Abstract
Current literature suggests that the incidence of cardiac involvement by lymphoma as identified by autopsy varies widely, ranging from 8.7% to 20%. Historically, many cases might have been clinically undetected; however, improved imaging techniques increasingly identify cardiac involvement incidentally. In addition, newer agents resulting in improved cancer therapy outcomes might alter the prevalence and location of metastatic deposits to include more unusual disease sites, including intracardiac locations. We present 2 cases and a review of the literature.
Keywords: Bortezomib, Diffuse large B-cell lymphoma, Metastasis, Mycosis fungoides
Introduction
Lymphoma deposits represent 13.6% of metastatic tumors to the heart.1 As imaging modalities and treatment options for lymphoma improve, more unusual disease presentations may be observed more frequently. We present 2 cases of cardiac lymphoma metastases in heavily pretreated patients and a review of the literature.
Case 1
A 58-year-old white man presented with a rapidly growing right-sided submandibular swelling. Biopsy and complete staging confirmed stage IIA diffuse large B-cell lymphoma. He commenced combination therapy with R-CHOP (rituximab plus cyclophosphamide/doxorubicin/vincristine/prednisone) for 8 cycles followed by involved-field radiation therapy. Although in complete remission after therapy, the disease-free interval was only 5 months. He developed a cutaneous recurrence within the previous radiation port. He proceeded to RICE (rituximab/ifosfamide/carboplatin/etoposide) therapy for 3 cycles with high-dose therapy and autologous stem cell support. In addition, he received extended-field radiation therapy and subsequent maintenance rituximab. A further local recurrence swiftly ensued, and he was commenced on gemcitabine and rituximab, but pancytopenia prevented optimal timely administration of therapy. He was instead treated with further electron beam radiation therapy. Disease progression occurred locally while the patient received radiation therapy.
The patient was referred to the National Cancer Institute (NCI) while awaiting an allogeneic transplantation match from the national donor pool. Staging computed tomography identified a 2.1-cm mass anterior to the submandibular gland and a 2.3-cm left ventricular mass (Figure 1). Echocardiography showed a nonobstructing mass lesion attached to the left ventricle (LV) septum with normal LV function, which was confirmed by cardiac magnetic resonance imaging (MRI). He was enrolled on an institutional review board–approved protocol (NCI 03-C-0096) and received bortezomib and EPOCH (infusional etoposide/vincristine plus doxorubicin/cyclophosphamide/prednisone) chemotherapy with complete resolution of the disease, including the cardiac mass, after 4 cycles of therapy. The patient proceeded to an allogeneic matched-unrelated donor hematopoietic stem cell transplantation. This resulted in a 6-month disease-free interval before recurrent disease was diagnosed. The patient died from his disease 1 month later.
Figure 1. Computed Tomography Images at Initial Review (Left) and After 2 Cycles of Bortezomib/EPOCH Therapy (Right) Demonstrating Complete Resolution of the Left Intraventricular Septal Wall Mass.
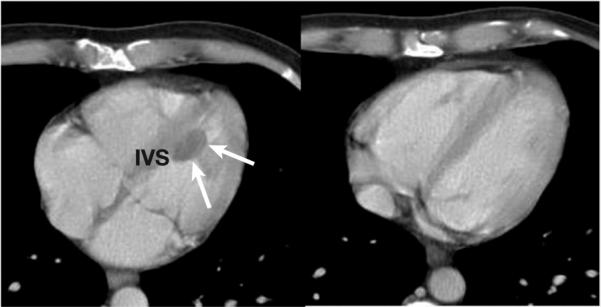
Abbreviations: EPOCH = infusional etoposide/vincristine plus doxorubicin/cyclophosphamide/prednisone; IVS = intraventricular septum
Case 2
A 58-year-old white man presented with a 20-year history of waxing and waning skin lesions before a mycosis fungoides (MF) diagnosis was established. At this time, he had clinically T3 N3 M0 stage IVa disease. He was initially treated with local therapies and, eventually, whole-body electron beam radiation therapy. Over a 2-year period, local radiation therapy, bexarotene, PUVA (psoralen plus UVA light), interferon, denileukin diftitox, and gemcitabine were attempted.
