INTRODUCTION
Anemia is the most common hematological complication in HIV-infected adults[1,2] and is positively associated with disease progression[3-5]. In adults anemia results primarily from reduced erythropoiesis[6-9]. Information about anemia mechanisms in HIV-infected children is scarce[10-13] and there have been no pediatric studies from sub-Saharan Africa.
We have previously reported that HIV infection was more common among severely anemic Malawian children than in a carefully selected control population (13% vs. 6%, p<0.001)[14]. The aim of the present study was to determine if HIV infection was associated with reduced erythroid precursor cells, or increased rates of apoptosis and dyserythropoiesis, and to investigate the role of cytokines, erythropoietin and plasma vitamin A in reducing apoptosis.
MATERIALS&METHODS
This study was part of a large case-control study investigating the etiology of severe anemia in southern Malawi[14]. All children aged 6-60 months with a primary diagnosis of severe anemia, (hemoglobin concentration<5g/dl) and no blood transfusion within the previous month were recruited prospectively between 2002 and 2004. HIV-uninfected children aged 6-60 months with no obvious signs of infection and undergoing elective operations were recruited as controls.
An automated full blood count, including reticulocytes, was performed on peripheral blood samples (Beckman Coulter, South Africa). Malaria slides were read by two independent microscopists. Stained bone marrow aspirate smears from all children were used to determine the myeloid:erythroid ratio[15] and assess dyserythropoiesis, which was defined and scored according to a published protocol[16].
C-reactive protein (CRP) and erythropoietin were determined using a Roche p800/e170 system (Roche, Switzerland). Inflammatory cytokine profiles were measured by Cytometric Bead Array flow cytometry (FACS-Calibur, BD Biosciences, USA). Serum vitamin A (retinol) was measured using high performance liquid chromatography[17]. HIV testing was performed using two rapid tests (Determine, Abbott-Laboratories, Japan; Unigold, Trinity-Biotech, Ireland). Reactive results in children less than 18 months and discordant results were resolved by PCR[18].
Fresh bone marrow aspirates underwent automated cell count (Coulter counter) and four color flow cytometry (FACS-Calibur, BD Biosciences, USA). Bone marrow cells were separated and incubated with different combinations of: CD14-PE-Cy5 (Tük4), CD34-FITC/PE (QBEND/10), CD36-PE (CLB-IVC7), CD235a-FITC (CLB-AME-1) (Sanquin Reagents, The Netherlands), Laser Dye Styril-751 (LDS, Applied Laser Technology, The Netherlands), and Annexin-V and Propidium-iodide (IQ-products, The Netherlands)[19]
Patient characteristics and hematological variables were compared using Chi-square and Fisher exact test, student t and Mann-Whitney U-tests. Correlations were assessed using Pearson or Spearman correlation coefficients. A two-sided significance level was set at p=0.05.
RESULTS
Complete data (bone marrow samples and HIV tests) for this study were available for 329 of 381 children enrolled in our original case-control study. The original study had shown that bacteremia, malaria, hookworm, HIV, G6PD, and vitamin A and B12 deficiency were associated with severe anemia. Iron deficiency was negatively associated with severe anemia. Folate deficiency and sickle cell disease were uncommon[14].
Forty of the 329 children (12%) were infected with HIV. Their median age was 25 compared to 16 months for HIV-uninfected children (p<0.01). No significant differences were found between HIV-infected and uninfected children with regard to other baseline characteristics, mean hemoglobin levels (p=0.67) or other erythrocytic indices (Table1).
Table1.
Characteristics and Hematological parameters in HIV-infected and uninfected children with severe anemia and a control population of children without HIV infection or severe anemia.
