1. Summary
Dendritic cells (DCs) are very important for the generation of long lasting immune responses against pathogens or the induction of anti-tumor responses. Targeting antigen to dendritic cells via monoclonal antibodies specific for DC cell surface receptors such as DEC205 was shown to elicit potent cellular and humoral immune responses in vivo. Therefore we investigated whether this novel strategy might also be useful for the generation of new monoclonal antibodies against molecules of choice. We show, that by targeting the extracellular domain of the human C-type lectin receptor ClecSF6/DCIR/LLIR (hDCIR) to DEC205 on DCs in vivo, we were able to generate highly specific monoclonal antibodies against hDCIR.
Keywords: dendritic cell subsets, DCIR, ClecSF6, monoclonal antibody generation, antibody targeting
2. Introduction
Dendritic cells (DCs) are the most important antigen presenting cells. They act as sentinels, take up and process antigens from the periphery such as the skin, airways, intestine, or interstitial spaces of various organs. Besides the uptake of soluble antigens, DCs are also able to ingest apoptotic cells, tumor cells, or infected cells. Antigen loaded DCs migrate from the periphery to the lymphoid tissues where they interact with antigen-specific T cells to present their antigens [1].
Antigen uptake is mediated either by macropinocytosis, phagocytosis or receptor mediated endocytosis followed by presentation of antigen derived peptides on major histocompatibility class I (MHC I) and II (MHC II) molecules to T cells [2–4]. Recently, this highly efficient antigen uptake and antigen presentation capacity of DCs has been used to initiate cellular and humoral immune responses against a variety of model antigens. To deliver the antigen to dendritic cells in vivo the antigen was fused to monoclonal antibodies recognizing endocytic cell surface receptors expressed on dendritic cells including the C-type lectin receptor DEC205 (CD205), DC-inhibitory receptor 2 (DCIR2), DC-NK lectin group receptor 1 (DNGR-1/Clec9a), CD11c, MHC-II and CD207 (Langerin) [5–10]. [11–13]. The potency of this strategy is reflected by the induction of tumor-, virus- and parasite-specific T cell responses upon targeting of the respective antigens to the C-type lectin receptor DEC205 in vivo [10,14–17]. More recent studies suggest that this approach might also be useful for induction of immune responses in humans. Thus, antigen targeting to human DEC205, MMR (macrophage mannose receptor, CD206), DC-SIGN (CD209), BDCA-2 (CD303), and hDCIR could induce CD4 and CD8 T cell responses in tissue culture [15,18–19], in non human primates [20] and humanized mice [21]. DCIR, also known as ClecSF6, or LLIR (lectin-like immunoreceptor) is the human homolog of murine DCIR1 and DCIR2 [22–24]. For the latter we could show that targeting antigens to murine CD11c+CD8− DCs induces strong CD4 T cell responses in vivo [6,17]. Thus, targeting of antigens to hDCIR might be a promising approach to generate immune responses in humans in the future.
As the hybridoma lines for currently existing hDCIR specific antibodies are not commercially available and some of these antibodies although potentially specific for the same antigen recognize different cell populations we decided to produce novel monoclonal antibodies against this promising receptor on human dendritic cells. As several attempts to generate hDCIR specific antibodies in mice by classic immunization protocols have failed we decided to target the extracellular domain of hDCIR to dendritic cells in vivo via the DEC205 receptor to generate hDCIR specific monoclonal antibodies. We show that this immunization strategy resulted in the generation of several hDCIR specific antibodies and suggests that this strategy will be a useful technique to generate other monoclonal antibodies against type II transmembrane receptors.
3. Materials and Methods
3.1 Mice
All experiments were performed with 6–8 week old female C57BL/6 mice purchased from Jackson. All mice were kept according to guidelines of the institutional animal care and use committee of the Rockefeller University and the University of Erlangen-Nuremberg.
3.2 Cloning of fusion molecules
Total RNA was prepared from human PBMCs using an RNeasy Mini kit (Qiagen). Single stranded cDNA was synthesized from 5 μg total RNA by reverse transcription using an oligo-dT primer and Superscript II™ (both from Invitrogen). PCR was performed with Pfu Polymerase (Roche) using the following oligonucleotides for hDCIR: 5′-GCGGGGAAGCTTGCCACCATGACTTCGGAAA-TCACTTATGCTGAAG-3′ and 5′-CCCCGGGCGGCCGCTCATAAGTGGATCTT-CATCATCTCACAAAC-3′ at 95°C, 5 min, 38 cycles with 95°C, 30 sec, 57°C, 30 sec, 72°C, 1 min, followed by a final extension step at 72°C for 10 min. The PCR product was cloned into pcDNA3.1 vector (Invitrogen) and sequenced. For production of monoclonal anti hDCIR antibodies we produced a recombinant chimeric anti mouse DEC205 antibody that contains the extracellular part of hDCIR in the very C-terminus of the heavy chain of the mDEC205 antibody in accordance to other targeting antibodies [6]. The extracellular domain of hDCIR was amplified with the following oligonucleotides 5′-CCCCGGGCTAGC-GGCGGAGGCGGGAGCGGCGGGGGCGGAAGCTTCTTTCAAAAATATTCTCA-GCTTCTTG-3′ and 5′-CCCCGGGCGGCCGCTCAAGCGTAGTCTGGCACGTC-GTATGGGTAGCTTCCGCCCCCGCCGCTCCCGCCTCCGCCTAAGTGGATCT-TCATCATCTCACAAAC-3′ and afterwards cloned via NheI/NotI into the very C-terminus of the DEC205 heavy chain [6]. To produce a soluble His-tagged hDCIR construct, a mouse IgG1 signaling peptide followed by a sequence of Histidin residues (His-tag) was put in front of the extracellular domain of hDCIR resulting in a soluble 5′His-hDCIR molecule. Therefore, two PCR rounds were performed using the following oligonucleotides: first PCR, 5′-CCCCGGAAGCTTGCCAC-CATGGGATGGTCATGTATCATCCT-3′ and 5′-ATTTTTGAAAGAATCTA-GATCCGCCCCCGCCGCTCCCGCCTCCGCCCGTAGAATCGAGA-CCGAGGAG-3′ on the plasmid CD11c-hDEC205 containing a mouse IgG1 signaling sequence [6], second PCR on a previously cloned full length hDCIR: 5′-GCGGGGGAATTCTTCTTTCAAAAATATTCTCAGC-3′ and 5′-CCCCGGGC-GGCCGCTCAGTGATGGTGATGGTGATGAGATCCGCCCCCGCCGCTCC-CGCCTCCGCCTAAGTGGATCTTCATCATCTC-3′. Murine DCIR2-His was subcloned from mDCIR2 full length cDNA [6]. A mIgG1 signaling peptide was subcloned from the CD11c-promotor mDEC205 construct [6] by overlap PCR with Pfu Polymerase with the following primers: 5′-GCGGGGGAATTCTATTTT-CAAAAGTACTCTCAAC-3′ and 5′-CCCCGGGCGGCCGCTCAGTGATGGTGAT-GGTGATGAGATCCGCCCCCGCCGCTCCCGCCTCCGCCTAAGTATATTTTCT-TCACCTG-3′. A His-tag separated by a G4S-Linker was cloned by PCR at the C-terminal end of the extracellular region of mDCIR2 using the following oligonucleotides: 5′-GCGGGGGAATTCTATTTTCAAAAGTACTCTCAAC-3′ and 5′-CCCCGGGCGGCCGCTCAGTGATGGTGATGGTGATGAGATCCTAAGTAT-ATTTTCTTCACCTG-3′. The PCR fragment was cloned into pcDNA3.1 via EcoRI/NotI. Alternatively to the human 5′His-hDCIR molecule a second construct was cloned consisting of the mouse IgG1 signaling peptide and the extracellular domain of hDCIR followed by a His-tag at the 3′-site. The following oligonucleotides were applied: 5′-GCGGGGGAATTCTTCTTTCAAAAATATTC-TCAGC-3′ and 5′-CCCCGGGCGGCCGCTCAGTGATGGTGATGGTGATGAGA-TCCTAAGTGGATCTTCATCATCTC-3′.
