Abstract
AIM: To investigate the benefits of insulin like growth factor-1 (IGF-1) supplementation to serum-free institut georges lopez-1 (IGL-1)® solution to protect fatty liver against cold ischemia reperfusion injury.
METHODS: Steatotic livers were preserved for 24 h in IGL-1® solution supplemented with or without IGF-1 and then perfused “ex vivo” for 2 h at 37°C. We examined the effects of IGF-1 on hepatic damage and function (transaminases, percentage of sulfobromophthalein clearance in bile and vascular resistance). We also studied other factors associated with the poor tolerance of fatty livers to cold ischemia reperfusion injury such as mitochondrial damage, oxidative stress, nitric oxide, tumor necrosis factor-α (TNF-α) and mitogen-activated protein kinases.
RESULTS: Steatotic livers preserved in IGL-1® solution supplemented with IGF-1 showed lower transaminase levels, increased bile clearance and a reduction in vascular resistance when compared to those preserved in IGL-1® solution alone. These benefits are mediated by activation of AKT and constitutive endothelial nitric oxide synthase (eNOS), as well as the inhibition of inflammatory cytokines such as TNF-α. Mitochondrial damage and oxidative stress were also prevented.
CONCLUSION: IGL-1® enrichment with IGF-1 increased fatty liver graft preservation through AKT and eNOS activation, and prevented TNF-α release during normothermic reperfusion.
Keywords: AKT, Institut georges lopez-1® solution, Insulin like growth factor-1, Ischemia reperfusion injury, Nitric oxide, Oxidative stress, Steatotic graft preservation
INTRODUCTION
One of the major challenges in liver transplantation is to increase the use of marginal organs. Steatotic, or fatty, livers are increasingly transplanted, in spite of the associated risk of graft dysfunction or non-function as a result of cold ischemia reperfusion injury (IRI)[1,2]. Steatotic livers show poor tolerance to IRI due to severe mitochondrial damage, impaired energy metabolism[3], increased reactive oxygen species (ROS) and release of inflammatory cytokines such as tumour necrosis factor-α (TNF-α), which impairs microcirculation[4,5]. All these factors render steatotic livers more vulnerable to cold IRI.
Liver preservation is a crucial step in maintaining graft quality after prolonged ischemic periods, especially steatotic livers. University of Wisconsin (UW) is the most widely used serum-free preservation solution for transplantation but it does not fully protect liver grafts during prolonged storage[6-8]. Recently, the new institut georges lopez-1 (IGL-1)® solution has been proposed as an effective alternative to UW in clinical kidney transplantation[9,10], and in experimental orthotopic liver transplantation models[11,12]. IGL-1® solution is characterized by inversion of K+ and Na+ concentrations in the UW solution and contains polyethylene glycol as osmotic support instead of HES. In addition, we have previously demonstrated that IGL-1® is more suitable than UW solution for fatty liver preservation[13]. Its benefits are associated in part, with the prevention of oxidative stress and its capacity to generate nitric oxide (NO)[13]. NO is a vasodilator with anti-inflammatory properties that prevents microcirculatory alterations[5,13] and the release of pro-inflammatory cytokines, such as TNF-α, during IRI[14-16].
Insulin like growth factor-1 (IGF-1) is a vascular protective factor that is mainly synthesized and released by the liver[17,18]. We have recently reported impaired synthesis of IGF-1 in steatotic livers subjected to warm IRI, and that exogenous administration of recombinant IGF-1 reduced IRI in steatotic livers[18]. IGF-1 prevents oxidative stress[17] and induces NO generation by eNOS activation, as a consequence of AKT kinase phosphorylation[19,20].
Several trophic factors (TF), including IGF-1, have been added to UW solution in an attempt to improve the survival of pig orthotopic liver allografts after 18 h of cold storage[21]. In vascular endothelial cells, the benefits of TF supplementation are associated with limitation of mitogen-activated protein kinase (MAPK) activities after cold ischemia/rewarming injury[22].
Taking this into account, we explored the effects of the addition of IGF-1 to IGL-1® solution on fatty liver preservation during cold IRI. We examined the mechanisms responsible for such effects, including AKT phosphorylation and NO generation, and the prevention of ROS production and TNF-α release after reperfusion. These latter factors have been implicated in the poor tolerance of steatotic livers to cold IRI.
MATERIALS AND METHODS
Animals and liver procurement
Isolated perfused rat liver was used to evaluate hepatic function in isolation from the influence of other organ systems (undefined plasma constituents and neural/hormonal effects). Hepatic architecture, microcirculation and bile production were preserved in this experimental model, as previously reported[23]. Homozygous obese (Ob) Zucker rats, aged 16-18 wk were purchased from Iffa-Credo (L’Abresle, France) and were housed at 22°C[23]. All procedures were performed under isoflurane inhalation anaesthesia. Experiments were conducted according to European Union regulations for animal experiments (Directive 86/609 CEE).
Liver procurement and experimental groups
The surgical technique was performed as previously reported[23,24]. After cannulation of the common bile duct, the portal vein was isolated and the splenic and gastroduodenal veins were ligated. All animals were randomly distributed into groups as described below. The steatotic livers were flushed and preserved in cold IGL-1® solution for 24 h with or without the addition of IGF-1 (10 μg/L), as described elsewhere[21,22,25].
