Abstract
Autoimmunity to type V collagen (col(V)) is a major risk factor for lung allograft rejection. Although col(V)-induced oral tolerance abrogates rejection of minor histoincompatible lung transplants, its ability to prevent rejection of fully MHC incompatible lung allografts is unknown. Rat lung allografts fully incompatible at MHC class I and II loci (Brown Norway (RT1n)) were transplanted into untreated Wistar Kyoto rat recipients (WKY, RT1l), or WKY rats were fed col(V) pretransplantation. To determine whether col(V) enhanced cyclosporine (CsA)-mediated immune suppression, WKY rats were treated with low-dose CsA (5 mg/kg), post-transplant, or oral col(V) plus CsA. The data showed that in contrast to col(V) or CsA, col(V) plus low-dose CsA significantly prevented rejection pathology, down-regulated alloantigen-induced production of IFN-γ and IL-17A, and suppressed chemotaxis for lung macrophages in allograft bronchoalveolar lavage fluid that was associated with lower local levels of MCP-1 (CCL2). Col(V) plus CsA was associated with alloantigen-induced expression of IL-10 in mediastinal lymph node or splenic T cells, intragraft expression of IL-10 and Foxp3 in perivascular and peribronchiolar mononuclear cells, and constitutive production of IL-10 from allograft alveolar macrophages. These data demonstrate that col(V) enhances low-dose CsA-mediated immune suppression, and suggest a role for oral col(V) in immune modulation in lung transplantation.
Lung transplantation is an established treatment for many end-stage pulmonary diseases. However, chronic rejection, the pathologic entity known as obliterative bronchiolitis or the clinical correlate, bronchiolitis obliterans syndrome (BOS),3 is the major impediment to the long-term survival of lung transplant patients (1). The main risk factors for the development of BOS have been debated, and include alloimmune mechanisms associated with frequency of acute rejection and lymphocytic bronchiolitis. Recently, we reported that cellular immune responses to a minor collagen, type V collagen (col(V)), by CD4+ T cells producing IL-17A are also a risk factor associated with onset of BOS (2). These data and reports from our laboratory and other investigators demonstrated the immunopathogenesis of lung transplant rejection involves both alloimmunity (anti-donor) and auto-immunity to col(V) (2–7).
Data showing immune responses to col(V) had key roles in lung allograft rejection in humans and preclinical models of lung transplantation in rats suggested that immunity to col(V) could be used to induce immune tolerance to lung allografts. Indeed, we reported that col(V)-induced oral tolerance was highly effective in preventing both acute and chronic rejection in rat models of orthotopic lung transplantation (3, 4, 8). In addition, oral col(V)-induced regulatory T cells (Tregs) that were able to suppress rejection of lung allografts transplanted into naive recipients in a TGF-β-dependent manner (8).
The rat models of col(V)-induced oral tolerance involved transplantation of lung grafts with minor histoincompatibilities to the recipient (3, 4, 8). However, in clinical lung transplantation, the donor lung is highly incompatible at both MHC class I and II loci relative to the recipient. This setting presents formidable immunologic barriers to immune suppression and tolerance induction. The ability of col(V)-induced oral tolerance to prevent rejection of fully MHC incompatible rat lung allografts is unknown.
Calcineurin inhibition mediated by cyclosporine (CsA) at doses >25 mg/kg has been reported to be effective in preventing rejection of fully MHC incompatible rat lung allografts Brown Norway (BN, RT1n) when transplanted into Wistar Kyoto (WKY, RT1l) rat recipients (9, 10). Although CsA is able to suppress alloimmunity in clinical transplantation, administration of this drug at doses sufficient to prevent rejection is also associated with multiple complications such as fibrosis and hypertension, among others. Indeed, it is known that conventional immunosuppressive drugs such as CsA exert their immunosuppressive effects at doses also associated with toxicities. Accordingly, modalities that allow for lower doses of CsA or similar drugs that retain suppression of alloimmunity would be a major advance in lung transplantation. Because oral col(V) induces suppression of alloimmunity (3, 4, 8), then the current study tests the hypothesis that col(V)-induced oral tolerance will augment CsA-induced suppression of alloimmunity, allowing for the use of much lower CsA dosing.
In the current study, WKY rats underwent col(V)-induced oral tolerance before transplanting fully MHC incompatible lungs from BN, followed by parenteral administration of low-dose CsA (5 mg/kg). The data showed that in contrast to col(V) or CsA, alone, col(V) plus low dose CsA significantly prevented rejection pathology, down-regulated alloantigen-induced production of IFN-γ and IL-17A, and suppressed chemotaxis for lung macrophages in allograft bronchoalveolar lavage (BAL) fluid that was associated with lower local levels of MCP-1 (CCL2). Col(V) plus CsA was associated with alloantigen-induced expression of IL-10 in mediastinal lymph node and splenic T cells, intragraft expression of IL-10 and Foxp3 in perivascular and peribronchiolar mononuclear cells, and constitutive production of IL-10 from allograft alveolar macrophages (AM).
Materials and Methods
Animals
Inbred, pathogen-free, MHC (RT1) incompatible male rats are used for the study, as follows: BN (RT1n) and WKY (RT1l) rats (250–300 g at the time of transplantation). Rats were purchased from Charles River Laboratories and Harlan Sprague-Dawley and housed in the Laboratory Animal Resource Center at Indiana University School of Medicine in accordance with institutional guidelines. All studies were approved by the Laboratory Animal Resource Center at Indiana University School of Medicine.
Preparation of collagen
Col(V) was extracted and purified similar to our prior reports (3).
Administration of collagen and CsA
WKY rats were fed 10 μg of col(V) dissolved in 0.5 ml of saline by a gastric gavage using a 16-gauge ball-point stainless steel animal feeding needle (Braintree Scientific), as previously reported (3, 4, 8). Animals were fed every other day for eight feedings, and 7 days after the last feeding, these rats were used as recipients of BN rat lung allografts. In some studies, lung allograft recipients were treated with CsA, alone, or CsA following oral col(V). CsA (5 mg/kg/day) was administrated s.c. daily for 3 days beginning on the first postoperative day.