After assessment at the NCI, including echocardiography, the patient was enrolled in a phase II trial using romidepsin. He received his first cycle of romidepsin 14 mg/m2 on days 1, 8, and 15 of a 28-day cycle without incident. After administration of the first dose on cycle 2, the patient developed a fever to 38.9°C. Examination revealed a right sternal border grade 2/6 systolic ejection murmur with an audible S4. Blood work demonstrated a minimally elevated creatine kinase (CK) level of 393 U/L (normal range, 52−386 U/L), a CK-myocardial band fraction of 6%, and a troponin I at 115.5 ng/mL (normal range, 0.03−0.10 ng/mL). Electrocardiogram demonstrated nonspecific ST segment changes, while 3 brief runs of nonsustained ventricular tachycardia were captured on cardiac monitoring. An ischemic workup was performed. In view of his acetylsalicylic acid allergy, he was started on clopidogrel and atenolol. The troponin and CK parameters returned to normal within 24 hours. The patient refused coronary angiography. A 2-dimensional echocardiogram demonstrated mild pulmonary hypertension but normal LV size and function, with no regional wall motion abnormalities. A nonstress contrast-enhanced cardiac MRI scan demonstrated a 3.4-cm mass within the LV wall suspicious for cardiac metastasis (Figure 2). He was counseled for further workup, but the patient refused.
Figure 2. Cardiac MRI Scans Before and After Intravenous Contrast Identifies a Solitary Cardiac Metastasis.
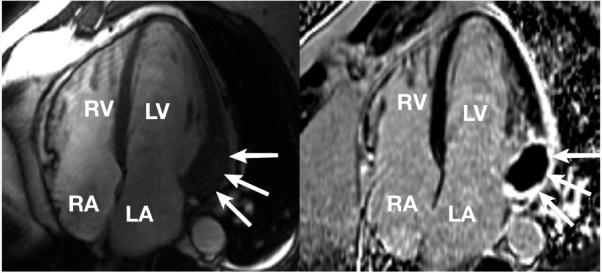
The image on the right is from a cine MRI scan in a 4-chamber view using a steady-state free precession technique. Note that the basal lateral wall is focally hypertrophied or thicker than other walls of the LV (arrows). This localization is atypical for familial hypertrophic cardiomyopathy or other secondary hypertrophic processes. Delayed-enhancement images (on the left) obtained 20 minutes after administration of gadolinium contrast using an inversion recovery gradient echo technique validated for imaging myocardial infarction. The region of localized hypertrophy in the basal lateral wall shows a surrounding region of enhancement (white), while the core of the mass is much darker than the surrounding tissue, suggesting low perfusion or low contrast accumulation. The findings are consistent with metastasis to the heart.
Abbreviations: LA = left atrium; MRI = magnetic resonance imaging; RA = right atrium; RV = right ventricle
Based on the MRI appearance, it was believed that the cardiac mass was consistent with a lymphomatous deposit. While we do not know the cause of troponin elevation, we presume it must have been caused by a bystander effect in myocardial cells associated with romidepsin-mediated tumor cytolysis. Because the cardiac enzyme increase might have been related to focal myocardial damage, it was decided to remove the patient from study. The patient returned to his local oncologist for further management. Two months later, the patient presented to the local emergency room with chest pain. Cardiac arrest ensued, and the patient could not be resuscitated. Autopsy confirmed the MRI findings of a large pericardial lymphomatous deposit. The distal circumflex artery had evidence of an acute occlusive thrombus and compression by lymphomatous deposits, while the left ventricle was largely replaced by necrotic tumor cells with prominent granulation tissue and fibrosis.
Discussion
Cardiac masses arising from the heart or the pericardium are potentially lethal whether defined as benign or malignant. Almost 75% of primary cardiac masses are benign. The most common primary malignant tumors are sarcomas and lymphomas.2 Metastatic deposits represent the vast majority of cardiac malignancies; the common primary sources include cancers of lung, esophagus, and breast as well as lymphoma, leukemia, and melanoma.3 Historically, data on cardiac masses have been obtained from autopsy studies.1,4-8 The largest population series reviewed > 12,000 consecutive autopsies; metastases to the heart were identified in 1.23% of cases, yielding a prevalence of primary cardiac tumors at 0.056%. Another series examining patients with cancer demonstrated metastases to the heart and pericardium in 10%−12% of cases.4,5 Cardiac involvement by lymphoma at autopsy has been described in 16% of patients with Hodgkin disease and 18% of patients with non-Hodgkin lymphoma (NHL), occurring at a median of 20 months after initial diagnosis.6
New agents resulting in improved cancer therapy outcomes might alter the prevalence and location of metastatic deposits to include more unusual disease sites, including intracardiac locations. Therapies might result in changes to underlying disease biology over time or an increase in the cancer-bearing interval and the random deposition of cardiac metastases. High-dose therapy has been reported to alter patterns of relapse in multiple myeloma, while all-trans retinoic acid has shown to have a similar effect in promyelocytic leukemia.9-11 There has been the suggestion that central nervous system metastases are increasing because of longer disease control in breast cancer.12 A similar scenario might be true for NHL.