All animals were randomized according to the experimental protocols, as follows.
Cold storage: After 24 h of cold storage at 4°C, livers from 8 Zucker rats preserved in IGL-1 solution with (IGL-1 + IGF-1) or without IGF-1 were flushed with Ringer’s lactate solution. Control livers (Cont 1) from 8 Zucker rats (Ob) were flushed with Ringer’s lactate solution at room temperature immediately after laparotomy via the portal vein without cold storage. Aliquots of the effluent flush were sampled for measurements of cumulative AST and ALT after 24 h cold storage.
Cold storage and reperfusion: Briefly, after 24 h cold storage at 4°C, livers from 8 Zucker rats (Ob) preserved in IGL-1 and IGL-1 + IGF-1 solutions were subjected to 2 h reperfusion at 37°C, as previously reported[13,24]. Control livers (Cont 2) from 8 Zucker rats (Ob) were flushed with Ringer’s lactate at room temperature and immediately perfused ex vivo without ischemic preservation.
Transaminase assay
Hepatic injury was assessed in terms of transaminase levels with commercial kits from RAL (Barcelona, Spain)[26]. Briefly, 200 μL of effluent perfusate were added to the substrate provided by the commercial kit and then alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels were determined at 365 nm with a UV spectrometer and calculated following the supplier’s instructions.
Hepatic clearance
As with bile output, hepatic clearance was considered as another parameter of hepatic function. Thirty minutes after the onset of perfusion (t30), 1 mg of sulfobromophthalein (BSP) (Sigma, Madrid, Spain) was added to the perfusate. The concentration of BSP in bile samples (t120) was measured at 580 nm with a UV-visible spectrometer. Bile BSP excretion was expressed as a percentage of perfusate content (t120 bile/t30 perfusate*100)[23].
Vascular resistance
Liver circulation was assessed by measuring perfusion flow rate and vascular resistance. Perfusion flow rate was assessed continuously throughout the reperfusion period and expressed as mL/min per gram of liver. Vascular resistance was defined as the ratio of portal venous pressure to flow rate and expressed in mmHg/min per gram of liver/mL[26].
Glutamate dehydrogenase activity
Glutamate dehydrogenase (GLDH) was used as an indirect measure of mitochondrial damage. GLDH was measured in the perfusate as described elsewhere[26].
Lipid peroxidation assay
Lipid peroxidation in liver was used as an indirect measure of the oxidative injury induced by ROS. Lipid peroxidation was determined by measuring the formation of malondialdehyde (MDA) with the thiobarbiturate reaction[13].
Determination of nitrite and nitrate
NO production in liver was determined by tissue accumulation of nitrite and nitrate[13].
Western blotting analysis of eNOS, AKT, P38 and ERK 1/2
Liver tissue was homogenized as previously described and proteins were separated by SDS-PAGE and transferred to PVDF membranes[27-29]. Membranes were immunoblotted using the following antibodies: eNOS (Transduction Laboratories, Lexington, KY, USA), total and phosphorylated AKT, total and phosphorylated P38, and total and phosphorylated ERK 1/2 (Cell Signaling, Beverly, MA, USA) and β-actin (Sigma Chemical, St. Louis, MO, USA). Signals were detected by the enhanced chemiluminescence kit (Bio-Rad Laboratories, Hercules, CA, USA) and quantified by scanning densitometry as previously described[26,28].
TNF-α determination
Perfusate TNF-α levels were measured after 120 min of reperfusion using a commercial immunoassay kit for rat TNF-α from Biosource (Camarillo, CA, USA)[30,31].
Statistical analysis
Data are expressed as means ± SE, and were compared statistically by variance analysis, followed by the Student-Newman-Keuls test (Graph Pad Prism software). P < 0.05 was considered significant.
RESULTS
Protective effect of IGF-1 against liver injury and function
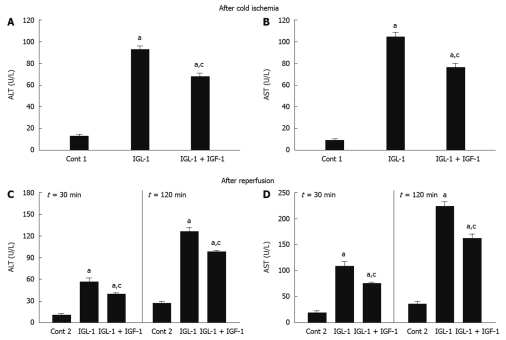
As shown in Figure 1A and B, the AST and ALT levels observed for steatotic livers preserved for 24 h at 4°C in IGL-1® solution confirmed their poor tolerance to cold ischemia injury. IGF-1 addition prevented AST/ALT release from steatotic livers when compared with IGL-1 alone.
Figure 1.
Alanine aminotransferase (A) and aspartate aminotransferase (B) levels in perfusate after 24 h cold storage, alanine aminotransferase (C) and aspartate aminotransferase (D) levels in perfusate after 30 and 120 min of normothermic reperfusion. Cont 1: Liver flushed without cold preservation; IGL-1: Livers preserved in IGL-1 solution; IGL-1 + IGF-1: Livers preserved in IGL-1 with IGF-1. aP < 0.05 vs Cont 1, cP < 0.05 vs IGL-1. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; IGF-1: Insulin like growth factor-1; IGL-1: Institut georges lopez-1.