| HIV+ n=40 |
HIV− n=289 |
p | Control n=18 |
||
|---|---|---|---|---|---|
| CHARACTERISTICS | |||||
|
| |||||
| Age median, IQR in months |
24.9 (15.6-38.4) |
15.8 (10.2-25.5) |
<0.01 | 24.0 (11.3-32.8) |
|
|
| |||||
| Boys | 17/40 (43%) |
144/289 (50%) |
0.39 | 15/18 (83%) |
|
|
| |||||
| Prior transfusion | 7/40 (18%) |
41/287 (14%) |
0.59 | 0/18 (0%) |
|
|
| |||||
| Wasting | 6/33 (18%) |
37/261 (14%) |
0.54 | 3/12 (25%) |
|
|
| |||||
| Iron deficiency | 5/21 (24%) |
34/155 (22%) |
0.85 | 4/12 (33%) |
|
|
| |||||
| Malaria parasitemia | 23/39 (59%) |
170/289 (59%) |
0.99 | 2/17 (12%) |
|
|
| |||||
| CRP median, IQR in mg/L |
117 (47-193) n=38 |
95 (42-153) n=269 |
0.73 | 2.5 (1.8-4.4) n=18 |
|
|
| |||||
|
| |||||
| AUTOMATED COUNT | |||||
|
| |||||
| Hemoglobin concentration mean ±SD in g/dL |
3.6 ±0.7 n=40 |
3.6 ±0.8 n=289 |
0.67 | 9.7 ±1.8 n=18 |
|
|
| |||||
| MCV mean ±SD in fL |
81.1 ±13.7 n=35 |
83.3 ±15.6 n=247 |
0.27 | 71.6 ±7.1 n=16 |
|
|
| |||||
| MCHC mean ±SD in g/dL |
32.4 ±3.1 n=35 |
32.5 ±7.2 n=245 |
0.68 | 33.0 ±3.6 n=16 |
|
|
| |||||
| RDW mean ±SD in % |
25.2 ±8.7 n=35 |
24.4 ±7.4 n=246 |
0.50 | 18.0 ±3.8 n=16 |
|
|
| |||||
| Reticulocytes median and IQR in 109/L |
58.6 (30.3-88.2) n=32 |
52.7 (30.2-91.7) n=209 |
0.85 | 70.7 (54.3-117.9) n=13 |
|
|
| |||||
|
| |||||
| LIGHT MICROSCOPY | |||||
|
| |||||
| MYELOID:ERYTHROID RATIO | |||||
| Decreased (<2.0:1) | 29/34 (85%) |
194/261 (74%) |
0.38 | 4/18 (22%) |
|
| Normal (2.0-4.9:1) | 4/34 (12%) |
54/261 (21%) |
10/18 (56%) |
||
| Increased (≥;5.0:1) | 1/34 (3%) |
13/261 (5%) |
4/18 (22%) |
||
|
| |||||
| ERYTHROID CELLS | |||||
| Pro-erythroblasts median and IQR in % of nucleated cells |
0.8 (0.0-1.6) n=34 |
0.4 (0.0-1.5) n=261 |
0.33 | 0.0 (0.0-0.8) n=18 |
|
| Basophilic erythroblast median and IQR in % of nucleated cells |
0.8 (0.0-2.4) n=34 |
0.8 (0.0-1.6) n=261 |
0.55 | 0.8 (0.0-1.6) n=18 |
|
| Ortho & Polychromatic erythroblast mean ±SD in % of nucleated cells |
37 ±15 n=34 |
36 ±16 n=261 |
0.69 | 18.8 ±12.3 n=18 |
|
|
| |||||
| DYSERYTHROPOIESIS | |||||
| Dyserythropoietic cells mean ±SD in % of erythrocytic precursors |
2.8 ±2.2 n=25 |
3.8 ±3.0 n=213 |
0.12 | 1.2 ±1.6 (n=13) |
|
|
| |||||
|
| |||||
| COULTER COUNTER | |||||
|
| |||||
| CELLULARITY | |||||
| Nucleated bone marrow cells median and range in 109/L |
62.2 (42.6-108.3) n=32 |
76.6 (45.6-119.6) n=246 |
0.37 | 91.6 (61.2-114.0) n=16 |
|
|
| |||||
|
| |||||
| FLOW CYTOMETRY | |||||
|
| |||||
| CELLS | |||||
| All CD34+ hematopoietic progenitors median and IQR in 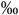 of ofmononucleated fraction |
CD34+ | 10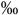 (5-20 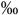 ) )n=34 |
15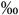 (7-30  ) )n=242 |
0.044 | 4.4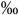 (2.8-8.1 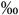 ) )n=17 |
| Erythroid progenitor cells median and IQR in 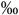 of ofmononucleated fraction |
CD34+ CD36+ CD14− |
2.2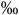 (0.8-4.4 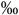 ) )n=27 |
3.4 (1.5-6.6 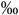 ) )n=210 |
0.05 | 0.5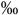 (0.4-0.7 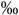 ) )n=16 |
| Erythroid precursor cells median and IQR in % of mononucleated fraction |
CD235+ LDS+ |
17.9% (13.0-30.8%) n=35 |
25.6% (14.9-38.3%) n=248 |
0.06 | 12.4% (10.9-18.6%) n=17 |
|
| |||||
|
APOPTOSIS OF ERYTHROID
PRECURSORS |
|||||
| Viable cells median and IQR |
CD235+ Annexin− PI− |
87% (73-94%) n=15 |
85% (68-91%) n=78 |
0.25 | 81.7% (65-96%) n=15 |
| Early apoptotic median and IQR |
CD235+ Annexin+ PI− |
