Abstract
Background/Aims
Fabry disease is an X-linked recessive and progressive disease caused by α-galactosidase A (α-GaL A) deficiency. We sought to assess the prevalence of unrecognized Fabry disease in dialysis-dependent patients and the efficacy of serum globotriaosylceramide (GL3) screening.
Methods
A total of 480 patients of 1,230 patients among 17 clinics were enrolled. Serum GL3 levels were measured by tandem mass spectrometry. Additionally, we studied the association between increased GL3 levels and cardiovascular disease, cerebrovascular disease, or left ventricular hypertrophy.
Results
Twenty-nine patients had elevated serum GL3 levels. The α-GaL A activity was determined for the 26 patients with high GL3 levels. The mean α-GaL A activity was 64.6 nmol/hr/mg (reference range, 45 to 85), and no patient was identified with decreased α-GaL A activity. Among the group with high GL3 levels, 15 women had a α-GaL A genetics analysis. No point mutations were discovered among the women with high GL3 levels. No correlation was observed between serum GL3 levels and α-GaL A activity; the Pearson correlation coefficient was 0.01352 (p = 0.9478). No significant correlation was observed between increased GL3 levels and the frequency of cardiovascular disease or cerebrovascular disease.
Conclusions
Fabry disease is very rare disease in patients with end-stage renal disease. Serum GL3 measurements as a screening method for Fabry disease showed a high false-positive rate. Thus, serum GL3 levels determined by tandem mass spectrometry may not be useful as a screening method for Fabry disease in patients with end stage renal disease.
Keywords: Fabry disease, Globotriaosylceramide, End-stage renal disease
INTRODUCTION
Fabry disease is an X-linked recessive and progressive multiorgan lysosomal storage disease caused by α-galactosidase A (α-GaL A) deficiency. Because the disease is X-linked, males (hemizygotes) are predominantly affected, and females (heterozygotes) are obligate carriers. The partial or complete deficiency of the lysosomal enzyme leads to an accumulation of neutral glycosphingolipids, especially globotriaosylceramide (GL3), in the vascular endothelium and visceral tissues throughout the body. This lipid accumulation leads to significant morbidity, especially in affected males. Many female carriers can also be affected, but because of random X-inactivation, they usually present with a more variable phenotype [1]. Therefore, females usually manifest the disease at a later age, although a homozygous female has been described with severe onset of classical disease at 8 years of age [2].
The prevalence of Fabry disease has been estimated to be 1 in 40,000 to 117,000 males, and the prevalence may be higher in patients with end-stage renal disease (ESRD) [3-8].
Fabry disease presents with a characteristic phenotype that varies and may include angiokeratoma, corneal opacity, acroparesthesia, cerebrovascular disease, ischemic heart disease, and chronic kidney disease. However, in addition to the classical Fabry disease phenotype, variants of Fabry disease exist. For example, the cardiac and renal variants show manifestations limited to the heart and kidneys, respectively. The renal variant of Fabry disease is characterized by proteinuria and chronic renal insufficiency in the absence of other clinical symptoms associated with classical Fabry disease. The cardiac variant of Fabry disease has primarily cardiac manifestations, including left ventricular hypertrophy (LVH), valvular involvement, and arrhythmias, but no other classical symptoms of Fabry disease. This variant was first noted in pathological studies of endomyocardial biopsy specimens and autopsy specimens of the heart [9]. For patients with the renal variant type, the renal manifestations generally occur later in life, and a greater residual α-GaL A activity is found in these patients [10].
Recently, enzyme replacement therapy for Fabry disease has shown that renal pathology and function can be improved with treatment. Therefore, early detection of renal involvement of Fabry disease is important for the prevention of progression to ESRD. Furthermore, patients with ESRD can be protected from cardiovascular and cerebrovascular complications by treatment with enzyme replacement therapy [11-13]. Therefore, an accurate and affordable screening method is needed to diagnose the disease as early as possible for prompt intervention.
Some screening methods for Fabry disease are available that determine the α-GaL A activity from a variety of sources. However, the α-GaL A activity method is not widely available for screening. Therefore, the goal of this study was to assess the prevalence of unrecognized Fabry disease in patients with ESRD on maintenance dialysis by measuring serum GL3 levels by tandem mass spectrometry, and the efficacy of serum GL3 as a screening method for Fabry disease was evaluated.
