 and 4/8 at the 4-cell and 8-cell stages respectively) were used. Isolated blastomeres of the same derivation group at the 4- or 8-cell stages were pooled, randomly distributed and individually plated in order to avoid their aggregation. In the second series of experiments 25 embryos for each test group (4-cell and 8-cell whole embryos) were used. In both series of experiments the positive controls were morula and blastocyst stages, traditionally used in ESC derivation.
Statistical analysis
Results were statistically analyzed by X2 or Fisher exact test using the GraphPad InStatTM program (La Jolla, California, USA). Values with a p < 0.05 were considered statistically significant.
Results
Influence of the derivation method on ESC derivation efficiency
Three different derivation methods (the standard method, the pre-adhesion method and the defined culture medium method) were used in order to determine which one provided the highest efficiency in terms of ESC derivation. The influence of the method applied was tested in experiments of ESC derivation from isolated blastomeres and from whole embryos at different developmental stages in two different series of experiments, as previously mentioned. A total of 227 ESC lines consisting in colonies with an ESC-like morphology were obtained in these two series of experiments of which 200 (88%) tested positive for all pluripotency markers analyzed by immunofluorescence. ESC derivation efficiencies were calculated based on these positive ESC lines. Data showing these results from isolated blastomeres and whole embryos are illustrated in Tables 2 and 3.
Table 2.
Influence of the method on the percentage of embryonic stem cell lines derived from isolated blastomeres
| Derivation groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1/4 (n) | 2/4 (n) | 1/8 (n) | 2/8 (n) | 3/8 (n) | 4/8 (n) | Morula (n) | Blastocyst (n) | |
| Standard method | 3.8 (3/78)a | 1.9 (1/53)a | 0 (0/57)a | 6.5 (2/31)a | 20 (5/25)a | 28 (7/25)ab | 15.6 (5/32)a | 12.5 (3/25)a |
| Pre-adhesion method | 2.3 (1/44)a | 4 (1/25)a | 3.7 (1/27)a | 4 (1/25)a | 8 (2/25)a | 8 (2/25)a | 20 (5/25)ab | 24 (6/25)ab |
| Defined culture medium method | 12 (3/25)a | 32 (8/25)b | 4 (1/25)a | 8 (2/25)a | 24 (6/25)a | 44 (11/25)b | 48 (12/25)b | 48 (12/25)b |
a, b Values with different superscripts within the same column differ significantly among derivation methods
Table 3.
Influence of the method on the percentage of embryonic stem cell lines derived from whole embryos
| Derivation groups | ||||
|---|---|---|---|---|
| 4-cell stage (n) | 8-cell stage (n) | Morula (n) | Blastocyst (n) | |
| Standard method | 8 (2/25)a | 16 (4/25)ab | 44 (11/25)ab | 44 (11/25)ab |
| Pre-adhesion method | 8 (2/25)a | 4 (1/25)a | 24 (6/25)a | 28 (7/25)a |
| Defined culture medium method | 36 (9/25)b | 44 (11/25)b | 72 (18/25)b | 72 (18/25)b |
a, b Values with different superscripts within the same column differ significantly among derivation methods
In both series of experiments, the defined culture medium method turned out to be the most efficient for ESC derivation from post-compaction control groups, i.e. morula and blastocyst (Tables 2 and 3). It is important to note that, in general, ESC derivation efficiencies from these control groups were lower in the first series of experiments (Table 2) than in the second one (Table 3), but differences between them were only significant for the standard method (morula p = 0.0357; blastocyst p = 0.0255).
When isolated blastomeres were used to start the ESC derivation process, significant differences among methods did not appear until at least half of the embryo was used to start the ESC derivation (Table 2). Particularly, the most efficient method for the derivation from the 2/4 group turned out to be the defined culture medium method (p = 0.0003 and p = 0.0232 when comparing with the standard and the pre-adhesion methods, respectively). Similarly, the defined culture medium method provided the highest percentage of ESC derivation for the 4/8 group, but in this case significant differences were only found when compared to the pre-adhesion method (p = 0.0083).
Interestingly, whole pre-compaction embryos at the 4- and 8-cell stages showed a similar pattern to their half-embryos counterparts in terms of the influence of the method on ESC derivation efficiency (Table 3). Thus, the defined culture medium method was the most efficient in terms of ESCs production both for 4- and 8-cell stage embryos, although not significantly different from the standard method at the 8-cell stage.
