Abstract
Primary graft dysfunction (PGD) is a major complication following lung transplantation. We reported that anti-type V collagen (col(V)) T cell immunity was strongly associated with PGD. However, the role of preformed anti-col(V) Abs and their potential target in PGD are unknown. Col(V) immune serum, purified IgG or B cells from col(V) immune rats were transferred to WKY rat lung isograft recipients followed by assessments of lung pathology, cytokines, and PaO2/FiO2, an index of lung dysfunction in PGD. Immune serum, purified IgG, and B cells all induced pathology consistent with PGD within 4 days posttransfer; up-regulated IFN-γ, TNF-α, and IL-1β locally; and induced significant reductions in PaO2/FiO2. Depleting anti-col(V) Abs before transfer demonstrated that IgG2c was a major subtype mediating injury. Confocal microscopy revealed strong apical col(V) expression on lung epithelial, but not endothelial cells; which was consistent with the ability of col(V) immune serum to induce complement-dependent cytotoxicity only in the epithelial cells. Examination of plasma from patients with or without PGD revealed that higher levels of preformed anti-col(V) Abs were strongly associated with PGD development. This study demonstrates a major role for anti-col(V) humoral immunity in PGD, and identifies the airway epithelium as a target in PGD.
Lung transplantation is considered a definitive therapy for many patients with end-stage pulmonary disease. However, chronic rejection, known as obliterative bronchiolitis, is the leading cause of death in these patients, and the primary reason why the 5-year survival rate posttransplant is poor (~50%) (1). Many risk factors have been associated with obliterative bronchiolitis or the clinical correlate, bronchiolitis obliterans syndrome (BOS),5 including acute cellular rejection and CMV infection (1). Recently, we reported that T cell-mediated autoimmunity to a native type V collagen (col(V)) was a major risk factor for BOS, the level of reactivity increasing with increased BOS severity (2).
Primary graft dysfunction (PGD) is a major cause of morbidity and mortality that occurs early in the posttransplant period (3, 4). In fact, PGD accounts for nearly 50% of early deaths posttransplantation (5, 6), and survivors of PGD have worse long-term mortality, consistent with the hypothesis that the injured allograft is more prone to BOS (7). Although a specific trigger for PGD has not been identified, recent reports demonstrating that PGD is a risk factor for BOS suggest a common immunologic basis for these processes (8). Recently, we reported in a rat model of lung transplantation and in humans that memory T cell immunity to col(V) in the pretransplant period was associated with PGD (9). Cellular (T cell) mediated immunity may give rise to Ag-specific humoral responses. Accordingly, data showing the presence of anti-col(V) T cell-dependent cellular immunity in PGD suggest that anti-col(V) humoral immunity could also have a key role in this disease process. A report from Lau et al. (10) demonstrated the presence of panel reactive Abs may be associated with longer postoperative mechanical ventilation. However, the potential role of preformed Abs against an autoantigen, or any Ag, including col(V), in the pathogenesis of PGD has not been reported.
Col(V) is a minor collagen, intercalated within fibrils of type I collagen, a major collagen in the lung. Due to its location in the perivascular and peribronchiolar tissues, we have considered col(V) a “sequestered Ag” in the normal lung. However, interstitial col(V) is readily exposed in response to ischemia reperfusion injury, which occurs during the transplantation procedure (11), and ischemia reperfusion injury is associated with release of antigenic col(V) fragments in bronchoalveolar lavage (BAL) fluid (12). Although classically described as an interstitial collagen, a report from Haralson and colleagues (13) demonstrated that col(V) may be expressed by an epithelial cell line. The study raises the intriguing possibility that anti-col(V) Abs could in part mediate PGD by recognition of the target Ag on lung epithelial cells. Although endothelial cell pathology is known to occur in PGD (14), a recent report from Calfee and colleagues (15) has linked markers of epithelial, but not endothelial, injury to PGD pathogenesis. However, the ability of primary lung epithelial cell to express col(V) is unknown, and the ability of anti-col(V) Abs to induce cytotoxicity in these cells has not been reported.
Using our model of systemic immunity to col(V) in WKY rats (2, 9, 11, 12), we conducted passive and adoptive transfer studies of col(V) immune serum or B cells, respectively, from col(V) immune rats to WKY rat lung isograft recipients. Transfer of col(V) immune serum, purified anti-col(V) IgG, or B cells from col(V) immunized rats induced lung pathology and impaired systemic oxygenation, consistent with PGD in lung isograft recipients. Lung injury was associated with increased local levels of TNF-α, IL-1β, and IFN-γ. Confocal imagining demonstrated that rat and human airway epithelial, but not endothelial, cells express col(V) apically, and that these cells were susceptible to anti-col(V) Ab-mediated complement-dependent cytotoxicity. Translational studies revealed that the presence of preformed serum anti-col(V) Abs in the pretransplant period was strongly associated with developing severe (grade 3) PGD posttransplantation. Collectively, these data support the concept that humoral, as well as cell-mediated immunity to col(V) is a major risk factor for PGD, and that preformed anti-col(V) Abs have a key role in this process.
Materials and Methods
Animal studies
Pathogen-free male Wistar Kyoto (WKY) rats were used for the study. All animals weighed between 250 and 300 g. All rats were purchased from Taconic Farms or Charles River Breeding Laboratories and housed in the Laboratory Animal Resource Center at Indiana University School of Medicine (Indianapolis, IN) in accordance with institutional guidelines. All studies were approved by the Laboratory Animal Resource Center at Indiana University School of Medicine.
Collagens
Bovine col(V) was isolated and used for all immunizations as reported previously (11). Because the current studies were conducted in rats, rat-derived col(V) was considered for these studies. However, similar to our prior studies (11), bovine col(V), and not rat col(V), was used due to the ability of isolating sufficient quantities of bovine col(V), but not rat col(V), for in vivo use.
Immunization with col(V) or hen egg lysozyme (HEL)
WKY rats were immunized at the base of the tail with 200 µg of bovine col(V) or HEL emulsified in 200 µl of CFA (Difco) as reported previously (11). To boost the initial immunization, rats received an injection of 200 µg of col(V) emulsified in 200 µl of IFA (Difco) at the base of the tail 21 days after the initial immunization. Ten days after boosting, rats were sacrificed, serum isolated from blood, and inguinal lymph nodes harvested. Serum was pooled from 10 individual rats and used in this study. Individual B cells (CD45RA+) were isolated from lymph node cells. In brief, lymph nodes were mechanically digested by mincing with scissors in medium (RPMI 1640; Invitrogen), passed through a cell strainer to remove large particles, and washed. Any remaining RBCs were lysed with ammonium chloride. CD45RA+ cells (>90% pure) were selected by autoMACS using rat CD45RA Micro Beads (Miltenyi Biotec). The cells were resuspended in 1% sterile (PBS) before adoptive transfer. The serum obtained from centrifuged blood was stored at −80°C until serum transfer.
Flow cytometry detection of serum anti-col(V) and anti-HEL Abs
Flow cytometric examination of anti-col(V) or anti-HEL coated beads were used to confirm that immunization induced systemic Ab production. In brief, after washing in PBS, streptavidin-coated beads (5 µm, binding capacity 10–20 µg, 1 × 107 beads; Polysciences) were suspended in 100 µl of PBS with human col(V) (40 µg/ml) or HEL (2 µg/ml). After 60 min of incubation at 4°C, beads were washed in PBS containing 10% FCS and stored at 4°C until use. For each assay, 1 × 106 conjugated beads were washed two times in PBS, and incubated in 100 µl of PBS plus 50 µl of human or rat serum, incubated for 30 min at room temperature, and washed in PBS containing 10% FCS. After incubation with anti-human or anti-rat PE-conjugated IgG Abs (Sigma-Aldrich), the beads were washed and analyzed on a FACSCalibur cytofluorograph (BD Biosciences). Positive controls were generated by following the same procedure described, but incubating the beads with biotinylated rabbit anti-human col(V) Ab or biotinylated anti-HEL (20 µg/ml) Abs.
Quantitation of serum total IgG and anti-col(V) IgG subtypes
Total IgG in the immunized or untreated rats was quantitated by ELISA using rat IgG ELISA quantitation kit (Bethyl Laboratories) per the manufacturer’s protocol. Anti-col(V) or HEL specific IgG, IgM, and IgG subclasses were measured using ELISA starter accessory kit (Bethyl Laboratories) with modifications. In brief, microtiter plates were coated 1 h at room temperature with bovine col(V) or HEL (5 µg/ml) in coating buffer (0.05 M carbonate-bicarbonate (pH 9.6)). Subsequently, the plates were blocked with blocking solution (50 mM Tris, 0.14 M NaCl, 1% BSA (pH 8.0)) for 30 min at room temperature and washed with wash solution (50 mM Tris, 0.14 M NaCl, 0.05% Tween 20 (pH 8.0)). Serum samples were diluted 1/56,000 ~ 5600 in sample/conjugate diluent (50 mM Tris, 0.14 M NaCl, 1% BSA, 0.05% Tween 20; Sigma-Aldrich) and 100 µl of diluted sample were added to each well. Purified rabbit anti-human col(V) IgG-biotin conjugate (Santa Cruz Biotechnology) was used as a standard. Plates were incubated for 1 h at room temperature, washed, and incubated with 100 µl (in sample/conjugate diluent) polyclonal goat anti-rat IgG-HRP conjugate (Bethyl Laboratories) or polyclonal goat anti-rat IgG1, IgG2a, IgG2b, IgG2c, or IgM-HRP conjugate (AbD Serotec) and avidin-HRP conjugate (eBioscience) for standard for 1 h. Plates were washed again, and 100 µl of tetramethylbenzidine (Bethyl Laboratories) were added to each well. After a 5- to 10-min incubation in the dark, absorbance was read at 450 nm on a microplate reader (Spectra max; Molecular Devices).
