Abstract
Background/Aims
Underlying cardiac pathology and atrial fibrillation (AF) affect the molecular remodeling of ion channels in the atria. Changes in the expression of these molecules have not been demonstrated in Korean patients with mitral valvular heart disease. Thus, the purpose of this study was to analyze ion channel expression in patients with chronic AF and mitral valvular heart disease.
Methods
A total of 17 patients (eight males and nine females; mean age, 57 ± 14 years [range, 19 to 77]) undergoing open-heart surgery were included in the study. Twelve patients (seven with coronary artery disease and five with aortic valvular disease) had sinus rhythm, and five patients (all with mitral valvular disease) had chronic, permanent AF. A piece of right atrial appendage tissue (0.5 g) was obtained during surgery. RT-PCR was used to evaluate the expression of L-type Ca2+ channels, ryanodine receptor (RyR2), sarcoplasmic reticular Ca2+-ATPase (SERCA2), gene encoding the rapid component of the delayed rectifier Ikr (HERG), gene encoding calcium-independent transient outward current Ito1 (Kv4.3), gene encoding the ultrarapid component of the delayed rectifier Iku (Kv1.5), K+ channel-interacting protein 2 (KChIP2), hyperpolarization-activated cation channel 2 associated with the pacemaker current If (HCN2), and gene encoding Na+ channel (SCN5A).
Results
Reduced L-type Ca2+ channel, RyR2, SERCA2, Kv1.5, and KChIP2 expression and borderline increased HCN2 expression were observed in the patients with AF and mitral valvular heart disease. Left atrial diameter was negatively correlated with RyR2 and KChIP2 expression. Fractional area shortening of the left atrium was positively correlated with RyR2 and KChIP2 expression.
Conclusions
Alterations in ion channel expression and the anatomical substrate may favor the initiation and maintenance of AF in patients with mitral valvular heart disease.
Keywords: Atrial fibrillation, Electrical remodeling, Ion channels
INTRODUCTION
Atrial fibrillation (AF) begets AF; that is, AF itself alters atrial electrophysiological properties in a manner that favors the induction and maintenance of AF (i.e., electrical remodeling) [1]. The major mechanisms of electrical remodeling are initiated by an increased atrial rate. The roughly 10-fold atrial rate increase caused by AF substantially increases cellular Ca2+ loading by Ca2+ intake through ICa [2]. Progressive Ca2+ loading threatens cell viability, and cells respond to minimize the impact of the increased rate on the intracellular Ca2+ load. Thus, defense mechanisms include voltage-dependent and intracellular Ca2+ concentration-dependent ICa inactivation. Decreases in ICa reduce Ca2+ entry and help to prevent Ca2+ overload; however, because ICa is a key contributor to the action potential plateau, reductions in ICa decrease the action potential duration (APD), reduce the refractory period, and promote the induction and maintenance of AF by multiple-circuit reentry [3]. Changes in a number of ionic currents have been detected in persistent and permanent human AF. An increased IK1 [4], lack of change in INa [5], decreased Ito [4], and decreased ICa [5,6] have been reported. Corresponding changes in IKur (no change [5], decreased [4]), IKr, IKs, and If are not well established [7].
Changes in the gene or protein expression of the channels responsible for action potential generation have been reported in AF. Changes in the expression of molecules such as gene encoding calcium-independent transient outward current Ito1 (Kv4.3) [8], α1c (ICa) [9], gene encoding the ultrarapid component of the delayed rectifier IKu (Kv1.5) [4], gene encoding the rapid component of the delayed rectifier Ikr (HERG) [8], and hyperpolarization-activated cation channel 2 associated with the pacemaker current If (HCN) [10] have also been reported. Molecules related to Ca2+ homeostasis in cardiomyocytes include ryanodine receptor (RyR2), sarcoplasmic reticular Ca2+-ATPase (SERCA2), phospholamban, calsequestrin, and L-type Ca2+ channels. Decreased SERCA2 and L-type Ca2+ channel expression has been reported in AF, and the expression of other molecules may be unaltered [9].