9.3% (4.4-19.8%) n=15 |
12.1% (6.3-22.6%) n=78 |
0.23 | 14.7% (3.4-30.1%) n=15 |
| Late apoptotic median and IQR |
CD235+ Annexin+ PI+ |
2.1% (1.2-4.9%) n=15 |
2.6% (1.0-5.5%) n=78 |
0.67 | 1.8% (0.3-2.7%) n=15 |
Wasting was defined as a weight for height Z-score of less than −2[20]. Dyserythropoiesis was defined as: (a) multinuclearity; (b) karyorrhexis; (c) intercellular chromatin bridging; and (d) incomplete mitoses. Early apoptosis refers to the expression of Phosphatidylserine only, whilst in late apoptosis also Propidium iodide was detected. In viable cells neither of these dies were detected[19]. IQR: Inter-Quartile Range, CRP: C-Reactive Protein, MCV: Mean Corpuscular Volume; MCHC: Mean Corpuscular Hemoglobin Concentration; RDW: Red cell Distribution Width., SD: Standard Deviation. LDS: Laser Dye Styril-751, stains DNA, PI: Propidium Iodide.
HIV-infected children had fewer bone marrow CD34+ hematopoietic progenitors, erythroid progenitor cells and erythroid precursor cells than HIV-uninfected children, but numbers of bone marrow pro-erythroblasts, basophilic erythroblasts and polychromatic erythroblasts, and peripheral blood reticulocytes were similar (Table1). Correction for age or malaria did not alter the results (data not displayed).
Dyserythropoiesis occurred in 2.8% and 3.8% of erythroid precursors in HIV-infected and uninfected children respectively (p=0.12, Table1). The proportions of viable erythroid precursor cells and those at various stages of apoptosis were similar between the two groups (Table1). The proportions of dyserythropoietic cells and red cells undergoing early apoptosis were positively correlated (r= 0.34, p=0.01). There were no correlations (range r=−0.14 – +0.15) between the proportion of either dyserythropoietic or apoptotic cells and the peripheral blood concentrations of cytokines TNF-α (p=0.90 and 0.28), IFN-γ (p=0.15 and 0.36), IL-10 (p=0.74 and 0.19), erythropoietin (p=0.22 and 0.83), or vitamin A (p=0.83 and 0.22).
DISCUSSION
This study is the first detailed prospective analysis of erythropoiesis using bone marrow samples and flow cytometry in HIV-infected children. HIV-infected children with severe anemia had 33% fewer CD34+ hematopoietic progenitors and 35% less erythroid progenitors in their bone marrow than uninfected children. This supports the hypothesis that red cell production failure is an important cause of severe anemia in HIV-infected children and may be caused by a reduced stem cell capacity[21]. However the proportion of more mature erythroid precursor cells in bone marrow or peripheral blood (reticulocytes) did not differ between the two groups, suggesting that HIV-uninfected children had less efficient later stages of erythropoiesis than HIV-infected children. This is supported by the trend towards less dyserythropoiesis and apoptosis in HIV-infected children, but is in contrast to previous reports suggesting that anemia due to dyserythropoiesis is more common in later stages of HIV disease[2,10]. Alternatively the lost CD34 cells in HIV-infected children may have been precursors that were not committed to erythropoiesis.
HIV infection affects hematopoietic processes[22] possibly through abnormal expression of cellular genes and cytokines. The African HIV-IC subtype can directly infect CD34+ hematopoietic progenitors[23]. Unlike previous studies[24,25] we found no association between dyserythropoiesis or apoptosis and altered cytokine levels or vitamin A deficiency[26,27], despite 90% of children having vitamin A deficiency[14]. More intensive investigations might identify cytokines that affect regulatory signals and could potentially be therapeutic targets to reduce hemopoietic inhibition in HIV patients.