Similar AST/ALT profiles were obtained when fatty livers were subjected to normothermic reperfusion for 30 and 120 min, respectively. Reduced AST/ALT levels were observed when IGL-1 was enriched with IGF-1 (Figure 1C and D). Similar profiles were seen for 60 and 90 min of reperfusion (data not shown).
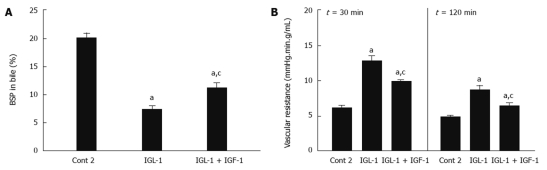
The mechanisms by which IGF-1 protects fatty livers against the harmful consequences of cold I/R damage were also investigated. For this reason, liver function was evaluated by the alterations in BSP clearance and vascular resistance, respectively. A reduction in BSP clearance was found in IGL-1 solution when compared to Cont 2. The highest BSP clearance in bile was observed after 2 h of normothermic reperfusion when IGF-1 was present (Figure 2A).
Figure 2.
Percentage of sulfobromophthalein in bile (A) and vascular resistance (B) of steatotic livers after 30 and 120 min of normothermic reperfusion. Cont 2: Liver flushed and perfused ex vivo without cold preservation; IGL-1: Livers preserved in IGL-1 solution; IGL-1 + IGF-1: Livers preserved in IGL-1 with IGF-1. aP < 0.05 vs Cont 2, cP < 0.05 vs IGL-1. BSP: Sulfobromophthalein; IGF-1: Insulin like growth factor-1; IGL-1: Institut georges lopez-1.
Vascular resistance was increased by IGL-1 solution (Figure 2B), but this was reversed by the addition of IGF-1. These alterations in vascular resistance were also seen in all groups at 30 and 120 min of reperfusion (Figure 2B).
Beneficial effects of IGF-1 on AKT and NO after reperfusion
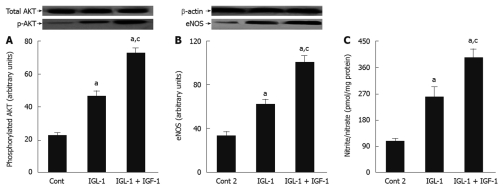
In order to explore whether the beneficial effects of IGF-1 in IGL-1 solution are associated with other protective cell signalling involved in the protective mechanisms against IRI, we evaluated the changes in AKT and NO levels. As shown in Figure 3, IGF-1 increased AKT phosphorylation when compared to IGL-1 alone and Cont 2, respectively (Figure 3A). This was concomitant with a significant generation of NO, as revealed by the nitrites/nitrates levels, as well as eNOS activation (Figure 3B and C).
Figure 3.
Beneficial effects of insulin like growth factor-1 on AKT and nitric oxide in steatotic liver graft preservation. A: Representative Western blottings of total and p-AKT at the top and densitometric analysis at the bottom after 24 h of cold storage and 120 min of normothermic reperfusion; B: Endothelial nitric oxide synthase (eNOS) protein levels in liver after 120 min of normothermic reperfusion. Representative Western blottings at the top and densitometric analysis at the bottom; C: Nitrite and nitrate levels after 120 min of normothermic reperfusion. Cont 2: Liver flushed and perfused ex vivo without cold preservation; IGL-1: Liver preserved in IGL-1 solution; IGL-1 + IGF-1: Livers preserved in IGL-1 with IGF-1. aP < 0.05 vs Cont 2, cP < 0.05 vs IGL-1. IGF-1: Insulin like growth factor-1; IGL-1: Institut georges lopez-1.
Role of IGF-1 on liver oxidative stress, mitochondrial damage and TNF-α release after reperfusion
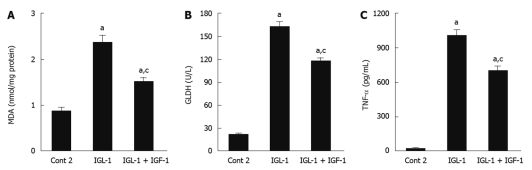
In order to examine the relationship between the parameters studied and others associated with the poor tolerance of fatty livers to reperfusion injury, we evaluated lipid peroxidation (MDA) and mitochondrial damage (GLDH). Steatotic livers preserved in IGL-1 showed significant MDA augments when compared to Cont 2. These increases were significantly prevented by the addition of IGF-1 (Figure 4A). In accordance with this, IGF-1 also diminished liver mitochondrial damage, as shown by the reduction in GLDH (Figure 4B). These beneficial effects of IGF-1 were also accompanied by a significant reduction in pro-inflammatory cytokine TNF-α level when compared to IGL-1 alone after 2 h normothermic reperfusion (Figure 4C).
Figure 4.
Role of insulin like growth factor-1 on oxidative stress, mitochondrial damage and tumor necrosis factor-α release in steatotic liver. A: Hepatic malondialdehyde (MDA) levels after 120 min of reperfusion; B: Glutamate dehydrogenase (GLDH) activity levels after 120 min of normothermic reperfusion; C: Tumor necrosis factor-α (TNF-α) levels in perfusate after 120 min of normothermic reperfusion. Cont 2: Liver flushed and perfused ex vivo without cold preservation; IGL-1: Livers preserved in IGL-1 solution; IGL-1 + IGF-1: Livers preserved in IGL-1 with IGF-1. aP < 0.05 vs Cont 2, cP < 0.05 vs IGL-1. IGF-1: Insulin like growth factor-1; IGL-1: Institut georges lopez-1.