The assembly of four voltage-gated K+ channel Kv α-subunits into a tetrameric structure creates a functional Ito channel. Reduced Ito levels have been reported in various conditions, including heart failure [11] and myocardial infarction [12] as well as AF, and the magnitude of Ito may affect APD [13]. Kv4.3 is responsible for most of the Ito current in the human atrium. Assembly of the β-subunits into pore-forming tetramers is sufficient to generate functional K+ channels. Kv expression is modulated by cytoplasmic proteins such as β-subunits, K+ channel-interacting protein (KChIP) 2, frequenin, and K+ channel-associated protein (KChAP). KChIP was recently shown to associate with Kv4 [14]. Of the three KChIP types known (KChIP1, KChIP2, and KChIP3), KChIP2 is expressed in the human heart. KChIP2 may be a regulatory subunit of Kv4.3 [15].
Expression changes in these molecules have not been demonstrated in Korean patients with mitral valvular heart disease. Therefore, the purpose of this study was to analyze the expression status of these molecules in patients with chronic AF and mitral valvular heart disease.
METHODS
Patients and atrial tissue collection
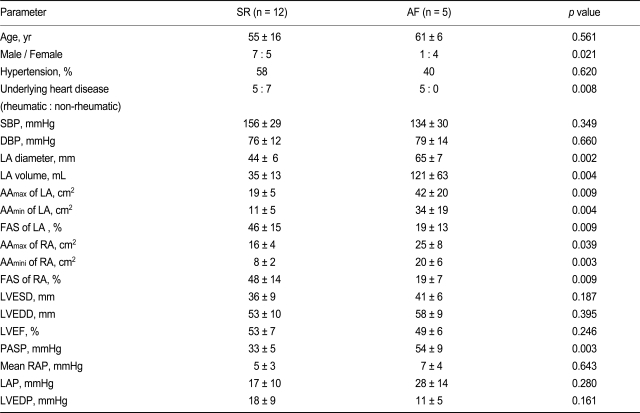
A total of 17 patients (eight males and nine females; mean age, 57 ± 14 years [range, 19 to 77]) undergoing open heart surgery for coronary artery bypass, valve repair, or replacement were included in the study. Twelve patients had sinus rhythm (SR): coronary artery disease (n = 7, CAD-SR) and aortic stenosis/stenoinsufficiency (n = 5, AVD-SR). Five patients had permanent AF (longer than 6 months) and mitral stenosis (MVD-AF). Written informed consent was obtained from each patient before all procedures. Echocardiography was performed within 1 week, and cardiac catheterization was performed within 1 month prior to surgery. The clinical characteristics of each group are listed in Table 1. A piece of right atrial appendage tissue (0.5 g) was obtained during surgery. The excised samples were immediately frozen in liquid nitrogen and stored at -80℃ until use.
Table 1.
Clinical, echocardiographic, and hemodynamic parameters
Values are presented as mean ± SD.
SR, sinus rhythm; AF, atrial fibrillation; SBP, systolic blood pressure; DBP, diastolic blood pressure; LA, left atrium; RA, right atrium; AA, atrial area measured in the apical four-chamber view; FAS, fractional area shortening; LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure; RAP, right atrial pressure; LAP, left atrial pressure; LVEDP, left ventricular end-diastolic pressure.
Transthoracic echocardiography
A 2.5-MHz phased array transducer and standard echocardiographic system (Acuson Sequoia C256, Siemens, Washington, DC, USA)were used to measure cardiac structural and functional parameters. Three diameters within the left atrium (LA) were measured: height in the parasternal long-axis view (LA1), width in the tilted parasternal short-axis view (LA2), and height in the apical four-chamber view (LA3). The left atrial volume was calculated as (π × LA1 × LA2 × LA3)/8. The apical four-chamber view was obtained and recorded on videotape for subsequent off-line measurements. For the LA and right atrium (RA), the largest atrial area (AAmax) during ventricular systole and smallest area (AAmin) during ventricular diastole were measured. Atrial fractional area shortening (FAS) was calculated as (AAmax - AAmin)/AAmax × 100. In the AF group, the average of three consecutive cardiac cycles was used for each echocardiographic measurement. Pulmonary artery systolic pressure (PASP) was estimated from the trans-tricuspid pressure gradient by Doppler examination.