In common with previous studies we did not find any differences in peripheral blood erythrocytic indices or bone marrow microscopy in HIV-infected compared to uninfected children[6-8,10,28], possibly because of the multi-factorial etiology of anemia in African children[14,29].
None of the children were on anti-retroviral therapy, which can exacerbate blood and bone marrow abnormalities[30]. Although not all tests were done on all children the large sample size increases confidence that the study sample was representative.
The findings in these severely anemic Malawian children indicate that despite an HIV-associated reduction in early red-cell precursors, subsequent erythropoiesis appears to proceed similarly in HIV-infected and HIV-uninfected children with severe anemia.
ACKNOWLEDGEMENTS
Funded by the Wellcome Trust and supported by independent grants of the Nutricia Research Foundation and the Ter Meulen Fund, Royal Netherlands Academy of Arts and Sciences.
We thank the parents and guardians of the children admitted to the study, the SEVANA study team, the staff of the Queen Elizabeth Central Hospital, Chikwawa district Hospital and Wellcome Trust Research Laboratories, and in particular, SM Graham, EM Molyneux, M Cornelissen, M Beld, L van Lieshout, FA Wijnberg and WJ van Lüling for their contributions to the study.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Spivak JL, Bender BS, Quinn TC. Hematologic abnormalities in the acquired immune deficiency syndrome. Am J Med. 1984;77(2):224–228. doi: 10.1016/0002-9343(84)90695-8. [DOI] [PubMed] [Google Scholar]
- 2.Zon LI, Arkin C, Groopman JE. Haematologic manifestations of the human immune deficiency virus (HIV) Br J Haematol. 1987;66(2):251–256. doi: 10.1111/j.1365-2141.1987.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13(8):943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 4.Moore RD, Keruly JC, Chaisson RE. Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(1):29–33. doi: 10.1097/00042560-199809010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91(1):301–308. [PubMed] [Google Scholar]
- 6.Bain BJ. The haematological features of HIV infection. Br J Haematol. 1997;99(1):1–8. doi: 10.1046/j.1365-2141.1997.2943111.x. [DOI] [PubMed] [Google Scholar]
- 7.Bain BJ. Pathogenesis and pathophysiology of anemia in HIV infection. Curr Opin Hematol. 1999;6(2):89–93. doi: 10.1097/00062752-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Moses A, Nelson J, Bagby GC., Jr. The influence of human immunodeficiency virus-1 on hematopoiesis. Blood. 1998;91(5):1479–1495. [PubMed] [Google Scholar]
- 9.Volberding PA, Baker KR, Levine AM. Human immunodeficiency virus hematology. Hematology Am Soc Hematol Educ Program. 2003:294–313. doi: 10.1182/asheducation-2003.1.294. [DOI] [PubMed] [Google Scholar]
- 10.Ellaurie M, Burns ER, Rubinstein A. Hematologic manifestations in pediatric HIV infection: severe anemia as a prognostic factor. Am J Pediatr Hematol Oncol. 1990;12(4):449–453. doi: 10.1097/00043426-199024000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Meira DG, Lorand-Metze I, Toro ADC, Silva MTN, Vilela MMDS. Bone marrow features in children with HIV infection and peripheral blood cytopenias. J Trop Pediatr. 2005;51(2):114–119. doi: 10.1093/tropej/fmh096. [DOI] [PubMed] [Google Scholar]
- 12.Mueller BU, Tannenbaum S, Pizzo PA. Bone marrow aspirates and biopsies in children with human immunodeficiency virus infection. J Pediatr Hematol Oncol. 1996;18(3):266–271. doi: 10.1097/00043426-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Sandhaus LM, Scudder R. Hematologic and bone marrow abnormalities in pediatric patients with human immunodeficiency virus (HIV) infection. Pediatr Pathol. 1989;9(3):277–288. doi: 10.3109/15513818909037732. [DOI] [PubMed] [Google Scholar]