Transplantation model and experimental groups
The orthotopic transplantation of left lung allografts (BN to WKY) was performed, as previously reported (2–5, 11). All surgeries were performed by Y.Y. Survival exceeded 90% in all transplantation groups. All transplants were harvested on day 7 posttransplantation.
Four groups of animals were studied as follows: Allo group, untreated allografts in which no rats received any type of immunosppression; col(V) group, WKY rats were fed col(V) before transplantation, as described above; CsA group, CsA was administered to transplant recipients, as described above; and col(V) plus CsA group, rats in this group received oral col(V) before transplantation plus s.c. CsA posttransplantation, as described above.
Pathological grading
Transplanted lungs from each group were harvested on day 7 posttransplantation, fixed, sectioned, stained, and graded for rejection pathology using standard criteria (12), and as reported previously (2–5, 11): grade 0, no infiltrates; grade 1, minimal perivascular and peribronchiolar mononuclear cell infiltrates; grade 2, mild perivascular and peribronchiolar mononuclear cell infiltrates; grade 3, moderate perivascular and peribronchiolar mononuclear cell infiltrates; and grade 4, severe perivascular and peribronchiolar mononuclear cell infiltrates.
Collection of BAL fluid
BAL was obtained from native and transplanted lungs, as previously reported. Cells in BAL and cell-free BAL were obtained from centrifuged specimens.
ELISPOT
Using enzymatic and mechanical digestion, T cells (CD3+, >98% pure) were isolated by magnetic beads (Miltenyi Biotec) from the spleens and mediastinal lymph nodes from the transplant recipients in each experimental group (13). After washing in RPMI 1640, cells were resuspended in complete medium (RPMI 1640, 2 mM L-glutamine, 5 × 10−5 M 2-ME, 100 U/ml penicillin, 100 μl/ml streptomycin, and 10% heat-inactivated FBS). Splenocytes, as a source for APCs, were isolated from normal BN rats, and depleted of T cells via negative selection with magnetic beads (anti-CD3 beads; Miltenyi Biotec). After washing, cells were resuspended in complete medium until use.
Ninety-six-well ELISPOT plates (Millipore) were coated with 100 μl/well capture Abs in sterile PBS overnight at 4°C. Capture Ab and detection Ab for IFN-γ were from the ELISPOT development module for rat IFN-γ and used per manufacturer’s protocol (R&D Systems). IL-10 capture Ab (4 μg/ml, A5-7; BD Biosciences), and IL-17A capture Ab (4 μg/ml, eBio17CK15A5; eBioscience) were also used for ELISPOT. After blocking plates for 2 h with 1% BSA and 5% sucrose in PBS, the plates were washed four times using 0.05% Tween 20 (Sigma-Aldrich) in PBS. Purified mediastinal lymph node T cells or purified splenic T cells (2 × 105/well) from the experimental groups were cocultured with APCs (T cell-depleted splenocytes from normal BN rats (5 × 104/well)). After incubating at 37°C, 5% CO2 for 72 h, the supernatants were collected from each well. To detect cytokines bound to the plates, biotinylated Abs specific to each cytokine were added to each well (2 μg/ml anti-IL-10 (A5-6; BD Biosciences), 2 μg/ml anti-IL-17A (eBio17B7; eBioscience), and anti-IFN-γ per manufacturer’s protocol (R&D Systems)). After an overnight incubation at 4°C, the plates were developed by adding streptavidin-alkaline phosphatase conjugate (Millipore), washing, and adding NBT/5-bromo-4-chloro-3-indolyl phosphate (Thermo Scientific) for 15-min incubation. After washing, the plates were read on an automated ELISPOT reader and image analysis system (KS ELISPOT automated image analysis system; Zeiss).
Adoptive transfer
Splenocytes or pure CD4+ splenic T cells were isolated from the spleens of WKY rats that received BN lung transplants and treated with col(V) plus CsA. All cells were isolated 7 days posttransplantation and were adoptively transferred by tail vein injection (1 × 107 cells) to untreated WKY rats 24 h prior transplantation BN lungs (8). All rats were sacrificed, and tissue was harvested on the seventh day posttransplantation in these studies.
Flow cytometry for Foxp3 expression
Three-color flow cytometry analysis was conducted to identify CD4+CD25+Foxp3+ T cells. Magnetic beads were used to purify CD3+ cells from mediastinal lymph node cells or splenocytes isolated from the lung transplant recipients. Cells were stained with anti-rat allophycocyanin-conjugated anti-CD4 mAb and FITC-conjugated anti-CD25 mAb (BD Biosciences). Subsequently, cells were fixed and permeabilized with the buffers of the Foxp3 staining set per protocol (eBioscience), and stained with anti-rat PE-conjugated Foxp3 mAb. Cells were analyzed on a Cytomics TM FC 500 (Beckman Coulter) using three-color analysis.
Immunohistochemistry
Transplanted lungs were fixed with glutaraldehyde, paraffin embedded, and processed for immunohistochemistry, as reported (8). Sections were stained with the following Abs, and isotype- and species-matched Abs served as controls: anti-rat Foxp3 mAb (FJK-16s; eBioscience), rat MHC class I RT1Aa mAb (R2/15s; Serotec), IL-10 polyclonal Ab (R&D Systems), and IFN-γ mAb (BD1; eBioscience). In brief, immunohistochemistry on paraffin sections was performed using the pressure cooker method of Ag retrieval (2 min, citrate buffer (pH 6.0)). Before staining, endogenous peroxidase was blocked, and the slides were incubated for 40 min with the primary Ab (50 μg/ml) and washed with PBS. Immunodetection was performed with biotinylated anti-mouse secondary Abs, followed by peroxidase-labeled streptavidin and diaminobenzidine chromogen as a substrate per manufacturer’s protocol (Universal immunoperoxidase polymer, anti-mouse; Nichirei Bioscience).