METHODS
Patients
Seventeen hemodialysis clinics in the Seoul and Gyeonggi-do regions of Korea participated in the Fabry disease screening program during 2005. From among patients receiving maintenance dialysis for more than 3 months, each of the clinics randomly selected male patients more than 40 years of age and female patients more than 50 years of age. The enrolled patients had no specific diagnosis for their ESRD.
Patient characteristics
We collected information on the characteristics of the screened participants including duration of renal disease, duration of dialysis, history of cardiovascular (myocardial infarction and angina pectoris) and cerebrovascular disease (stroke), and the presence of LVH on electrocardiogram (ECG).
Screening test by serum globotriaosylceramide (GL3) measurement
Blood samples were collected before dialysis, and the serum was separated. The GL3 levels were measured by tandem mass spectrometry using an API4000 instrument (MDS Sciex, Ontario, Canada). The serum GL3 level reference range in a normal person is 3.88 to 9.87 µg/mL. We defined patients with a serum GL3 level in the reference range as the "normal GL3 group" and patients with serum GL3 levels above the reference range as the "high GL3 group."
Confirmation test by α-GaL A activity measure-ment
For the patients in the high GL3 group, we measured the α-GaL A activity on peripheral blood leukocytes by a fluorescence assay with 4-methylumbelliferyl. The reference range of α-GaL A activity in normal persons is 45 to 85 nmol/hr/mg in leukocytes.
Molecular analysis of the α-GaL A gene
We confirmed the presence of Fabry disease by analyzing patients for the α-GaL A mutation using PCR-sequence analysis. Additionally, we assessed the clinical manifestations of patients with significantly decreased α-GaL A activity, as well as female patients with high GL3 levels. Genomic DNA was isolated from peripheral blood leukocytes using the PUREGENE blood kit (Genta, Minneapolis, MN, USA).
Mutation analysis was performed by polymerase chain reaction (PCR) to amplify each of the seven α-GaL A exon and intron sequences. The amplification was performed over 30 cycles; each cycle consisted of denaturing at 95℃ for 30 seconds, annealing at 55℃ for 30 seconds, and extension at 72℃ for 45 seconds. The PCR was conducted in reaction volumes of 20 µL, containing 100 ng of genomic DNA template, 1 µM of each primer, 200 µM each of dNTP, 1.5 mM MgCl2, 50 mM KCl, and 10 mM Tris-HCl (pH 8.3), and 1.0 unit of Taq polymerase (Promega, Madison, WI, USA). The PCR was performed in a PTC-200 thermocycler (MJ Research, Watertown, MA, USA). Double-stranded DNA was directly sequenced from the PCR products using the same primers as the PCR with the BigDye Termination version 3.0 (ABI Systems, Forester, CA, USA).
The sequences were diluted in eight volumes of enzyme dilution buffer (400 mM Tris-HCl; 10 mM MgCl2; pH 9.0). After the reaction was finished, the reactants were electrophoresed on an ABI3100 Genetic Analyzer (ABI Systems), with the POP-6 polymer as the running matrix. The sequences were analyzed with ABI PRISM Sequence Analysis version 3.7 (ABI Systems).
Statistical analysis
Qualitative variables were compared by the chi-square test, and the Pearson correlation analysis was also used. Analyses were performed using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Measurement of serum GL3
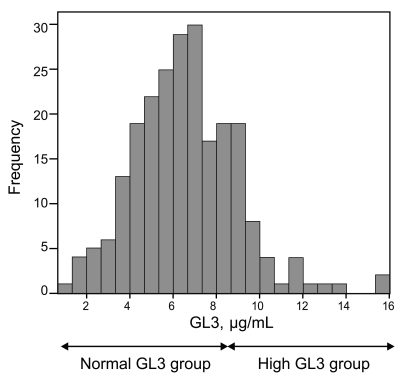
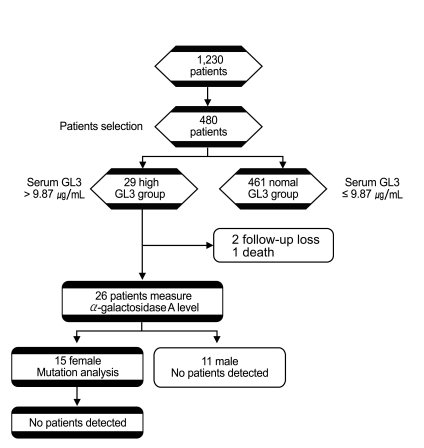
Four-hundred eighty patients (311 men and 169 women) were selected from 1,230 patients (670 men and 560 women) among the 17 hemodialysis clinics. Patients with clinically identified causes of ESRD were excluded from the study, such as patients with polycystic kidney disease, biopsy-proven disease, and diabetic nephropathy. The serum GL3 levels ranged from 0.96 µg/mL to 15.85 µg/mL. The mean serum GL3 level was 6.45 ± 2.28 µg/mL (males, 6.14 ± 2.16 µg/mL; females, 7.00 ± 2.37 µg/mL) (Fig. 1).