Influence of the number of isolated blastomeres on ESC derivation efficiency
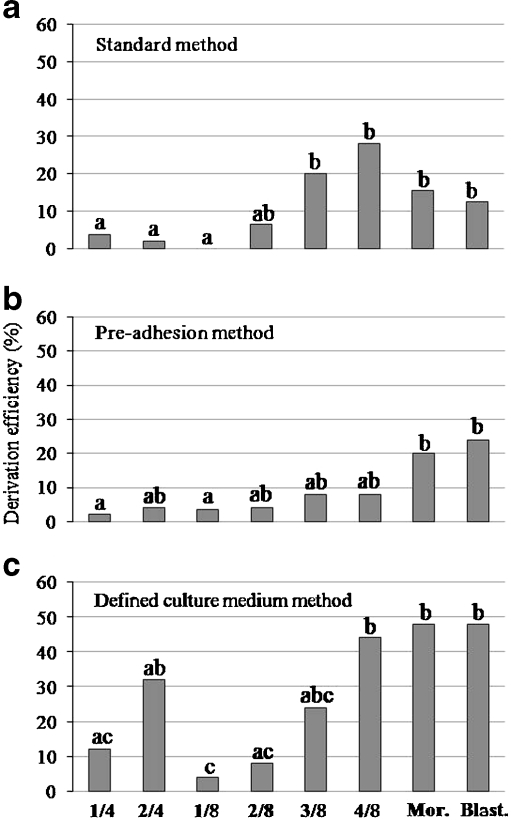
In the first set of experiments, the influence of the number of isolated blastomeres on ESC derivation was assessed using the three derivation methods previously analyzed. In this case, results are expressed in graphs and data is statistically analyzed between derivation groups of a particular method to observe the trend while increasing the number of isolated blastomeres (Fig. 2). A significant correlation between the number of isolated blastomeres and the number of positive ESC lines derived was found for all the three derivation methods analyzed (standard method p < 0.0001; pre-adhesion method p = 0.0241; defined culture medium method p = 0.0001). Figure 3 (a–c) shows an example of an ESC line in culture derived from two isolated blastomeres at the 8-cell stage (2/8 group). Positive ESCs from this derivation group confirmed by immunofluorescence are illustrated in Fig. 4 (Panel a, A–D). Moreover, the confirmation of the in vitro differentiation of an ESC line derived from 4/8 blastomeres by the standard method is shown in Fig. 4 (Panel b, A–C). As previously mentioned, the differentiated lines were immunostained with three markers of differentiation and also with Oct4 to detect undifferentiated cells remaining inside ESC colonies. The combination of both kinds of markers showed the distribution of the newly differentiated cells with respect to the remaining undifferentiated cells inside the ESC colonies. Particularly, Nestin-positive cells were uniformly distributed around the colonies of ESCs at low density, AFP-positive cells were found inside some ESC colonies, either as a group or isolated; and, finally, α-SMA-positive cells were also uniformly distributed around ESC colonies like Nestin-positive cells, but at higher density.
Fig. 2.
Influence of the number of isolated blastomeres on embryonic stem cell derivation by the standard (a), the pre-adhesion (b) and the defined culture medium method (c)
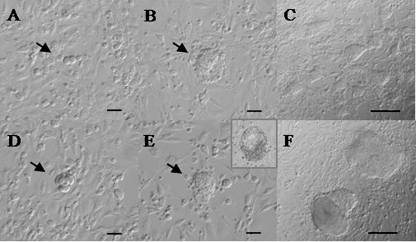
Fig. 3.
A–C. Embryonic stem cell (ESC) derivation from 2 blastomeres at the 8-cell stage (2/8 group) by the standard method. A. Two blastomeres from an embryo at the 8-cell stage onto a monolayer of feeder cells (Scale bar: 50 μm). B. A 3-day outgrowth (Scale bar: 50 μm). C. 30-day ESC colonies (Scale bar: 100 μm). D-F. ESC derivation from a whole embryo at the 4-cell stage by the defined culture medium method. D. A 4-cell stage embryo without zona pellucida onto a feeder cell monolayer (Scale bar: 50 μm). E. A 4-day outgrowth (Scale bar: 50 μm). In this method, embryos used to initiate the process by forming a blastocyst and then attaching to the feeder cells (inset) F. 30-day ESC colonies (Scale bar: 100 μm)
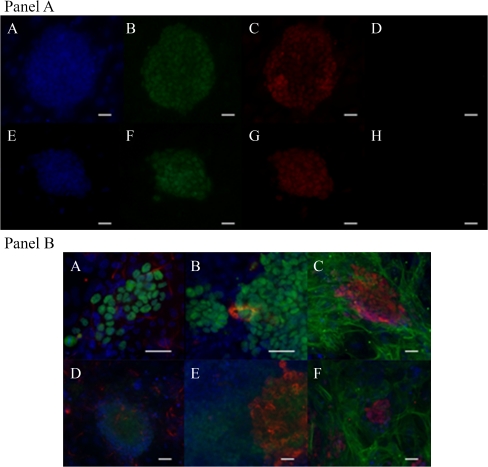
Fig. 4.