Cardiac catheterization
All cardiac catheterization procedures were performed as a preoperative evaluation. In cases of rheumatic valvular heart disease, right and left heart catheterization was performed to obtain hemodynamic profiles of each chamber and cardiac function. In cases of coronary artery disease, left heart catheterization was performed before coronary angiography. Pressure profiles were measured using a standard fluid-filled catheter manometer.
Semiquantitative RT-PCR
Total RNA was extracted from the harvested atrial tissues using the following procedure. Briefly, the frozen tissues were homogenized with Ultraspec-II (Biotecx, Houston, TX, USA), and the supernatant was collected from the mixture with chloroform by centrifugation. RNA was extracted using isopropanol, RNA Tack Resin (Biotecx), and ethanol precipitation. First-strand cDNA was synthesized from the RNA using the PolyATract cDNA Synthesis System (Promega, Madison, WI, USA). The sequences and sizes of the sense and antisense primers used for each molecule are listed in Table 2. cDNA amplification was performed in a thermal cycler (GeneAmp PCR System 9600, Perkin-Elmer Cetus, Waltham, MA, USA) using Taq polymerase (Promega). Following denaturation at 94℃ for 5 minutes, the samples were subjected to 30 cycles of denaturation at 94℃ for 30 seconds, annealing (see Table 2 for the temperature) for 30 seconds, and extension at 72℃ for 7 minutes. The amplified products were electrophoresed in 1.5% agarose gels containing ethidium bromide and examined under a UV transilluminator. The intensity of each band was quantified using image analysis software (TINA version 2.10, Raytest, Straubenhardt, Germany), and the expression levels were calculated by normalization against the intensity of β-actin.
Table 2.
Primer sequences
L-type Ca2+ channel, voltage-gated L-type Ca2+ channel subunit α1c; RyR2, ryanodine receptor; SERCA2, sarcoplasmic reticular calcium adenosine triphosphatase; HERG, gene encoding the rapid component of the delayed rectifier IKr; Kv4.3, gene encoding calcium-independent transient outward current Ito1; Kv1.5, gene encoding the ultrarapid component of the delayed rectifier IKur; KChIP2, K+ channel-interacting protein 2; HCN2, hyperpolarization-activated cation channel 2 associated with the pacemaker current If; SCN5A, gene encoding Na+ channel.
Statistical analysis
All numerical values are expressed as the mean ± standard deviation. Statistical significance was determined using the Mann-Whitney U test for group-to-group mean comparisons. A p value of less than 0.05 was considered to be significant.
RESULTS
Clinical parameters of the enrolled patients