Purification of serum IgG Abs and depletion of anti-col(V) IgG Abs
IgG was purified from serum of immunized rats using ImmunoPure IgG Purification kit, per the manufacturer’s protocol (Pierce). In some experiments, anti-col(V) Abs were depleted from the col(V) immune serum by incubating with col(V)-coated beads (described earlier). In brief, the beads were placed into a protein A column (Pierce) and washed three times with PBS buffer. One milliliter of immune serum was applied to the column and fractions of purified serum were collected.
Rat lung transplantation
The orthotopic transplantation of left lungs were performed as previously described (2, 9, 11, 16). Survival exceeded 90% in all transplantation groups. No immunosuppressive therapy was given at any time during the experimental period.
Serum and Ab transfer
One milliliter of col(V) immune serum was injected by tail vein twice daily into lung isograft recipients for 2 days, beginning on the first postoperative day. This dosing schedule was determined based on preliminary studies showing that single injections did not induce lung pathology. In some studies, IgG (1 mg) purified from col(V) immune serum was transferred by tail vein injection once daily beginning on the first postoperative day for three consecutive days. Serum was isolated from rats on the third or fourth day posttransplantation. Controls for these studies used serum transfer to normal WKY rats (not transplanted or immunized).
Adoptive transfer
In parallel studies, pure B cells (CD45RA+ lymphocytes) were isolated from inguinal lymph nodes of col(V)-immunized rats by magnetic beads (Miltenyi Biotec) and were adoptively transferred by tail vein injection (2 × 107 cells) of WKY rats, 24 h after isograft lung transplantation to WKY rats. CD45RA+ cells were pooled from two rats for injection into two recipient rats. Adoptive transfer procedures were conducted similarly to prior studies (11, 16).
Arterial blood gas analysis
In parallel studies, arterial blood gas analyses were conducted and used to determine the arterial oxygen tension per fraction of inspired oxygen (PaO2/FiO2) ratio as a measure of acute lung injury, as previously reported (9). In brief, all animals were studied 72 h posttransplantation. Rats were anesthetized with an i.m. injection of ketamine and xylazine, intubated through a tracheostomy, and cannulated via the left carotid artery. The rats were mechanically ventilated with a rodent ventilator (model 683; Harvard Apparatus) with 100% oxygen and isoflurane at a minute ventilation to maintain the PaCO2 level at 40 torr. Arterial blood samples were assayed by using an IRMA SL blood analysis system (Diametrics Medical).
Collection of BAL fluid
BAL from native and transplanted lungs was performed in ketamine-anesthetized rats on the day of sacrifice as reported (9, 11, 12, 17). Cell-free BAL fluid supernatants obtained from centrifuged specimens were stored at −80°C until use.
Pathological grading
Native and transplanted lungs were harvested, fixed by an intratracheal instillation of 6% glutaraldehyde, sectioned, stained with H&E, and examined under light microscopy. Grading for rejection pathology was performed in a blinded manner using standard criteria as reported (11).
Quantitation of cytokines
IL-1β, IFN-γ, and TNF-α, were measured in unconcentrated isograft BAL fluid by ELISA using Cytoscreen immunoassay kits (BioSource International) per the manufacturer’s protocol.
Anti-col(V) Ab ELISA
The 96-well microtiter plates (Immunolon II, Thermo Fisher Scientific) were coated with col(V) (1 µg/ml), diluted in coating buffer (0.2 M sodium phosphate (pH 6.5)). Plates were covered and incubated overnight at 4°C. After aspirating wells and washing with PBS-Tween 20 (0.05% PBS in Tween 20; Sigma-Aldrich), plates were blocked with PBS-10% FBS (Hy-Clone Laboratories) for 1 h at room temperature. Patient serum samples (100 µl) were added to duplicate wells, incubated for 2 h at room temperature followed by washing with wash buffer. Anti-human IgG HRP conjugated Abs (1/1000 dilution; Sigma-Aldrich) were added to each well, and aspirated after a 1-h incubation at room temperature. Plates were developed by addition of TMB Substrate Reagent (BD Pharmingen) to each well (100 µl/well). Reactions were stopped after 30 min of incubation in the dark by addition of 2 N H2SO4 to each well, and absorbance was read at 450 nm.
Immunocytochemistry and confocal imaging
Primary rat lung epithelial cells (L2 cells) from American Type Culture Collection (ATCC) were seeded at 1.2 × 105 cells/ml Ham’s/F-12 medium (HyClone Laboratories) plus 10% FBS. Primary rat microvascular lung endothelial cells, a gift from Dr. T. Stevens (University of South Alabama College of Medicine, Mobile, AL), were seeded at 1 × 105 cells/ml DMEM with 4.5 g/L glucose (Mediatech) plus 10% FBS into Lab-Tek Permanox chamber slides (Nalge Nunc International).
After a 24-h incubation (37°C, 5% CO2), cells were washed, fixed with methanol, and blocked by incubating in 10% goat serum (Invitrogen). Each well of cells were incubated with a primary Ab to type I, IV, or V collagen diluted in PBS/1.5% normal goat serum (Rabbit anti-collagen I; Abcam); chicken polyclonal Ab to Col4A3BP (C-178; Novus Biologicals); and rabbit polyclonal Ab to rat collagen type V (Biodesign International). For type I and type V collagens, the cells were incubated with goat anti-rabbit IgG Rhodamine red (Invitrogen); and to detect for type IV collagen, the cells were incubated with goat anti-chicken IgG FITC (Novus Biologicals). After washing and attaching coverslips, the slides were examined by fluorescent microscopy.
In parallel studies, L2 cells were examined by confocal microscopy to determine the distribution of collagens I and V in the cells. In brief, slides were incubated with chicken polyclonal Ab to Col4A3BP(C-178), and detected with goat anti-chicken IgG FITC (Novus Biologicals). To detect col(V) on in the same specimen, slides were washed and incubated with rabbit anti-col(V) Ab (Biodesign International), and detected by an incubation with goat anti-rabbit IgG Rhodamine red (Invitrogen). The cover-slips were mounted and slides examined with a Zeiss UV LSM-510 confocal microscope system that is mounted on a Zeiss Axiovert 100 inverted microscope. A C-Apochromat 40X/1.2 W Corr objective was used to acquire Z-stack images.
For immunostaining of lung tissue sections, 5-µm thick paraffin-embedded tissue sections were dewaxed, hydrated, rinsed with TBS, and digested by a 5-min incubation in proteinase K (DakoCytomation) (11). After rinsing and incubation in 3% H2O2 for 10 min, slides were incubated with primary rabbit anti-rat col(V) Abs or control Ab (1/40 dilution; Chemicon International) for 60 min, and rinsed. Ab detection was performed by 30 min of incubation with biotinylated donkey anti-rabbit Abs, rinsed, then incubated with streptavidin-HRP (DakoCytomation) for 30 min, and developed by 5 min of incubation with diaminobenzidine (DakoCytomation). Slides were then rinsed and counterstained with hematoxylin. Tissues were then examined by light microscopy.
Complement-dependent cytotoxicity assays
Primary rat airway epithelial cells (L2 cells; ATCC) were grown to confluence in complete medium at 37°C, 5% CO2 (F-12 complete medium; HyClone Laboratories). The optimal conditions for these assays were determined by using varying quantities of serum or complement in preliminary studies. The 100 µl of heat-inactivated col(V) or HEL immune serum was added to each 1 ml of medium. After 20 min of incubation, 10 µl or preadsorbed rabbit complement (Pel-Freez Biologicals) was added to each 1 ml of medium. Following 12 h of incubation, cells were stained with propidium iodide (Sigma-Aldrich) and examined by flow cytometry to detect dead cells (propidium iodide-positive) per the manufacturer’s protocol (Beckman Coulter).
Apoptosis assay
To investigate whether treatment with col(V) Ab or complement-induced L2 cell apoptosis, we measured the activation levels of executioner caspases in cell lysates. Caspase-3 activity was measured with the EnzChek Caspase-3 Assay kit No. 1 (Invitrogen) using a specific aminomethylcoumarin-derived casaspe-3 substrate Z-DEVD-AMC, which yields a bright, blue fluorescent product upon proteolytic cleavage. Following the manufacturer’s instructions and buffers, after treatment cells were lysed in the cell lysis buffer, the debris were pelleted after centrifugation at 5000 rpm for 5 min, and the supernatant was incubated in a multiwell plate with the substrate for 30 min at room temperature. The fluorescence was measured using a fluorometer at 342/441 nm. The results were expressed as relative fluorescence units.