Panel a: characterization of embryonic stem cell (ESC) lines by immunofluorescence. A–D ESC line derived from 2/8 blastomeres stained with Hoechst (A), positive for Oct4 (B) and Nanog (C) markers and negative for the expression of Cdx2 marker (D). E–H. ESC line derived from a whole 4-cell embryo stained with Hoechst (E), positive for Oct4 (F) and Sox2 (G) markers and negative for the expression of SSEA3 marker (H). Scale bars: 30 μm. Panel b: characterization of ESC lines differentiated in vitro. A–C In vitro differentiation of an ESC line derived from 4/8 blastomeres by the standard derivation method positive for Nestin (A), AFP (B) and α-SMA (C). D–F In vitro differentiation of an ESC line derived from a whole 4-cell stage embryo by the defined culture medium method positive for Nestin (D), AFP (E) and α-SMA (F). Oct4 is detected in green in pictures A, B, D and E and in red in picture C and F. Scale bars: 30 μm
The comparison, in terms of ESC derivation efficiency, between both control stages (morula and blastocyst) turned out to be not statistically significant for any of the three methods used. ESC derivation from one or two blastomeres at the 4-cell stage (1/4 and 2/4 groups) was similarly efficient in all the three methods. However, when compared to control groups (morula and blastocyst), derivation from the 1/4 group provided significantly lower efficiencies in the three methods whereas the 2/4 group resulted in similar derivation efficiencies as the controls, except for the standard method. When the ESC derivation was started with 1–4 isolated blastomeres at the 8-cell stage a general tendency towards higher derivation efficiencies was observed when increasing the number of isolated blastomeres, but the significance of this tendency varied according to the derivation method used. In the standard method, derivation from 2, 3 or 4 blastomeres turned out to be equivalent to the control groups, while derivation from a single blastomere was null (Fig. 2a). A similar trend was observed in the pre-adhesion method though, in this case, derivation from the 1/8 group, while still less efficient than from the control groups, was not significantly different from the 2/8, 3/8 and 4/8 groups (Fig. 2b). Finally, in the defined culture medium method, only the 3/8 and 4/8 groups resulted in a similar number of ESC lines as the control groups, and significant differences between both the 1/8 and 2/8 groups and the 4/8 group were found (Fig. 2c).
It is interesting to point out that independently of the derivation method applied and the embryonic stage from which the blastomeres were obtained, derivation efficiencies from single blastomeres (1/4 and 1/8) were low (<12%) and in all cases significantly lower than from whole post-compaction control embryos. Moreover, for the 1/8 group in particular, no ESC lines could be obtained when using the standard method (Fig. 2a). Despite the two-fold difference in cell volume between the 1/4 and the 1/8 groups, they provided similar derivation efficiencies under the three derivation methods applied.
To try to gain some insight into the cause for such low derivation efficiency from the 1/4 and 1/8 groups, the rates of blastomere division after the first 24 hours in culture were compared among all the groups. As shown in Table 4, the division capacity of the 1/8 group resulted to be extremely significantly lower than the one of the 1/4 group (p < 0.0001) in all the methods tested except for the standard one. Moreover, the rate of division of the 1/8 group was significantly lower than those of the rest of the derivation groups with a higher number of blastomeres at the 8-cell stage (2/8, 3/8 and 4/8 groups) for all three derivation methods. By contrast, when comparing the division capacity of the 1/4 group to the one of the 2/4 group, differences only appeared in the standard method.
Table 4.