Parameters such as sex, underlying heart disease, atrial diameter, left atrial volume, and PASP were significantly different between the SR and AF groups (Table 1). Briefly, more female patients, more patients with rheumatic valvular heart disease, a larger atrium, lower FAS of the LA, and higher PASP were common among the patients in the AF group.
Molecules related to Ca2+ homeostasis
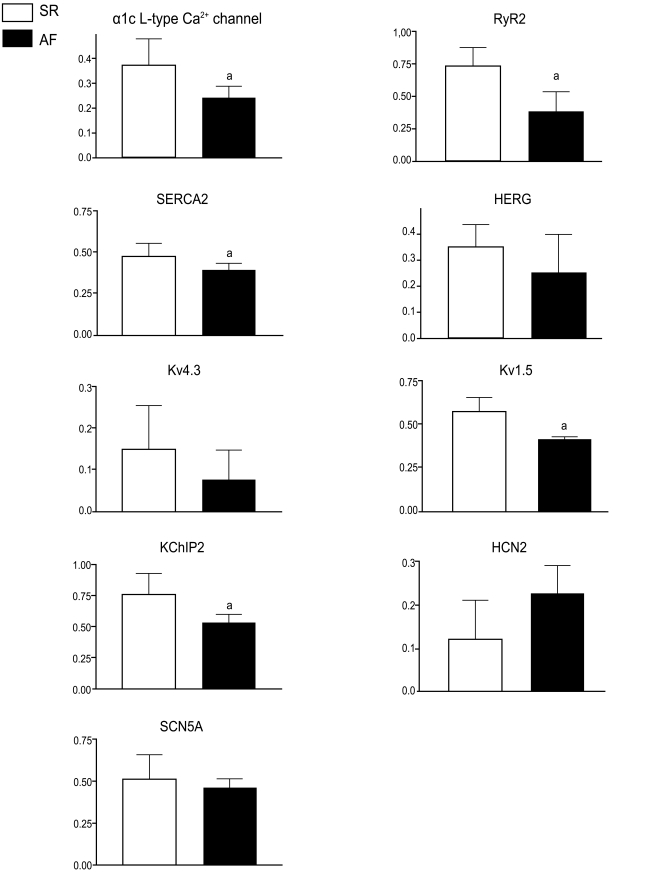
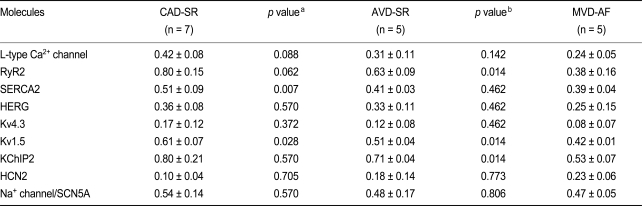
Reduced L-type Ca2+ channel, RyR2, and SERCA2 expression was observed in the AF group (Fig. 1). Atrial samples obtained from patients in the MVD-AF group showed reduced RyR2 expression compared with the AVD-SR group; however, no significant difference in L-type Ca2+ channel and SERCA2 expression was noted between the AVD-SR and MVD-AF groups (Table 3). A significant reduction in SERCA2 expression and borderline significance in the differences in the expression of L-type Ca2+ channels and RyR2 were noted between the CAD-SR and AVD-SR groups (Table 3).
Figure 1.
Molecular remodeling: mRNA expression levels in patients with sinus rhythm (SR; blank boxes) and atrial fibrillation (AF; filled boxes). Error bars indicate the standard deviation. ap < 0.05. L-type Ca2+ channel, voltage-gated L-type Ca2+ channel subunit α1c; RyR2, ryanodine receptor; SERCA2, sarcoplasmic reticular calcium adenosine triphosphatase; HERG, gene encoding the rapid component of the delayed rectifier IKr; Kv4.3, gene encoding calciumin-dependent transient outward current Ito1; Kv1.5, gene encoding the ultrarapid component of the delayed rectifier IKur; KChIP2, K+ channel-interacting protein 2; HCN2, hyperpolarization-activated cation channel 2 associated with the pacemaker current If; SCN5A, gene encoding Na+ channel.
Table 3.
mRNA expression levels between the patient groups: coronary artery disease with SR vs. aortic valvular disease with SR vs. mitral valvular disease with AF
Values are presented as mean ± SD.
SR, sinus rhythm; AF, atrial fibrillation; CAD, coronary artery disease; AVD, aortic valvular disease; MVD, mitral valvular disease; L-type Ca2+ channel, voltage-gated L-type Ca2+ channel subunit α1c; RyR2, ryanodine receptor; SERCA2, sarcoplasmic reticular calcium adenosine triphosphatase; HERG, gene encoding the rapid component of the delayed rectifier IKr; Kv4.3, gene encoding calcium-independent transient outward current Ito1; Kv1.5, gene encoding the ultrarapid component of the delayed rectifier IKur; KChIP2, K+ channel-interacting protein 2; HCN2, hyperpolarization-activated cation channel 2 associated with the pacemaker current If; SCN5A, gene encoding Na+ channel.
ap value, CAD-SR vs. AVD-SR.
bp value, AVD-SR vs. MVD-AF.