Clinical presentation of cardiac metastases is determined by numerous factors such as tumor location, size, growth rate, degree of invasion, and friability. Obstruction of blood flow or valve function by a cardiac mass, invasion of the conduction pathways causing arrhythmias, invasion of pericardium producing pericardial effusion, or tamponade, tumor embolization, or constitutional symptoms are some of the mechanisms of presentation. Troponin elevations as a result of cardiac ischemia from lymphomatous deposits are a more unusual presentation. However, this was the finding in our patient treated with romidepsin that prompted further investigation. It should be noted that in > 590 troponin levels analyzed in patients treated with romidepsin, no evidence of authentic troponin elevation was observed.13 Modern imaging techniques increasingly identify cardiac lesions incidentally. Plain chest radiographs lack sensitivity and specificity as an initial diagnostic tool but can demonstrate cardiomegaly, signs of heart failure, and abnormalities of cardiac contour or specific chamber hypertrophy. Echocardiography was the first noninvasive mechanism for examining the chambers of the heart and pericardium, but the restricted acoustic window of the transthoracic approach remains a significant limitation.14 The larger imaging field afforded by transesophageal echocardiography (TEE) makes this a more sensitive technique for assessing patients.15 Computed tomography adequately demonstrates morphology, location, and extent of cardiac neoplasms with a larger field view, while magnetic resonance signal intensity with contrast enhancement results in superior images identifying anatomy, blood flow, and cardiac function.16,17 [18F]Fluorodeoxyglucose positron emission tomography imaging has recently been reported to reveal previously unsuspected cardiac involvement.18,19
The correct pathologic diagnosis is essential to management of cardiac masses. Although traditionally this required a thoracotomy, less invasive procedures are now readily available such as TEE-guided biopsy, endomyocardial biopsy, or percutaneous intracardiac biopsy with combined fluoroscopy and TEE or pericardial fluid sampling.20-24 There have been many single-case reports in the literature documenting management of these patients; however, because of rarity, data are lacking to produce definitive guidelines regarding management. The available literature suggests systemic chemotherapy is the only effective therapy.20 There is a theoretical risk of cardiac wall perforation in response to chemotherapy; however, only 1 case of prophylactic pericardial patching is reported in the literature, while wall rupture is infrequently reported.25,26 The majority of cases are treated with combination chemotherapy with varying results.27-37
Advances in drug development, including the addition of monoclonal therapies to chemotherapy, have resulted in improved response and survival rates.38 Radiation as a therapeutic modality risks pericarditis, cardiomyopathy, diastolic dysfunction, conduction defects, and coronary artery disease. The indication for this therapy appears limited to the cardiac mass that progresses despite chemotherapy. Radical surgery is not advised. Considering the rarity of cardiac lymphoma metastases, it might be prudent to consider the establishment of a specific tumor registry to collect data and potentially guide future management approaches.
Conclusion
Lymphomatous involvement of the heart, previously a rarity, is now being reported with greater frequency. Extranodal lymphomas are increasingly seen with acquired immunodeficiencies such as HIV or after bone marrow and solid organ transplantation. The increased incidence might be partly a result of increased clinical awareness, improved imaging techniques, and a change in cellular biology.