First division percentages of isolated blastomeres
| Derivation groups | ||||||
|---|---|---|---|---|---|---|
| 1/4 (n) | 2/4 (n) | 1/8 (n) | 2/8 (n) | 3/8 (n) | 4/8 (n) | |
| Standard method | 39.7 (31/78)ac | 77.4 (41/53)b | 28.1 (16/57)c | 61.3 (19/31)ab | 72.0 (18/25)b | 84.0 (21/25)b |
| Pre-adhesion method | 70.5 (31/44)ac | 60.0 (15/25)ac | 22.2 (6/27)b | 76.0 (19/25)ac | 56.0 (14/25)a | 88.0 (22/25)c |
| Defined culture medium method | 84.0 (21/25)a | 92.0 (23/25)a | 16.0 (4/25)b | 84.0 (21/25)a | 88.0 (22/25)a | 100.0 (25/25)a |
a, b, c Values with different superscripts within the same row differ significantly among derivation methods
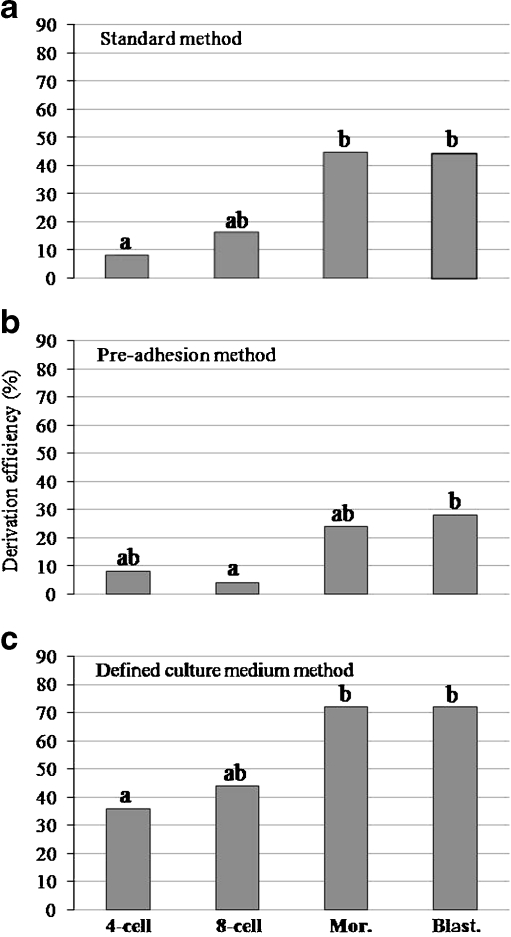
Influence of the embryonic stage on ESC derivation efficiency
In the second set of experiments, whole embryos at different developmental stages were used to assess the influence of the embryonic stage on ESC derivation. In this case, results have been also expressed in graphs in order to show the trend as development proceeds and statistical analysis has been performed between embryonic stages among a particular derivation method (Fig. 5). A significant association between the embryonic stage and the number of positive ESC lines derived was found for the standard and the defined culture medium methods (p = 0.0044 and p = 0.0134, respectively). Figure 3 (D–F) shows an example of ESC colonies in culture derived from a whole 4-cell stage embryo. An example of a positive ESC line from a 4-cell whole embryo, confirmed by immunofluorescence, is shown in Fig. 4 (E–H). Moreover, the confirmation of the in vitro differentiation of an ESC line derived from a whole 4-cell stage embryo by the defined culture medium method is shown in Fig. 4 (Panel b, D–F). In this case, the distribution of the markers of differentiation with respect to Oct4 followed the same trend described before.
Fig. 5.
Influence of the embryonic stage on embryonic stem cell derivation by the standard (a), the pre-adhesion (b) and the defined culture medium method (c)
As shown in Fig. 5, no significant differences in the ESC derivation efficiency were found between post-compaction control stages (morula and blastocyst) or between pre-compaction stages (4-cell and 8-cell stages) for any of the three methods used. However, comparing pre- and post-compaction stages, the derivation efficiency obtained from 4-cell embryos was significantly lower than from post-compaction control stages for both the standard (p = 0.0083; Fig. 5a) and the defined culture medium methods (p = 0.0222; Fig. 5c). By contrast, 8-cell stage embryos resulted in an equivalent percentage of ESC colonies than control post-compaction embryos, with the only exception of a significantly lower derivation efficiency in comparison to blastocysts in the pre-adhesion method (p = 0.0488; Fig. 5b). Therefore, derivation of ESC lines from post-compaction embryos is in general more efficient than from pre-compaction stages, even though 8-cell stage embryos are more similar to the post-compaction groups than 4-cell embryos.
Discussion
The aim of the present study was to develop mouse ESC lines from isolated blastomeres and whole embryos at the 4- and 8-cell stages by comparing three already described derivation methods and to analyze the influence of the number of blastomeres and the embryonic stage on the derivation efficiency.
ESC lines could be derived from all groups of blastomeres and whole embryos at the 4- and 8-cell stages with a reasonable efficiency (1.9–44%) using the three derivation methods compared. There was only one exception, the 1/8 group which did not produce any ESC line when using the standard method. Moreover, pluripotency of ESC lines described in our study was confirmed by the expression of specific markers of undifferentiated cells and by the ability of these lines to differentiate in vitro into cells of the three germ layers.