3.3 Production of fusion proteins
Recombinant proteins were produced by transient transfection of HEK293T cells using CaCl2 method as described [6]. Proteins were enriched by ammonium sulfate precipitation. After dialysis to PBS, enriched supernatants of His-tagged proteins 5′His-hDCIR, 3′His-hDCIR and 5′His-mDCIR were incubated with NiNTA beads (Qiagen), and eluted with Imidazol on affinity chromatography columns [25]. Chimeric antibody anti mouse DEC205-hDCIR-HA was incubated with Protein G beads over night and eluted from affinity chromatography columns using glycine buffer (0.1 M, pH 3.0) [6]. All proteins were loaded onto 4–20% Tris/Glycine polyacrylamid gels (Cambrex/Fisher) and Coomassie staining or western blot were performed to verify the protein production. Afterwards they were tested for LPS contamination (Fisher-Cambrex) and decontaminated when necessary (Pierce).
3.4 Antibody generation
C57BL/6 mice were injected i.p. with 10 μg of anti murine DEC205-hDCIR-HA protein together with 50 μg of anti CD40 antibody (clone 1C10) and 50 μg of Poly(I:C) (Invivogen) as described [14]. Two months later mice were boosted with 10 μg of soluble hDCIR-His-tagged protein. After 4 days fusion of mouse splenocytes with myeloma cells was performed at the Monoclonal Antibody Core Facility (MACF) of the Memorial Sloan-Kettering Cancer Center (MSKCC, New York). Antibody supernatants from hybridoma cells were tested in ELISA, FACS and Western blot.
3.5 ELISA
The Enzyme-Linked ImmunoSorbent Assay (ELISA) was performed 1) to screen for antibodies specific for hDCIR, 2) to develop a method for identification of soluble hDCIR. 1) To screen for specific anti hDCIR antibodies, 96-well flat bottom plates (Costar; high-binding capacity) were coated in PBS with different forms of hDCIR protein (each 2.5 μg/ml) as anti murine DEC205-hDCIR-HA antibody construct, soluble 5′His-hDCIR, soluble 3′His-hDCIR, or soluble mDCIR2-His (not shown) and incubated with hybridoma supernatant containing antibodies. Additionally, supernatants were also tested on hDEC-OVA coated plates to exclude hDEC-specific antibodies (data not shown). Serum from the terminal bleeding was used as a positive control. Plates were blocked (1x PBS; 0.02% Tween-20; 1% BSA) and afterwards incubated with HRP conjugated secondary antibodies. IgG1, IgG2a, IgG2b, IgG3, IgA and IgM (all 1:500) were purchased from Southern Biotech, whole IgG antibody (1:5000) was obtained from Jackson ImmunoResearch. ELISA plates were developed using 100 μl developing reagent (1-Step™ ABTS, Pierce). The reaction was stopped with 100 μl 10% SDS solution and measured with a microplate reader VERSAmax (Molecular Devices) at a wavelength of 416 nm. 2) To detect soluble hDCIR, 96-well flat bottom plates (Greiner bio-one; high binding capacity) were coated with 1.25 μg/ml 15E12, I3-612 (Alexa-647 labeled), 216110, polyclonal serum or isotype controls in PBS o/n at room temperature. Plates were washed (PBS, 0.02% Tween-20) and blocked (PBS; 0.02% Tween-20; 1% BSA) at room temperature for 1 hour and after a second wash incubated with 2.5 μg/ml of soluble hDCIR (5′hDCIR-His in PBS + 0.25% BSA) for 2 hours. Plates were washed and incubated with 5 μg/ml (1:200) biotinylated 15E12 antibody (PBS; 0.25% BSA) for another 2 hours. After washing all wells were incubated with Streptavidin-HRP (Trevigen; 1:800 in PBS; 0.25% BSA) for additional 2 hours. ELISAs were developed using 50 μl horseradish peroxidase substrate (TMB; Sigma), followed by 50 μl stop reagent (6% ortho-phosphoric acid in PBS). OD495nm was measured using a VERSAmax microplate reader (Molecular Devices).
3.6 Coomassie and Western blot
Purified 5′His or 3′His-tagged hDCIR proteins or anti mouse DEC205-hDCIR-HA antibody fusion protein were loaded in loading dye (0.5 M Tris/HCl pH 6.8, 10% SDS, 87% glycerol, 1% bromophenol blue, 0.2 μM EDTA pH 8.0 in dH2O) at a ratio of 1:5 onto 4–20% Tris/Glycine gels (Fisher/Cambrex) with either 0.5 μg (Western blot) or 2 μg (Coomassie) protein per lane. Gels were run in Tris/Glycine/SDS buffer. SDS-PAGE marker was from BioRAD. For Coomassie staining: gel was stained for 1 hour in Coomassie Blue buffer (30% MeOH; 10% acetic acid; Coomassie Brilliant Blue R in dH2O), destained for 2–3 hours with destaining buffer (30% MeOH; 10% acetic acid in dH2O) and afterwards dried on a gel dryer (BioRAD). Western blot was performed as described before [25]. Briefly, after transfer of the separated proteins onto PVDF-membranes (Millipore), membranes were washed with TBST (10 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1% Tween 20) for 5 minutes. Blocking was done for 1 h in TBST buffer containing 5% milk (Merck). Membranes were incubated with either anti hDCIR hybridoma antibody supernatants (1:100 to 1:5000), anti His antibody (1:500; Roche), or anti HA-antibody (Roche), respectively in TBST containing 3% dry milk o/n at 4°C. After washing, membranes were incubated with HRP-labeled goat anti mouse IgG antibody (1:2000 to 1:5000; Southern Biotech), or HRP-labelled goat anti rat antibody (Dianova) for 1 h at room temperature. Finally, blots were washed three times. Proteins were visualized using the ECL Western blotting detection system (Pierce).