Acknowledgement
This research was supported, in part, by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
References
- 1.Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027–31. [PubMed] [Google Scholar]
- 2.Grebenc ML, Rosado de Christenson ML, Burke AP, et al. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000;20:1073–103. doi: 10.1148/radiographics.20.4.g00jl081073. quiz 110−1, 112. [DOI] [PubMed] [Google Scholar]
- 3.Chiles C, Woodard PK, Gutierrez FR, et al. Metastatic involvement of the heart and pericardium: CT and MR imaging. Radiographics. 2001;21:439–49. doi: 10.1148/radiographics.21.2.g01mr15439. [DOI] [PubMed] [Google Scholar]
- 4.Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol. 1990;3:195–8. [PubMed] [Google Scholar]
- 5.Klatt EC, Heitz DR. Cardiac metastases. Cancer. 1990;65:1456–9. doi: 10.1002/1097-0142(19900315)65:6<1456::aid-cncr2820650634>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Petersen CD, Robinson WA, Kurnick JE. Involvement of the heart and pericardium in the malignant lymphomas. Am J Med Sci. 1976;272:161–5. doi: 10.1097/00000441-197609000-00005. [DOI] [PubMed] [Google Scholar]
- 7.McAllister HA. Atlas of tumour pathology. Armed Forces Institute of Pathology; Washington, DC: 1978. Tumours of the cardiovascular system. [Google Scholar]
- 8.Barbaro G, Di Lorenzo G, Grisorio B, et al. Cardiac involvement in the acquired immunodeficiency syndrome: a multicenter clinical-pathological study. Gruppo Italiano per lo Studio Cardiologico dei pazienti affetti da AIDS Investigators. AIDS Res Hum Retroviruses. 1998;14:1071–7. doi: 10.1089/aid.1998.14.1071. [DOI] [PubMed] [Google Scholar]
- 9.Sanfructuoso C, Caballero MD, Garcia-Sanz R, et al. Relapse of multiple myeloma in extramedullary sites after autologous bone marrow transplantation. Eur J Haematol. 1996;56:181–3. doi: 10.1111/j.1600-0609.1996.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 10.Alegre A, Granda A, Martinez-Chamorro C, et al. Different patterns of relapse after autologous peripheral blood stem cell transplantation in multiple myeloma: clinical results of 280 cases from the Spanish Registry. Haematologica. 2002;87:609–14. [PubMed] [Google Scholar]
- 11.Menendez A, Gonzalez A, Cabrera H, et al. Clinical spectrum of extramedullary acute promyelocytic leukemia. Eur J Haematol. 2000;64:201–3. doi: 10.1034/j.1600-0609.2000.70424.x. [DOI] [PubMed] [Google Scholar]
- 12.Aragon-Ching JB, Zujewski JA. CNS metastasis: an old problem in a new guise. Clin Cancer Res. 2007;13:1644–7. doi: 10.1158/1078-0432.CCR-07-0096. [DOI] [PubMed] [Google Scholar]
- 13.Piekarz RL, Frye AR, Wright JJ, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res. 2006;12:3762–73. doi: 10.1158/1078-0432.CCR-05-2095. [DOI] [PubMed] [Google Scholar]
- 14.Majano-Lainez RA. Cardiac tumors: a current clinical and pathological perspective. Crit Rev Oncog. 1997;8:293–303. doi: 10.1615/critrevoncog.v8.i4.10. [DOI] [PubMed] [Google Scholar]
- 15.Meng Q, Lai H, Lima J, et al. Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases. Int J Cardiol. 2002;84:69–75. doi: 10.1016/s0167-5273(02)00136-5. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann U, Globits S, Schima W, et al. Usefulness of magnetic resonance imaging of cardiac and paracardiac masses. Am J Cardiol. 2003;92:890–5. doi: 10.1016/s0002-9149(03)00911-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang ZJ, Reddy GP, Gotway MB, et al. CT and MR imaging of pericardial disease. Radiographics. 2003 doi: 10.1148/rg.23si035504. 23 spec No:S167−80. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen JD, Carrasquillo JA, Little RF, et al. Fluorodeoxyglucose positron emission tomography in the presence of cardiac metastases. Clin Nucl Med. 2003;28:979–80. doi: 10.1097/01.rlu.0000099808.30653.06. [DOI] [PubMed] [Google Scholar]
- 19.Romer W, Garbrecht M, Fuchs C, et al. Images in cardiovascular medicine. Metabolic imaging identifies non-Hodgkin's lymphoma infiltrating heart. Circulation. 1998;97:2577–8. doi: 10.1161/01.cir.97.25.2577. [DOI] [PubMed] [Google Scholar]
