Abstract
Purpose
To evaluate whether the duration of gonadotropin stimulation predicts the likelihood of live birth after ART.
Methods
All IVF or ICSI cycles using fresh autologous oocytes at our institution between January 2004 and December 2007 were analyzed.
Results
Out of 699 cycles resulting in oocyte retrieval, 193 produced a live birth (27.6%). Women who achieved a live birth had a significantly shorter stimulation phase (11.1 vs. 11.5 days, respectively). Multivariable analysis suggested that 13 days or longer of stimulation decreased the likelihood of a live birth by 53% as compared to cycles that were 10–12 days long (odds ratio [OR] 0.47; 95% confidence interval [CI]: 0.30–0.75) after adjustment for female age, maximum historical FSH, total dose of gonadotropin received, oocytes retrieved, embryos transferred, antagonist suppression and PCOS diagnosis.
Conclusions
Prolonged duration of gonadotropin stimulation is an independent negative predictor of ART success in our cohort.
Keywords: Assisted reproduction, Gonadotropins, IVF/ICSI outcome, Infertility, Ovarian stimulation
Introduction
Since the first human conceived in vitro birth in 1978, assisted reproductive technologies (ART) have been estimated to create between one and three million babies worldwide [1, 2] and now represent over 1% of all births in developed countries [3]. Identification of predictors of ART success is paramount for proper treatment and counseling [4, 5]. Female chronologic and reproductive ageing are recognized as leading factors that determine the probability of live birth after infertility treatment [6, 7]. However, these parameters are not modifiable and cannot aid in the active management of a gonadotropin stimulation cycle.
A critical clinical question that remains is whether anything can be done to optimize the outcome of ART either immediately prior or during infertility treatment. Obesity, smoking, and presence of hydrosalpinges reduce the odds of pregnancy and are potentially amenable to pre-treatment adjustment [8–10]. Protocols for controlled ovarian stimulation may constitute another aspect of therapy that influences outcome [11], however, there is a paucity of studies focused on the impact of specific aspects of gonadotropin stimulation on success with ART.
Using a univariate analysis, a recent study reported that the length of stimulation did not affect clinical pregnancy outcomes following ART [12]. A number of parameters may influence both the duration of gonadotropin stimulation and the likelihood of success, and, thus, constitute potential confounders. For example, a meta-analysis of data from 3,865 women demonstrated that the use of gonadotropin-releasing hormone (GnRH) antagonists shortened the ovarian response time and was associated with diminished chance for clinical pregnancy [13]. Similarly, obesity is associated with prolonged stimulation phase [14] and reduced pregnancy rates [10]. Further, in a pooled report of 330 cycles, women with polycystic ovary syndrome (PCOS) had significantly prolonged (1.2 days on average) gonadotropin stimulation cycles, when compared to non-PCOS controls [15].
A multivariable approach is best suited to evaluate whether a relationship exists between the duration of ovarian stimulation and the probability of live birth following ART. The objective of this study was to determine whether the duration of gonadotropin stimulation predicts live birth after ART.
Materials and methods
Patients
All IVF and ICSI cycles at Montefiore’s Institute for Reproductive Medicine and Health for the period between January 2004 and December 2007 were identified. Analysis was restricted to 545 women undergoing 794 fresh autologous oocyte embryo transfer (ET) cycles. Patient and cycle characteristics were evaluated, including age, female body mass index (BMI), maximum historical baseline follicle-stimulating hormone (FSH) level, and prior reproductive history. One hundred and eighty women had multiple cycles and each cycle was counted separately. Sensitivity analysis was conducted by analyzing a subset of patients who had only one cycle (i.e. first cycle analysis) as described below in detail. The study was approved by the Institutional Review Board, Montefiore Medical Center in accordance with the Declaration of Helsinki for Medical Research.