3.7 Flow cytometry
Freshly isolated PBMCs were incubated in FACS buffer (1% BSA in PBS or 2%FCS in PBS) with the following antibodies: CD3 (UCHT1), CD4 (SK3), CD8 (SK1), CD9 (M-L13), CD10 (H11Oa), CD11a (HI 111), CD11b (ICRF44), CD11c (3.9), CD14 (MφP9), CD15 (HI98), CD16 (3G8), CD19 (SJ25-CI), CD20 (2H7), CD21 (BU 32), CD22 (HIB22), CD23 (EBVCS2), CD25 (2A3), CD28 (L293), CD31 (WM59), CD34 (4H11), CD38 (HIT2), CD45RA (HI100), CD45RO (UCHL1), CD56 (MEM188), CD62L (Dreg56), CD83 (HB15A), CD94 (DX22), CD123 (6H6), CD138 (DL-101), BDCA1 (AD5-8E7), BDCA2 (AC144), BDCA3 (AD5-14H12), BDCA4 (AD5-17F6), HLA-DR (LN3), BAFF-R (11C1). All antibodies were from Becton Dickinson, e-Bioscience, BioLegend or Miltenyi. Isotype controls for IgG1 (MOPC-21), IgG2a (MOPC-173) were from Becton Dickinson, goat serum was obtained from Jackson ImmunoResearch. Cells were analyzed using FACS Canto or FACS Calibur (Becton Dickenson). Data were evaluated using FlowJo (Treestar) software. Our newly generated hDCIR antibody clone 15E12 was either labeled with Alexa-488 or Alexa-647 (Invitrogen/Molecular Probes). The isotype controls IgG1, IgG2a, goat serum as well as the antibody clones I3-612 (Becton Dickinson), 216110 (R&D Systems), and the polyclonal hDCIR antibody (R&D Systems) were either bought or labeled with Alexa-647 (Invitrogen/Molecular Probes). To analyze 293T cells transiently transfected with hDCIR-HA or hDCIR (Lipofectamin, Invitrogen and Fugene, Roche), hybridoma antibody supernatants were incubated with the transfected 293T cells, washed and further incubated with anti mouse whole IgG PE antibody (Southern Biotech). In some experiments the cells were stained with anti HA-FITC antibody (Roche).
3.8 Cells
Hybridoma cells were grown in supplemented Hyclone Hybridoma SFM medium with 1% Hybridoma cloning factor (Invitrogen), 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 2 mM L-glutamine. Later, cells were transferred into ISF-1 serum free medium (Biochrom). 293T cells were cultured in DMEM containing 5% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 2 mM L-glutamine. Stable transfectants of CHO cells (CHO:hDEC205, CHO:mDEC205, kindly provided by Chae Gyu Park, Rockefeller University, New York) were cultured in RPMI medium containing 5% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, 0.8 mg/ml geneticin (G418, Biochrom) and 2 mM L-glutamine.
3.9 Preparation of PBMCs for FACS analysis
The study was approved by the Institutional Review Board of the University Hospital of Erlangen and the Rockefeller University, and written informed consent was obtained from all blood donors in accordance with the Declaration of Helsinki (Ethical approval, University Hospital of Erlangen, 3761 and 3762). PBMCs were prepared as described before [25]. After Ficoll gradient (GE-Healthcare) the leukocyte fraction was washed twice with ice cold PBS and afterwards resuspended in FACS buffer (1% BSA in PBS). The cells were stained with directly conjugated anti human antibodies or isotype controls which were either from BD, e-Bioscience, or Miltenyi.
4. Results
4.1 Generation of anti human DCIR antibodies
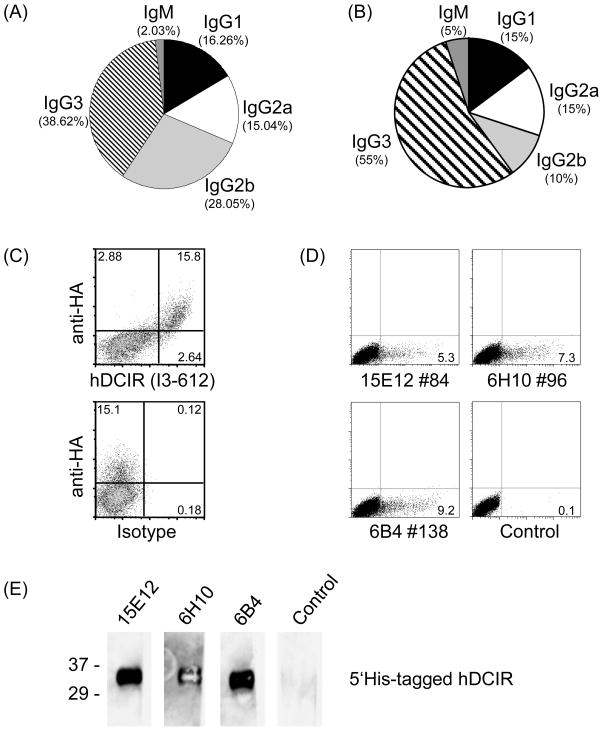
For antigen targeting of human dendritic cells (DCs), antibodies against human endocytosis receptors are necessary. In mice we found that targeting antigens to the endocytosis and C-type lectin receptor DCIR2 induced strong CD4 T cell responses in vivo. Therefore, we decided to generate antibodies against the human counterpart hDCIR. For efficient antigen presentation and to optimize the immune responses against hDCIR we targeted the extracellular domain of hDCIR directly to murine CD11c+CD8+ DCs. For this, we produced a recombinant chimeric antibody consisting of the variable regions of the anti mouse DEC205 light and heavy chains fused to a mutated (that is non-Fc-receptor binding) mouse IgG1 Fc region, the extracellular part of the hDCIR C-type lectin domain and a hemagglutinin-tag for detection (Fig. 1 A, B). We injected anti mouse DEC205-hDCIR-HA together with anti CD40 stimulating antibody and poly(I:C), a TLR3 ligand, to achieve full DC maturation in C57BL/6 mice [14]. Two months later mice were boosted with a soluble His-tagged hDCIR (5′His hDCIR) protein (Fig. 1 C, D). In the beginning, 890 hybridoma clones were screened by ELISA using the anti mDEC205-hDCIR-HA antibody, anti mDEC205-Ovalbumin, 5′-His-tagged soluble hDCIR, 3′-His-tagged soluble hDCIR and 3′-His-tagged soluble mDCIR2 fusion proteins, ensuring the identification of specific antibodies exclusively directed against the extracellular domain of hDCIR (Fig. 1 C, D, and data not shown). Only those hybridomas were further cultured that showed a binding to all three human DCIR fusion molecules but not to mouse DCIR2, Ovalbumin, hemagglutinin, or the His-tag. After initial identification of 246 hybridoma clones from which 2.03% were IgM, 16.26% IgG1, 15.04% IgG2a, 28.05% IgG2b and 38.62% IgG3 (Fig. 2A), we finally focused our attention on 36 antibodies. These antibodies bound specifically and constantly over two selection rounds to the extracellular domain of hDCIR in ELISA, from which 5% were IgM, 15% IgG1, 15% IgG2a, 10% IgG2b, 55% were IgG3 (Fig. 2B).