Effects of IGF-1 on p-P38 and p-ERK MAPKs after reperfusion
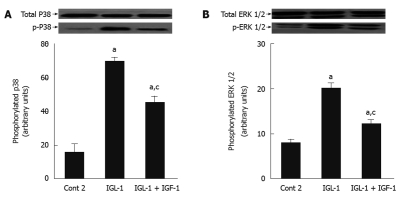
Finally, we examined the effects of IGL-1 with and without IGF-1 on P38 and ERK 1/2 MAPKs, whose activation is closely related with hypothermic conditions. Figure 5 shows increased phosphorylation of ERK 1/2 and P38 MAPK in IGL-1 solution when compared to Cont 2, but again this increase was offset by the addition of IGF-1.
Figure 5.
Effects of insulin like growth factor-1 on p-P38 and p-ERK in steatotic livers subjected to cold ischemia reperfusion. A: P38 protein levels in liver after 120 min of normothermic reperfusion. Representative Western blottings of total and p-P38 at the top and densitometric analysis at the bottom after 24 h of cold storage and 120 min of normothermic reperfusion; B: ERK 1/2 protein levels in liver after 120 min of normothermic reperfusion. Representative Western blottings of total and p-ERK 1/2 at the top and densitometric analysis at the bottom. Cont 2: Liver flushed and perfused ex vivo without cold preservation; IGL-1: Lliver preserved in IGL-1 solution; IGL-1 + IGF-1: Livers preserved in IGL-1 with IGF-1. aP < 0.05 vs Cont 2, cP < 0.05 vs IGL-1. IGF-1: Insulin like growth factor-1; IGL-1: Institut georges lopez-1.
DISCUSSION
IGF-1 is a 70-amino-acid polypeptide, mainly synthesized by the liver, with protective effects against IRI in a variety of tissues[32-35], including the liver[18]. We recently reported that IGF-1 protects steatotic livers subjected to warm IRI, but its role in fatty liver preservation has been poorly investigated[18]. For this reason, we explored the effect of IGF-1 supplementation of serum free IGL-1® solution, which has been shown to protect fatty livers against cold IRI[13]. We used the concentration of IGF-I (10 μg/L) according to previous studies carried out in different experimental models. In these studies, IGF-I it was used to supplement UW solution in dog kidney transplantation, as well as, primary canine kidney tubule and human umbilical vein endothelial cell cultures[21,22,25,36]. The results reported here show that the addition of IGF-1 to IGL-1® solution significantly improved fatty liver preservation, as evidenced by the reduction in AST/ALT levels. These results are consistent with previous reports by other authors, who demonstrated that the addition of TF to UW solution improves the survival of orthotopic liver allografts[21].
BSP clearance in bile is a useful tool for assessing liver function after prolonged cold ischemia[23,24]. It is well established that complications in biliary structures appear in more than 25% of liver transplant recipients. The increases in BSP clearance observed in IGL-1 + IGF-1 solution revealed that IGF-1 supplementation enhances steatotic graft function.
Steatosis is the result of intracytoplasmic fat accumulation, which is associated with an increase in hepatocellular volume, induced distortion and narrowing of sinusoids with a reduction in the luminal diameter by up to 50% when compared to normal liver[5]. This provokes severe alterations in hepatic blood flow and microcirculation, and prevents appropriate revascularization of the graft. The benefits of the use of IGL-1® solution for fatty liver preservation are associated with its capacity to generate NO[13], a potent vasodilator involved in the regulation of hepatic microcirculation, through eNOS activation, as previously reported by us[24]. Moreover, it has been established that IGF-1 up-regulates eNOS activity by interacting with a tyrosine kinase membrane receptor which activates the AKT signalling pathway[19,37,38]. The results of the present study show that IGF-1 increases AKT phosphorylation and enhance eNOS activation induced by IGL-1® alone. This in turn induces NO generation, which reduces vascular resistance following reperfusion. This is in line with several reported studies demonstrating that a low concentration of eNOS-derived NO maximizes blood perfusion, promotes cell survival and protects the liver against IRI[39]. In contrast, the sustained presence of iNOS-derived NO might become detrimental by increasing toxic reactive oxygen species, thus leading to liver injury[39-41]. In our experimental model, the benefits of NO were not associated with iNOS activation (data not show). Moreover, other studies demonstrate that while increasing e-NOS, IGF-1 inhibits inducible NO[19,42].
It is clear that fatty accumulation resulting from steatosis induces ultra-structural and biochemical changes in liver mitochondria[43,44], which may render these organelles intrinsically more susceptible to IRI injury. Given that mitochondria are the main sites of ROS production in IRI, the increased oxidative stress observed in steatotic livers after cold IRI could be attributed to mitochondrial damage. It is well known that IGF-1 prevents mitochondrial damage and oxidative stress following IRI[17,33,34]. Our results indicate that the addition of IGF-1 to IGL-1® preservation solution increases liver mitochondria protection, and prevents ROS generation associated with reperfusion injury.