To quantify stained cells, microscopy was performed on stained lung tissue sections using a Nikon Eclipse (TE200S) inverted fluorescence system. Images were captured in a blinded fashion such that primarily small airways and vessels were sampled separately from the acinar parenchyma. Quantitative intensity (expression) data were obtained by using a macro in the Metamorph Imaging software (Universal), as previously described (14). Metamorph was used to quantitate all cells except macrophages, which were counted manually and identified by their typical morphology and expression of MHC class I by RT1Aa mAb staining.
MLRs for cytokine production
In some studies, AM (CD68+) were purified from normal BAL fluid of normal BN rats or BN allografts from the experimental groups and used as source of APCs. In brief, unseparated fresh BAL fluid was incubated with mouse anti-rat CD68 Ab (ED1; Serotec), followed by an incubation with rat anti-mouse IgG1 magnetic beads (Miltenyi Biotec). The purity of the cell isolated was confirmed by examining cytospin specimens for cells with macrophage morphology. Analyses revealed that >90% of the isolated cells were AM. Pure T cells (CD3+) were isolated from mediastinal lymph nodes of normal WKY rats or those that received lung allografts. AM (5 × 104) or T cells (2 × 105) were cultured, alone, or cocultured in 96-well microtiter plates in complete medium. Supernatants were collected after 72-h incubation and stored at −80°C until cytokine assays.
Quantitation of cytokines
IFN-γ, IL-10, and IL-17A were assayed in MLR supernatants by ELISA using the paired Abs for capture and detection per manufacturer’s protocol (BD Biosciences for IL-10; eBioscience for IFN-γ and IL-17A). MCP-1 (CCL-2) was assayed in BAL fluid using ELISA kit per manufacturer’s directions (PeproTech).
Chemotaxis assays
Chemotaxis assays were performed, as previously reported (15). In brief, chemotactic activity in BAL from transplanted donor lungs was determined using a 48-well chemotaxis microchamber (NeuroProbe). BAL fluid was placed in the lower chamber. A polycarbonate polyvinylpyrolidone-free membrane (NeuroProbe) perforated with 3-μm-diameter pores was placed on top of the lower chamber. The upper chamber was attached, and AM from normal WKY rats were placed into each upper chamber (2 × 104 AM in 50 μl of complete medium). After 3-h incubation at 37°C (5% CO2), the membrane was removed, stained with Diff-Quick (Biochemical Sciences), and examined by light microscopy at ×400 magnification. Migrating cells embedded in the membrane were counted in each group. The chemotactic index for BAL was determined using the following formula: (average number of migrating cells to BAL)/(average number of cells to the background control). In some experiments, a neutralizing Ab to MCP-1 (5 μg/ml; PeproTech) or an isotype/species-matched control Ab (rabbit IgG; Pepro-Tech) was added at the start of the study.
Statistics
All data are expressed as the mean ± SEM. Statistical analysis was conducted using one-way ANOVA test and posthoc comparisons using Tukey’s test for multiple comparisons. A p value of <0.05 was considered significant. All analyses were performed using a statistics software package (GraphPad).
Results
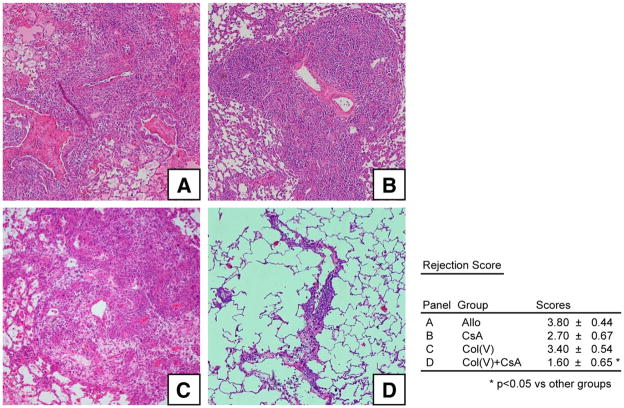
To determine whether low-dose CsA plus col(V)-induced oral tolerance would prevent rejection of fully incompatible grafts, we examined the following experimental groups: Allo, untreated allograft recipients; col(V), WKY rats fed col(V) before lung transplantation of BN lung grafts; CsA, WKY rat lung allograft recipients treated with CsA (5 mg/kg); and col(V) plus CsA, WKY rats fed col(V) before BN rat lung transplantation, followed by CsA treatment. All rats were sacrificed, and tissues were analyzed 7 days posttransplantation. As expected, transplanted lungs in the Allo group developed acute severe rejection (grade 4), as shown by extensive perivascular and peribronchiolar infiltrates (Fig. 1A). Treatment with col(V) or CsA, alone, did not prevent the rejection response (Fig. 1, B and C, respectively). In contrast, col(V) plus CsA reduced significantly the severity of rejection pathology (Fig. 1D). Using a scoring system for clinical acute rejection, col(V) plus CsA significantly reduced acute rejection scores compared with all other groups (Fig. 1; p < 0.05).
FIGURE 1.
Col(V) plus CsA prevents acute rejection. Photomicrographs show histology of transplanted lungs from each group reported in Materials and Methods. A, Allo; B, CsA; C, col(V); D, col(V) plus CsA. Acute rejection was graded A0 to A4 according to the presence and extent of perivascular mononuclear infiltrates. Col(V) plus CsA group grafts had significantly less severe rejection pathology compared with Allo, CsA, and col(V) groups (*, p < 0.05). Photomicrographs are representative of the histology of five allografts in each group (original magnification: ×100). Scores represent the mean ± SEM of the pathologic score of five rats in each group.