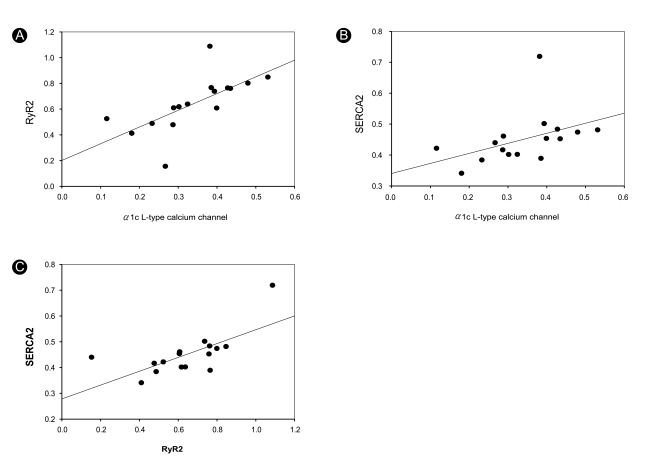
A positive correlation was found between the expression of L-type Ca2+ channels, RyR2, and SERCA2 (Fig. 2): L-type Ca2+ channels vs. RyR2 (p = 0.004, r2 = 0.455) and RyR2 vs. SERCA2 (p = 0.004, r2 = 0.467). L-type Ca2+ channel and SERCA2 expression (p = 0.096, r2 = 0.185) did not exhibit a robust correlation.
Figure 2.
Correlations among L-type Ca2+ channel, RyR2, and SERCA2 expression. L-type Ca2+ channel, voltage-gated L-type Ca2+ channel subunit α1c; RyR2, ryanodine receptor; SERCA2, sarcoplasmic reticular calcium adenosine triphosphatase.
Molecules encoding K+ and Na+ channels
The expression of Kv1.5 was reduced in AF, and borderline increased expression of HCN2 was observed in AF; however, the expression of HERG, Kv4.3, and SCN5A was unchanged (Fig. 1). Atrial samples obtained from patients in the AVD-SR group showed reduced SERCA2 and Kv1.5 expression compared with the CAD-SR group, and the MVD-AF group showed decreased RyR2 and Kv1.5 expression compared with the AVD-SR group (Table 3).
KChIP2 expression
The AF group showed significantly reduced KChIP2 expression (Fig. 1). In contrast, the expression of Kv4.3, which is responsible for Ito, was not decreased in the patients with AF enrolled in the present study (Fig. 1); moreover, KChIP2 expression was not correlated with that of Kv4.3 (p = 0.839, r2 = 0.003).
Correlation between molecular remodeling and structural and functional parameters
Left atrial diameter was negatively correlated with RyR2 (p = 0.010, r2 = 0.979) and KChIP2 expression (p = 0.018, r2 = 0.965). A correlation between L-type Ca2+ channel expression and left atrial diameter showed borderline significance (p = 0.090, r2 = 0.828). Left atrial volume was negatively correlated with KChIP2 expression, with borderline significance (p = 0.099, r2 = 0.811). Left ventricular end-systolic diameter (LVESD) was negatively correlated with RyR2 (p = 0.007, r2 = 0.985), Kv1.5 (p = 0.006, r2 = 0.987), and KChIP2 expression (p = 0.049, r2 = 0.904). Left ventricular end-diastolic diameter (LVEDD) was negatively correlated with RyR2 (p = 0.014, r2 = 0.973) and Kv1.5 (p = 0.002, r2 = 0.997).
FAS of the LA was positively correlated with RyR2 (p = 0.023, r2 = 0.954) and KChIP2 expression (p = 0.013, r2 = 0.975), and with Kv1.5 expression, with borderline significance (p = 0.062, r2 = 0.879). FAS of the RA was positively correlated with RyR2 (p = 0.097, r2 = 0.816) and Kv1.5 expression (p = 0.052, r2 = 0.899), with borderline significance. LVEDP was negatively correlated with HERG expression, with borderline significance (p = 0.069, r2 = 0.866).
DISCUSSION
Molecular remodeling
The decreased expression of L-type Ca2+ channels and SERCA2 found among the patients with AF in this study is similar to the findings of previous reports [9,19-22]. Decreased expression of RyR2 in AF was observed in this study; however, this finding is controversial in that unchanged [9] and decreased [19] expression have been reported previously. Further investigation is needed to resolve this issue. Kv4.3 expression has been shown to be unchanged in AF [8]. Decreased mRNA expression in AF has been reported [23]; however, that finding is not compatible with the data produced in this experiment. Therefore, the expression status of Kv4.3 in AF remains controversial. Kv1.5 expression was reduced in AF, and this finding is compatible with findings in a previous report [4].