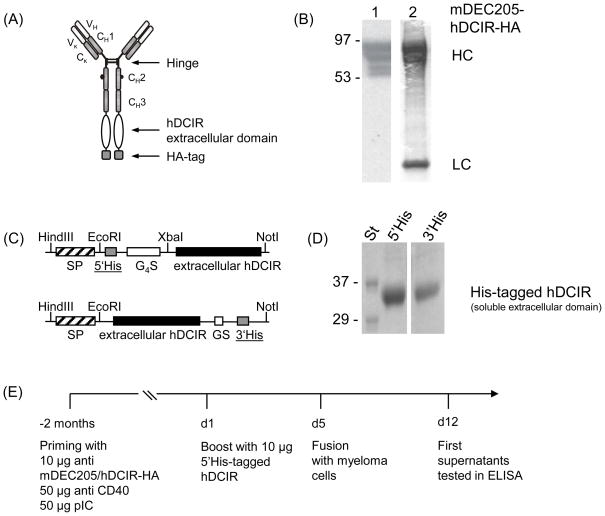
Fig. 1.
Generation of anti hDCIR antibodies. (A) Schematic drawing of the recombinant chimeric anti mouse DEC205/hDCIR-HA antibody. (B) 0.5 μg (left lane, 1), or 2 μg (right lane, 2) of recombinant anti mouse DEC205/hDCIR-HA protein were loaded onto a 4–20% SDS-Page. Protein was detected by Western blot using an anti rat HRP conjugated secondary antibody (1) or directly stained with Coomassie blue (2). The upper bands show the antibody heavy chain (HC), which migrates slower than a normal IgG heavy chain due to the hDCIR fusion. The lower band shows the antibody light chain (LC). The experiment was repeated twice. (C) Scheme of the recombinant 5′ (upper part) and 3′ His-tagged soluble hDCIR expression cassettes (lower part) consisting of mouse IgG1 signaling peptide (SP) ( ), His-tag (6xHis) (
), His-tag (6xHis) ( ), (G4S)2, or GS linker (□), and extracellular hDCIR domain (■). (D) Coomassie gels show purified 5′His-tagged (left) and 3′His-tagged soluble hDCIR protein (right). (E) Timeline of anti hDCIR antibody production. C57BL/6 mice were immunized with 10 μg mDEC205/hDCIR-HA antibody in the presence of 50 μg poly (I:C) and 50 μg anti CD40 stimulating antibody. Mice were boosted with 10 μg soluble 5′His-tagged hDCIR.
), (G4S)2, or GS linker (□), and extracellular hDCIR domain (■). (D) Coomassie gels show purified 5′His-tagged (left) and 3′His-tagged soluble hDCIR protein (right). (E) Timeline of anti hDCIR antibody production. C57BL/6 mice were immunized with 10 μg mDEC205/hDCIR-HA antibody in the presence of 50 μg poly (I:C) and 50 μg anti CD40 stimulating antibody. Mice were boosted with 10 μg soluble 5′His-tagged hDCIR.
Fig. 2.
Selection of anti hDCIR antibodies. ELISA was performed on supernatants of (A) 246 clones from selection round one and (B) remaining 36 clones from the second selection round. Pie-charts show percentage of antibody subclasses IgM ( ), IgG (■), IgG2a (□), IgG2b (
), IgG (■), IgG2a (□), IgG2b ( ), IgG3 (
), IgG3 ( ) present in the individual rounds of selection. (C) 293T cells were transiently transfected with hDCIR-HA and analyzed by FACS. Dot blots show living cells which were stained with anti HA-FITC (y-axis) and hDCIR-A647 antibody (clone I3-612 from BD, x-axis, upper panel), or anti HA-FITC antibody and Alexa-647 labeled isotype control IgG1 (y-axis, lower panel). (D) ELISA positive antibody supernatants were used for FACS staining of 293T cells transiently transfected with hDCIR. As a control unspecific isotype matched control antibodies were used. Shown are dot blots on living cells. The experiment was repeated 2 times. (E) Soluble 5′His-tagged hDCIR (2 μg/lane) protein was loaded onto a 4–16% Tris-Glycine gel, blotted and developed with anti hDCIR antibody supernatants (clones 15E12, 6H10, 6B4), or unspecific antibody supernatant and HRP-conjugated anti mouse whole IgG antibodies. Western blots were developed with enhanced luminescence (Pierce). The experiment was repeated twice.
) present in the individual rounds of selection. (C) 293T cells were transiently transfected with hDCIR-HA and analyzed by FACS. Dot blots show living cells which were stained with anti HA-FITC (y-axis) and hDCIR-A647 antibody (clone I3-612 from BD, x-axis, upper panel), or anti HA-FITC antibody and Alexa-647 labeled isotype control IgG1 (y-axis, lower panel). (D) ELISA positive antibody supernatants were used for FACS staining of 293T cells transiently transfected with hDCIR. As a control unspecific isotype matched control antibodies were used. Shown are dot blots on living cells. The experiment was repeated 2 times. (E) Soluble 5′His-tagged hDCIR (2 μg/lane) protein was loaded onto a 4–16% Tris-Glycine gel, blotted and developed with anti hDCIR antibody supernatants (clones 15E12, 6H10, 6B4), or unspecific antibody supernatant and HRP-conjugated anti mouse whole IgG antibodies. Western blots were developed with enhanced luminescence (Pierce). The experiment was repeated twice.
4.2 Identification of specific anti human DCIR antibodies
To determine if the antibodies recognize cell surface expressed hDCIR, we performed FACS analysis on 293T cells transiently transfected with hDCIR (Fig. 2C, D). For confirmation that hDCIR is expressed after transfection we inserted a hemagglutinin-tag into the very C-terminal region of the extracellular domain of hDCIR (hDCIR-HA), and detected the latter one with an anti HA-antibody and the commercially available clone I3-612 (Fig. 2C). All 36 antibodies were tested on hDCIR and hDCIR-HA transfected 293T cells. Out of 36 antibodies 6 antibodies recognized hDCIR by flow cytometry (Fig. 2D and data not shown). Further, we investigated if some of these antibodies would be able to detect hDCIR also under reducing conditions. Therefore, we performed Western blot analysis in which we loaded soluble 5′His-hDCIR onto reducing SDS gels (Fig. 2E). We found that 3 out of these 6 antibodies also recognized hDCIR under reducing conditions (Fig. 2E). Thus, using an antibody coupled to the extracellular C-type lectin domain successfully produced hDCIR specific antibodies which are able to recognize the protein under non-reducing as well as under reducing conditions.