Figure 1.
Frequency of globotriasoylceramide (GL3) levels. Patients with serum GL3 levels in the reference range as the 'normal GL3 group' and patients with serum GL3 levels above the reference range as the 'high GL3 group' (reference range of serum GL3, 3.88 to 9.87 µg/mL). Serum GL3 levels ranged 0.96 to 15.85 µg/mL. The mean serum GL3 level was 6.45 ± 2.28 µg/mL.
Characteristics of the patients with a high GL3
Among the 480 selected patients, 29 patients (6.0%) had high serum GL3 levels. Twelve patients (2.5%) were male and 17 patients (3.5%) were female. The mean age was 63.4 ± 13.1 years in the high GL3 group and 53.1 ± 13 years in the normal GL3 group (p < 0.05). Dividing the high GL3 group by sex revealed that the mean age was 64.0 ± 11.3 years in men and 63.1 ± 14.5 years in women. The mean duration of renal disease was 93.2 ± 51.5 months in the high GL3 group and 109.8 ± 80.8 months in the normal GL3 group (p = 0.045). The mean duration of dialysis was 57.1 ± 40.8 months in the high GL3 group and 89.7 ± 74.1 months in the normal GL3 group (p = 0.046).
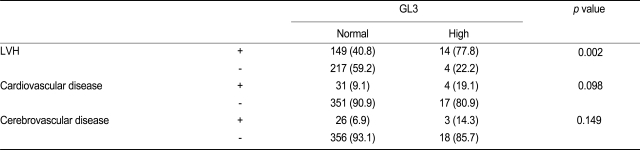
We investigated the relationship between increased GL3 levels and cardiovascular disease, cerebrovascular disease, and LVH. A significant increase in the frequency of LVH in the high GL3 group was observed. The prevalence of LVH in patients in the high GL3 group was 77.8%, whereas patients without LVH accounted for 22.2% (p = 0.002). Therefore, a significant increase in the frequency of LVH was observed in the high GL3 group. However, no significant increase in the frequency of cardiovascular or cerebrovascular disease was observed (Table 1).
Table 1.
The relationship between serum GL3 levels and cardiovascular disease, cerebrovascular disease, and LVH
Values are presented as number (%).
GL3, globotriaosylceramide; LVH, left ventricular hypertrophy.
Interview of patients in the high GL3 group
Among 29 patients in the high GL3 group, one patient died (GL3 = 15.85 µg/mL) and two patients were lost to follow-up (GL3 = 11.13 and 10.34 µg/mL). Thus, we interviewed 26 patients with high GL3 levels. We asked about family history of renal disease of unknown cause and about symptoms and signs related to Fabry disease, such as angiokeratoma and acroparesthesia. However, no patients identified a family history suggesting Fabry disease.
Measurement of α-GaL A activity
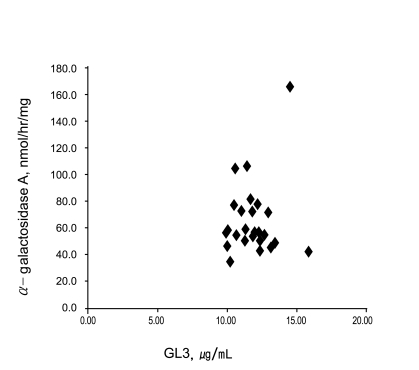
The mean α-GaL A activity of the 26 patients with high GL3 levels was 64.6 nmol/hr/mg (range, 34.6 to 165.1 ). No patients were found in whom α-GaL A activity had decreased significantly, and, therefore, no cases of Fabry disease were identified. The correlation between serum GL3 levels and α-GaL A is shown in Fig. 2. The Pearson's correlation coefficient between the serum GL3 and α-GaL A activity was 0.01352 (p = 0.9478).