We next determined whether the col(V) plus CsA effect in the current study could be transferred to untreated WKY rats before transplantation of BN lungs. To conduct these studies, unseparated splenocytes or pure CD4+ splenic T cells were isolated from WKY lung transplant recipients rats in the col(V) plus CsA group 7 days postlung transplantation. Splenocytes or pure CD4+ T cells (1 × 107 cells) were transferred to untreated WKY rats 24 h before transplantation of BN lungs. Review of lung pathology revealed severe (grade 4) rejection in all groups (data not shown, n = 4 in each group). These data indicated that, in contrast to our minor mismatch model, tolerance was not transferrable to fully MHC incompatible lungs.
The ability to transfer col(V)-induced oral tolerance in the minor MHC incompatibility model was dependent on induction of systemic Tregs (8). The failure to transfer tolerance in the current model could have been due to lack of systemic induction of cells able to function as regulators, or that these cells were present within the transplanted lung, and not systemically. To address these possibilities, we determined the frequency of Tregs (CD4+CD25+Foxp3+) in the mediastinal lymph node lymphocytes or splenocytes from the four groups. Interestingly, although the number of Tregs was increased compared with normal rats, these percentages were the same in all treatment groups and did not vary significantly relative to the tissue examined, i.e., lymph node or spleen (Fig. 2).
FIGURE 2.
Flow cytometric analysis of T cells with a regulatory phenotype. CD3+ T cells were isolated from the mediastinal lymph nodes (A) and spleens (B) from rats of each group and normal WKY rats. Cells were analyzed by three-color flow cytometry to detect the percentages of CD4+ CD25+Foxp3+ T cells (Tregs) in each group. There were no significant differences in Treg population among all groups (p > 0.05). Data represent the mean ± SEM of the percentages of Tregs within the CD3+ population of three or four rats in each group.
We next determined whether cells with a regulatory phenotype were present with the transplanted lungs. Immunohistochemical studies revealed that Foxp3+ cells were readily detected in the perivascular and peribronchiolar tissues of the col(V) plus CsA group (Fig. 3D; p < 0.05), and that the quantity of these cells was significantly greater in the col(V) or col(V) plus CsA groups compared with the Allo group (Fig. 3).
FIGURE 3.

Immunohistochemistry for Foxp3 expression in lung allografts. Lung transplantation and treatments were performed, as described in Materials and Methods. Seven days posttransplantation, allograft lungs were harvested, fixed, sectioned, and stained for Foxp3, and Foxp3-expressing cells were quantified. A, Allo; B, CsA; C, col(V); D, col(V) plus CsA. Foxp3+ cells (see arrows) were significantly greater in the col(V) group (C) and col(V) plus CsA group (D) compared with others (p < 0.05). Photomicrographs are representative of immunohistochemistry of three or four allografts in each group (original magnification: ×400). Quantity represents the mean ± SEM of the quantity of Foxp3+ cells of three or four rats in each group.
Consistent with the protective effects of col(V) plus CsA reported above, additional immunohistochemical analysis of lung allograft sections showed that col(V) plus CsA resulted in significantly greater quantities of perivascular and peribronchiolar mononuclear cells expressing IL-10, which is known to have potent immunosuppressive effects (p < 0.05; Fig. 4D). In addition, IFN-γ, known to have key roles in rejection immunity and readily detected in the Allo and CsA groups, was not detected in the col(V) or col(V) plus CsA groups (p < 0.05; Fig. 5, C and D). Collectively, these data suggest the col(V) plus CsA may prevent rejection in part by abrogating local IFN-γ production, while up-regulating mononuclear cells that express Foxp3 and IL-10.
FIGURE 4.
Immunohistochemistry for IL-10 expression in lung allografts. Lung transplantation and treatments were performed, as described in Materials and Methods. Seven days posttransplantation, allograft lungs were harvested, fixed, sectioned, and stained for IL-10, and IL-10-expressing cells were quantified. A, Allo; B, CsA; C, col(V); D, col(V) plus CsA. IL-10+ (see arrows) cells were significantly greater in the col(V) plus CsA group (D) compared with Allo group (p < 0.05). Photomicrographs are representative of immunohistochemistry of three or four allografts in each group (original magnification: ×400). Quantity represents the mean ± SEM of the quantity of IL-10+ cells of three or four rats in each group.
FIGURE 5.
Immunohistochemistry for IFN-γ expression in lung allografts. Lung transplantation and treatments were performed, as described in Materials and Methods. Seven days posttransplantation, allograft lungs were harvested, fixed, sectioned, and stained for IFN-γ, and IFN-γ-expressing cells (see arrows) were quantified. A, Allo; B, CsA; C, col(V); D, col(V) plus CsA. IFN-γ+ cells were not detected in the col(V) or col(V) plus CsA groups (*, p < 0.05 compared with Allo). Photomicrographs are representative of immunohistochemistry of three or four allografts in each group (original magnification: ×400). Quantity represents the mean ± SEM of the quantity of IFN-γ+ cells.
Alloimmune responses early postlung transplantation are believed to be initiated by APCs of the donor lung interacting with recipient T cells, i.e., direct allorecognition (16). Data showing that col(V) plus CsA induced intragraft expression of IL-10, but suppressed IFN-γ, suggested that the col(V) plus CsA was regulating the cytokine response that occurred during direct allorecognition. To address this question, pure T cells (CD3+) from mediastinal nodes or splenocytes of normal WKY rats or those of the transplant groups were cocultured with T cell-depleted splenocytes from normal BN rats as a source of donor-derived APCs. The frequency of alloantigen-responsive cells that produced IFN-γ and IL-10 was determined by ELISPOT. IL-17A, recently reported to have key roles in col(V)-related immunity, including lung transplant rejection (2, 5, 11, 17), was also studied. As expected, donor-derived APCs (BN) induced high frequencies of IFN-γ+ mediastinal lymph node or splenic T cells isolated from the Allo group (untreated allograft recipients). Although there was a trend toward fewer IFN-γ-producing cells in the col(V) or CsA group, col(V) plus CsA treatments resulted in host T cells with a reduced ability to synthesize IFN-γ in response to donor-derived APCs (p < 0.05; Fig. 6, A and B).