4.3 The novel human DCIR antibody does not crossreact with other C type lectins
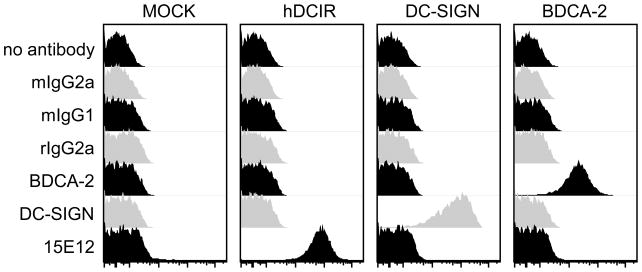
The most promising hDCIR specific antibody clone (15E12, IgG2a subclass) was further tested for cross reactivity to other homologous C-type lectin family members. Therefore, we transiently transfected 293T cells with 28 different human and murine C-type lectin receptors (Table 1). In addition, we tested CHO cells stably transfected with murine or human DEC205 for binding of the 15E12/hDCIR antibody clone (Fig. 3, and data not shown). We found that besides hDCIR none of the other tested C-type lectin receptors was recognized by the clone 15E12, suggesting a highly specific binding of the antibody to the extracellular domain of hDCIR.
Table 1.
| CLRa | RSb | 5′Primer 3′Primer |
|---|---|---|
| human | ||
| ASGR2 NM_001181.3 |
E/Nc | 5′-GCGGGGGAATTCGCCACCATGGCCAAGGACTTTCAAGATATCCAGCAGCTG-3′ 5′-CCCCGGGCGGCCGCTCAGGCCACCTCGCCGGTGGCATTCCGCCT-3′ |
| BDCA-2/CD303/CLEC4C NM_130441.2 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGTGCCTGAAGAAGAGCCTCAAG-3′ 5′-CCCCGGGCGGCCGCTTATATGTAGATCTTCTTCATCTTGC-3′ |
| CD301/CLEC10A NM_182906.2 |
E/N | 5′-GCGGGGGAATTCGCCACCATGACAAGGACGTATGAAAACTTCCAGTACTTGG-3′ 5′-CCCCGGGCGGCCGCTCAGTGACTCTCCTGGCTGGTCTGACCCAGGC-3′ |
| CLEC4D/CLECSF8 NM_080387.4 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGGGCTAGAAAAACCTCA-3′ 5′-CCCCGGGCGGCCGCCTATTCAATGTTGTTCCAGGTATTTTAC-3′ |
| DCAL-1/CLECL1 NM_172004.2 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGTTAGTAATTTCTTCCATGTCATACAAGTATTC-3′ 5′-CCCCGGGCGGCCGCCTAAATGTTAAATCTCACCATAGCAGTAATGT-3′ |
| DCAL-2/MICL/CLEC12A NM_138337.5 |
E/N | 5′-GCGGGGGAATTCGCCACCATGTCTGAAGAAGTTACTTATGCAGATCTTCAAT-3′ 5′-CCCCGGGCGGCCGCTCATGCCTCCCTAAAATATGTAGAACCAAGC-3′ |
| DCIR/CLECSF6 NM_016184.3 |
H/N | 5′-GCGGGGAAGCTTGCCACCATGACTTCGGAAATCACTTATGCTGAAG-3′ 5′-CCCCGGGCGGCCGCTCATAAGTGGATCTTCATCATCTCACAAAC-3′ |
| DC-SIGN/CD209 NM_021155 |
E/N | 5′-GCGGGGGAATTCGCCACCATGAGTGACTCCAAGGAACCA-3′ 5′-CCCCGGGCGGCCGCCTACGCAGGAGGGGGGTTTGGGGTGG-3′ |
| DC-SIGNR/CLEC4M NM_014257.4 |
E/N | 5′-GCGGGGGAATTCGCCACCATGAGTGACTCCAAGGAACCAAG-3′ 5′-CCCCGGGCGGCCGCCTATTCGTCTCTGAAGCAGGCTGC-3′ |
| DECTIN-1/CLECSF12/CLEC7A NM_197947.2 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGAATATCATCCTGATTTAGAAAATTTG-3′ 5′-CCCCGGGCGGCCGCTTACATTGAAAACTTCTTCTCACAAATACT-3′ |
| DECTIN-2/CLECSF10 NM_001007033.1 |
B/N | 5′-GCGGGGGGATCCGCCACCATGATGCAAGAGCAGCAACC-3′ 5′-CCCCGGGCGGCCGCTCATAGGTAAATCTTATTCATCTCAC-3′ |
| DNGR-1/CLEC9A NM_207345.2 |
E/N | 5′-GCGGGGGAATTCGCCACCATGCACGAGGAAGAAATATA-3′ 5′-CCCCGGGCGGCCGCTCAGACAGAGGATCTCAACGCATACT-3′ |
| LANGERIN//CLEC4K/CD209 NM_015717.2 |
Nh/N | 5′-GCGGGGGCTAGCGCCACCATGACTGTGGAGAAGGAGGC-3′ 5′-CCCCGGGCGGCCGCTCACGGTTCTGATGGGACTAAGGGT-3′ |
| MINCLE/CLEC4É/CLECSF9 NM_014358.2 |
H/N | 5′-GCGGGGAAGCTTGCCACCATGAATTCATCTAAATCATCTG-3′ 5′-CCCCGGGCGGCCGCTTAAAGAGATTTTCCTTTGTTCAAAG-3′ |
| NKG2A/CD159A/KLRC1 NM_002259.4 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGATAACCAAGGAGTAATCTACTCAGACCTG-3′ 5′-CCCCGGGCGGCCGCCTAAAGCTTATGCTTACAATGATATATTATTGA-3′ |
| NKG2D/CD314/KLRK1 NM_007360.2 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGGGTGGATTCGTGGTCG-3′ 5′-CCCCGGGCGGCCGCTTACACAGTCCTTTGCATGCAGATGT-3′ |
| mouse | ||
| CD209a/Cire/DC-Sign NM_133238.5 |
E/N | 5′-GCGGGGGAATTCGCCACCATGAGTGATTCTAAGGAAATGGGGAAGA-3′ 5′-CCCCGGGCGGCCGCTCACTTGCTAGGGCAGGAAGTTGAAAG-3′ |
| Clec1b/Clec2 NM_019985.2 |
E/N | 5′-GCGGGGGAATTCGCCACCATGCAGGATGAAGATGGGTATATCACTTTAAA-3′ 5′-CCCCGGGCGGCCGCTTAAAGCAGTTGGTCCACTCTTGTCATGCCA-3′ |
| Clec4g NM_029465.3 |
E/N | 5′-GCGGGGGAATTCGCCACCATGAACACTGGTGAATACAACAAGC-3′ 5′-CCCCGGGCGGCCGCTTAGTAGCAACTGCTCCTCTTCTCAC-3′ |
| Dcar/Clec4b1 NM_027218.2 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGTTCAGGAAAGACAGCTACAAGGGA-3′ 5′-CCCCGGGCGGCCGCTCATAAGTTTATTTTCTTCATCTGACA-3′ |