Figure 2.
Correlation between the serum globotriasoyl ceramide (GL3) and α-galactosidase A activity. The pearson correlation coefficient between the serum GL3 and α-galactosidase A activity was 0.01352 (p = 0.9478). There was no correlation between the serum GL3 levels and the α-galactosidase A activity.
Molecular analysis of the α-GaL A gene
Among 26 patients (9 males and 17 females) with high GL3 levels, 15 women (two lost to follow-up) were evaluated by restriction analysis to confirm whether a point mutation of the α-GLA gene was present. The genetic analysis showed no point mutations among the women with high GL3 levels (Fig. 3).
Figure 3.
Flow chart illustrating the study. Among 1,230 patients, there was no Fabry patient detected. GL3, globotriaosylceramide.
DISCUSSION
The current Fabry disease model describes the accumulation of GL3 in different organs leading to severe organ damage [14]. Therefore, effective screening methods are needed for the early detection of Fabry disease before it causes irreversible harm.
Several screening methods for Fabry disease have been introduced. One method is detecting α-GaL A activity. α-GaL A activity screening can be performed on a variety of biological materials including leukocytes, dried whole blood spots, serum, and plasma. The limitation of this method is that abnormal values must be confirmed by a more reliable source such as blood leukocytes, and measurement of α-GaL A activity cannot detect all female carriers. For female heterozygous patients, a very low level of α-GaL A is diagnostic of the Fabry carrier state. However, a normal or subnormal enzyme activity level does not rule out the possibility that a woman is a carrier because of random X-chromosome inactivation. Therefore, all women at risk for carrying the abnormal gene should have DNA studies [15].
In the newborn, results from dried blood spots and plasma provide an effective screening method and concordant results. However, insufficient information is available to determine whether blood spots are more reliable than plasma for screening adult patient populations with renal and/or cardiac disease. It is reasonable to suspect that the circulating enzyme levels in adult populations might be influenced by secondary confounders (dietary, biologic, pathologic, or iatrogenic) compared with neonates [16].
Another possible method is detecting levels of GL3 in plasma. Glycosphingolipids (GSLs) are components of plasma cell membranes, which are synthesized in the endoplasmic reticulum and Golgi apparatus. A deficiency of the GSL catalyzing enzymes or cofactors leads to a specific storage disorder; in the case of Fabry disease, the enzyme is α-GaL A, and the accumulated substance is GL3 [17,18].
In advanced Fabry disease, the levels of GL3 in urine and plasma and non-neural tissues increase. However, accurately measuring GL3 in biological samples is difficult due to the inherent heterogeneity of the GL3 molecule, which increases the overall complexity of GL3 measurements. Current analytic methods reduce the complexity of GL3 determinations by measuring "total GL3," which is the sum of all GL3 isoforms [19]. The methods used to measure total GL3 include thin-layer chromatography, ELISA, liquid chromatography, C-UV, and gas chromatography-flame ionization. However, routine use of such methods has been limited by the long processing time. In contrast, tandem mass spectrometry requires a relatively short time for analysis, and it can quantify the isoforms separately.
In this study, 480 patients with ESRD were screened by assaying serum GL3 using tandem mass spectrometry, and the α-GaL A activity was determined in the blood leukocytes of patients with a high GL3. Furthermore, genetic mutation analysis in female patients with high GL3 was performed to determine the prevalence of Fabry disease in an at-risk population of patients with ESRD. We did not identify any patients with Fabry disease in these patients on dialysis in our community. We can explain these results in four ways.
First, the sample size of our study might be too small to detect Fabry disease considering the relatively low prevalence of this disease, as reported previously. The number of male patients on dialysis in our study was 311 among 670 male patients. In previous studies, the number of male dialysis patients ranged from 440 to 1,516. If we found only one male with Fabry disease in our study, the prevalence would have been 0.32%. Therefore, the number of enrolled male patients should ideally be in accordance with the prevalence of this disease in a given region.