Control post-compaction stages (morula and blastocyst) exhibited variable derivation efficiencies ranging from 12.5% to 72%. Particularly, in the first series of experiments, ESC derivation efficiencies from morulae and blastocysts were relatively low (12.5–48%) when compared to those reported by other authors, specially for the standard method. It is well known that ESC lines can be derived from up to 50% of the blastocysts by using the same culture method [22] and in a more recent study, using a method equivalent to our defined culture medium mehtod, Wakayama et al. (2007) [14] reported 88% of derivation from blastocysts (vs. 48% in our study). These previous data suggested us that ESC derivation efficiencies were susceptible to be increased by changing the batch of feeder cells. Due to their long term culture (for more than 200 passages), the feeder cells could have a reduced capacity to sustain the ESC derivation process, therefore producing low rates of ESC derivation. The new batch of feeder cells used to perform the second set of experiments increased the efficiency of ESC derivation from control post-compacted embryos reaching percentages more similar to those found by the previous aforementioned studies. Moreover, due to the fact that differences between the two series of experiments were statistically significant only for the standard method, it could be deduced that the contact between blastomeres or embryos and feeder cells could be more crucial in this method than in the others. By contrast, in the defined culture medium method, which uses the same technical conditions but a different composition of the medium, the contact with feeder cells seems to be less important suggesting that the composition of the medium is the crucial aspect to produce ESC lines. As previously described [14], the use of KSR results in a more defined composition of the medium that may overcome the differentiating phenomena of the putative ESC induced by the uncontrolled factors provided by the FCS. Moreover, the introduction of the ACTH hormone supports the derivation from single blastomeres as well as the propagation of the ESC line from single cells.
When analyzing the influence of the derivation method on the derivation efficiencies obtained from pre-compacted embryos, significant differences were found between the three methods compared only when the derivation process was initiated by at least half of the embryo (2/4 and 4/8 groups) or by the whole embryo (4- and 8-cell stages). This could be due to the fact that a low number of blastomeres involved in the derivation process may result in low efficiency rates that could conceal differences among methods.
Several attempts have been done in the last few years to increase the derivation efficiency from single blastomeres by testing different technical and culture conditions. Delhaise et al. (1996) [20] used the conventional derivation method, equivalent to the standard method used in our experiments, to derive mouse ESC lines from disaggregated uncompacted 8-cell stage embryos (1/8 group) and obtained a derivation efficiency of 1.9% using the 129/Sv strain. More recently, Wakayama et al. (2007) [14] established mouse ESCs from isolated blastomeres at early 4-cell (28–40%), late 4-cell (22%) and 8-cell (14–16%) stages from (B6D2F1 × 129/Sv)F1 and (129/Sv × 129/Sv)F1 embryos using equivalent culture conditions to the defined culture medium method in our experiments. The rates of ESC lines production in the aforementioned studies are higher than those obtained in our experiments and it could be attributed to differences in the genetic background of the embryos used to derive the ESC lines. It is well established that the mouse strains used to obtain the embryos have an influence on the derivation process [23].
The technical conditions of the ESC culture were also compared in our experiments by using the pre-adhesion method, equivalent to the one set up by Tesar (2005) [8] in which denuded 4- and 8-cell stage mouse embryos were first attached to the culture dish by incubating them in a protein free PBS solution. After the attachment, the feeder cell suspension was poured on 5 min later. Using this technique, the author claims that ESC derivation efficiency from pre-compacted stages increased over 50%. He suggested that the first adhesion to the culture dish, like morulae do spontaneously after they are seeded onto the feeder cell monolayer, was crucial to increase ESC derivation efficiency. In our study, we were able to attach isolated blastomeres to the culture dish using the pre-adhesion method, in addition to whole embryos at the 4- and 8-cell stages as were used in Tesar’s experiments. However, this pre-adhesion method resulted the least efficient among all the derivation groups analyzed at the 4- and 8-cell stages, maybe because it involves a more extensive manipulation of the blastomeres and embryos which could decrease their viability. Moreover, our results suggest that this initial attachment is not required to increase the derivation efficiency and that the crucial aspect may be the first contact with the feeder cells.
Therefore, from the analysis of the effect of the ESC derivation method we can conclude that the higher rates of ESC lines derivation are obtained with the defined culture medium method, probably because of the use of KSR and ACTH, compounds not present in the culture medium used in the standard method, and the more gentle manipulation it involves as compared to the pre-adhesion method. It seems that the adhesion to the substrate at the beginning of the derivation process in the pre-adhesion method is not so important in the efficiency of the method.