| Dcir1/Clec4a2/ClecSF6 NM_001170333.1 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGCTTCAGAAATCACTTAT-3′ 5′-CCCCGGGCGGCCGCTCATAAGTTTATTTTCTTCATCTGACA-3′ |
| Dcir2/Clec4a4 NM_001005860.1 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGCTTCAGAAATCACTTATGCAG-3′ 5′-CCCCGGGCGGCCGCTCATAAGTATATTTTCTTCACCTGAC-3′ |
| Dcir3/Clec4a3 NM_153197.3 |
E/N | 5′-GCGGGGGAATTCGCCACCATGTTTTCAGAAAACATTTATGTTAAC-3′ 5′-CCCCGGGCGGCCGCTCATAAGTATATTTTTTTCACATGGC-3′ |
| Dcir4/Clec4a1 NM_199311.1 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGCATTACCAAACATTTATACTGACGTG-3′ 5′-CCCCGGGCGGCCGCTCATACATAGAGCTGCCTCATCTCACAAATC-3′ |
| Dectin-2/ClecSF10/Clec4n NM_020001.1 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGTGCAGGAAAGACAATCC-3′ 5′-CCCCGGGCGGCCGCTCATAGGTAAATCTTCTTCAT-3′ |
| Dngr-1/Clec9a NM_172732.2 |
E/N | 5′-GCGGGGGAATTCGCCACCATGCATGCGGAAGAAATATATACCT-3′ 5′-CCCCGGGCGGCCGCTCAGATGCAGGATCCAAATGCCTTCTT-3′ |
| CD161/NKRP1/Klrb1b NM_001159904.1 |
E/N | 5′-GCGGGGGAATTCGCCACCATGGATTCAACAACACTGGTC-3′ 5′-CCCCGGGCGGCCGCTCAGGAGTCATTACACGGGGTTTCA-3′ |
| Siglec-E/Siglecl1/CD170 NM_031181 |
E/N | 5′-GCGGGGGAATTCGCCACCATGCTGCTGTTGCTGCTGCTG-3′ 5′-CCCCGGGCGGCCGCTCATGGCCATGCGGTCCTTTG-3′ |
CLR: C-type lectin receptor,
RS: restriction site,
indicates accession ID of corresponding cDNAs,
E/N: EcoRI/NotI,
H/N: HindIII/NotI,
B/N: BamHI/NotI,
Nh/N: NheI/NotI
Fig. 3.
New anti hDCIR antibody clone is highly specific to hDCIR. 293T cells were transiently transfected with hDCIR, DC-SIGN, or BDCA-2 together with a GFP expression plasmid. One day later binding of hDCIR antibody clone 15E12, as well as binding of anti DC-SIGN, and anti BDCA-2 and their isotype controls (mouse IgG1, mouse IgG2a, rat IgG2a) was tested. Shown are histogram overlays of GFP positive 293T cells. The experiment was repeated three times.
4.4 The anti hDCIR antibody recognizes CD11c+ peripheral blood cells
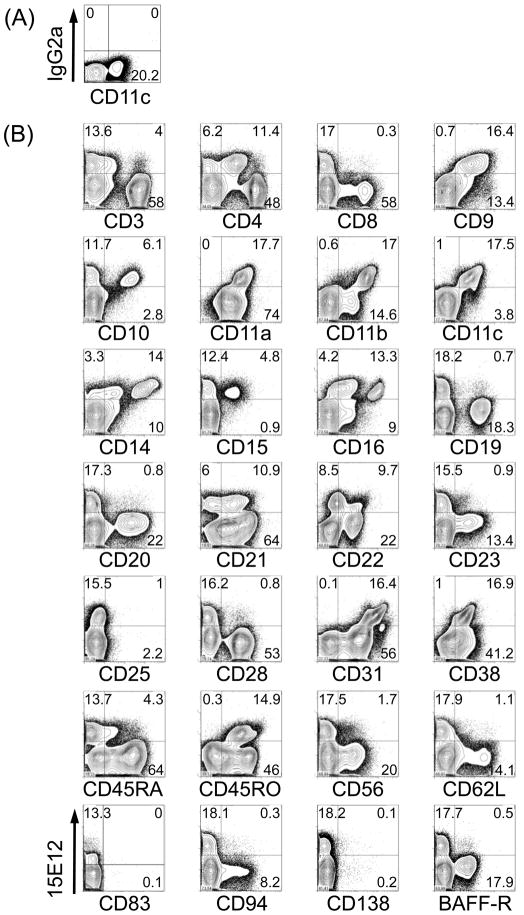
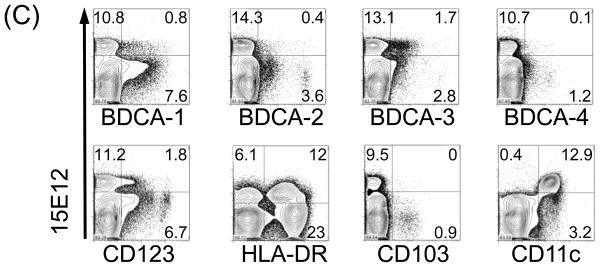
To identify which cell types are recognized by our newly generated anti hDCIR antibody clone 15E12 we used human peripheral blood mononuclear cells (PBMCs) for FACS analysis. Roughly 15% of all PBMCs were recognized by the 15E12 antibody, but not by the isotype control (Fig. 4 A, B). A further in-depth analysis revealed that the hDCIR positive cells also express CD9, CD11a and CD11c (Fig. 4B). In addition, the 15E12 antibody was binding to subpopulations of CD4, CD10, CD11b, CD14, CD15, CD16, CD21, CD22, CD31, CD38, and CD45RO (Fig. 4B) and HLA-DR positive cells (Fig. 4C). Very low binding to a fraction of CD3, CD19, CD23, CD28, CD45RA, and CD56 positive cells was observed. We could not or only marginally detect binding to CD8, CD20, CD25, CD62L, CD83, CD94, CD138, and BAFF-R positive cells (Fig. 4B). Furthermore, we analyzed if the generated anti hDCIR antibody would also recognize peripheral blood DCs and found that the antibody binds to BDCA1+ (MDC1) and BDCA3+ (MDC2) DCs, respectively, but not to BDCA2+, BDCA4+, CD123+ plasmacytoid DCs (Fig. 4C). Thus, hDCIR expression in human PBMCs is restricted to CD11c+ peripheral blood monocytes, granulocytes, neutrophils as well as myeloid DCs and shows binding similarity to the earlier published antibody from Bates et al., [22].
Fig. 4.
Expression of hDCIR is restricted to monocytes, neutrophiles, granulocytes and dendritic cells. FACS-Density blots show PBMCs that were stained with Alexa-647 labeled (A) isotype control antibody (IgG2a), (B, C) hDCIR antibody clone 15E12 (y-axis) and antibodies characterizing (B) different lymphocytes or (C) DC-subpopulations (x-axis). Shown are living cells. The experiment was repeated three times with different donors.