Human studies
We performed a nested case control study, within a prospective cohort study of patients undergoing first lung transplantation at a single center. Specimen collection was conducted according to Declaration of Helsinki principles. All subjects had standard immunosuppression including induction with IL-2R antagonist, followed by maintenance with a calcineurin inhibitor, azathioprine and methylprednisolone at 0.5 mg/kg dose. PGD cases were defined as International Society of Heart and Lung Transplantation (ISHLT) grade 3 PGD at 72 h with the following: chest radiographs with diffuse infiltrates involving the lung allografts; PaO2/FiO2 less than 200 mm Hg; and no other secondary cause of graft dysfunction identified (4). This definition is similar to the acute respiratory distress syndrome and has been used in prior studies illustrating association with poor outcomes (3, 6). Controls were subjects who did not have any evidence of lung injury during the posttransplant course (ISHLT PGD grade 0). A total of five PGD cases and five non-PGD controls were chosen to provide 80% power to detect a difference of 2 SDs in the col(V) Ab assay between cases and controls, at an alpha level of significance of 0.05. To control for possible confounding, controls were selected as a population to contain the same number of subjects within a diagnosis category (i.e., three subjects with chronic obstructive pulmonary disease (COPD), two with idiopathic pulmonary fibrosis (IPF), and one with idiopathic pulmonary arterial hypertension (IPAH)), no patients underwent cardiopulmonary bypass, and all ischemic times were below 330 min (18, 19). Informed consent for this study was obtained before organ transplantation. Blood samples were obtained in citrated Vacutainers at 6, 24, 48, and 72 h after removal of the pulmonary arterial cross-clamp. Samples were centrifuged within 30 min of collection and aliquoted plasma was stored at −80°C. Clinical variables were categorized and defined using methods previously published (20, 21).
Statistical analyses
Data are expressed as the mean ± SEM, or as medians with intraquartile ranges if non-normally distributed. Statistical analysis was conducted using one-way ANOVA test and post hoc comparisons using Tukey’s test for multiple comparisons. Wilcoxon rank sum test was used for analyses of skewed data. A value of p < 0.05 was considered significant. Statistical comparisons were performed using STATA (version 9.2).
Results
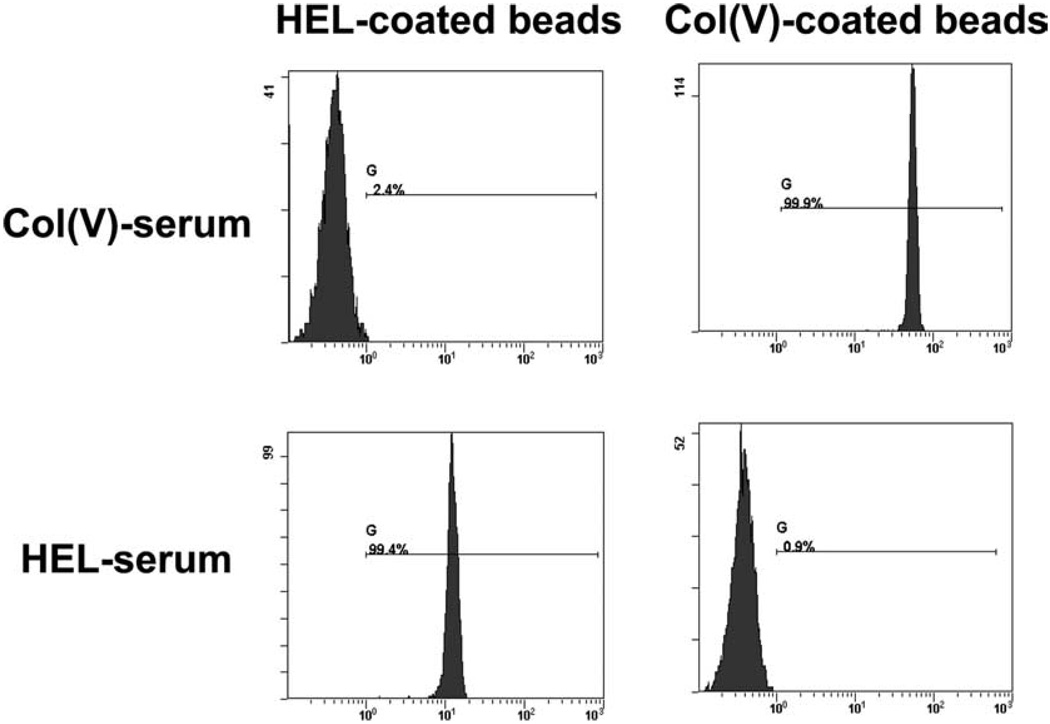
We initially determined that immunization with col(V) or HEL can induce systemic anti-col(V) or anti-HEL Abs in rats (Fig. 1A). Using a flow cytometry based bead assay, as described in Materials and Methods, Fig. 1A shows that immunization with col(V) or HEL induces Ag-specific Ab production systemically. Specifically, anti-col(V)-coated beads only detected anti-col(V) Abs from col(V)-immunized rats, and anti-HEL Abs only detected anti-HEL Abs in HEL-immunized rats.
FIGURE 1.
Detection of systemic anti-col(V) or anti-HEL Abs. Serum was collected from rats there were immunized with col(V) or HEL and incubated with Sepharose beads coated with col(V) or HEL. Flow cytometry was used to detect IgG Abs bound to col(V) or HEL-coated beads. Data shown are derived from serum collected from an individual rat immunized with col(V) or HEL and are representative of n = 5–6 rats immunized with each Ag.
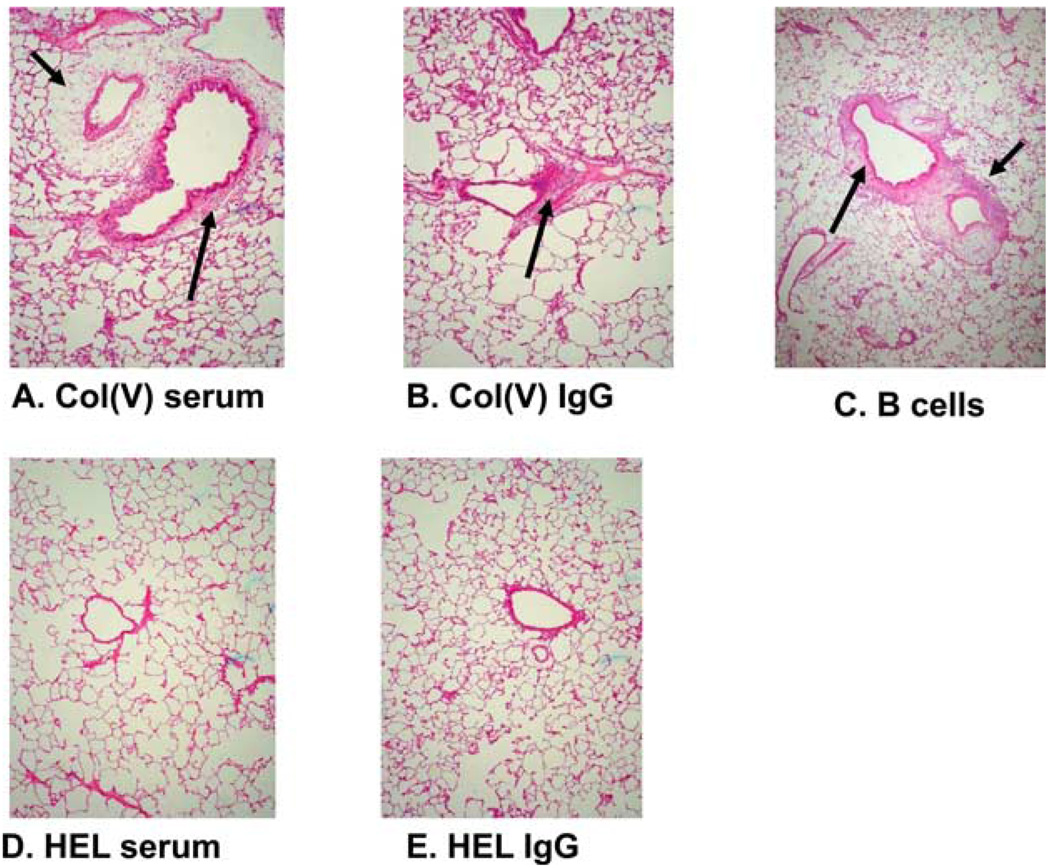
Our prior reports demonstrated that adoptive transfer of col(V)-reactive lymphocytes from col(V)-immunized rats induced “rejection-like” pathology in WKY rat lung isografts (11). To determine whether humoral immunity could induce lung injury and histology possibly consistent with PGD, col(V) or HEL immune serum was passively transferred to WKY lung isograft recipients followed by an assessment of lung pathology. Col(V) immune serum induced perivascular, peribronchiolar, and alveolar neutrophilic infiltrates in the isograft but not native lung (Fig. 2A). To determine whether the soluble mediator in serum was IgG, these studies were repeated by passively transferring IgG affinity purified from col(V) immune serum to isograft recipients. Indeed, purified IgG also induced similar pathologic lesions (Fig. 2B). Consistent with a role for Abs in the process, adoptive transfer of splenic B cells from col(V)-immunized rats recapitulated rejection-like pathology in isograft lungs (Fig. 2C). In contrast, HEL immune serum or IgG purified from HEL immune serum did not induce any pathologic lesions (Fig. 2, D and E, respectively).