Concerning the influence of the number of isolated blastomeres on ESC derivation, studies with different number of isolated blastomeres at different development stages had never been performed, but previous experiments [24, 25] suggested the importance of cell number reduction on the viability of the embryo after transfer. He reported that both a single blastomere at the 4-cell stage and two blastomeres at the 8-cell stage formed a pseudoblastocyst with a clear and distinguishable ICM resulting in an equivalent embryonic survival rate after transfer. By contrast, one single blastomere at the 8-cell stage formed a pseudoblastocyst which showed a reduced viability due to the lack of an evident ICM. He suggested that it was not due to a reduced developmental potential of the single blastomere but a matter of the remaining proportion of the embryo volume to form the whole embryo. Assuming that embryos that reach cavitation with a higher number of ICM cells have higher chances to produce ESC lines, Tarkowski et al. (2005) [26] suggested a relationship between the number of blastomeres involved in the ESC derivation and the efficiency of the process. In groups starting the derivation with a higher embryo volume, the number of blastomeres at the ICM was higher too thus obtaining a higher efficiency of ESC lines derivation. Altogether, these data seem to indicate that the remaining proportion of the embryo volume and the number of isolated blastomeres are two aspects intimately linked and that the number of blastomeres involved is the main aspect to ensure both embryo viability and ESC derivation.
Consistent with these data, our study shows a general tendency towards similar derivation efficiencies among groups that have different cell number and different embryonic stage but the same remaining embryo volume. These results, obtained by the comparison of groups of 1/4 vs. 2/8 and 2/4 vs. 4/8, suggest an important relationship between the remaining embryo volume used to derive ESCs and the efficiency of the process. Moreover, the comparison between derivation groups which have different embryonic stage and embryo volume but the same number of isolated blastomeres (1/4 vs. 1/8 and 2/4 vs. 2/8) reveals equivalent derivation efficiencies for the three derivation methods also suggesting an important role of the number of blastomeres on the derivation process. Therefore, these two sets of comparisons between groups with the same embryo volume first, and with the same cell number second, suggest again the relationship between the embryo volume and the cell number to ensure the ESC derivation process. The fact of having a higher number of ICM-like blastomeres, key to the ESC derivation process, could explain the higher efficiency of this process when starting with a higher proportion of the embryo volume. Furthermore, cell to cell adhesion and communication could be responsible for the influence of the cell number on the derivation capacity as will be further discuss below.
It is important to note that no ESC lines could be derived from the 1/8 group by using the standard method and that low derivation rates were obtained from this group using the pre-adhesion (3.7%) and the defined culture medium methods (4%). This low efficiency could be attributed to the reduced division capacity of the 1/8 group which in turn could be due to the lack of contact with neighboring blastomeres. In fact, intercellular junctions between blastomeres at the 8-cell stage are thought to be involved in multiple processes such as cell differentiation, cell signaling and cell proliferation [27]. Therefore, further research will be needed to determine the possible role of such junctions in ESC derivation.
Finally, the analysis of the effect of the embryonic stage on the derivation process revealed significant differences between pre- and post-compaction stages, being the latter the most efficient to derive ESC lines. However, recent studies have pointed out the possibility that early cleavage stages provide higher derivation efficiencies due to a higher degree of pluripotency of the blastomeres at these stages [14]. Despite this, the blastocyst is the stage which has traditionally provided the highest derivation efficiencies [28] and, moreover, Wakayama and co-workers in the aforementioned study obtained the highest ESC derivation rates (88%) from blastocysts. This fact agrees with other authors’ suggestion that the key aspect to derive ESCs is to have blastomeres with a defined ICM phenotype [29]. Moreover, it has been suggested that cell fate decisions could be earlier made at 2- and 4-cell stages [30–32]. On the other hand, early studies on artificial twinning [33, 34] suggested the importance of having a sufficient amount of ICM-like cells from the isolated blastomeres before cavitation to ensure embryo viability. From these studies it can be deduced that early embryonic stages could have higher chances to produce ESC lines than late embryonic stages, not because of an increased development potential but as a matter of a higher amount of ICM-like cells due to the superior number of cleavages they can perform. This issue has to be further studied.
Our results from isolated blastomeres show that ESC derivation efficiency increases as development progresses suggesting that late embryonic stages, which have a more committed and defined ICM phenotype, contribute more efficiently to form ESC lines than early embryonic stages. In this situation, the higher number of ICM-like blastomeres formed from early cleavage stages seems to be less important in the derivation process, suggesting that the real important event is to have a more committed and defined ICM phenotype provided by late stages. When using whole embryos to obtain ESCs, however, all blastomeres are involved in the derivation process so the number of blastomeres is not a limiting factor to produce enough cells with an ICM phenotype. In this case, the influence of the embryonic stage can be properly analyzed and again our results point out to higher derivation efficiencies as development progresses, when blastomeres have the possibility to acquire a more defined ICM phenotype.