Vairetti et al[45] using the same “ex vivo” experimental model, have shown that TNF-α is released when fatty livers are subjected to cold ischemia reperfusion. In our conditions, TNF-α release was also evidenced in response to cold ischemia-reperfusion insult.
IGF-1 supplementation prevented the release of TNF-α[46], which has a pivotal role in the progression of liver reperfusion damage. This finding is consistent with the accumulating evidence of crosstalk between IGF-1 and TNF-α during ischemia reperfusion injury[47]. NO generated by IGF-1 could decrease TNF-α release, as occurs in liver ischemic preconditioning, in which the induced hepatoprotection is also mediated by the inhibitory action of NO on TNF-α release through eNOS activation[48].
In addition, several intracellular signalling pathways, including the extracellular signal-regulated kinases 1/2 and P38 mitogen-activated protein kinase, are activated during hypothermia[49-52], In these conditions, a mutual activation effect between TNF-α and MAPKs may also occur thus exacerbating the liver damage[53]. Our results indicate that cold storage in IGL-1® solution alone activates P38 and ERK 1/2, and that IGF-1 addition prevented this activation, which is consistent with a TNF-α reduction that leads to an improvement in liver injury and function. This is in line with previous reports that the addition of TF to UW improved endothelial cell preservation by reducing ERK 1/2 and P38 activation[22].
In conclusion, we have demonstrated that IGF-1 addition to IGL-1® solution protects fatty liver against cold IRI. The beneficial action of IGF-1 appears to be mediated by AKT activation and NO generation, with concomitant prevention of pro-inflammatory cytokines, such as TNF-α. However, further studies will be required to examine the underlying mechanisms. This may increase the use of steatotic livers, which partially compensates the shortage of organs for transplant.
COMMENTS
Background
Static preservation solution is crucial for graft viability; especially when steatosis is present. Serum-free preservation solutions deprive the donor organ of essential trophic factors such as insulin like growth factor-1 (IGF-1) which is associated with deleterious effects including cell cycle arrest and death. The addition of IGF-1 to University of Wisconsin (UW) solution improves the capacity of this preservation solution for protecting liver grafts subjected to a prolonged ischemic period. Serum-free institut georges lopez-1 (IGL-1) solution has been proposed as an effective alternative to UW for steatotic liver preservation.
Research frontiers
In this study, the authors focused on the importance of IGF-1 as a biological additive to IGL-1 preservation solution. The induction of nitric oxide (NO) synthesis by the modified IGL-1 solution improves microcirculatory disorders in fatty livers. IGL-1 supplementation with IGF-1 increases graft protection against ischemia reperfusion injury through the activation of protein kinase AKT and a concomitant reduction in tumour necrosis factor (TNF) release and P38/ERK 1/2 MAPKs activation which play an important cryoprotective role in liver graft preservation.
Innovations and breakthroughs
The enrichment of IGL-1 solution with IGF-1 increases NO generation through AKT activation and prevents pro-inflammatory cytokine release such as TNF-α during steatotic liver graft reperfusion.
Applications
The use of modified IGL-1 solutions should be a useful strategy for increasing steatotic liver graft preservation.
Peer review
This manuscript describes the beneficial effects of IGF-1 when added to IGL-1 solution in the preservation of (fatty) liver.
Acknowledgments
We are grateful to Robin Rycroft at the Language Advisory Service of the University of Barcelona for revising the English text.
Footnotes
Supported by The Ministry of Health and Consumption (PI 081988), CIBER-ehd, Carlos III Institute, Madrid, Spain; Ministry of Foreign Affairs and International Cooperation (A/020255/08 and A/02987/09); Mohamed Amine Zaouali is fellowship-holder from the Catalan Society of Transplantation
Peer reviewers: Sharon DeMorrow, Assistant Professor, Division of Research and Education, Scott and White Hospital and The Texas A&M University System, Health Science Center College of Medicine, Temple, TX 76504, United States; Filip Braet, Associate Professor, Australian Key Centre for Microscopy and Microanalysis, Madsen Building (F09), The University of Sydney, Sydney NSW 2006, Australia
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH
References
- 1.Casillas-Ramirez A, Amine-Zaouali M, Massip-Salcedo M, Padrissa-Altés S, Bintanel-Morcillo M, Ramalho F, Serafín A, Rimola A, Arroyo V, Rodés J, et al. Inhibition of angiotensin II action protects rat steatotic livers against ischemia-reperfusion injury. Crit Care Med. 2008;36:1256–1266. doi: 10.1097/CCM.0b013e31816a023c. [DOI] [PubMed] [Google Scholar]