- 20.Ceresoli GL, Ferreri AJ, Bucci E, et al. Primary cardiac lymphoma in immunocompetent patients: diagnostic and therapeutic management. Cancer. 1997;80:1497–506. doi: 10.1002/(sici)1097-0142(19971015)80:8<1497::aid-cncr18>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Abramowitz Y, Hiller N, Perlman G, et al. The diagnosis of primary cardiac lymphoma by right heart catheterization and biopsy using fluoroscopic and transthoracic echocardiographic guidance. Int J Cardiol. 2007;118:e39–40. doi: 10.1016/j.ijcard.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Chim CS, Chan AC, Kwong YL, et al. Primary cardiac lymphoma. Am J Hematol. 1997;54:79–83. doi: 10.1002/(sici)1096-8652(199701)54:1<79::aid-ajh13>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Kang SM, Rim SJ, Chang HJ, et al. Primary cardiac lymphoma diagnosed by transvenous biopsy under transesophageal echocardiographic guidance and treated with systemic chemotherapy. Echocardiography. 2003;20:101–3. doi: 10.1046/j.1540-8175.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 24.Jurkovich D, de Marchena E, Bilsker M, et al. Primary cardiac lymphoma diagnosed by percutaneous intracardiac biopsy with combined fluoroscopic and transesophageal echocardiographic imaging. Catheter Cardiovasc Interv. 2000;50:226–33. doi: 10.1002/(sici)1522-726x(200006)50:2<226::aid-ccd19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Molajo AO, McWilliam L, Ward C, et al. Cardiac lymphoma: an unusual case of myocardial perforation--clinical, echocardiographic, haemodynamic and pathological features. Eur Heart J. 1987;8:549–52. doi: 10.1093/oxfordjournals.eurheartj.a062317. [DOI] [PubMed] [Google Scholar]
- 26.Beckwith C, Butera J, Sadaniantz A, et al. Diagnosis in oncology. Case 1: primary transmural cardiac lymphoma. J Clin Oncol. 2000;18:1996–7. doi: 10.1200/JCO.2000.18.9.1996. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda H, Nakamura S, Nishimaki H, et al. Primary lymphoma of the heart: case report and literature review. Pathol Int. 2004;54:187–95. doi: 10.1111/j.1440-1827.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- 28.Koehler F, Borges AC, Fotuhi PC. Large B cell lymphoma with cardiac infiltration. Heart. 2003;89:1282. doi: 10.1136/heart.89.11.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alzeerah MA, Singh R, Jarrous A. Large B-cell lymphoma of the atria. Tex Heart Inst J. 2003;30:74–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Jang JJ, Danik S, Goldman M. Primary cardiac lymphoma: diagnosis and treatment guided by transesophageal echocardiogram perfusion imaging. J Am Soc Echocardiogr. 2006;19:1073, e7–9. doi: 10.1016/j.echo.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Morillas P, Quiles J, Nunez D, et al. Complete regression of cardiac non-Hodgkin lymphoma. Int J Cardiol. 2006;106:426–7. doi: 10.1016/j.ijcard.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Hirabayashi T, Tanabe M, Onishi K, et al. Cardiac malignant lymphoma with atrial arrhythmias. Int J Cardiol. 2007;114:E42–4. doi: 10.1016/j.ijcard.2006.07.214. [DOI] [PubMed] [Google Scholar]
- 33.Piccaluga PP, Vigna E, Placci A, et al. Primary cardiac non-Hodgkin lymphoma presenting with atrial flutter and pericardial effusion. Br J Haematol. 2006;134:356. doi: 10.1111/j.1365-2141.2006.06168.x. [DOI] [PubMed] [Google Scholar]
- 34.Nonami A, Takenaka K, Kamezaki K, et al. Successful treatment of primary cardiac lymphoma by rituximab-CHOP and high-dose chemotherapy with autologous peripheral blood stem cell transplantation. Int J Hematol. 2007;85:264–6. doi: 10.1532/IJH97.06197. [DOI] [PubMed] [Google Scholar]
- 35.Kaul P, Javangula K. Burkitt lymphoma masquerading as cardiac tamponade. J Cardiothorac Surg. 2007;2:30. doi: 10.1186/1749-8090-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang YY, Fan K, Lam YM, et al. Primary cardiac lymphoma presenting with right heart mass and bradycardia. Ann Hematol. 2007;86:685–6. doi: 10.1007/s00277-007-0285-7. [DOI] [PubMed] [Google Scholar]
- 37.Knowles JW, Elliott AB, Brody J. A case of complete heart block reverting to normal sinus rhythm after treatment for cardiac invasive Burkitt's lymphoma. Ann Hematol. 2007;86:687–90. doi: 10.1007/s00277-007-0290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagawa Y, Ikeda U, Hirose M, et al. Successful treatment of primary cardiac lymphoma with monoclonal CD20 antibody (rituximab). Circ J. 2004;68:172–3. doi: 10.1253/circj.68.172. [DOI] [PubMed] [Google Scholar]