FIGURE 6.
ELISPOT for cells producing IFN-γ, IL-17A, and IL-10. Purified T cells (CD3+) derived from mediastinal lymph nodes (MLN) or splenocytes from each group were cultured alone (2 × 105), or cocultured with T cell-depleted splenocytes from normal BN rats (5 × 104) as a source of APCs. A, IFN-γ assay with MLN T cells; B, IFN-γ assay with splenic T cells; C, IL-17A assay with MLN T cells; D, IL-17A assay with splenic T cells; E, IL-10 assay with MLN T cells; F, IL-10 assay with splenic T cells. The frequency of spot-forming cells was determined for each group after 72-h incubation. Data represent the mean ± SEM of four separate experiments in each group. Greater IFN-γ+ cells were present in the Allo group of MLN T cells compared with the col(V) plus CsA group (A; *, p < 0.05). A similar trend for IFN-γ+ cells was present in the splenic T cell group (B). There were significantly greater IL-10+ cells in col(V) or col(V) plus CsA splenocyte groups compared with Allo or CsA groups (F; *, p < 0.05).
Donor-derived APCs induced IL-17A production from normal WKY rat T cells (Fig. 6, C and D). In addition, mediastinal and splenic T cells from all of the treatment groups produced IL-17A in response to donor-derived APCs (Fig. 6, C and D). In contrast, IL-10-producing cells, detected at lower levels in normal, Allo, and CsA groups, were significantly increased in the col(V) and col(V) plus CsA groups (p < 0.05; Fig. 6, E and F). Our prior studies demonstrated that col(V)-induced oral tolerance was associated with alloantigen-induced production of TGF-β (8). Although reagents were not available to detect TGF-β-producing rat cells by ELISPOT, we did not consistently detect significant quantities of active TGF-β in the culture supernatants by ELISA from all treatment groups (data not shown). In addition, immunohistochemical staining of lung grafts did not show differential expression of TGF-β among all groups (data not shown).
We next determined whether the protective effect of col(V) plus CsA regulated either T cells from the recipient or AM from the allograft in producing IFN-γ, IL-17A, or IL-10. To conduct these studies, CD3+ T cells from mediastinal lymph nodes of the Allo, col(V), CsA, or col(V) plus CsA groups (derived from WKY rats) were cocultured with AM from normal BN rats. Parallel studies used allograft AM from the Allo, col(V), CsA, or col(V) plus CsA groups in coculture with pure T cells (CD3+) from mediastinal lymph nodes of normal WKY rats. Cytokines produced in the supernatants were determined by ELISA.
T cells from the Allo group produced copious amounts of IFN-γ in response to allogeneic (BN) normal AM (Fig. 7B); this response was significantly reduced in T cells from the col(V) or CsA group. Notably, T cells from the col(V) plus CsA group did not produce IFN-γ in response to the macrophages, suggesting a synergistic effect of col(V) and CsA on T cells (Fig. 7B). These data are consistent with the immunohistochemical staining (Fig. 5), which detected copious IFN-γ+ cells in the Allo, but not col(V) plus CsA groups. Whereas T cells in the Allo group produced low levels of IL-17A constitutively, normal AM induced significantly higher levels of IL-17A from Allo T cells (Fig. 7D; p < 0.001), a finding consistent with alloantigen inducing a trend toward a higher frequency of IL-17A-producing T cells as shown by ELISPOT (Fig. 6, C and D). AM from the Allo group, but not those from other groups, were able to induce IL-17A production from normal WKY-derived T cells (Fig. 7C). These data show that T cells derived from untreated allograft recipients (Allo group) are primed to produce IL-17A in response to alloantigen, and that AM from this same group are able to induce IL-17A synthesis from normal T cells. In contrast, AM conditioned by exposure to col(V), CsA, or col(V) plus CsA are not able to induce IL-17A production.
FIGURE 7.
Cytokine profiles in supernatants of MLRs. A, C, and E, Pure T cells (CD3+, 2 × 105) from mediastinal lymph nodes of normal WKY rats were cultured, alone, or cocultured with normal AM from BN rats (CD68+, 5 × 104), or AM isolated from Allo, CsA, col(V), or col(V) plus CsA groups. B, D, and F, Normal AM from BN rats (5 × 104) were cultured, alone, or with T cells (CD3+, 2 × 105) from the mediastinal lymph nodes of Allo, CsA, col(V), or col(V) plus CsA groups. IFN-γ, IL-17A, and IL-10 were assayed by ELISA after 72-h incubation. In contrast to cocultures of normal T cells or T cells from the col(V) plus CsA group, Allo group T cells produced high levels of IFN-γ in response to normal AM (*, p < 0.001; B). Normal T cells or Allo group T cells produced IL-17A in response to Allo AM or normal AM (C and D, respectively; D, *, p < 0.001). AM from the col(V) plus CsA group produced higher levels of IL-10, constitutively, compared with AM from normal or Allo groups (E; *, p < 0.001). Normal macrophages did not induce IL-10 in T cells derived from allograft recipients under all conditions (F).
Only AM from the col(V) plus CsA group produced very high levels of IL-10 constitutively (Fig. 7E; p < 0.001). Notably, T cells from the col(V), CsA, col(V) plus CsA, or Allo groups did not produce IL-10 under any conditions, and AM from the col(V) plus CsA group did not induce further IL-10 from T cells (Fig. 7E). Normal AM were not able to induce IL-10 from T cells derived from allograft recipients under any conditions tested, and these T cells, alone, did not produce IL-10 constitutively (Fig. 7F). Collectively, these coculture data in Fig. 7 show that col(V) plus CsA-mediated prevention of allograft rejection is associated with suppression of IFN-γ and IL-17A production and markedly enhanced constitutive production of AM-derived IL-10.