FIGURE 2.
Passive transfer of col(V) immune serum or purified IgG, or adoptive transfer of purified B cells from to isograft recipients induces rejection-like pathology in isograft lungs. Col(V) or HEL immune serum or purified IgG or B cells from col(V)-immunized rats were isolated and transferred to lung isograft recipients and lung tissues collected posttransfer as described in Materials and Methods. A, Passive transfer of col(V) immune serum. B, Passive transfer of IgG purified from col(V) immune serum. C, Adoptive transfer of B cells from col(V) immunized rats. D, Passive transfer of HEL immune serum. E, Passive transfer of IgG purified from HEL immune serum. No pathologic lesions are observed in isograft lungs of rats that received HEL immune sera or IgG purified from HEL-immunized rats (A and B, respectively). Arrows indicate perivascular and peribronchiolar infiltrates (A–C). Data are representative of n = 4–6 rats in each group. Magnification of H&E stained lung sections is ×20.
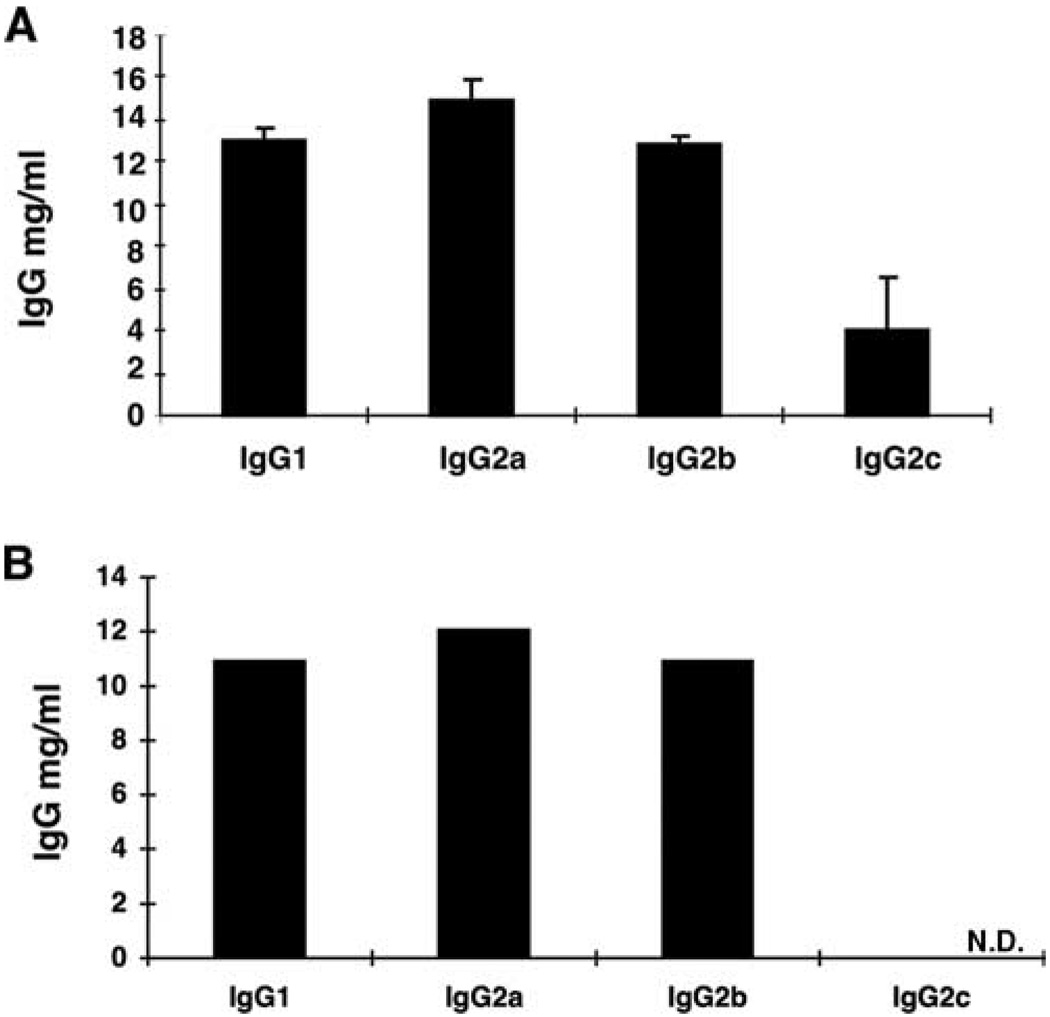
IgG subclasses may vary in their ability to induce injury in many animal models. To investigate which subtypes could induce injury after passive transfer, we first determined the IgG subtypes present in col(V) immune serum. The relative proportions of IgG1, IgG2a, IgG2b, and Ig2c in these samples were similar to those observed in the normal rat (Fig. 3A), although the concentration of total IgG tended to be higher in immunized compared with normal WKY rats (data not shown). To determine the subtypes of anti-col(V) Abs, we used the col(V)-coated beads to deplete anti-col(V) Abs from immune serum, followed by an assessment of IgG subtypes. The quantity of total col(V)-specific IgG was 12.2 mg/ml. The concentrations of IgG1, IgG2a, and IgG2b were reduced slightly after treatment with col(V)-coated beads. However, the IgG2c level, originally at 4 mg/ml was reduced after adsorption to below the limit of detection in the assay, suggesting that this may be the predominant subclass induced by col(V) immunization (Fig. 3B). However, we cannot totally exclude that nonspecific absorption to the beads could have contributed, in part, to the reduction of IgG2c observed.
FIGURE 3.
Quantitation of IgG subtypes col(V) immune serum. IgG subtypes were quantified in serum from col(V)-immunized rats by ELISA as reported in Materials and Methods. A, IgG subtypes in immune serum. Data representative of mean ± SEM of IgG subtypes present in serum isolated from n = 4 immunized rats. B, IgG subtypes present in IgG-purified from immune serum then incubated with anti-col(V) beads to deplete anti-col(V) Abs. Data derived from serum pooled from n = 4 col(V)-immunized rats. N.D., Not detected.
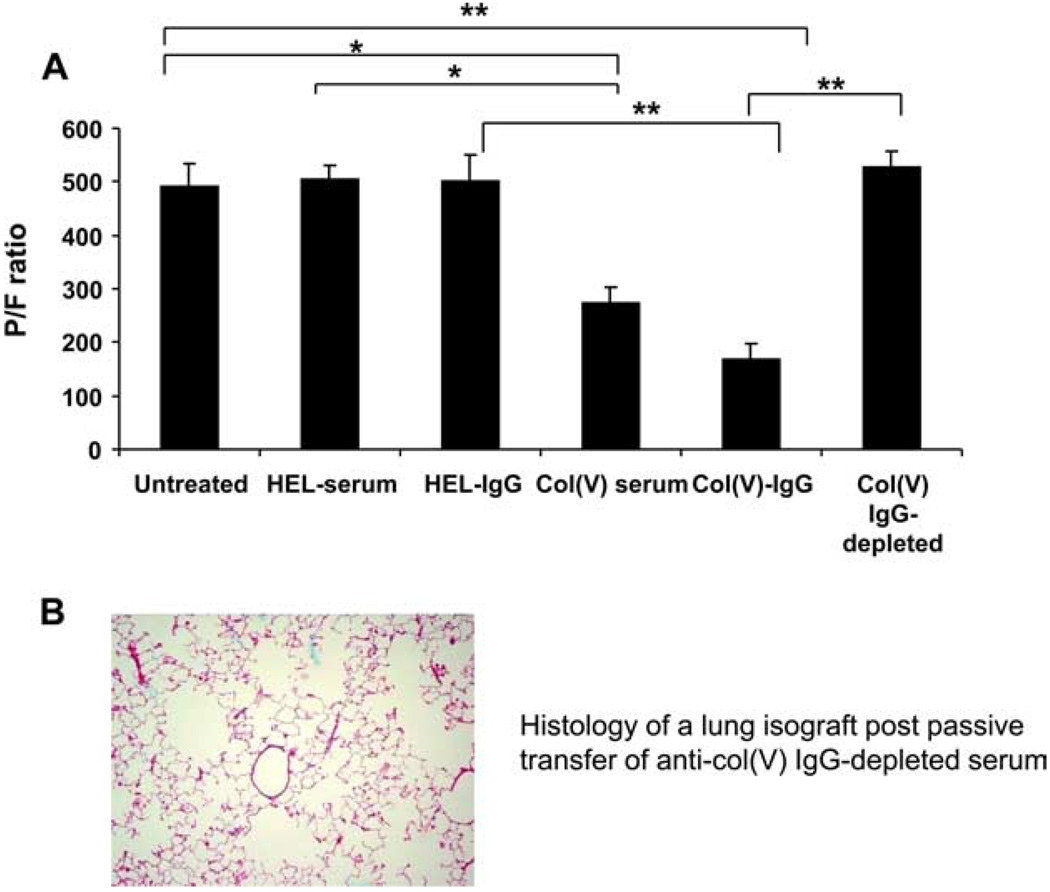
Data described demonstrate that passive transfer of anti-col(V) immune sera or IgG induces acute injury in lung isografts. To determine whether the pathologic lesions correlated with altered physiology, we next determined the PaO2/FiO2, a commonly used index of acute lung injury (4), in untreated isograft recipients and in isograft recipients after passive transfer of col(V) immune serum preparations. Similar to our prior report (9), the PaO2/FiO2 ratio approached 500 in untreated isograft recipients, indicating a mild degree of lung injury that results from ischemia reperfusion injury at the time of transplantation (the PaO2/FiO2 in a normal rat approaches 600, data not shown). Transfer of HEL immune serum or purified IgG from HEL immune serum did not affect the PaO2/FiO2 compared with untreated isograft recipients (Fig. 4). In contrast, passive transfer of col(V) immune serum, or IgG purified from immune serum, resulted in significant reductions in the PaO2/FiO2 compared with untreated isograft recipients or isograft recipients posttransfer of HEL immune serum or HEL-IgG (p < 0.01 and p < 0.001 for col(V) serum or col(V)-IgG, respectively). In contrast, transfer of anti-col(V) Ab-depleted serum restored the PaO2/FiO2 to baseline levels (Fig. 4A), and did not induce lung pathology (Fig. 4B) strongly implicating a role for anti-col(V) Abs in the pathogenesis of lung injury.