- 2.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 3.Selzner M, Rüdiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–1288. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 4.Serafín A, Roselló-Catafau J, Prats N, Gelpí E, Rodés J, Peralta C. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology. 2004;39:688–698. doi: 10.1002/hep.20089. [DOI] [PubMed] [Google Scholar]
- 5.Ramalho FS, Fernandez-Monteiro I, Rosello-Catafau J, Peralta C. Hepatic microcirculatory failure. Acta Cir Bras. 2006;21 Suppl 1:48–53. doi: 10.1590/s0102-86502006000700012. [DOI] [PubMed] [Google Scholar]
- 6.El-Gibaly AM, Scheuer C, Menger MD, Vollmar B. Improvement of rat liver graft quality by pifithrin-alpha-mediated inhibition of hepatocyte necrapoptosis. Hepatology. 2004;39:1553–1562. doi: 10.1002/hep.20243. [DOI] [PubMed] [Google Scholar]
- 7.Klar E, Angelescu M, Zapletal C, Kraus T, Bredt M, Herfarth C. Definition of maximum cold ischemia time without reduction of graft quality in clinical liver transplantation. Transplant Proc. 1998;30:3683–3685. doi: 10.1016/s0041-1345(98)01193-2. [DOI] [PubMed] [Google Scholar]
- 8.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711–714. [PMC free article] [PubMed] [Google Scholar]
- 9.Badet L, Petruzzo P, Lefrançois N, McGregor B, Espa M, Berthillot C, Danjou F, Contu P, Aissa AH, Virieux SR, et al. Kidney preservation with IGL-1 solution: a preliminary report. Transplant Proc. 2005;37:308–311. doi: 10.1016/j.transproceed.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 10.Badet L, Ben Abdennebi H, Petruzzo P, McGregor B, Espa M, Hadj-Aissa A, Ramella-Virieux S, Steghens JP, Portoghese F, Martin X. Effect of IGL-1, a new preservation solution, on kidney grafts (a pre-clinical study) Transpl Int. 2005;17:815–821. doi: 10.1007/s00147-004-0789-1. [DOI] [PubMed] [Google Scholar]
- 11.Franco-Gou R, Mosbah IB, Serafin A, Abdennebi HB, Roselló-Catafau J, Peralta C. New preservation strategies for preventing liver grafts against cold ischemia reperfusion injury. J Gastroenterol Hepatol. 2007;22:1120–1126. doi: 10.1111/j.1440-1746.2006.04495.x. [DOI] [PubMed] [Google Scholar]
- 12.Ben Abdennebi H, Elrassi Z, Scoazec JY, Steghens JP, Ramella-Virieux S, Boillot O. Evaluation of IGL-1 preservation solution using an orthotopic liver transplantation model. World J Gastroenterol. 2006;12:5326–5330. doi: 10.3748/wjg.v12.i33.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben Mosbah I, Roselló-Catafau J, Franco-Gou R, Abdennebi HB, Saidane D, Ramella-Virieux S, Boillot O, Peralta C. Preservation of steatotic livers in IGL-1 solution. Liver Transpl. 2006;12:1215–1223. doi: 10.1002/lt.20788. [DOI] [PubMed] [Google Scholar]
- 14.Phillips L, Toledo AH, Lopez-Neblina F, Anaya-Prado R, Toledo-Pereyra LH. Nitric oxide mechanism of protection in ischemia and reperfusion injury. J Invest Surg. 2009;22:46–55. doi: 10.1080/08941930802709470. [DOI] [PubMed] [Google Scholar]
- 15.Kuriyama N, Isaji S, Hamada T, Kishiwada M, Ohsawa I, Usui M, Sakurai H, Tabata M, Hayashi T, Suzuki K. The cytoprotective effects of addition of activated protein C into preservation solution on small-for-size grafts in rats. Liver Transpl. 2010;16:1–11. doi: 10.1002/lt.21923. [DOI] [PubMed] [Google Scholar]
- 16.Laroux FS, Pavlick KP, Hines IN, Kawachi S, Harada H, Bharwani S, Hoffman JM, Grisham MB. Role of nitric oxide in inflammation. Acta Physiol Scand. 2001;173:113–118. doi: 10.1046/j.1365-201X.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 17.García-Fernández M, Castilla-Cortázar I, Díaz-Sanchez M, Navarro I, Puche JE, Castilla A, Casares AD, Clavijo E, González-Barón S. Antioxidant effects of insulin-like growth factor-I (IGF-I) in rats with advanced liver cirrhosis. BMC Gastroenterol. 2005;5:7. doi: 10.1186/1471-230X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casillas-Ramírez A, Zaouali A, Padrissa-Altés S, Ben Mosbah I, Pertosa A, Alfany-Fernández I, Bintanel-Morcillo M, Xaus C, Rimola A, Rodés J, et al. Insulin-like growth factor and epidermal growth factor treatment: new approaches to protecting steatotic livers against ischemia-reperfusion injury. Endocrinology. 2009;150:3153–3161. doi: 10.1210/en.2008-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, Andreotti F. Insulin-like growth factor-1 as a vascular protective factor. Circulation. 2004;110:2260–2265. doi: 10.1161/01.CIR.0000144309.87183.FB. [DOI] [PubMed] [Google Scholar]
- 20.Yang AL, Chao JI, Lee SD. Altered insulin-mediated and insulin-like growth factor-1-mediated vasorelaxation in aortas of obese Zucker rats. Int J Obes (Lond) 2007;31:72–77. doi: 10.1038/sj.ijo.0803364. [DOI] [PubMed] [Google Scholar]