Stimulation protocols
Prevention of premature LH surge was achieved with ovarian down regulation via long luteal GnRH agonist protocol, GnRH agonist flare or, alternatively, with GnRH antagonist (59.8%, 1% and 39.2% of all cycles, respectively). Gonadotropin stimulation was initiated after down-regulation was established or after discontinuing oral contraceptives (typically, a standard course of 14 days). Standard formulations of gonadotropins (75 units per ampoule) were used for stimulation with initial dosing ranging from 150–450 IU per day. Ovarian response was monitored by serial follicular ultrasound and serum estradiol assessments, and gonadotropin dose was adjusted as needed as per routine clinical practice. The decision to proceed with oocyte retrieval was made when a minimum of 2 dominant follicles attained a size of 17 mm or more. Ultrasound-guided oocyte retrieval was performed approximately 34 h following ovulation trigger with human chorionic gonadotropin (hCG). Oocytes were inseminated with or without ICSI, as per our clinical practice and in accordance with general recommendations for cases of severe male factor infertility or previous failed or poor fertilization [16]. Evidence for fertilization was assessed approximately 18 h post insemination. ET was performed under ultrasound guidance on day 3 or 5 post insemination. Indications for day 5 transfer: at least six embryos on day two that are at least four cells with 15 or less percent fragmentation. The number of embryos transferred was based on the number and quality of embryos available, the age of the women and previous pregnancy history. Luteal support was provided by intramuscular progesterone supplementation until the first pregnancy test and was continued in all cycles with a positive serum βhCG until documentation of fetal cardiac activity. The ART cycle outcomes of interest were live born rate (delivery of a live born infant after 24 completed weeks of gestation) and clinical pregnancy rate (defined as an intrauterine gestational sac by ultrasound).
In addition to analyzing the duration of gonadotropin stimulation as a continuous variable, it was also categorized into three groups: less than or equal to 9 days, 10 to 12 days, and greater than or equal to 13 days of gonadotropin stimulation. The duration data was first categorized by quintiles in order to have roughly equal numbers of observations in each group, and then the three middle quintiles were combined to represent a clinically relevant intermediate length of stimulation for our cohort, giving rise to the final three groups.
Statistical analysis
Live birth after ART was regarded as the primary outcome. For continuous variables, associations between demographic and clinical characteristics and the outcome were assessed using Student’s t-test or ANOVA for normally distributed data and Mann–Whitney U test or Kruskal-Wallis for skewed data as appropriate. Chi-square test was used for categorical variables. In addition to bivariate analyses, multivariable logistic regression was conducted to determine predictors of live birth with duration of stimulation as the independent variable of interest. Logistic regression models were specified using model building strategies suggested by Hosmer and Lemeshow [17]. Appropriate regression diagnostics were performed to examine whether the model fit was supported over the entire set of covariate patterns. Because of the relatively small numbers for live birth in the longest duration group (n = 30), a propensity score analysis was used to adjust for covariates of importance without unduly burdening the statistical models [18]. Briefly, a propensity score was derived from a separate multivariable model (linear or logistic, as appropriate) incorporating the adjustment covariates of interest. Subsequently, this score was used as a single adjustment variable (summarizing the included covariates) in the logistic regression models that were used to determine the association between the ART outcome and the independent variable of interest (duration of stimulation). Separate sensitivity analyses were conducted by excluding those who underwent day 5 transfer, withdrawal of gonadotropin stimulation (“coasting”), had multiple cycles, or represented the outliers for the length of simulation (the lowest or highest 5% of distribution). The strength of association between the likelihood of live birth after ART and duration of stimulation is presented as an odds ratio (OR) with 95% confidence interval (CI). All statistical tests used a two-tailed alpha of 0.05. Analyses were performed using STATA 9.2 (StataCorp LP, College Station, TX).