In addition to T cells, macrophages may be recruited to the transplanted lung and participate in allograft pathology. We next determined whether host-derived macrophages were increased in the transplanted lung, and whether the protective effect of col(V) plus CsA was associated with down-regulated macrophage recruitment. We used anti-MHC class I Abs specific for WKY rats (anti-RT1Aa) and cellular morphology to identify macrophages in transplanted lungs. Data in Fig. 8 show a large number of recipient-derived (WKY) macrophages in the alveolar space of transplanted lungs from the Allo group. In contrast, treatment with col(V) plus CsA was associated with significantly fewer recipient-derived macrophages (Fig. 8; p < 0.01).
FIGURE 8.
Immunohistochemistry for rat MHC class I, RT1Aa (WKY) in transplanted lungs of Allo group (A) and col(V) plus CsA group (B). Seven days after transplantation, BN lungs were stained with anti-RT1Aa Ab, and the quantity of cells with a macrophage morphology in acini was determined (*, p < 0.01 compared with Allo group). Data represent the mean ± SEM of cells detected with macrophage morphology (n = 3; ×400 magnification).
We have reported previously that lung allograft rejection in the rat is associated with increased local chemotaxis for graft-infiltrating mononuclear cells (15). We next determined whether this effect involved macrophage recruitment and whether col(V) plus CsA prevented this response. Compared with normal rats, BAL fluid from the Allo group had significantly greater chemotactic activity for macrophages (Fig. 9A; *, p < 0.001), and this response was due to MCP-1 because blocking this chemokine abrogated macrophage chemotaxis (Fig. 9A; †, p < 0.001, compared with Allo). Notably, compared with Allo, the chemotactic index was significantly reduced in the col(V) and col(V) plus CsA group (Fig. 9A; ‡, p < 0.05). Because MCP-1 is strongly chemotactic for macrophages (18, 19), and our prior report showed up-regulated MCP-1 in allograft BAL fluid (15), we next determined whether col(V), CsA, or col(V) plus CsA suppressed local MCP-1 levels. Consistent with fewer macrophages in the allograft lung and diminished macrophage chemotaxis, col(V) plus CsA treatment resulted in a significant reduction in bronchoalveolar fluid levels of MCP-1 (Fig. 9; †, p < 0.001 compared with Allo).
FIGURE 9.
Chemotaxis index and chemokines. A, Chemotaxis index in BAL fluid from the normal, or transplanted lungs from untreated Allo, CsA, col(V), or col(V) plus CsA groups. Neutralizing MCP-1 Abs or iso-type control Abs were added to Allo BAL fluid. The chemotaxis index was determined with the following formula: (average number of migrating cells to BAL)/(average number of cells to the background control) (*, p < 0.001 compared with normal; †, p < 0.001 compared with Allo; and ‡, p < 0.05 compared with Allo). B, MCP-1 levels in BAL fluid from normals, Allo, CsA, col(V), and col(V) plus CsA group were determined by ELISA (*, p < 0.001 compared with normal, and †, p < 0.001 compared with Allo). All data in A and B represent the mean ± SEM of three or four experiments in each group.
Discussion
We have reported that lung allograft rejection in humans and rats involves an immune response to col(V), and that col(V)-induced oral tolerance prevented acute and chronic rejection of minor histoincompatible lung allografts (3, 4, 8). CsA at doses >25 mg/kg, but not lower doses, prevents rejection of fully MHC incompatible lung allografts. The current study demonstrates that col(V) plus low-dose CsA (5 mg/kg) down-regulated the rejection of fully MHC incompatible lung grafts. Col(V) plus CsA was associated with perivascular and peribronchiolar cells that expressed Foxp3+ and IL-10, but not IFN-γ. In addition, col(V) plus CsA induced constitutive production of IL-10 from allograft AM, and abrogated local chemotactic stimuli for macrophages.
In contrast to our prior studies in minor histoincompatible rat lung grafts, immune tolerance in clinical lung transplantation is challenged by major MHC incompatibilities between donor and recipient. In this setting, oral col(V) would be less likely to fully prevent the rejection response. Indeed, oral col(V), alone, although inducing Foxp3 and abrogating IFN-γ in the graft, was insufficient to prevent severe rejection pathology. In contrast, col(V) plus CsA strongly induced intragraft IL-10-expressing cells. The association of IL-10 expression with preservation of graft histology is in contrast to our prior col(V) tolerance studies, which demonstrated a role for TGF-β-expressing Tregs that suppressed alloimmunity and anti-col(V) immunity in response to oral col(V) (8). The explanation for the lack of association with TGF-β production and col(V) in the current studies is unclear, but could be related to the vigorous nature of the immune response induced due to the degree of MHC disparity between donor and recipient. Indeed, studies from Mohanakumar and colleagues (6, 7) reported that IL-10, but not TGF-β, had a key role in suppressing IFN-γ-mediated anti-col(V) immunity in human lung allograft recipients. In fact, loss of IL-10-mediated immune regulation to col(V) preceded graft rejection (6, 7).
IL-17A has been reported to have key roles in autoimmune mediated diseases, and we and others have demonstrated a major role for IL-17A during chronic rejection in human lung transplant recipients (2, 17). Data in the current study showed that T cells from all groups of rats, including those derived from normal WKY rats, produced IL-17A in response allogeneic APCs. These data are consistent with a very recent report showing that direct allorecognition of allogeneic MHC class II molecules is sufficient to induce IL-17A production from CD4+ T cells, a finding potentially related to IL-17A-associated autoimmunity (20). Interestingly, normal AM from BN rats were potent inducers of IL-17A from T cells derived from the Allo group, which is consistent with the role of allogeneic macrophages reported to induce IL-17A from T cells reported in other studies (21). Differentiation of Th17 cells and regulatory T cells requires TGF-β, but each cell type is dependent on different transcription factors: RORγt for Th17 cells and Foxp3 for Tregs. Data showing high local expression of Foxp3 in lung allografts and high levels of IL-17A in mediastinal lymph node T cells could suggest differential expression levels of TGF-β in each compartment. Indeed, the induction of Th17 cell as compared with Tregs has been shown recently to depend on the degree of exposure to TGF-β (22). This question will be addressed in future studies. Col(V) plus CsA abrogated the ability of AM to induce IL-17A from T cells, suggesting col(V) plus CsA may be an effective strategy to prevent deleterious IL-17A production in vivo.