- 14.Calis JCJ, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, et al. Factors associated with severe anemia in Malawian children. N Engl J Med. 2008;358(9):888–899. doi: 10.1056/NEJMoa072727. [DOI] [PubMed] [Google Scholar]
- 15.Bain BJ, Clark DM, Lampert IA, Wilkins ES. Bone Marrow Pahtology. Blackwell Science; Oxford: 2007. [Google Scholar]
- 16.Newton CR, Warn PA, Winstanley PA, Peshu N, Snow RW, Pasvol G, et al. Severe anaemia in children living in a malaria endemic area of Kenya. Trop Med Int Health. 1997;2(2):165–178. doi: 10.1046/j.1365-3156.1997.d01-238.x. [DOI] [PubMed] [Google Scholar]
- 17.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979;32(10):2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- 18.Molyneux EM, Walsh AL, Malenga G, Rogerson S, Molyneux ME. Salmonella meningitis in children in Blantyre, Malawi, 1996-1999. Ann Trop Paediatr. 2000;20(1):41–44. doi: 10.1080/02724930092057. [DOI] [PubMed] [Google Scholar]
- 19.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84(5):1415–1420. [PubMed] [Google Scholar]
- 20.Dibley MJ, Goldsby JB, Staehling NW, Trowbridge FL. Development of normalized curves for the international growth reference: historical and technical considerations. Am J Clin Nutr. 1987;46(5):736–748. doi: 10.1093/ajcn/46.5.736. [DOI] [PubMed] [Google Scholar]
- 21.Redd AD, Avalos A, Phiri K, Essex M. Effects of HIV type 1 infection on hematopoiesis in Botswana. AIDS Res Hum Retroviruses. 2007;23(8):996–1003. doi: 10.1089/aid.2006.0283. [DOI] [PubMed] [Google Scholar]
- 22.Koka PS, Reddy ST. Cytopenias in HIV infection: Mechanisms and alleviation of hematopoietic inhibition. Current HIV Research. 2004;2(3):275–282. doi: 10.2174/1570162043351282. [DOI] [PubMed] [Google Scholar]
- 23.Redd AD, Avalos A, Essex M. Infection of hematopoietic progenitor cells by HIV-1 subtype C, and its association with anemia in southern Africa. Blood. 2007;110(9):3143–3149. doi: 10.1182/blood-2007-04-086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellaurie M, Rubinstein A. Elevated tumor necrosis factor-alpha in association with severe anemia in human immunodeficiency virus infection and Mycobacterium avium intracellulare infection. Pediatr Hematol Oncol. 1995;12(3):221–230. doi: 10.3109/08880019509029563. [DOI] [PubMed] [Google Scholar]
- 25.Testa U. Apoptotic mechanisms in the control of erythropoiesis. Leukemia. 2004;18(7):1176–1199. doi: 10.1038/sj.leu.2403383. [DOI] [PubMed] [Google Scholar]
- 26.Herault O, Domenech J, Georget MT, Clement N, Colombat P, Binet C. All-trans retinoic acid prevents apoptosis of human marrow CD34+ cells deprived of haematopoietic growth factors. Br J Haematol. 2002;118(1):289–295. doi: 10.1046/j.1365-2141.2002.03573.x. [DOI] [PubMed] [Google Scholar]
- 27.Zauli G, Visani G, Vitale M, Gibellini D, Bertolaso L, Capitani S. All-trans retinoic acid shows multiple effects on the survival, proliferation and differentiation of human fetal CD34+ haemopoietic progenitor cells. Br J Haematol. 1995;90(2):274–282. doi: 10.1111/j.1365-2141.1995.tb05147.x. [DOI] [PubMed] [Google Scholar]
- 28.Galli L, de MM, Rossi ME, Panza B, Farina S, Vierucci A. Hemochrome parameters during the first two years of life in children with perinatal HIV-1 infection. Pediatr AIDS HIV Infect. 1995;6(6):340–345. [PubMed] [Google Scholar]
- 29.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;131(2S-2):636S–645S. doi: 10.1093/jn/131.2.636S. [DOI] [PubMed] [Google Scholar]
- 30.Harris CE, Biggs JC, Concanon AJ, Dodds A. Peripheral blood and bone marrow findings in patients with acquired immune deficiency syndrome. Pathology. 1990;22(4):206–211. doi: 10.3109/00313029009086664. [DOI] [PubMed] [Google Scholar]