Second, measuring serum GL3 as a screening method might have been inappropriate. In another study, serum GL3 levels increased four-fold in patients with classical Fabry disease compared with normal persons [20]. Among 26 patients who had blood samples drawn for α-GaL A activity and genetic mutation analysis, we did not find any cases of Fabry disease, even in those with high GL3 levels. In our preliminary study, we measured serum GL3 levels pre-hemodialysis and post-hemodialysis in the same five patients. The serum GL3 levels in the pre-hemodialysis compared to post-hemodialysis samples were not different (data not shown), suggesting that serum GL3 was not significantly altered by hemodialysis. Therefore, we used the pre-hemodialysis serum GL3 data for our study. Serum GL3 was analyzed by tandem mass spectrometry screening. However, a prior study reported that serum GL3 levels were not an appropriate screening method for Fabry disease because the serum GL3 levels could be within the normal range in heterozygotes with Fabry disease. Young et al. [21] found that only 33% of proven heterozygotes (15 of 44) had elevated plasma GL3, and six male patients (6/6) with the N125S mutation had normal GL3 levels in plasma. In another study, GL3 levels were within the normal range in two patients (2/11) [13] and overlapped between male heterozygotes and a healthy person [22]. Measuring serum GL3 levels for screening Fabry disease continues to be debated. We performed a molecular analysis of the α-GaL A gene in female patients with high GL3 levels. The patients with high GL3 levels in this study might have had a cofactor deficiency, or an unknown enzyme cascade may have existed. Therefore, further study is needed to identify other relevant metabolic pathways.
There are other causes for elevated GL3 levels. High GL3 levels may be associated with many other conditions such as inflammation, nutritional status, or limited excretion, or they may be falsely elevated [23]. Therefore, other factors that influence GL3 levels should be studied.
Third, a selection bias might have occurred in this study. The enrolled patients were chosen based on the attending physician's judgment that there was no specific diagnosis for the ESRD. Because Fabry disease is a low-prevalence disease, if only a few patients were missed, the data would be inaccurate. Fourth, three patients were lost to follow-up in the high GL3 group.
Interestingly, the high GL3 group included older patients, those with a shorter duration of renal disease, and a shorter duration of dialysis compared with the normal GL3 group. Additionally, patients with high GL3 levels had a significantly increased prevalence of LVH. Therefore, further study is needed to explain this relationship between high GL3 levels and LVH, as well as high GL3 levels and age. However, the results may be coincidental.
In summary, we did not identify any patients with Fabry disease in our cohort with ESRD on maintenance dialysis using the serum GL3 levels determined by tandem mass spectrometry. The reason may be that Fabry disease is very rare, and that the GL3 method showed a high false-positive rate.
Some patients on maintenance dialysis had increased serum GL3 levels. However, the reason for the high serum GL3 levels was not identified, and the elevation of serum GL3 levels may be due to some other factor than Fabry disease in patients on maintenance dialysis. The GL3 levels appear not to be useful for screening patients for Fabry disease who are on maintenance hemodialysis. The significance of high GL3 levels with advancing age as well as the progression of ESRD should be further studied.
Acknowledgements
We express our special thanks to the following collaborators: Young Mo Lee, Jun Kwa Wang, Sang Won Park, Jung Sun Kim (Division of Nephrology, Department of Internal Medicine, Korea University).
This study was supported by the extramural grant designated research donation number: R0609431.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bekri S, Enica A, Ghafari T, et al. Fabry disease in patients with end-stage renal failure: the potential benefits of screening. Nephron Clin Pract. 2005;101:c33–c38. doi: 10.1159/000085709. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Mari A, Coll MJ, Chabas A. Molecular analysis in Fabry disease in Spain: fifteen novel GLA mutations and identification of a homozygous female. Hum Mutat. 2003;22:258. doi: 10.1002/humu.9172. [DOI] [PubMed] [Google Scholar]