4.5 The new antibody against hDCIR binds to a different epitope than commercially available anti hDCIR antibodies
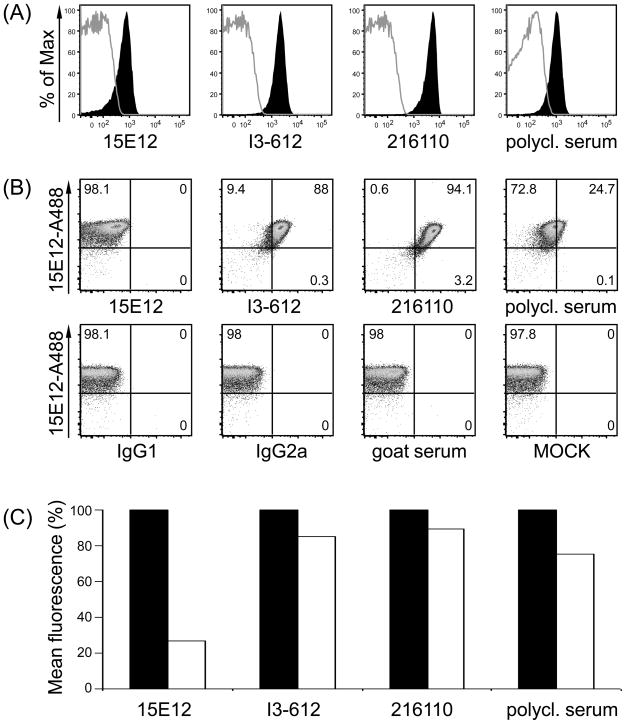
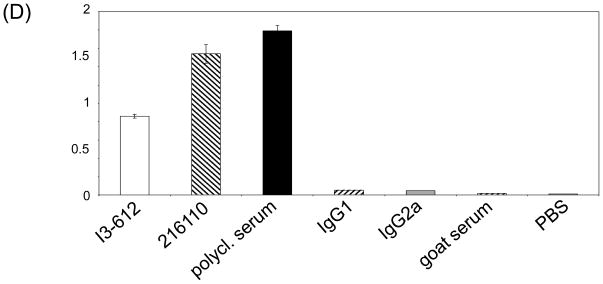
In order to identify if commercially available antibodies recognize the same or different epitopes in the extracellular domain of hDCIR we performed competitive binding studies with either membrane-bound or soluble hDCIR. FACS analyses on freshly isolated PBMCs or transiently transfected 293T cells (data not shown) showed that all antibodies recognized different epitopes on CD11c+HLA-DR+ DCs or hDCIR transfected 293T cells, respectively (Fig. 5A-C and data not shown). To test whether the combination of 15E12 with any of the other hDCIR specific antibodies might be used as pairs of capture and detection antibodies for identification of soluble hDCIR we performed an ELISA with the commercially available antibodies as capture antibodies and the clone 15E12 as detection antibody. As shown in Figure 5B the antibody clones I3-612, and 216110 as well as the polyclonal serum against hDCIR worked well in combination with 15E12 to detect soluble 5′His-tagged hDCIR in ELISA (Fig. 5D)
Fig. 5.
Anti hDCIR antibodies recognize different epitopes. (A-C) Freshly isolated PBMCs were analyzed by flow cytometry. (A) Histograms show antibody staining of 15E12, I3-612, 216110, the polyclonal hDCIR serum under non competitive conditions on CD11c+HLA-DR+ living cells. (B) Shown are CD11c+HLA-DR+ cells that were primarily incubated with Alexa 488-labeled anti hDCIR antibody clone 15E12. After washing, cells were further incubated with commercially available anti hDCIR antibodies (I3-612, 216110, the polyclonal hDCIR serum), or isotype controls (IgG1, IgG2a, goat serum) or left untreated (MOCK) which were directly conjugated with Alexa-dye 647. (C) Bar histogram illustrates relative mean fluorescences intensity (geom. mean) of (A) and (B) in percent, non competitive conditions are set to 100%. (A-C) Shown is one of three experiments. (D) ELISA was performed using commercially available hDCIR antibodies and isotype controls for coating (1.25 μg/ml), followed by incubation with soluble His-tagged hDCIR (2.5 μg/ml), and incubation with biotinylated antibody clone 15E12 (1:200) for detection. Elisa was developed using Streptavidin-HRP. The experiments were repeated three times in triplicates with similar results.
5. Discussion
DCs are key players in the induction of immune responses and the maintenance of peripheral tolerance [26]. Thus, recent approaches use an in vivo targeting strategy with antibodies recognizing DC associated cell surface proteins. We and others have shown that targeting antigens under inflammatory conditions to murine DCs in vivo induces strong CD4 and CD8 T cell responses and also antibody responses [5–11,14,17]. Especially targeting antigens to the DEC205 receptor seems to be a promising strategy for the generation of strong antibody responses against antigens of choice [14].
Using this strategy, we fused the extracellular domain of the type II transmembrane protein hDCIR to the C-terminal domain of the DEC205 heavy chain. As few as 10 μg of the fusion protein were enough to generate specific monoclonal antibodies directed against the extracellular domain of hDCIR. This strategy might also be a useful approach to generate antibodies against other molecules. Especially, the generation of antibodies against the extracellular domain of type II transmembrane proteins might be difficult, as a signaling peptide is needed for secretion of the N-terminal extracellular domain of type II transmembrane proteins. In our case the extracellular domain was cloned in frame to the C-terminal part of the DEC205 antibody heavy chain and the addition of a signaling peptide was not necessary. As C-type lectin receptors are highly glycosylated, their glycosylation might be very important for proper folding of the extracellular domain [27]. Therefore, we generated the fusion proteins in a eukaryotic system (HEK-293T cells) to ensure proper glycosylation. With a stringent antibody screening protocol we excluded antibodies that were not binding to the extracellular domain of hDCIR. We finally identified three antibodies that recognized hDCIR under native and under reducing conditions.
As C-type lectin receptors show a close homology to each other we investigated if our new anti hDCIR antibody (clone 15E12) might recognize other C-type lectin receptors. Therefore, we cloned human and mouse C-type lectin receptor family members that showed strong similarities in their C-type lectin domain (data not shown). In these experiments we found that none of the cloned C-type lectin receptors was recognized by our anti hDCIR antibody clone 15E12 indicating a high specificity of the antibody. By staining the transfected 293T cells with e.g. mDCIR2, hDC-SIGN, Langerin, DEC205 antibodies and cotransfection with a GFP expression vector we could exclude that the antibody was not binding due to low transfection efficiency or cell surface expression of the target antigen.