Inducing IL-10 production locally has been shown to prevent lung allograft rejection in rats. For example, Stammberger et al. (23) reported that local IL-10 overexpression was effective in down-regulating rejection of fully MHC incompatible rat lung allografts. Data in the current study showed a higher frequency of alloantigen-induced IL-10+ T cells from the col(V) and col(V) plus CsA groups, the latter of which was associated with preventing graft rejection. Notably, col(V) plus CsA induced very high constitutive production of IL-10 from allograft AM (Fig. 7). This effect could have been mediated by col(V); however, it is unlikely because very few IL-10-expressing cells were detected by immunostaining in the col(V), alone, group, whereas copious IL-10-expressing mononuclear cells were present in the col(V) plus CsA group. It is likely that col(V) enhanced CsA-induced IL-10 expression because IL-10+ cells were detected by immunohistochemistry in the CsA group, but at a lower level than observed in the col(V) plus CsA group (Fig. 4). These data are also consistent with a report from Jiang et al. (24), who reported that CsA is able to induce IL-10 expression in macrophages. The mechanism of IL-10 induction in macrophages may involve macrophage interactions with other cells in vivo, such as Tregs, that have been shown to induce macrophage-derived IL-10 (25). Indeed, cells expressing Foxp3, a marker of Tregs, were increased in lung of rats treated with col(V) plus CsA. Data showing that col(V) plus CsA, but not col(V), alone, induced IL-10 expression in allograft mononuclear cells (Fig. 4) could potentially contradict data in Fig. 6, which showed that col(V) and col(V) plus CsA were both associated with induction of IL-10 via ELISPOT. The differences could be related to the source of cells expressing IL-10 and the stimulus inducing IL-10. Mediastinal node and splenic T cells were the source of IL-10 in Fig. 6, and the APCs were T cell-depleted splenocytes. Macrophages may constitute a significant portion of the IL-10+ cells shown in Fig. 4. This is consistent with data shown in Fig. 7 that demonstrated macrophages from the col(V) plus CsA group constitutively produced IL-10.
Acute lung allograft rejection is characterized by varying degrees of perivascular and peribronchiolar mononuclear cell infiltrates. Belperio et al. (19) and our prior studies (15) reported that mononuclear cell influx during lung allograft rejection is in part the result of increased chemotactic stimuli, such as MCP-1 (CCL2). Data in the current study showing increased chemotaxis for macrophages are consistent with up-regulated MCP-1 levels in BAL fluid in untreated allografts (Allo group). We believe a novel finding is that col(V) plus CsA was associated with significantly reduced macrophage chemotaxis and levels of MCP-1. Indeed, CsA may reduce MCP-1 production from different cell types, including AM (26, 27). However, data in Fig. 9B show that col(V), alone, was associated with reduced MCP-1 levels in BAL fluid, and that the effect of col(V) plus CsA was additive in reducing MCP-1. Suppressing MCP-1 is likely to be a desirable effect in lung transplant rejection. Indeed, elegant studies from Belperio et al. (19) showed that locally up-regulated MCP-1 was strongly associated with preclinical models of obliterative bronchiolitis and clinical BOS.
Although lung transplantation is considered a definitive treatment for many patients, the outcomes remain poor, and immunosuppression-induced complications remain a major cause of morbidity. Accordingly, immunosuppressive regimens that are effective to prevent rejection and are associated with minimal morbidity would be a major advance in the treatment of transplant patients. Preclinical data in the current study suggest that col(V)-induced oral tolerance plus low doses of calcineurin inhibitors could be an effective strategy to prevent lung allograft rejection. In addition, that ability of col(V) to allow for the use of very low doses of CsA suggests that this regimen may also prevent common calcineurin inhibitor-associated complications such as fibrosis and hypertension, among others. Future clinical trials are planned to address these questions.
Acknowledgments
We thank Eriko Kubo for her contribution in pathology and immunohistochemistry.
Footnotes
This work was supported by National Institutes of Health Grants HL081350, HL60797, and HL/Al67177 (to D.S.W.); National Institutes of Health RO1 Grant HL090950 (to I.P.); Department of Veterans Affairs Research Grant (to D.B.B.); and grants-in-aid for science research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (15591466 to Y.S. and 19591612 to S.Y.).
Abbreviations used in this paper: BOS, bronchiolitis obliterans syndrome; AM, alveolar macrophage; BAL, bronchoalveolar lavage; BN, Brown Norway; col(V), type V collagen; CsA, cyclosporine; Treg, regulatory T cell; WKY, Wistar Kyoto.
Disclosures
D.S.W. is cofounder and Chief Scientific Officer of ImmuneWorks, a company developing therapeutics for immune mediated lung diseases. The remaining authors have no financial conflict of interest.