Results
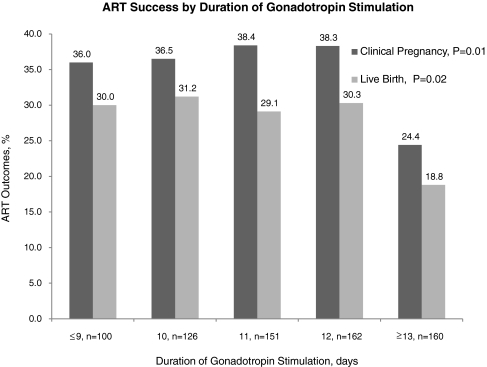
Of the 794 fresh autologous oocyte cycles undertaken during the study period, 699 cycles (88%) proceeded to oocyte retrieval. 241 cycles resulted in a clinical pregnancy (34.5% per retrieval) and 193 cycles produced a live birth (27.6% per retrieval). Table 1 describes the patient and cycle characteristics categorized by ART outcome. Patients with a live birth following ART were younger, had lower baseline FSH, fewer antagonist suppression cycles, shorter stimulation phases, used fewer ampoules of medication, and produced significantly more oocytes at retrieval than patients with no live birth. When duration of gonadotropin stimulation was considered by quintiles, only the longest duration (13 days or longer) was associated with a significantly lower clinical pregnancy and live birth rates when compared with the overall sample (p = 0.01 and p = 0.02, respectively, see Fig. 1). As the outcomes were nearly identical among patients in the intermediate ranges of duration (10, 11 or 12 days), three categories of length of stimulation phase were created: less than or equal to 9 days, 10 to 12 days, and greater than or equal to 13 days.
Table 1.
Mean (standard deviation) or prevalence (n) of demographic and cycle characteristics by ART outcome
| No live birth (n = 506) | Live birth (n = 193) | P-value | |
|---|---|---|---|
| Female Age, years | 35.7 (4.3) | 33.6 (3.9) | <0.01 |
| Primary infertility,% | 43.1 (218) | 44.0 (85) | 0.82 |
| History of prior live birth,% | 25.9 (131) | 29.0 (56) | 0.40 |
| Current smoking,% | 5.1 (26) | 6.7 (13) | 0.41 |
| Female BMI, kg/m2 | 25.6 (5.4) | 25.8 (6.1) | 0.56 |
| First ART cycle,% | 54.4 (275) | 59.6 (115) | 0.21 |
| Max historical FSH, mIU/ml | 8.1 (2.8) | 7.1 (2.3) | <0.01 |
| Duration of stimulation, days | 11.5 (1.9) | 11.1 (1.6) | 0.02 |
| ICSI,% | 67.4 (341) | 68.9 (133) | 0.70 |
| Suppression,% | |||
| Long luteal GnRH agonist | 55.9 (283) | 70.0 (135) | <0.01 |
| GnRH antagonist | 43.1 (218) | 29.0 (56) | |
| GnRH agonist flare | 1.0 (5) | 1.0 (2) | |
| Total dose of gonadotropins, IU | 3,593.5 (1,868) | 2,565.8 (1,274) | <0.01 |
| Coasting,% | 1.0 (5) | 1.6 (3) | 0.53 |
| Oocytes retrieved | 11.7 (6.9) | 13.9 (6.7) | <0.01 |
| Embryos transferred | 2.6 (1.0) | 2.7 (0.0) | 0.31 |
Fig. 1.
ART success by duration of gonadotropin stimulation
Cycles with at least 13 days of stimulation were associated with significantly higher doses of gonadotropin used per cycle and were more likely in women with a PCOS diagnosis (Table 2). Additionally, there was a statistically significant increase in the number of cycles that had undergone gonadotropin withdrawal (“coasting”), in the longest duration group. However, the absolute magnitude of this difference was small (ranging from 1 to 5 cycles). There was no significant difference in prevalence of cycles complicated by ovarian hyperstimulation syndrome (defined as bilateral ovarian enlargement, accompanied by abdominal discomfort and/or sudden weight gain) by the duration of stimulation (Table 2). However, it is conceivable that the sample was underpowered to detect a difference in this regard, as there were only 15 cases during the study period.
Table 2.