- 21.Ambiru S, Uryuhara K, Talpe S, Dehoux JP, Jacobbi L, Murphy CJ, McAnulty JF, Gianello P. Improved survival of orthotopic liver allograft in swine by addition of trophic factors to University of Wisconsin solution. Transplantation. 2004;77:302–319. doi: 10.1097/01.TP.0000100468.94126.AF. [DOI] [PubMed] [Google Scholar]
- 22.Kwon YS, Foley JD, Russell P, McAnulty JF, Murphy CJ. Prevention of cold ischemia/rewarming-induced ERK 1/2, p38 kinase and HO-1 activation by trophic factor supplementation of UW solution. Cryobiology. 2008;57:72–74. doi: 10.1016/j.cryobiol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Ben Mosbah I, Casillas-Ramírez A, Xaus C, Serafín A, Roselló-Catafau J, Peralta C. Trimetazidine: is it a promising drug for use in steatotic grafts? World J Gastroenterol. 2006;12:908–914. doi: 10.3748/wjg.v12.i6.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben Mosbah I, Massip-Salcedo M, Fernández-Monteiro I, Xaus C, Bartrons R, Boillot O, Roselló-Catafau J, Peralta C. Addition of adenosine monophosphate-activated protein kinase activators to University of Wisconsin solution: a way of protecting rat steatotic livers. Liver Transpl. 2007;13:410–425. doi: 10.1002/lt.21059. [DOI] [PubMed] [Google Scholar]
- 25.McAnulty JF, Reid TW, Waller KR, Murphy CJ. Successful six-day kidney preservation using trophic factor supplemented media and simple cold storage. Am J Transplant. 2002;2:712–718. doi: 10.1034/j.1600-6143.2002.20805.x. [DOI] [PubMed] [Google Scholar]
- 26.Ben Mosbah I, Roselló-Catafau J, Alfany-Fernandez I, Rimola A, Parellada PP, Mitjavila MT, Lojek A, Ben Abdennebi H, Boillot O, Rodés J, et al. Addition of carvedilol to University Wisconsin solution improves rat steatotic and nonsteatotic liver preservation. Liver Transpl. 2010;16:163–171. doi: 10.1002/lt.21968. [DOI] [PubMed] [Google Scholar]
- 27.Massip-Salcedo M, Casillas-Ramirez A, Franco-Gou R, Bartrons R, Ben Mosbah I, Serafin A, Roselló-Catafau J, Peralta C. Heat shock proteins and mitogen-activated protein kinases in steatotic livers undergoing ischemia-reperfusion: some answers. Am J Pathol. 2006;168:1474–1485. doi: 10.2353/ajpath.2006.050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massip-Salcedo M, Zaouali MA, Padrissa-Altés S, Casillas-Ramirez A, Rodés J, Roselló-Catafau J, Peralta C. Activation of peroxisome proliferator-activated receptor-alpha inhibits the injurious effects of adiponectin in rat steatotic liver undergoing ischemia-reperfusion. Hepatology. 2008;47:461–472. doi: 10.1002/hep.21935. [DOI] [PubMed] [Google Scholar]
- 29.Alfany-Fernandez I, Casillas-Ramirez A, Bintanel-Morcillo M, Brosnihan KB, Ferrario CM, Serafin A, Rimola A, Rodés J, Roselló-Catafau J, Peralta C. Therapeutic targets in liver transplantation: angiotensin II in nonsteatotic grafts and angiotensin-(1-7) in steatotic grafts. Am J Transplant. 2009;9:439–451. doi: 10.1111/j.1600-6143.2008.02521.x. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Ari Z, Hochhauser E, Burstein I, Papo O, Kaganovsky E, Krasnov T, Vamichkim A, Vidne BA. Role of anti-tumor necrosis factor-alpha in ischemia/reperfusion injury in isolated rat liver in a blood-free environment. Transplantation. 2002;73:1875–1880. doi: 10.1097/00007890-200206270-00004. [DOI] [PubMed] [Google Scholar]
- 31.Fernández L, Carrasco-Chaumel E, Serafín A, Xaus C, Grande L, Rimola A, Roselló-Catafau J, Peralta C. Is ischemic preconditioning a useful strategy in steatotic liver transplantation? Am J Transplant. 2004;4:888–899. doi: 10.1111/j.1600-6143.2004.00447.x. [DOI] [PubMed] [Google Scholar]
- 32.Yamauchi T, Sakurai M, Abe K, Takano H, Sawa Y. Neuroprotective effects of activated protein C through induction of insulin-like growth factor-1 (IGF-1), IGF-1 receptor, and its downstream signal phosphorylated serine-threonine kinase after spinal cord ischemia in rabbits. Stroke. 2006;37:1081–1086. doi: 10.1161/01.STR.0000206280.30972.21. [DOI] [PubMed] [Google Scholar]
- 33.Pi Y, Goldenthal MJ, Marín-García J. Mitochondrial involvement in IGF-1 induced protection of cardiomyocytes against hypoxia/reoxygenation injury. Mol Cell Biochem. 2007;301:181–189. doi: 10.1007/s11010-007-9410-0. [DOI] [PubMed] [Google Scholar]
- 34.Davani EY, Brumme Z, Singhera GK, Côté HC, Harrigan PR, Dorscheid DR. Insulin-like growth factor-1 protects ischemic murine myocardium from ischemia/reperfusion associated injury. Crit Care. 2003;7:R176–R183. doi: 10.1186/cc2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haglind E, Malmlof K, Fan J, Lang CH. Insulin-like growth factor-I and growth hormone administration in intestinal ischemia shock in the rat. Shock. 1998;10:62–68. doi: 10.1097/00024382-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Kwon YS, Foley JD, Murphy CJ, McAnulty JF. The effect of trophic factor supplementation on cold ischemia-induced early apoptotic changes. Transplantation. 2007;83:91–94. doi: 10.1097/01.tp.0000242524.35562.4b. [DOI] [PubMed] [Google Scholar]