FIGURE 4.
PaO2/FIO2 as a function of treatment groups. A, Ratio determined as reported in Materials and Methods for untreated (normal) WKY rats and WKY isograft recipients posttransfer of serum or purified IgG. Data represent mean ± SEM of n = 3–4 rats in each group. *, p < 0.01 and **, p < 0.001. B, Histology of isograft lungs posttransfer of purified IgG depleted of anti-col(V) Abs. Data are representative of n = 3 rats per group. magnification with H&E stain is ×20.
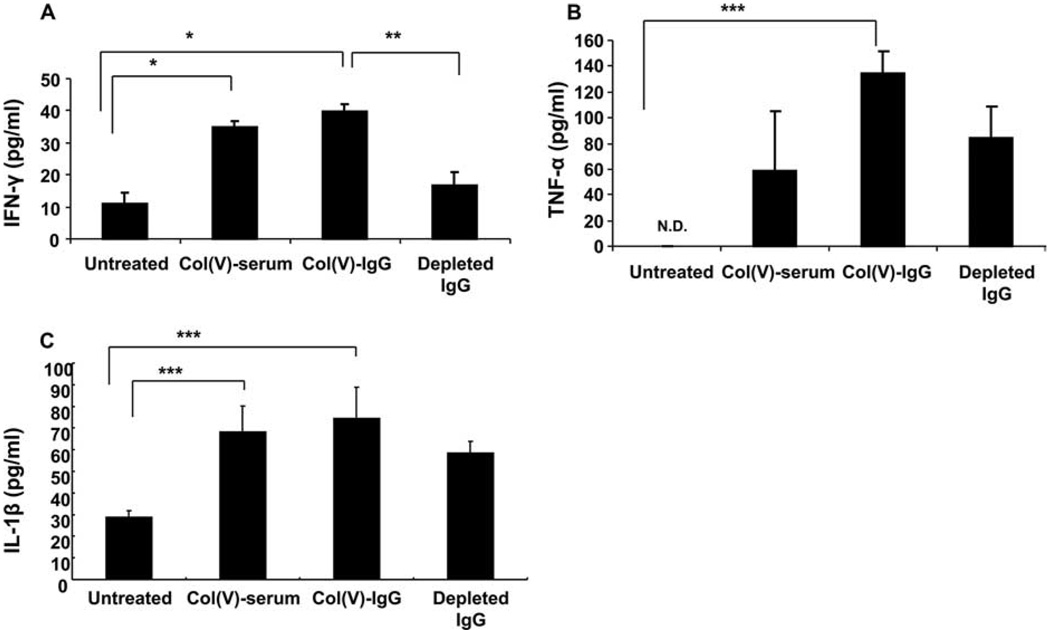
We have reported that clinical PGD that was associated with col(V)-reactive T cells resulted in up-regulation of IFN-γ, TNF-α, and IL-1β (9). To determine whether anti-col(V) humoral immunity was associated with a similar cytokine profile, we quantified these cytokines in BAL fluid 3 days after isograft transplantation. Although modest levels of IFN-γ were detected in controls, transfer of immune serum or purified IgG each significantly elevated IFN-γ levels (p < 0.001 for each) (Fig. 5A), whereas depletion of anti-col(V) Abs significantly reduced local production of IFN-γ compared with other passive transfer groups (p < 0.01) to levels similar to those of untreated isograft recipients. Similarly, transfer of col(V) immune serum or IgG purified from immune serum significantly induced TNF-α expression in BAL fluid (p < 0.05) compared with untreated isograft recipients, which had no detectable TNF-α. Depleting anti-col(V) Abs from serum before transfer lowered the local levels of TNF-α (Fig. 5B), but did not abrogate the increase in IL-1β induced by the transfer of anti-col(V) immune serum or purified IgG (p < 0.05 for each) (Fig. 5C).
FIGURE 5.
Cytokine profiles in BAL fluid. Cytokine IFN-γ (A), TNF-α (B), and IL-1β (C) levels in BAL fluid in untreated isograft recipients and isograft recipients passive posttransfer of serum or IgG preparations. Data represent mean ± SEM of n = 3 rats in each group. *, p < 0.001; **, p < 0.01; and ***, p < 0.05. N.d., Not detected.
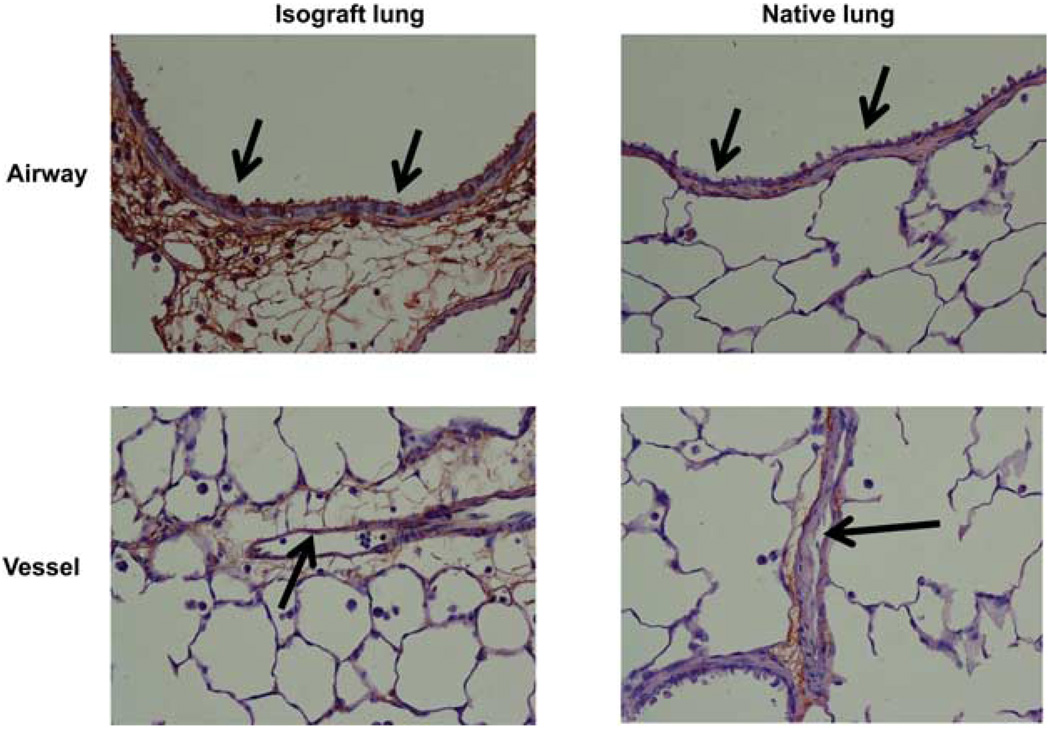
We reported previously that col(V) is considered a sequestered Ag in the lung interstitium, but that it becomes highly exposed after ischemia reperfusion injury in the transplanted lung (2, 11). Although endothelial injury is an established component in PGD, very recent studies report that epithelial cell injury may occur within 4 h of postreperfusion in lung transplant recipients, and that this pathologic process may be more closely associated with morbidity than endothelial cell injury in PGD (15). Our prior data demonstrating that anti-col(V) immunity is associated with PGD suggest that col(V) expression by lung epithelial may explain why these cells are a target of attack during PGD. However, there are no studies reporting that col(V) is expressed by lung epithelial cells in situ. To address this question, we used immunohistochemistry to detect col(V) expression in native and isograft lungs. Notably, col(V) was expressed strongly on the apical surface of the bronchiolar epithelium in lung isografts (Fig. 6, see arrows showing brown deposits), but such staining was detected inconsistently in the native lung. As expected, col(V) was detected in the sub-epithelial, subendothelial, and other interstitial tissues (Fig. 6). We did not detect staining for col(V) on endothelial cells (Fig. 6).
FIGURE 6.
Immunohistochemical staining for col(V) in native and isograft lungs. Native and isograft lungs were harvested from WKY rats 4 days posttransplantation and stained for col(V) performed as described in Materials and Methods. Arrows indicate airway epithelium or vascular endothelium. Col(V) staining identified by brown. Data are representative of n = 3 rats per group. Magnification is ×40.