Mean (standard deviation) or prevalence (n) of demographic and cycle characteristics by duration of gonadotropin stimulation
| ≤ 9 days, n = 100 | 10–12 days, n = 439 | ≥13 days, n = 160 | P-valuea | |
|---|---|---|---|---|
| Female age, years | 34.6 (4.5) | 35.5 (4.3) | 34.5 (4.2) | 0.81 |
| Primary infertility,% | 40.0 (40) | 41.2(181) | 51.3 (82) | 0.07 |
| Female BMI, kg/m2 | 26.0 (6.3) | 25.4 (5.3) | 26.1 (5.8) | 0.36 |
| First ART cycle,% | 53.0 (53) | 54.9 (241) | 60.0 (96) | 0.45 |
| Max historical FSH, mIU/ml | 7.6 (2.7) | 7.7 (2.5) | 8.0 (3.0) | 0.62 |
| PCOS,% | 12.0 (12) | 8.9 (39) | 17.5 (28) | 0.01 |
| ICSI,% | 68.0 (68) | 66.3 (291) | 71.9 (115) | 0.43 |
| Suppression,% | ||||
| Long luteal GnRH agonist | 49.0 (49) | 63.1 (277) | 58.5 (92) | 0.12 |
| GnRH antagonist | 50.0 (50) | 36.0 (158) | 41.3 (66) | |
| GnRH agonist flare | 1.0 (1) | 0.9 (4) | 1.3 (2) | |
| Total dose of gonadotropins, IU | 2,401 (1,188) | 3,142 (1,615) | 4,319 (2,065) | <0.01 |
| Coasting,% | 1 (1) | 0.5 (2) | 3.1 (5) | 0.03 |
| OHSS,% | 2 (2) | 1.6 (7) | 3.8 (6) | 0.27 |
| Oocytes retrieved | 11.3 (6.3) | 12.8 (7.1) | 11.7 (6.6) | 0.21 |
| Embryos transferred | 2.6 (1) | 2.6 (0.9) | 2.5 (0.9) | 0.62 |
| Day 5 transfer,% | 2 (2) | 9.3 (41) | 6.9 (11) | 0.04 |
| Clinical pregnancy,% | 36.0 (36) | 37.8 (166) | 24.4 (39) | <0.01 |
| Live birth,% | 30.0 (30) | 30.3 (133) | 18.8 (30) | 0.02 |
aComparisons across three durations of stimulation were made by using chi-square for categorical variables or ANOVA or Kruskal-Wallis for continuous variables as appropriate
Multivariable logistic models were specified to estimate the effect of the duration of stimulation on the likelihood of live birth while adjusting for age, maximum historical FSH, total gonadotropin dose utilized, oocytes retrieved, and number of embryos transferred, antagonist suppression and PCOS diagnosis. It was noteworthy that the effect of the length of stimulation was similar whether the duration was used as a continuous or categorical variable (Table 3). Duration of stimulation of 13 days or longer was associated with reduction of the likelihood of a live birth by 53% as compared to cycles that were 10–12 days long (odds ratio [OR] 0.47; confidence interval [CI]: 0.30–0.75). Of note, antagonist suppression was also significantly associated with a reduction in live birth by 45%, while PCOS diagnosis was significantly associated with an increase in the odds of a live birth by 2-fold. Nonetheless, the effect of the gonadotropin stimulation duration in our sample was independent of these prognosticators (Table 3).
Table 3.