Moreover, the standard and defined culture medium methods showed equivalent derivation efficiencies from the 8-cell stage and control stages. These results again support the previous hypothesis since inner blastomeres at the 8-cell stage should have a more defined fate to produce ICM cells than 4-cell stage blastomeres. In the pre-adhesion method this tendency is not observed perhaps because the low derivation efficiencies of this method could be concealing differences between embryonic stages.
Overall, from the study we present, we can conclude that mouse ESC lines from isolated blastomeres and whole embryos can be efficiently derived by using the defined culture medium method which has provided the highest rates of ESC lines production. Particularly, when deriving from isolated blastomeres, ESC derivation efficiency increases by using the highest proportion of the embryo volume and therefore, the maximum number of isolated blastomeres at the 4- and 8-cell stages that can be removed without compromising embryo viability. Likewise, ESC derivation from whole embryos was more efficient as embryonic development progresses, when blastomeres acquire a more defined ICM phenotype.
Conclusions
Overall, from the study we present, we can conclude that mouse ESC lines from isolated blastomeres can be efficiently derived by using the highest proportion of the embryo volume and therefore the maximum number of isolated blastomeres at the 4- and 8-cell stages that can be removed without compromising embryo viability. Moreover, the method which provides the highest rates of ESC lines production is the defined culture medium method.
Acknowledgements
This work received financial support from the Spanish Ministerio de Educación y Ciencia (MEC) projects BIO 2005-04341 and BIO2006-11792, the Generalitat de Catalunya DGR project #2005SGR-0047 and MEC FPU fellowship AP2006-02038. We thank Marc Puigcerver and Jonatan Lucas their technical assistance.
Footnotes
Capsule
Influence of the embryonic stage and the number of isolated blastomeres on the derivation of mouse embryonic stem cells using three described derivation methods.
References
- 1.Rossant J. Stem cells and early lineage development. Cell. 2008;132:527–31. doi: 10.1016/j.cell.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 2.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 3.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–7. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 5.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 6.Niwa H. Molecular mechanism for cell-fate determination in ES cells. Tanpakushitsu Kakusan Koso. 2000;45:2047–55. [PubMed] [Google Scholar]
- 7.Eistetter HR. Pluripotent embryonal stem cell lines can be established from disaggregated mouse morulae. Develop Growth Differ. 1989;31:275–82. doi: 10.1111/j.1440-169X.1989.00275.x. [DOI] [PubMed] [Google Scholar]
- 8.Tesar PJ. Derivation of germ-line-competent embryonic stem cell lines from preblastocyst mouse embryos. Proc Natl Acad Sci. 2005;102:8239–44. doi: 10.1073/pnas.0503231102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strelchenko N, Verlinsky O, Kukharenko V, Verlinsky Y. Morula-derived human embryonic stem cells. Reprod Biomed Online. 2004;9:623–9. doi: 10.1016/S1472-6483(10)61772-5. [DOI] [PubMed] [Google Scholar]
- 10.Chung Y, Klimanskaya I, Becker S, Marh J, Lu SJ, Johnson J, et al. Embryonic and extraembryonic stem cell lines derived from single mouse blastomeres. Nature. 2006;439:216–9. doi: 10.1038/nature04277. [DOI] [PubMed] [Google Scholar]
- 11.Chung Y, Klimanskaya I, Becker S, Maserati M, Lu SJ, Zdravkovic T, et al. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell. 2008;2:113–7. doi: 10.1016/j.stem.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Geens M, Mateizel I, Sermon K, Rycke M, Spits C, Cauffman G, et al. Human embryonic stem cell lines derived from single blastomeres of two 4-cell stage embryos. Hum Reprod. 2009;24:2709–17. doi: 10.1093/humrep/dep262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444:481–5. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- 14.Wakayama S, Hikichi T, Suetsugu R, Sakaide Y, Bui HT, Mizutani E, et al. Efficient establishment of mouse embryonic stem cell lines from single blastomeres and polar bodies. Stem Cells. 2007;25:986–93. doi: 10.1634/stemcells.2006-0615. [DOI] [PubMed] [Google Scholar]