As Bates et al. showed binding of their anti hDCIR antibody (I3-612) on myeloid cells including monocytes, granulocytes, neutrophils as well as DCs [22] we tested if our antibody recognizes similar subsets of peripheral blood cells. In accordance to their binding studies we found that the clone 15E12 bound very well to monocytes, neutrophiles, granulocytes and DCs. However, we only found little binding to B cells which is in slight contrast to the antibody generated by Bates et al. [22]. Recently, it was published that the hDCIR antibody clone 216110 as well as a polyclonal anti hDCIR antibody serum is binding to peripheral blood pDCs. We were unable to detect binding to pDCs with our newly generated anti hDCIR antibody clone 15E12 (Fig. 4C) and with the antibody clone I3-612 [19,28]. Analysis of the RNA expression of different C-type lectin receptors on pDCs from peripheral blood confirms our finding [29]. This suggests that although cell surface hDCIR protein is recognized by the antibody 216110 and the polyclonal hDCIR serum (Fig. 5A-C) there might be an additional homologous C-type lectin receptor that these antibodies could recognize as well. Testing our array of C-type lectin receptors we did not find binding of the antibody clone 260110 or the polyclonal hDCIR serum to another C-type lectin receptor (data not shown), suggesting that other receptors not represented in our library might be recognized. With respect to the identification of soluble versions of hDCIR that might be secreted into the serum of patients with arthritis [24,30–31], the combination of 15E12 with other commercially available anti hDCIR antibodies might be a useful tool to detect these soluble variants by ELISA (Fig. 5D).
Taken together, we produced a highly specific antibody against the human type II transmembrane receptor hDCIR by specific antigen targeting to murine DCs. This technique might also be very helpful for the generation of antibodies against small peptides, or extracellular domains of type II transmembrane proteins, including other C-type lectin receptors.
Acknowledgments
We would like to thank Francis Garcia and Sylvia Boscardin for help in the generation of monoclonal antibodies (MSKCC, New York), Anna Baranska, Christian Lehmann for critical reading the manuscript, Lukas Heger and Kathrin Weidner for technical support. We also would like to thank Gerold Schuler for his continuous support. This work was supported by the German Research Foundation to D.D. (SFB643/II-TP-A7, DU-548/1-1, DU-548/2-1) and to F.N. (SFB643/II-TP-A8, FOR832); by the Bavarian State of Ministry of Sciences, Research and the Arts (BayGene) to D.D. and F.N. G.F.H. was partly supported by DAAD. D.D. is a fellow of the ‘Förderkolleg’ of the Bavarian Academy of Sciences. M.C. Nussenzweig has financial interests in Celldex, which is developing anti DEC205 antibodies for human use.
Footnotes
The other authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Burgdorf S, Kurts C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr Opin Immunol. 2008;20:89–95. doi: 10.1016/j.coi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 4.Lanzavecchia A. Mechanisms of antigen uptake for presentation. Curr Opin Immunol. 1996;8:348–354. doi: 10.1016/s0952-7915(96)80124-5. [DOI] [PubMed] [Google Scholar]
- 5.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 7.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, et al. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 9.Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro FV, Tutt AL, White AL, Teeling JL, James S, French RR, et al. CD11c provides an effective immunotarget for the generation of both CD4 and CD8 T cell responses. Eur J Immunol. 2008;38:2263–2273. doi: 10.1002/eji.200838302. [DOI] [PubMed] [Google Scholar]
- 12.Estrada A, McDermott MR, Underdown BJ, Snider DP. Intestinal immunization of mice with antigen conjugated to anti-MHC class II antibodies. Vaccine. 1995;13:901–907. doi: 10.1016/0264-410x(95)00012-p. [DOI] [PubMed] [Google Scholar]
- 13.van Bergen J, Ossendorp F, Jordens R, Mommaas AM, Drijfhout JW, Koning F. Get into the groove! Targeting antigens to MHC class II. Immunol Rev. 1999;172:87–96. doi: 10.1111/j.1600-065x.1999.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 14.Boscardin SB, Hafalla JC, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci U S A. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do Y, Park CG, Kang YS, Park SH, Lynch RM, Lee H, et al. Broad T cell immunity to the LcrV virulence protein is induced by targeted delivery to DEC-205/CD205-positive mouse dendritic cells. Eur J Immunol. 2008;38:20–29. doi: 10.1002/eji.200737799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, et al. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 19.Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ, et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 20.Pereira CF, Torensma R, Hebeda K, Kretz-Rommel A, Faas SJ, Figdor CG, et al. In vivo targeting of DC-SIGN-positive antigen-presenting cells in a nonhuman primate model. J Immunother. 2007;30:705–714. doi: 10.1097/CJI.0b013e31812e6256. [DOI] [PubMed] [Google Scholar]
- 21.Gurer C, Strowig T, Brilot F, Pack M, Trumpfheller C, Arrey F, et al. Targeting the nuclear antigen 1 of Epstein-Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood. 2008;112:1231–1239. doi: 10.1182/blood-2008-03-148072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bates EE, Fournier N, Garcia E, Valladeau J, Durand I, Pin JJ, et al. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J Immunol. 1999;163:1973–1983. [PubMed] [Google Scholar]
- 23.Flornes LM, Bryceson YT, Spurkland A, Lorentzen JC, Dissen E, Fossum S. Identification of lectin-like receptors expressed by antigen presenting cells and neutrophils and their mapping to a novel gene complex. Immunogenetics. 2004;56:506–517. doi: 10.1007/s00251-004-0714-x. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Yuan Z, Chen G, Zhang M, Zhang W, Yu Y, et al. Cloning and characterization of a novel ITIM containing lectin-like immunoreceptor LLIR and its two transmembrane region deletion variants. Biochem Biophys Res Commun. 2001;281:131–140. doi: 10.1006/bbrc.2001.4322. [DOI] [PubMed] [Google Scholar]
- 25.Dudziak D, Nimmerjahn F, Bornkamm GW, Laux G. Alternative splicing generates putative soluble CD83 proteins that inhibit T cell proliferation. J Immunol. 2005;174:6672–6676. doi: 10.4049/jimmunol.174.11.6672. [DOI] [PubMed] [Google Scholar]
- 26.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jimenez D, Roda-Navarro P, Springer TA, Casasnovas JM. Contribution of N-linked glycans to the conformation and function of intercellular adhesion molecules (ICAMs) J Biol Chem. 2005;280:5854–5861. doi: 10.1074/jbc.M412104200. [DOI] [PubMed] [Google Scholar]
- 28.Meyer-Wentrup F, Cambi A, Joosten B, Looman MW, de Vries IJ, Figdor CG, et al. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J Leukoc Biol. 2009;85:518–525. doi: 10.1189/jlb.0608352. [DOI] [PubMed] [Google Scholar]
- 29.Cao W, Zhang L, Rosen DB, Bover L, Watanabe G, Bao M, et al. BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS Biol. 2007;5:e248. doi: 10.1371/journal.pbio.0050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronninger M, Eklow C, Lorentzen JC, Klareskog L, Padyukov L. Differential expression of transcripts for the autoimmunity-related human dendritic cell immunoreceptor. Genes Immun. 2008;9:412–418. doi: 10.1038/gene.2008.32. [DOI] [PubMed] [Google Scholar]
- 31.Eklow C, Makrygiannakis D, Backdahl L, Padyukov L, Ulfgren AK, Lorentzen JC, et al. Cellular distribution of the C-type II lectin DCIR and its expression in the rheumatic joint-identification of a subpopulation of DCIR+ T cells. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.076976. [DOI] [PubMed] [Google Scholar]