- 3.Mehta A, Ricci R, Widmer U, et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest. 2004;34:236–242. doi: 10.1111/j.1365-2362.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- 4.Linthorst GE, Hollak CE, Korevaar JC, Van Manen JG, Aerts JM, Boeschoten EW. alpha-Galactosidase A deficiency in Dutch patients on dialysis: a critical appraisal of screening for Fabry disease. Nephrol Dial Transplant. 2003;18:1581–1584. doi: 10.1093/ndt/gfg194. [DOI] [PubMed] [Google Scholar]
- 5.Kotanko P, Kramar R, Devrnja D, et al. Results of a nationwide screening for Anderson-Fabry disease among dialysis patients. J Am Soc Nephrol. 2004;15:1323–1329. doi: 10.1097/01.asn.0000124671.61963.1e. [DOI] [PubMed] [Google Scholar]
- 6.Tsakiris D, Simpson HK, Jones EH, et al. Report on management of renale failure in Europe, XXVI, 1995. Rare diseases in renal replacement therapy in the ERA-EDTA Registry. Nephrol Dial Transplant. 1996;11(Suppl 7):4–20. doi: 10.1093/ndt/11.supp7.4. [DOI] [PubMed] [Google Scholar]
- 7.Thadhani R, Wolf M, West ML, et al. Patients with Fabry disease on dialysis in the United States. Kidney Int. 2002;61:249–255. doi: 10.1046/j.1523-1755.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 8.Nakao S, Kodama C, Takenaka T, et al. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a "renal variant" phenotype. Kidney Int. 2003;64:801–807. doi: 10.1046/j.1523-1755.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakao S, Takenaka T, Maeda M, et al. An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–293. doi: 10.1056/NEJM199508033330504. [DOI] [PubMed] [Google Scholar]
- 10.Meroni M, Spisni C, Tazzari S, et al. Isolated glomerular proteinuria as the only clinical manifestation of Fabry's disease in an adult male. Nephrol Dial Transplant. 1997;12:221–223. doi: 10.1093/ndt/12.1.221. [DOI] [PubMed] [Google Scholar]
- 11.Brenner BM, Grünfeld JP. Renoprotection by enzyme replacement therapy. Curr Opin Nephrol Hypertens. 2004;13:231–241. doi: 10.1097/00041552-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Desnick RJ, Brady R, Barranger J, et al. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003;138:338–346. doi: 10.7326/0003-4819-138-4-200302180-00014. [DOI] [PubMed] [Google Scholar]
- 13.Choi JH, Cho YM, Suh KS, et al. Short-term efficacy of enzyme replacement therapy in Korean patients with Fabry disease. J Korean Med Sci. 2008;23:243–250. doi: 10.3346/jkms.2008.23.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eng CM, Fletcher J, Wilcox WR, et al. Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis. 2007;30:184–192. doi: 10.1007/s10545-007-0521-2. [DOI] [PubMed] [Google Scholar]
- 15.Desnick RJ, Brady RO. Fabry disease in childhood. J Pediatr. 2004;144(5 Suppl):S20–S26. doi: 10.1016/j.jpeds.2004.01.051. [DOI] [PubMed] [Google Scholar]
- 16.Fuller M, Lovejoy M, Brooks DA, Harkin ML, Hopwood JJ, Meikle PJ. Immunoquantification of alpha-galactosidase: evaluation for the diagnosis of Fabry disease. Clin Chem. 2004;50:1979–1985. doi: 10.1373/clinchem.2004.037937. [DOI] [PubMed] [Google Scholar]
- 17.Ginzburg L, Kacher Y, Futerman AH. The pathogenesis of glycosphingolipid storage disorders. Semin Cell Dev Biol. 2004;15:417–431. doi: 10.1016/j.semcdb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Pastores GM, Lien YH. Biochemical and molecular genetic basis of Fabry disease. J Am Soc Nephrol. 2002;13(Suppl 2):S130–S133. [PubMed] [Google Scholar]
- 19.Nelson BC, Roddy T, Araghi S, et al. Globotriaosylceramide isoform profiles in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;805:127–134. doi: 10.1016/j.jchromb.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Mills K, Johnson A, Winchester B. Synthesis of novel internal standards for the quantitative determination of plasma ceramide trihexoside in Fabry disease by tandem mass spectrometry. FEBS Lett. 2002;515:171–176. doi: 10.1016/s0014-5793(02)02491-2. [DOI] [PubMed] [Google Scholar]
- 21.Young E, Mills K, Morris P, et al. Is globotriaosylceramide a useful biomarker in Fabry disease? Acta Paediatr Suppl. 2005;94:51–54. doi: 10.1111/j.1651-2227.2005.tb02112.x. [DOI] [PubMed] [Google Scholar]
- 22.Yoon HR, Cho K, Yoo HW, et al. Determination of plasma C16-C24 globotriaosylceramide (Gb3) isoforms by tandem mass spectrometry for diagnosis of Fabry disease. J Genet Med. 2007;4:45–52. [Google Scholar]
- 23.Forni S, Fu X, Schiffmann R, Sweetman L. Falsely elevated urinary Gb3 (globotriaosylceramide, CTH, GL3) Mol Genet Metab. 2009;97:91. doi: 10.1016/j.ymgme.2009.01.011. [DOI] [PubMed] [Google Scholar]