- 15.Handyside AH, Lesko JG, Tarin JJ, Winston RM, Hughes MR. Birth of a normal girl after in vitro fertilization and preimplantation diagnostic testing for cystic fibrosis. N Engl J Med. 1992;327:905–9. doi: 10.1056/NEJM199209243271301. [DOI] [PubMed] [Google Scholar]
- 16.Veiga A, Calderon G, Barri PN, Coroleu B. Pregnancy after the replacement of a frozen-thawed embryo with less than 50% intact blastomeres. Hum Reprod. 1987;2:321–3. doi: 10.1093/oxfordjournals.humrep.a136542. [DOI] [PubMed] [Google Scholar]
- 17.Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci. 1975;72:1441–5. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci. 1997;94:5709–12. doi: 10.1073/pnas.94.11.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biggers JD, McGinnis LK, Raffin M. Amino acids and preimplantation development of the mouse in protein-free potassium simplex optimized medium. Biol Reprod. 2000;63:281–93. doi: 10.1095/biolreprod63.1.281. [DOI] [PubMed] [Google Scholar]
- 20.Delhaise F, Bralion V, Schuurbiers N, Dessy F. Establishment of an embryonic stem cell line from 8-cell stage mouse embryos. Eur J Morphol. 1996;34:237–43. doi: 10.1076/ejom.34.4.237.13046. [DOI] [PubMed] [Google Scholar]
- 21.Robertson EJ. Embryo-derived stem cell lines. In: Robertson EJ, editor. Teratocarcinomas and embryonic stem cells a practical approach. Oxford: IRL; 1987. pp. 71–112. [Google Scholar]
- 22.Lanza R, Weissman I, Thomson J, Pedersen R, Hogan B, Gearhart J, et al. Isolation and maintenance of murine embryonic stem cells. New York: Cold Spring Harbor; 2004. [Google Scholar]
- 23.Kawase E, Suemori H, Takahashi N, Okazaki K, Hashimoto K, Nakatsuji N. Strain difference in establishment of mouse embryonic stem (ES) cell lines. Int J Dev Biol. 1994;38:385–90. [PubMed] [Google Scholar]
- 24.Willadsen SM. The viability of early cleavage stages containing half the normal number of blastomeres in the sheep. J Reprod Fertil. 1980;59:357–62. doi: 10.1530/jrf.0.0590357. [DOI] [PubMed] [Google Scholar]
- 25.Willadsen SM. The development capacity of blastomeres from 4- and 8-cell sheep embryos. J Embryol Exp Morphol. 1981;65:165–72. [PubMed] [Google Scholar]
- 26.Tarkowski AK, Ozdzenski W, Czolowska R. Identical triplets and twins developed from isolated blastomeres of 8- and 16-cell mouse embryos supported with tetraploid blastomeres. Int J Dev Biol. 2005;49:825–32. doi: 10.1387/ijdb.052018at. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg MS, McNutt PM. Cadherins and their connections: adhesion junctions have broader functions. Curr Opin Cell Biol. 1999;11:554–60. doi: 10.1016/S0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 28.Rossant J. Stem cells from the Mammalian blastocyst. Stem Cells. 2001;19:477–82. doi: 10.1634/stemcells.19-6-477. [DOI] [PubMed] [Google Scholar]
- 29.Lorthongpanich C, Yang SH, Piotrowska-Nitsche K, Parnpai R, Chan AW. Development of single mouse blastomeres into blastocysts, outgrowths and the establishment of embryonic stem cells. Reproduction. 2008;135:805–13. doi: 10.1530/REP-07-0478. [DOI] [PubMed] [Google Scholar]
- 30.Gardner RL. Specification of embryonic axes begins before cleavage in normal mouse development. Development. 2001;128:839–47. doi: 10.1242/dev.128.6.839. [DOI] [PubMed] [Google Scholar]
- 31.Fujimori T, Kurotaki Y, Miyazaki J, Nabeshima Y. Analysis of cell lineage in two- and four-cell mouse embryos. Development. 2003;130:5113–22. doi: 10.1242/dev.00725. [DOI] [PubMed] [Google Scholar]
- 32.Piotrowska-Nitsche K, Zernicka-Goetz M. Spatial arrangement of individual 4-cell stage blastomeres and the order in which they are generated correlate with blastocyst pattern in the mouse embryo. Mech Dev. 2005;122:487–500. doi: 10.1016/j.mod.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Tarkowski AK, Wroblewska J. Development of blastomeres of mouse eggs isolated at the 4- and 8-cell stage. J Embryol Exp Morphol. 1967;18:155–80. [PubMed] [Google Scholar]
- 34.Rossant J. Postimplantation development of blastomeres isolated from 4- and 8-cell mouse eggs. J Embryol Exp Morphol. 1976;36:283–90. [PubMed] [Google Scholar]