AVD versus CAD in patients with SR
L-type Ca2+ channels, RyR2, SERCA2, and Kv1.5 all showed a tendency toward reduced expression in the AVD-SR group compared with the CAD-SR group. The two groups in this study showed no significant differences in structural and hemodynamic parameters, except for a higher PASP in the AVD-SR group. Of those molecules, L-type Ca2+ channel and SERCA2 expression was not different between the AVD-SR and MVD-AF groups; however, these groups showed significant differences in atrium size and FAS. Therefore, the expression status of L-type Ca2+ channels and SERCA2 might be related to underlying heart disease.
KChIP2 expression in AF
The precise meaning and mechanism of these findings are unclear. Kv4.3 is responsible for most human Ito current, and KChIP2 could be a physiological regulatory subunit of Kv4.3 [15]. Therefore, reduced KChIP2 expression may induce decreased Kv4.3 function. Although the expression status of Kv4.3 in AF is controversial, the decreased Ito current observed in AF4 may be related to decreased KChIP2 expression.
Clinical implications
Molecular remodeling may develop secondary to changes in the atrial environment, and expression changes in channel or channel-related molecules known to affect electrophysiological properties may favor the initiation and maintenance of atrial tachyarrhythmias, including AF. Classical therapeutic modalities for AF target the substrate or rhythm itself; however, recent progress in our understanding of the remodeling associated with AF may make "remodeling attenuation therapy" or "upstream therapy" possible. Some approaches, including the use of Ca2+ channel blockers, have been investigated without remarkable results. Remodeling attenuation therapy using blocking agents should target the molecules showing increased expression in AF. All of the molecules evaluated in this study except HCN2 showed reduced expression; therefore, they are not good therapeutic candidates. HCN2 may also not be a good candidate because its role in AF is unclear. In this respect, therapy based on structural remodeling is a reasonable substitute for channel blockade as a form of remodeling attenuation therapy. Recent trials targeting the reninangiotensin system have shown positive results for electrical remodeling [24] and prevention of the recurrence of AF [25].
Study limitations
We analyzed right appendage tissue in this study; however, the use of this tissue does not reveal changes occurring in the rest of the atrium. We cannot know a molecule's function based on its mRNA expression alone. Therefore, additional studies are required to evaluate ion channel function. In addition, the patient populations enrolled in this study were heterogeneous. Therefore, the comparisons made among the groups might have limitations.
Conclusions
Reduced L-type Ca2+ channel, RyR2, SERCA2, Kv1.5, and KChIP2 expression and borderline increased HCN2 expression were observed in all patients with AF and mitral valvular heart disease. RyR2, Kv1.5, and KChIP2 expression was correlated with structural/hemodynamic parameters. Alterations in ion channel expression and the anatomical substrate may favor the initiation and maintenance of AF in patients with mitral valvular heart disease.
Acknowledgements
This study was supported by a grant from the Seoul National University Hospital Research Fund (No. 09-2002-012).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Chartier D, Leblanc N, Nattel S. Intracellular calcium changes and tachycardia-induced contractile dysfunction in canine atrial myocytes. Cardiovasc Res. 2001;49:751–761. doi: 10.1016/s0008-6363(00)00294-7. [DOI] [PubMed] [Google Scholar]
- 3.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 4.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv15 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 5.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 6.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 7.Allessie MA, Boyden PA, Camm AJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769–777. doi: 10.1161/01.cir.103.5.769. [DOI] [PubMed] [Google Scholar]
- 8.Van Wagoner DR, Nerbonne JM. Molecular basis of electrical remodeling in atrial fibrillation. J Mol Cell Cardiol. 2000;32:1101–1117. doi: 10.1006/jmcc.2000.1147. [DOI] [PubMed] [Google Scholar]