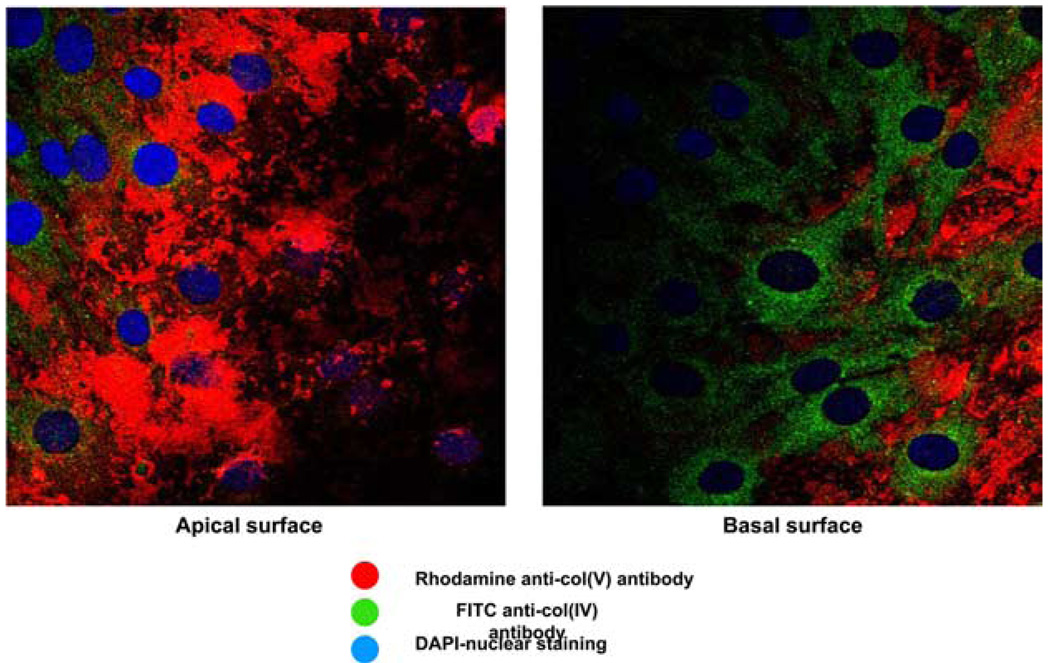
To address the potential relevance of the in vivo immunostaining described above, we next determined whether primary rat alveolar epithelial cells express col(V), and whether the distribution is either apical or basal. Fig. 7 shows col(V) staining on lung epithelial cells, but not lung endothelial cells. Moreover, confocal imaging confirms the apical location of col(V) on the airway cells (Fig. 7 and supplemental video).6 Notably, the epithelial cells did not express type I collagen, a major lung collagen.
FIGURE 7.
Confocal microscopy expression of col(V) on apical but not basal surface of airway epithelial cells. Primary rat airway epithelial cells were stained for col(V) (Rhodamine red), type IV collagen (col(IV) FITC, green), and nuclei identified by DAPI (blue) staining. Confocal imaging was used to scan sections from apical (left) to basal (right) surface as shown. Data are representative of three separate experiments with similar results.
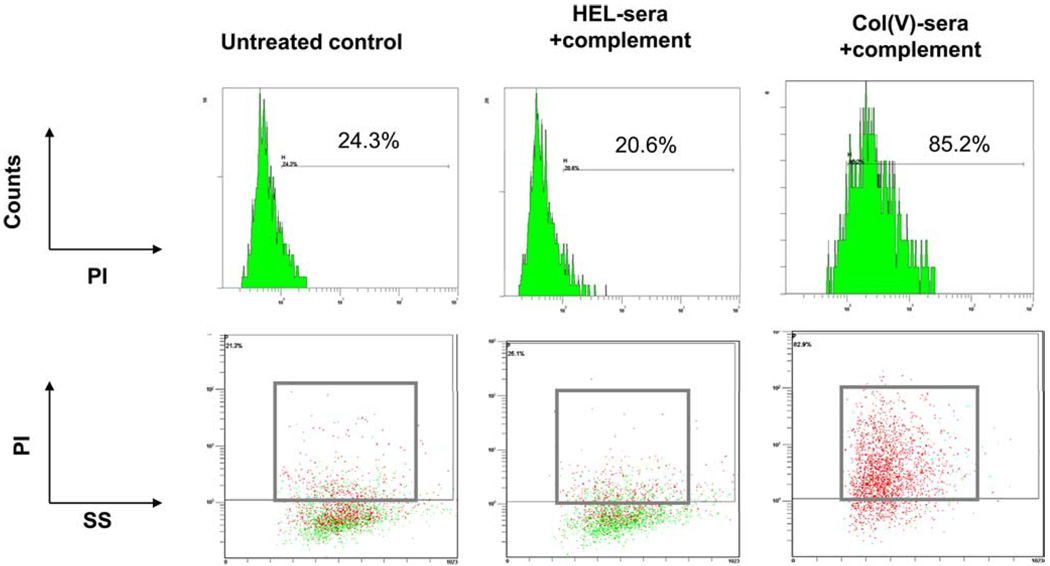
Data in the current study demonstrating that anti-col(V) Abs induce lung injury in vivo and that epithelial cells express col(V) suggest that col(V) immune serum could induce cytotoxicity in lung epithelial cells. To address this question, we incubated rat lung epithelial cells with col(V) or HEL immune serum, in the presence or absence of complement followed by an assessment of cytotoxicity. Our data show that col(V), but not HEL, immune serum induced complement-dependent cytotoxicity (Fig. 8). Lung endothelial cells, which do not express col(V), were not susceptible to Ab-induced cytotoxicity (data not shown). Ab-mediated injury may also be associated with induction of apoptosis in target cells. However, caspase-3/7 activity, a marker of apoptosis, was not significantly increased in cells undergoing Ab-mediated cytotoxicity (data not shown).
FIGURE 8.
Col(V) immune serum induction of complement-dependent cytotoxicity in airway epithelial cells. After growing to confluence, rat airway epithelial cells were incubated with HEL or col(V) immune serum in the presence or absence complement. After an incubation period, cells were stained with propidium iodide (PI) and analyzed by examined by flow cytometry to detect dead cells (propidium iodide-positive) by side scatter light (SS) analysis. Data are representative of two experiments with similar results.
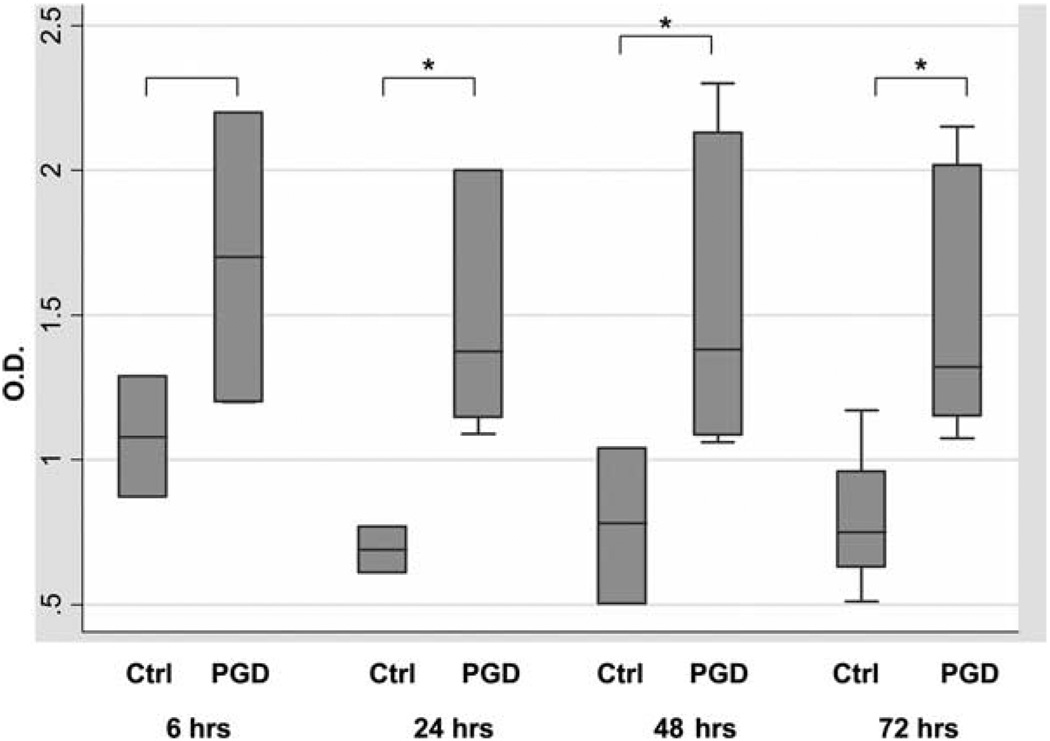
To determine the relevance of these findings in clinical lung transplantation, we examined plasma from patients with PGD hours after reperfusion for the presence of anti-col(V) Ab and compared results with patients with no PGD. The presence of higher levels of anti-col(V) Ab was strongly associated with PGD; for all time points combined, the median OD at was 1.38 (OD range 1.06 –2.30) in PGD subjects vs 0.76 (range 0.36 –1.29) in non-PGD controls (p < 0.001) and p ≤ 0.05 for each individual time point comparison beyond 24 h (Fig. 9 and Table I). Humoral responses to any Ag require several weeks to develop. Therefore, data showing detection of serum IgG anti-col(V) Abs within 24 h posttransplant in patients with PGD indicates the presence of preformed anti-col(V) Abs, and possibly activation of col(V)-specific memory B cells in the recipient.