References
- 1.Knoop C, Estenne M. Acute and chronic rejection after lung transplantation. Semin Respir Crit Care Med. 2006;27:521–533. doi: 10.1055/s-2006-954609. [DOI] [PubMed] [Google Scholar]
- 2.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasufuku K, Heidler KM, O’Donnell PW, Smith GN, Jr, Cummings OW, Foresman BH, Fujisawa T, Wilkes DS. Oral tolerance induction by type V collagen down-regulates lung allograft rejection. Am J Respir Cell Mol Biol. 2001;25:26–34. doi: 10.1165/ajrcmb.25.1.4431. [DOI] [PubMed] [Google Scholar]
- 4.Yasufuku K, Heidler KM, Woods KA, Smith GN, Jr, Cummings OW, Fujisawa T, Wilkes DS. Prevention of bronchiolitis obliterans in rat lung allografts by type V collagen-induced oral tolerance. Transplantation. 2002;73:500–505. doi: 10.1097/00007890-200202270-00002. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, Baldridge LA, Heidler KM, Cummings OW, Fujisawa T, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–735. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 6.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6:1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 7.Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T. Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. J Immunol. 2006;177:5631–5638. doi: 10.4049/jimmunol.177.8.5631. [DOI] [PubMed] [Google Scholar]
- 8.Mizobuchi T, Yasufuku K, Zheng Y, Haque MA, Heidler KM, Woods K, Smith GN, Jr, Cummings OW, Fujisawa T, Blum JS, Wilkes DS. Differential expression of Smad7 transcripts identifies the CD4+ CD45RChigh regulatory T cells that mediate type V collagen-induced tolerance to lung allografts. J Immunol. 2003;171:1140–1147. doi: 10.4049/jimmunol.171.3.1140. [DOI] [PubMed] [Google Scholar]
- 9.Francalancia NA, Wang SC, Thai NL, Aeba R, Simmons RL, Yousem SA, Hardesty RL, Griffith BP. Graft cytokine mRNA activity in rat single lung transplants by reverse transcription-polymerase chain reaction: effect of cyclosporine. J Heart Lung Transplant. 1992;11:1041–1045. [PubMed] [Google Scholar]
- 10.Hausen B, Boeke K, Berry GJ, Segarra IT, Christians U, Morris RE. Suppression of acute rejection in allogeneic rat lung transplantation: a study of the efficacy and pharmacokinetics of rapamycin derivative (SDZ RAD) used alone and in combination with a microemulsion formulation of cyclosporine. J Heart Lung Transplant. 1999;18:150–159. doi: 10.1016/s1053-2498(98)00020-5. [DOI] [PubMed] [Google Scholar]
- 11.Bobadilla JL, Love RB, Jankowska-Gan E, Xu Q, Haynes LD, Braun RK, Hayney MS, Munoz Del Rio A, Meyer K, Greenspan DS, et al. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. Am J Respir Crit Care Med. 2008;177:660–668. doi: 10.1164/rccm.200612-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, Tazelaar HD. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 13.Chiyo M, Iwata T, Webb TJ, Vasko MR, Thompson EL, Heidler KM, Cummings OW, Yoshida S, Fujisawa T, Brand DD, Wilkes DS. Silencing S1P1 receptors regulates collagen-V reactive lymphocyte-mediated immunobiology in the transplanted lung. Am J Transplant. 2008;8:537–546. doi: 10.1111/j.1600-6143.2007.02116.x. [DOI] [PubMed] [Google Scholar]
- 14.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr, Chiyo M, Lee J, Mickler E, Smith GN, Petrache I, Brand DB, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181:5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekine Y, Yasufuku K, Heidler KM, Cummings OW, Van Rooijen N, Fujisawa T, Brown J, Wilkes DS. Monocyte chemoattractant protein-1 and RANTES are chemotactic for graft infiltrating lymphocytes during acute lung allograft rejection. Am J Respir Cell Mol Biol. 2000;23:719–726. doi: 10.1165/ajrcmb.23.6.3825. [DOI] [PubMed] [Google Scholar]
- 16.Hornick P. Direct and indirect allorecognition. Methods Mol Biol. 2006;333:145–156. doi: 10.1385/1-59745-049-9:145. [DOI] [PubMed] [Google Scholar]
- 17.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, Meyts I, Bullens DM, Reynders V, Wuyts WA, Van Raemdonck DE, DuPont LJ, Verleden GM. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8:1911–1920. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 18.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belperio JA, Keane MP, Burdick MD, Lynch JP, III, Xue YY, Berlin A, Ross DJ, Kunkel SL, Charo IF, Strieter RM. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benghiat FS, Craciun L, De Wilde V, Dernies T, Kubjak C, Lhomme F, Goldman M, Le Moine A. IL-17 production elicited by allo-major histocompatibility complex class II recognition depends on CD25posCD4pos T cells. Transplantation. 2008;85:943–949. doi: 10.1097/TP.0b013e31816a5ae7. [DOI] [PubMed] [Google Scholar]
- 21.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1 β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing ROR γt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stammberger U, Bilici M, Gugger M, Gazdhar A, Hamacher J, Hyde SC, Schmid RA. Prolonged amelioration of acute lung allograft rejection by overexpression of human interleukin-10 under control of a long acting ubiquitin C promoter in rats. J Heart Lung Transplant. 2006;25:1474–1479. doi: 10.1016/j.healun.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Wynn C, Pan F, Ebbs A, Erickson LM, Kobayashi M. Tacrolimus and cyclosporine differ in their capacity to overcome ongoing allograft rejection as a result of their differential abilities to inhibit interleukin-10 production. Transplantation. 2002;73:1808–1817. doi: 10.1097/00007890-200206150-00019. [DOI] [PubMed] [Google Scholar]
- 25.Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, Bijlsma JW, Lafeber FP. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66:222–230. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satonaka H, Suzuki E, Nishimatsu H, Oba S, Takeda R, Goto A, Omata M, Fujita T, Nagai R, Hirata Y. Calcineurin promotes the expression of monocyte chemoattractant protein-1 in vascular myocytes and mediates vascular inflammation. Circ Res. 2004;94:693–700. doi: 10.1161/01.RES.0000118250.67032.5E. [DOI] [PubMed] [Google Scholar]
- 27.Naidu BV, Krishnadasan B, Byrne K, Farr AL, Rosengart M, Verrier ED, Mulligan MS. Regulation of chemokine expression by cyclosporine A in alveolar macrophages exposed to hypoxia and reoxygenation. Ann Thorac Surg. 2002;74:899–905. doi: 10.1016/s0003-4975(02)03746-3. discussion 905. [DOI] [PubMed] [Google Scholar]