Likelihood of live birth (odds ratio ±95% confidence interval) after ART, by specified determinant
| Determinant | Unadjusted OR (95% CI) | P value | Fully adjusteda OR (95% CI) | P value |
|---|---|---|---|---|
| Antagonist suppression | 0.53 (0.37–0.75) | <0.01 | 0.55 (0.38–0.80) | <0.01 |
| PCOS diagnosis | 2.22 (1.36–3.49) | <0.01 | 2.13 (1.28–3.52) | <0.01 |
| Gonadotropin stimulation | ||||
| As a continuous variable, days | 0.90 (0.82–0.98) | 0.02 | 0.87 (0.79–0.97) | 0.009 |
| As a categorical variable | ||||
| Gonadotropin stimulation ≥13 daysb | 0.54 (0.33–0.83) | <0.01 | 0.47 (0.30–0.75) | <0.01 |
| Gonadotropin stimulation ≤9 daysb | 0.98 (0.61–1.58) | 0.95 | 1.04 (0.63–1.69) | 0.89 |
aIncluding propensity score analysis, adjusting for age, maximum historical FSH, total gonadotropin dose utilized, number of oocytes retrieved, and number of embryos transferred
bCompared with duration of gonadotropin stimulation of 10 to 12 days
Sensitivity analyses were performed to determine whether several potential factors (coasting, repeat cycles, and the outliers for the duration or day 5 transfer) might have biased the observed association between the live birth rate and length of stimulation. For all tested subsets, the association between the 13 days of stimulation or longer and ART success remained statistically significant and the point estimates remained similar to that of the full cohort (Table 4).
Table 4.
Sensitivity analyses of the association of duration of gonadotropin stimulation ≥13 days and likelihood of live birth after ART
| Analytic samplea | Odds ratio(95% CI)b | P value |
|---|---|---|
| Full analytic sample | 0.47 (0.30–0.75) | <0.01 |
| Excluding women who had more than one cycle, n = 305 | 0.51 (0.24–0.77) | <0.01 |
| Excluding women who had undergone gonadotropin withdrawal (“coasting”), n = 8 | 0.47 (0.29–0.76) | <0.01 |
| Excluding women who were outliers for the length of stimulation (approximately the lowest and highest 5% of distribution), n = 38 | 0.48 (0.29–0.80) | <0.01 |
| Excluding women with day 5 transfer, n = 54 | 0.50 (0.31–0.82) | <0.01 |
aLogistic regression models with likelihood of live birth after ART as the model outcome, adjusted for mode of suppression (GnRH antagonist vs. agonist), PCOS diagnosis, and propensity score analysis, including age, maximum historical FSH, total gonadotropin dose utilized, number of oocytes retrieved, and number of embryos transferred
bOdds ratio for the association of the duration of gonadotropin stimulation ≥13 days with the model outcome after adjustment for multiple covariates
Discussion
In this multivariable analysis of 699 cycles, we demonstrate that prolonged duration of gonadotropin stimulation is associated with decreased take-home baby rates after ART. Our data contribute to the consensus in the literature suggesting detrimental prognostic impact of increased gonadotropin requirements during ART [11, 19–21]. While most studies of gonadotropin utilization focus on the total amount of medication administered per cycle, we specifically evaluated live birth outcomes in relation to the time needed to achieve follicular development appropriate for oocyte retrieval. Among the assets of our study is the ability to control for confounders with multivariable analysis and the use of rigorous sensitivity analyses. Moreover, our conclusions are strengthened by the choice of the primary outcome, take-home baby rate, that is the ultimate yardstick for evaluation of efficacy of fertility interventions and the most clinically relevant statistic [22].
Martin et al. recently reported no effect of the length of gonadotropin stimulation on clinical pregnancy following ART in a univariate analysis of 555 cycles [12]. Several potential differences between our studies may account for the disparate results. Nearly one fifth of our patients were obese, with the mean study BMI in the overweight range (Table 1). The body composition of patients included in Martin et al. study was not provided. Obesity had been reported to influence both stimulation length and cycle outcome [10, 14]; thus, lack of correspondence of body composition between the two studies may explain the lack of agreement. Further, the patient subset analyzed by Martin was limited to patients undergoing long luteal GnRH agonist suppression during 2001-2002, a time point that precedes the extensive use of GnRH antagonists. While GnRH antagonist utilization obviates pre-stimulation down-regulation, it is also associated with a significant reduction in clinically pregnancy rates that had been reported in randomized trials and meta-analyses [13, 23]. Finally, Martin et al. included patients with 12 days of stimulation into the longest duration subgroup, while we started seeing negative effect of prolonged length at 13 days (see Fig. 1).