FIGURE 9.
Circulating Ab levels in patients with PGD and in non-PGD controls during the first 3 days after lung transplant. Box plots represent medians with intraquartile ranges, with whiskers representing full ranges. *, p ≤ 0.05 for the comparison at each time point between PGD and non-PGD. p < 0.001 for comparisons across all time points.
Table I.
The median OD and range of anti-col(V) Aba
| Anti-col(V) ELISA OD | |||
|---|---|---|---|
| Time (h) | PGD | non-PGD | p Valueb |
| 6 | 1.70 (1.20, 2.20) | 1.08 (0.87, 1.29) | 0.25 |
| 24 | 1.37 (1.10, 2.00) | 0.69 (0.61, 0.77) | 0.05 |
| 48 | 1.38 (1.06, 2.30) | 0.78 (0.50, 1.04) | 0.02 |
| 72 | 1.32 (1.08, 2.15) | 0.75 (0.51, 1.17) | 0.05 |
Median OD and range of anti-col(V) Ab read at 450 nm for each time point, by PGD status.
The p value is derived using the Wilcoxon rank sum test.
Discussion
The key findings of this study are that anti-col(V) Abs induce pathology and physiology consistent with PGD in rat lung isografts in vivo, and that expression of col(V) by alveolar epithelial cells contributes to this process. Furthermore, translational studies demonstrated that preexisting anti-col(V) humoral immunity was associated strongly with PGD in lung transplant recipients. The findings reported in the current study may in fact represent a link between early lung injury and later BOS, perhaps explaining the novel observations of Daud and colleagues (8). In addition, our results and a recent report from Goers et al. (22) highlight the role of autoantibodies in the induction of lung allograft pathology.
Col(V) is considered a minor collagen in the lung and is sequestered within the fibrils of type I collagen, the major pulmonary collagen (23). We have reported previously that cellular immune responses to col(V) are a major risk factor for obliterative bronchiolitis/ BOS (2). Moreover, memory T cell responses to col(V) are also associated with onset of PGD (9). Because Ag-specific T cells may induce Ag-specific B cell immunity, we extended our studies to examine the possible involvement of anti-col(V) Abs in lung posttransplant pathology. Notably, anti-col(V) IgG Abs were readily detected in allograft BAL fluid in patients that developed anti-col(V) cellular immunity. Although specific for the α1 chain of col(V) (data not shown), the same Ag recognized by col(V) reactive T cells (2, 11), detection of these Abs was not associated with acute rejection or BOS during clinical lung transplantation (W. J. Burlingham and D. S. Wilkes, unpublished data).
Based on these studies and our prior report showing that anti-col(V) memory T cell responses are associated with PGD, and that the transplant procedure itself induces exposure of antigenic col(V) (2, 11), we hypothesized that systemic anti-col(V) humoral immunity present in the pretransplant period would be associated with lung injury posttransplantation. Our prior studies showed that col(V) immunization in rats induces vigorous cellular immune responses in regional lymph nodes (11, 16). The current studies extend these observations and demonstrate that systemic humoral immunity also results from the immunization procedure. Data showing that IgG2c, and not IgM, directed against col(V) could be related to the timing of serum collection postimmunization, to the types of adjuvants used for these procedures, or to genetics-dependent immunity in the rats used for these studies. Indeed, Firth et al. (24) reported that type II collagen immunization in rats induces IgG subtype specific responses other than IgG2c.
Ab-mediated injury during lung allograft rejection has been associated with complement activation, as evidenced by deposition of C4d on the endothelium and epithelium in animal models; and on the endothelium of human lung allografts (25–27). In the current study, Ab-mediated injury induced by passive transfer studies did not result in C4d deposits in the vascular endothelium, a finding that is consistent with our inability to detect col(V) by immunostaining in primary rat endothelial cells. Although lung epithelial cells express col(V) in vivo, and in vitro, and col(V) immune serum induces complement-dependent cytotoxicity in L2 cells, we did not consistently detect C4d deposits in our passive transfer studies (data not shown). Because complement activation could result in time-dependent complement deposition in tissues, then future studies will determine whether C4d deposits occurred either earlier or later passive posttransfer. However, the current studies do not exclude a role for immune complex-mediated injury and this question will be addressed in future studies.
Due to its associated morbidity and mortality, and lack of clear etiology, the pathogenesis of PGD remains an area of intense investigation. Our recent study and the current report support a major role for col(V) autoimmunity in this process. Furthermore, the current findings highlight the importance of col(V) location within the lung, in areas readily accessible to attack by the immune system. The interstitial distribution of col(V) has been well documented (2, 11). We believe a novel finding of the current study is the demonstration of col(V) expression on the apical surface of primary rat lung epithelial cells. This finding was not limited to this epithelial cell type, as we also observed apical col(V) expression in H441 cells, which are derived from human Clara cells (our unpublished observations). Interestingly, we did not detect col(V) staining in human or rat endothelial cells. The differential expression of col(V) could have implications in the PGD pathogenesis. For example, PGD is associated with varying degrees of noncardiogenic pulmonary edema suggesting endothelial cell dysfunction. However, a recent study demonstrated that increases in serum RAGE (Receptor for Advanced Glycation End-products), a marker of alveolar type I epithelial cells, but not endothelial cells, was associated with PGD (15). These data are consistent with findings in the current study showing that serum anti-col(V) Abs induced cytotoxicity in lung epithelial, but not endothelial cells. However, it is important to note that the relationship of systemic anti-col(V) Abs and epithelial cell expression of this Ag does not rule out a key role for endothelial cells in PGD. It is conceivable that the loss of endothelial cell integrity must occur first before serum rich in anti-col(V) Abs would have access to the epithelial surface where they could induce further injury.
In our prior study reporting the association of col(V)-specific memory T cells and PGD, we demonstrated that proinflammatory cytokines such as IL-1β and TNF-α were increased in both human lung transplant recipients as well as our rat model that mimics the human condition (9). IFN-γ, classically associated with delayed type hypersensitivity, was increased as well. Similar findings were reported in the current study, passive posttransfer of immune serum. Because immune cells were not present in the immune serum, the induction of cytokines is likely related, at least in part, to the Fc portion of the anti-col(V) Abs binding to and activating local immune cells. The specific mechanisms involved in this process are the subject of ongoing studies.
The risk factors for PGD have been reported extensively, and recent studies have refined the definition of this serious disease (4, 28, 29). However, a specific mechanism for PGD has been elusive but likely includes elements of the complement cascade, coagulation factors, and other pathways either acting alone or in concert to induce lung injury. Indeed our prior study invokes T cell mediated immunity to col(V) in PGD in clinical lung transplantation (9). In the current report we extend our human translational studies by examining a carefully matched phenotype representing the severest form of PGD. Future studies aimed at examining the clinical utility of autoimmunity to col(V) will include patients with the full spectrum of lung injury, and will focus on pretransplant samples not available in the current study. Our prior and current studies implicate recipient-derived cellular and humoral autoimmunity to col(V) collectively in the development of clinical PGD. However, these data do not exclude other pathways in PGD pathogenesis. Although the sample size of the patients reported in the current study is relatively small, the finding that anti-col(V) Abs were higher in all PGD cases is striking, as the PGD and non-PGD subjects had no overlap. Furthermore, we do not believe that preexistent clinical factors confounded our results, as an equal number of patients with COPD, IPF, and IPAH were represented in each group, and subjects who underwent cardiopulmonary bypass or had prolonged ischemic times were excluded from our analysis (28, 30). We therefore believe these data may have profound implications for the risk stratification and potential management or treatment of PGD. Before the significance of these findings are fully understood, ongoing studies will need to determine whether specific lung diseases are associated with anti-col(V) Abs, the titer of these Abs and their relationship to grade of PGD, and whether other autoimmune phenomena, other than col(V) reactivity are associated with PGD, by focusing on pretransplant samples not available in the current study. The answer to these questions may also yield insights into the pathogenesis of COPD, IPF, and pulmonary hypertension, which are the major indications for lung transplantation. Indeed, there have been recent reports of a potential role for autoimmunity in the pathogenesis of emphysema (31) and IPAH (32) (formerly known as primary pulmonary hypertension).
Although anti-col(V) Abs induced lung injury in the current study, the interpretation of these results is limited by the fact that rats do not spontaneously develop anti-col(V) Abs, but did so after immunization. However, anti-col(V) preformed Abs could participate in PGD pathogenesis. Because Ag-specific Ab production requires several weeks to develop after Ag exposure, then data showing detection of anti-col(V) Abs in the serum of patients within 6 h after onset of PGD suggests the presence of these Abs in the pretransplant period. In addition, these data also suggest that certain lung diseases that are indications for lung transplantation may be associated with production anti-col(V) Abs. Validation of our findings in other clinical populations may lead to targeted therapy trials of tolerance induction strategies in subjects with preexisting autoimmunity, and not limited to col(V).