- 37.Isenovic ER, Divald A, Milivojevic N, Grgurevic T, Fisher SE, Sowers JR. Interactive effects of insulin-like growth factor-1 and beta-estradiol on endothelial nitric oxide synthase activity in rat aortic endothelial cells. Metabolism. 2003;52:482–487. doi: 10.1053/meta.2003.50079. [DOI] [PubMed] [Google Scholar]
- 38.Isenovic ER, Meng Y, Divald A, Milivojevic N, Sowers JR. Role of phosphatidylinositol 3-kinase/Akt pathway in angiotensin II and insulin-like growth factor-1 modulation of nitric oxide synthase in vascular smooth muscle cells. Endocrine. 2002;19:287–292. doi: 10.1385/ENDO:19:3:287. [DOI] [PubMed] [Google Scholar]
- 39.Duranski MR, Elrod JW, Calvert JW, Bryan NS, Feelisch M, Lefer DJ. Genetic overexpression of eNOS attenuates hepatic ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H2980–H2986. doi: 10.1152/ajpheart.01173.2005. [DOI] [PubMed] [Google Scholar]
- 40.Abe Y, Hines I, Zibari G, Grisham MB. Hepatocellular protection by nitric oxide or nitrite in ischemia and reperfusion injury. Arch Biochem Biophys. 2009;484:232–237. doi: 10.1016/j.abb.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: a brief review. Mol Med. 2008;14:337–345. doi: 10.2119/2007-00134.Vardanian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schini-Kerth VB. Dual effects of insulin-like growth factor-I on the constitutive and inducible nitric oxide (NO) synthase-dependent formation of NO in vascular cells. J Endocrinol Invest. 1999;22:82–88. [PubMed] [Google Scholar]
- 43.Rashid A, Wu TC, Huang CC, Chen CH, Lin HZ, Yang SQ, Lee FY, Diehl AM. Mitochondrial proteins that regulate apoptosis and necrosis are induced in mouse fatty liver. Hepatology. 1999;29:1131–1138. doi: 10.1002/hep.510290428. [DOI] [PubMed] [Google Scholar]
- 44.Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, Parker WD Jr. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430–434. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 45.Vairetti M, Ferrigno A, Carlucci F, Tabucchi A, Rizzo V, Boncompagni E, Neri D, Gringeri E, Freitas I, Cillo U. Subnormothermic machine perfusion protects steatotic livers against preservation injury: a potential for donor pool increase? Liver Transpl. 2009;15:20–29. doi: 10.1002/lt.21581. [DOI] [PubMed] [Google Scholar]
- 46.Lorenzo-Zúñiga V, Rodríguez-Ortigosa CM, Bartolí R, Martínez-Chantar ML, Martínez-Peralta L, Pardo A, Ojanguren I, Quiroga J, Planas R, Prieto J. Insulin-like growth factor I improves intestinal barrier function in cirrhotic rats. Gut. 2006;55:1306–1312. doi: 10.1136/gut.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang M, Tsai B, Brown JW, Meldrum DR. Insulin-like growth factor-1 in myocardial tissue: interaction with tumor necrosis factor. Crit Care. 2003;7:417–419. doi: 10.1186/cc2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peralta C, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. Protective effect of liver ischemic preconditioning on liver and lung injury induced by hepatic ischemia-reperfusion in the rat. Hepatology. 1999;30:1481–1489. doi: 10.1002/hep.510300622. [DOI] [PubMed] [Google Scholar]
- 49.Roberts JR, Rowe PA, Demaine AG. Activation of NF-kappaB and MAP kinase cascades by hypothermic stress in endothelial cells. Cryobiology. 2002;44:161–169. doi: 10.1016/s0011-2240(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 50.Sakiyama S, Hamilton J, Han B, Jiao Y, Shen-Tu G, de Perrot M, Keshavjee S, Liu M. Activation of mitogen-activated protein kinases during human lung transplantation. J Heart Lung Transplant. 2005;24:2079–2085. doi: 10.1016/j.healun.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Diestel A, Roessler J, Pohl-Schickinger A, Koster A, Drescher C, Berger F, Schmitt KR. Specific p38 inhibition in stimulated endothelial cells: a possible new anti-inflammatory strategy after hypothermia and rewarming. Vascul Pharmacol. 2009;51:246–252. doi: 10.1016/j.vph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Kaizu T, Ikeda A, Nakao A, Tsung A, Toyokawa H, Ueki S, Geller DA, Murase N. Protection of transplant-induced hepatic ischemia/reperfusion injury with carbon monoxide via MEK/ERK1/2 pathway downregulation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G236–G244. doi: 10.1152/ajpgi.00144.2007. [DOI] [PubMed] [Google Scholar]
- 53.Bradham CA, Stachlewitz RF, Gao W, Qian T, Jayadev S, Jenkins G, Hannun Y, Lemasters JJ, Thurman RG, Brenner DA. Reperfusion after liver transplantation in rats differentially activates the mitogen-activated protein kinases. Hepatology. 1997;25:1128–1135. doi: 10.1002/hep.510250514. [DOI] [PubMed] [Google Scholar]