Although we have made a judicious effort to adjust for parameters that are recognized modifiers of ART success, our study is still limited by the retrospective design. AMH levels and antral follicle count were not measured during the time period of the study; yet this information may prove helpful with deciding a gonadotropin dose prior to cycle initiation. Moreover, we concede that our conclusions may be affected by the limited ability to control for unknown factors that could have played a role in clinical decisions. We did not evaluate the effects of the starting gonadotropin dose or the need to increase the dose during the treatment. However, we used a sensitivity analysis of first treatment cycles only to determine the impact of these potential confounders. We did not find a significant change in the observed associations between the duration of gonadotropin stimulation and live pregnancy outcome suggesting that prior gonadotropin dose or response to treatment did not confound our results. This finding is consistent with the absence of data supporting the concept that changing any specific parameter of a previously unsuccessful protocol can improve ART outcome [24].
The primary premise for our work was to answer an often-encountered clinical question “What potentially modifiable factors affect ART pregnancy outcome?” We show that prolonged stimulation imposes a detrimental prognostic effect that is independent of other influences. It should be stressed that our study agrees with the extant literature in that higher gonadotropin requirements correlate with reduced clinical pregnancy and live birth after ART [11, 19–21]. Additionally, it is possible that duration of gonadotropin stimulation during ART may not be an easily modifiable factor: once a controlled ovarian hyperstimulation cycle has been initiated, it may be too late to modify treatment in order to positively affect the outcome. Rather, prolonged stimulation may simply represent another marker of diminished ability of the ovaries to respond to exogenous gonadotropins. Specifically, the fact that patients with shorter stimulations had a higher incidence of day 5 embryo transfers might suggest that patients with prolonged stimulation had a higher incidence of compromised embryo quality, which is a reflection of the ovarian reserve.
Our data could be taken to mean that an evaluation of milder protocols aimed at recovery of fewer oocytes is warranted. While our work does not constitute evidence that shorter stimulation improves outcomes, this assertion is one of the possible explanations for the observed association of prolonged course of ovarian stimulation and poor live birth rates. Given the fact that any evidence-based guidance for strategies after a failed ART cycle is scarce [24], this represents a testable hypothesis. Namely, patients with failed cycles after a prolonged course of stimulation are candidates for a well-designed trial of mild stimulation. As suggested by others [19], “less may be more” as fewer medications for ART could be associated with better outcomes. In this vein, the recently advocated minimal stimulation protocols [25] should be formally tested with a randomized clinical trial for patients who failed a prior ART cycle and had a prolonged duration of ovarian stimulation.
Footnotes
Capsule
In this study of 699 ART cycles, ovarian stimulation for 13 days or longer was independently associated with lower live birth rates when compared to shorter cycles.