- 9.Lai LP, Su MJ, Lin JL, et al. Down-regulation of L-type calcium channel and sarcoplasmic reticular Ca (2+)-ATPase mRNA in human atrial fibrillation without significant change in the mRNA of ryanodine receptor, calsequestrin and phospholamban: an insight into the mechanism of atrial electrical remodeling. J Am Coll Cardiol. 1999;33:1231–1237. doi: 10.1016/s0735-1097(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 10.Lai LP, Su MJ, Lin JL, et al. Measurement of funny current (I(f)) channel mRNA in human atrial tissue: correlation with left atrial filling pressure and atrial fibrillation. J Cardiovasc Electrophysiol. 1999;10:947–953. doi: 10.1111/j.1540-8167.1999.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 11.Näbauer M, Beuckelmann DJ, Erdmann E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:386–394. doi: 10.1161/01.res.73.2.386. [DOI] [PubMed] [Google Scholar]
- 12.Jeck C, Pinto J, Boyden P. Transient outward currents in subendocardial Purkinje myocytes surviving in the infarcted heart. Circulation. 1995;92:465–473. doi: 10.1161/01.cir.92.3.465. [DOI] [PubMed] [Google Scholar]
- 13.Greenstein JL, Wu R, Po S, Tomaselli GF, Winslow RL. Role of the calcium-independent transient outward current I(to1) in shaping action potential morphology and duration. Circ Res. 2000;87:1026–1033. doi: 10.1161/01.res.87.11.1026. [DOI] [PubMed] [Google Scholar]
- 14.An WF, Bowlby MR, Betty M, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 15.Decher N, Uyguner O, Scherer CR, et al. hKChIP2 is a functional modifier of hKv4.3 potassium channels: cloning and expression of a short hKChIP2 splice variant. Cardiovasc Res. 2001;52:255–264. doi: 10.1016/s0008-6363(01)00374-1. [DOI] [PubMed] [Google Scholar]
- 16.Brundel BJ, Van Gelder IC, Henning RH, et al. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation. 2001;103:684–690. doi: 10.1161/01.cir.103.5.684. [DOI] [PubMed] [Google Scholar]
- 17.Ohya S, Morohashi Y, Muraki K, et al. Molecular cloning and expression of the novel splice variants of K(+) channel-interacting protein 2. Biochem Biophys Res Commun. 2001;282:96–102. doi: 10.1006/bbrc.2001.4558. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, Biel M. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J. 1999;18:2323–2329. doi: 10.1093/emboj/18.9.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohkusa T, Ueyama T, Yamada J, et al. Alterations in cardiac sarcoplasmic reticulum Ca2+ regulatory proteins in the atrial tissue of patients with chronic atrial fibrillation. J Am Coll Cardiol. 1999;34:255–263. doi: 10.1016/s0735-1097(99)00169-2. [DOI] [PubMed] [Google Scholar]
- 20.Grammer JB, Zeng X, Bosch RF, Kühlkamp V. Atrial L-type Ca2+-channel, beta-adrenorecptor, and 5-hydroxytryptamine type 4 receptor mRNAs in human atrial fibrillation. Basic Res Cardiol. 2001;96:82–90. doi: 10.1007/s003950170081. [DOI] [PubMed] [Google Scholar]
- 21.van der Velden HMW, van der Zee L, Wijffels MC, et al. Atrial fibrillation in the goat induces changes in monophasic action potential and mRNA expression of ion channels involved in repolarization. J Cardiovasc Electrophysiol. 2000;11:1262–1269. doi: 10.1046/j.1540-8167.2000.01262.x. [DOI] [PubMed] [Google Scholar]
- 22.Grammer JB, Bosch RF, Kuhlkamp V, Seipel L. Molecular and electrophysiological evidence for "remodeling" of the L-type Ca2+ channel in persistent atrial fibrillation in humans. Z Kardiol. 2000;89(Suppl 4):IV23–IV29. doi: 10.1007/s003920070060. [DOI] [PubMed] [Google Scholar]
- 23.Grammer JB, Bosch RF, Kühlkamp V, Seipel L. Molecular remodeling of Kv4.3 potassium channels in human atrial fibrillation. J Cardiovasc Electrophysiol. 2000;11:626–633. doi: 10.1111/j.1540-8167.2000.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima H, Kumagai K, Urata H, Gondo N, Ideishi M, Arakawa K. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation. 2000;101:2612–2617. doi: 10.1161/01.cir.101.22.2612. [DOI] [PubMed] [Google Scholar]
- 25.Madrid AH, Bueno MG, Rebollo JM, et al. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002;106:331–336. doi: 10.1161/01.cir.0000022665.18619.83. [DOI] [PubMed] [Google Scholar]