Identifying the Ag that triggers an immune response creates the opportunity to develop strategies to suppress this process or induce immune tolerance. We reported that acute and chronic lung allograft rejection could be prevented by col(V)-induced oral tolerance in our rat models (17, 33). Those studies were focused on examining regulation of cellular (T cell-mediated) immunity. Suppressing an Ag-specific T cell response is also likely to down-regulate humoral immunity to the same Ag. Therefore, we hypothesize that targeting sensitized lung transplant candidates with col(V)-based immunomodulation strategies in the pretransplant period may minimize the risk of PGD onset. Such techniques could potentially involve pretransplantation col(V)-induced tolerance, plasmapheresis, and B cell depletion. These approaches could also be combined with other strategies that may be involved in PGD such as targeting phagocytes or coagulation cascades, and immune modulation. These questions will be addressed in both ongoing preclinical and clinical studies.
Footnotes
This work was supported by Grants HL081350, HL60797, and HL/Al67177 (to D.S.W.), HL 077328 (to I.P.), HL087115 and HL081619 (to J.D.C.), AI048624 and AI066219 (to W.B.), and GM71679 and AR53815 (to D.S.G.) from the National Institutes of Health, and a Department of Veterans Affairs Research grant (to D.B.B.).
Abbreviations used in this paper: BOS, bronchiolitis obliterans syndrome; PGD, primary graft dysfunction; col(V), type V collagen; HEL, hen egg lysozyme; BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis; IPAH, idiopathic pulmonary arterial hypertension.
The online version of this article contains supplemental material.
Disclosures
D.S.W. is Founder of ImmuneWorks, LLC.
References
- 1.Arcasoy SM, Kotloff RM. Lung transplantation. N. Engl. J. Med. 1999;340:1081–1091. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 2.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J. Clin. Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christie JD, Bavaria JE, Palevsky HI, Litzky L, Blumenthal NP, Kaiser LR, Kotloff RM. Primary graft failure following lung transplantation. Chest. 1998;114:51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 4.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction. Part II: Definition. A consensus statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2005;24:1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 5.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Mohacsi PJ, Keck BM, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twentieth Official Adult Lung and Heart-Lung Transplant Report 2003. J. Heart Lung Transplant. 2003;22:625–635. doi: 10.1016/s1053-2498(03)00182-7. [DOI] [PubMed] [Google Scholar]
- 6.Christie JD, Sager JS, Kimmel SE, Ahya VN, Gaughan C, Blumenthal NP, Kotloff RM. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127:161–165. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 7.Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, DeMissie E, Kimmel SE. The effect of primary graft dysfunction on survival after lung transplantation. Am. J. Respir. Crit. Care Med. 2005;171:1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am. J. Respir. Crit. Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 9.Bobadilla JL, Love RB, Jankowska-Gan E, Xu Q, Haynes LD, Braun RK, Hayney MS, Munoz Del Rio A, Meyer K, Greenspan DS, et al. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. Am. J. Respir. Crit. Care Med. 2008;177:660–668. doi: 10.1164/rccm.200612-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau CL, Palmer SM, Posther KE, Howell DN, Reinsmoen NL, Massey HT, Tapson VF, Jaggers JJ, D’Amico TA, Davis RD., Jr Influence of panel-reactive antibodies on posttransplant outcomes in lung transplant recipients. Ann. Thorac. Surg. 2000;69:1520–1524. doi: 10.1016/s0003-4975(00)01224-8. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, Baldridge LA, Heidler KM, Cummings OW, Fujisawa T, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am. J. Transplant. 2006;6:724–735. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 12.Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, Woods K, Smith GN, Cummings OW, Heidler KM, et al. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J. Immunol. 2002;169:1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 13.Federspiel SJ, DiMari SJ, Howe AM, Guerry-Force ML, Haralson MA. Extracellular matrix biosynthesis by cultured fetal rat lung epithelial cells. III. Effects of chronic exposure to epidermal growth factor on growth, differentiation, and collagen biosynthesis. Lab. Invest. 1991;64:463–473. [PubMed] [Google Scholar]
- 14.Pinsky DJ. The vascular biology of heart and lung preservation for transplantation. Thromb. Haemost. 1995;74:58–65. [PubMed] [Google Scholar]
- 15.Calfee CS, Budev MM, Matthay MA, Church G, Brady S, Uchida T, Ishizaka A, Lara A, Ranes JL, deCamp MM, Arroliga AC. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J. Heart Lung Transplant. 2007;26:675–680. doi: 10.1016/j.healun.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiyo M, Iwata T, Webb TJ, Vasko MR, Thompson EL, Heidler KM, Cummings OW, Yoshida S, Fujisawa T, Brand DD, Wilkes DS. Silencing S1P1 receptors regulates collagen-V reactive lymphocyte-mediated immunobiology in the transplanted lung. Am. J. Transplant. 2008;8:537–546. doi: 10.1111/j.1600-6143.2007.02116.x. [DOI] [PubMed] [Google Scholar]
- 17.Yasufuku K, Heidler KM, Woods KA, Smith GN, Jr, Cummings OW, Fujisawa T, Wilkes DS. Prevention of bronchiolitis obliterans in rat lung allografts by type V collagen-induced oral tolerance. Transplantation. 2002;73:500–505. doi: 10.1097/00007890-200202270-00002. [DOI] [PubMed] [Google Scholar]
- 18.Thabut G, Vinatier I, Brugiere O, Leseche G, Loirat P, Bisson A, Marty J, Fournier M, Mal H. Influence of preservation solution on early graft failure in clinical lung transplantation. Am. J. Respir. Crit. Care Med. 2001;164:1204–1208. doi: 10.1164/ajrccm.164.7.2012135. [DOI] [PubMed] [Google Scholar]
- 19.Thabut G, Vinatier I, Stern JB, Leseche G, Loirat P, Fournier M, Mal H. Primary graft failure following lung transplantation: predictive factors of mortality. Chest. 2002;121:1876–1882. doi: 10.1378/chest.121.6.1876. [DOI] [PubMed] [Google Scholar]
- 20.Christie JD, Robinson N, Ware LB, Plotnick M, De Andrade J, Lama V, Milstone A, Orens J, Weinacker A, Demissie E, et al. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am. J. Respir. Crit. Care Med. 2007;175:69–74. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, Kimmel SE. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124:1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 22.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-α1 tubulin-specific antibodies: role in chronic lung allograft rejection. J. Immunol. 2008;180:4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linsenmayer TF, Gibney E, Igoe F, Gordon MK, Fitch JM, Fessler LI, Birk DE. Type V collagen: molecular structure and fibrillar organization of the chicken α1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J. Cell Biol. 1993;121:1181–1189. doi: 10.1083/jcb.121.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firth SA, Morgan K, Evans HB, Holt PJ. IgG subclasses in collagen-induced arthritis in the rat. Immunol. Lett. 1984;7:243–247. doi: 10.1016/0165-2478(84)90029-4. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima S, Qian Z, Rahimi S, Wasowska BA, Baldwin WM., III Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J. Immunol. 2002;169:4620–4627. doi: 10.4049/jimmunol.169.8.4620. [DOI] [PubMed] [Google Scholar]
- 26.Magro CM, Klinger DM, Adams PW, Orosz CG, Pope-Harman AL, Waldman WJ, Knight D, Ross P., Jr Evidence that humoral allograft rejection in lung transplant patients is not histocompatibility antigen-related. Am. J. Transplant. 2003;3:1264–1272. doi: 10.1046/j.1600-6143.2003.00229.x. [DOI] [PubMed] [Google Scholar]
- 27.Magro CM, Pope Harman A, Klinger D, Orosz C, Adams P, Waldman J, Knight D, Kelsey M, Ross P., Jr Use of C4d as a diagnostic adjunct in lung allograft biopsies. Am. J. Transplant. 2003;3:1143–1154. doi: 10.1034/j.1600-6143.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 28.Whitson BA, Nath DS, Johnson AC, Walker AR, Prekker ME, Radosevich DM, Herrington CS, Dahlberg PS. Risk factors for primary graft dysfunction after lung transplantation. J. Thorac. Cardiovasc. Surg. 2006;131:73–80. doi: 10.1016/j.jtcvs.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 29.Barr ML, Kawut SM, Whelan TP, Girgis R, Bottcher H, Sonett J, Vigneswaran W, Follette DM, Corris PA. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part IV: recipient-related risk factors and markers. J. Heart Lung Transplant. 2005;24:1468–1482. doi: 10.1016/j.healun.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Christie J, Keshavjee S, Orens J, Arcasoy S, DePerrot M, Barr M, Van Raemdonck D. Potential refinements of the International Society for Heart and Lung Transplantation primary graft dysfunction grading system. J. Heart Lung Transplant. 2008;27:138. doi: 10.1016/j.healun.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 31.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Cogswell S, Storness-Bliss C, Corry DB, Kheradmand F. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat. Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 32.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur. Respir. J. 2005;26:1110–1118. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 33.Yasufuku K, Heidler KM, O’Donnell PW, Smith GN, Jr, Cummings OW, Foresman BH, Fujisawa T, Wilkes DS. Oral tolerance induction by type V collagen downregulates lung allograft rejection. Am. J. Respir. Cell Mol. Biol. 2001;25:26–34. doi: 10.1165/ajrcmb.25.1.4431. [DOI] [PubMed] [Google Scholar]