References
- 1.Bonduelle M, Wennerholm UB, Loft A, Tarlatzis BC, Peters C, Henriet S, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20:413–9. doi: 10.1093/humrep/deh592. [DOI] [PubMed] [Google Scholar]
- 2.Mouzon J. IVF monitoring worldwide (ICMART) Hum Reprod. 2006;21:i76. [Google Scholar]
- 3.Sutcliffe AG, Ludwig M. Outcome of assisted reproduction. Lancet. 2007;370:351–9. doi: 10.1016/S0140-6736(07)60456-5. [DOI] [PubMed] [Google Scholar]
- 4.Templeton A, Morris JK, Parslow W. Factors that affect outcome of in-vitro fertilisation treatment. Lancet. 1996;348:1402–6. doi: 10.1016/S0140-6736(96)05291-9. [DOI] [PubMed] [Google Scholar]
- 5.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 6.Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360:236–43. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 7.Martin JS, Nisker JA, Tummon IS, Daniel SA, Auckland JL, Feyles V. Future in vitro fertilization pregnancy potential of women with variably elevated day 3 follicle-stimulating hormone levels. Fertil Steril. 1996;65:1238–40. doi: 10.1016/s0015-0282(16)58347-2. [DOI] [PubMed] [Google Scholar]
- 8.Johnson NP, Mak W, Sowter MC. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database Syst Rev 2004:CD002125. [DOI] [PubMed]
- 9.Younglai EV, Holloway AC, Foster WG. Environmental and occupational factors affecting fertility and IVF success. Hum Reprod Update. 2005;11:43–57. doi: 10.1093/humupd/dmh055. [DOI] [PubMed] [Google Scholar]
- 10.Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology–a systematic review. Hum Reprod Update. 2007;13:433–44. doi: 10.1093/humupd/dmm017. [DOI] [PubMed] [Google Scholar]
- 11.Hock DL, Louie H, Shelden RM, Ananth CV, Kemmann E. The need to step up the gonadotropin dosage in the stimulation phase of IVF treatment predicts a poor outcome. J Assist Reprod Genet. 1998;15:427–30. doi: 10.1007/BF02744936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin JR, Mahutte NG, Arici A, Sakkas D. Impact of duration and dose of gonadotrophins on IVF outcomes. Reprod Biomed Online. 2006;13:645–50. doi: 10.1016/S1472-6483(10)60654-2. [DOI] [PubMed] [Google Scholar]
- 13.Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev 2006;3:CD001750. [DOI] [PubMed]
- 14.Fedorcsak P, Dale PO, Storeng R, Ertzeid G, Bjercke S, Oldereid N, et al. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod. 2004;19:2523–8. doi: 10.1093/humrep/deh485. [DOI] [PubMed] [Google Scholar]
- 15.Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:13–21. doi: 10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- 16.Devroey P, Steirteghem A. A review of ten years experience of ICSI. Hum Reprod Update. 2004;10:19–28. doi: 10.1093/humupd/dmh004. [DOI] [PubMed] [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied logistic regression. New York: Wiley; 2000. [Google Scholar]
- 18.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–70. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 19.Pal L, Jindal S, Witt BR, Santoro N. Less is more: increased gonadotropin use for ovarian stimulation adversely influences clinical pregnancy and live birth after in vitro fertilization. Fertil Steril. 2008;89:1694–701. doi: 10.1016/j.fertnstert.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muasher SJ, Garcia JE. Fewer medications for in vitro fertilization can be better: thinking outside the box. Fertil Steril. 2009;92:1187–9. doi: 10.1016/j.fertnstert.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 21.Lekamge DN, Lane M, Gilchrist RB, Tremellen KP. Increased gonadotrophin stimulation does not improve IVF outcomes in patients with predicted poor ovarian reserve. J Assist Reprod Genet. 2008;25:515–21. doi: 10.1007/s10815-008-9266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min JK, Breheny SA, MacLachlan V, Healy DL. What is the most relevant standard of success in assisted reproduction? The singleton, term gestation, live birth rate per cycle initiated: the BESST endpoint for assisted reproduction. Hum Reprod. 2004;19:3–7. doi: 10.1093/humrep/deh028. [DOI] [PubMed] [Google Scholar]
- 23.Tarlatzis BC, Fauser BC, Kolibianakis EM, Diedrich K, Rombauts L, Devroey P. GnRH antagonists in ovarian stimulation for IVF. Hum Reprod Update. 2006;12:333–40. doi: 10.1093/humupd/dml001. [DOI] [PubMed] [Google Scholar]
- 24.Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006;21:3036–43. doi: 10.1093/humrep/del305. [DOI] [PubMed] [Google Scholar]
- 25.Barri PN, Tur R, Martinez F, Coroleu B. Mild stimulation in assisted reproduction. Gynecol Endocrinol. 2010;26(4):261–264. doi: 10.3109/09513590903511489. [DOI] [PubMed] [Google Scholar]