Summary
Objective
To estimate the lifetime risk of symptomatic hip osteoarthritis (OA).
Design
We analyzed data from the Johnston County Osteoarthritis Project (a longitudinal population-based study of OA in North Carolina, United States [n=3,068]). The weighted baseline sample comprised 18% blacks and 54% women, and the mean age was 63 years (range=45-93). Symptomatic hip OA was defined as a Kellgren-Lawrence (K-L) radiographic score of ≥2 (anterior-posterior pelvis x-rays) and pain, aching or stiffness on most days, or groin pain, in the same hip. Lifetime risk, defined as the proportion who developed symptomatic hip OA in at least one hip by age 85, among people who live to age 85, was modeled using logistic regression with repeated measures (through generalized estimating equations).
Results
Lifetime risk of symptomatic hip OA was 25.3% (95% confidence interval [CI] = 21.3–29.3). Lifetime risk was similar by sex, race, highest educational attainment, and hip injury history. We studied lifetime risk by body mass index (BMI) in three forms: at age 18; at baseline and follow-up; and at age 18, baseline and follow-up and found no differences in estimates.
Conclusion
The burden of symptomatic hip OA is substantial with one in four people developing this condition by age 85. The similar race-specific estimates suggest that racial disparities in total hip replacements are not attributable to differences in disease occurrence. Despite increasing evidence that obesity predicts an increased risk of both hip OA and joint replacement, we found no association between BMI and lifetime risk.
Introduction
Symptomatic hip osteoarthritis (OA) can be a highly disabling form of lower extremity OA that limits basic activities, such as walking a few blocks or climbing stairs1, and is the most common indication for total joint replacement of the hip2. In 2007, approximately 252,000 hip replacements were performed in the United States at an estimated total cost of $4 billion 3.
Lifetime risk is the probability of developing a condition over the course of a lifetime. Whereas prevalence and incidence convey the population burden of a condition, lifetime risk describes individual risk. Lifetime risk has been estimated for various chronic conditions (e.g., symptomatic knee osteoarthritis 4, breast cancer 5, coronary heart disease 6, diabetes 7). To our knowledge, the lifetime risk of symptomatic hip OA has not been reported. We present lifetime risk estimates – defined as the proportion of the population who live to age 85 that develop symptomatic hip OA by age 85 -- for symptomatic hip OA in Johnston County, North Carolina. We estimated the lifetime risk of symptomatic hip OA overall and stratified by six factors: age, sex, race, educational attainment, history of hip injury, and body mass index (BMI) — among participants of the Johnston County Osteoarthritis (JoCo OA) Project.
Methods
The study sample were participants (n = 3,068) in the JoCo OA Project, a longitudinal study of the onset and progression of hip and knee OA among semirural residents of Johnston County, North Carolina, USA. The JoCo OA Project is the largest population-based, longitudinal study in the United States to monitor the occurrence and natural history of hip OA among black and white males and females. Project methods are described elsewhere 8-10 . The JoCo OA Project cohort was selected to be representative of the civilian, noninstitutionalized, English-speaking black and white population aged ≥45 years who were residents of one of six selected townships of Johnston County for at least 1 year, and who were physically and mentally capable of completing the study’s protocol.
The study protocol at both baseline (1990–1997) and first follow up (1999–2003) included an initial home interview, a clinical examination (including x-rays), and a second home interview approximately 2 weeks after the clinical examination. X-rays included supine anteroposterior radiographs of the hip, which were read for radiographic hip OA using Kellgren-Lawrence (K-L) grades by one bone and joint radiologist (JBR) 11. The intra-rater and inter-rater reliability of the JBR were previously determined to be high with a weighted kappa of 0.89 (intra-rater) and 0.86 (inter-rater) 9. Pelvic radiographs were not obtained from women of reproductive age (i.e., <50 years), therefore these women were included in only the follow-up sample of this analysis. Weight and height were physically measured by staff at the baseline and first follow-up. Study participants completed an interviewer administered questionnaire that measured sociodemographic and clinical characteristics including age, sex, race, educational attainment, income, history of hip injury, and the presence of hip symptoms (“On MOST days do you have pain, aching or stiffness in your left (or right) hip?”). In the baseline questionnaire, participants reported their height and weight at age 18. Because symptoms of hip OA may manifest in a broader region of the hip 12, the trained examiner queried participants about their pain at numerous sites in the broader hip region (e.g., left and right groin pain) using a standardized history and examination protocol.
Multiple strategies were used to minimize cohort attrition between baseline and follow up (e.g., annual newsletters, advertisements in local media and medical and community settings, inquiries throughout the community). Participants’ deaths were identified by reviewing local obituaries, North Carolina state and local death records, and the US National Death Index (NDI) and through word of mouth. The NDI is the most complete and accurate source of US mortality data with the sensitivity ranging from 93 to 98% and specificity of virtually 100% 13, 14.
ANALYSIS
First, we examined the baseline sociodemographic characteristics, symptoms and OA status. Next, we estimated the lifetime risk for symptomatic hip OA. Symptomatic hip OA was defined as the presence of both radiographic K-L grade ≥2 (at least mild radiographic OA 11) in at least one hip and hip symptoms reported in the radiographically affected joint. To determine whether estimates varied with symptom definition, we estimated lifetime risk using two definitions: 1) the presence of pain, aching, or stiffness in the radiographically affected hip or 2) the presence of pain, aching, or stiffness or groin pain in the radiographically affected hip. The Estimates were similar for the two definitions (reported in Results), and so we used the second, or broader, definition for the remainder of the analyses. The analysis was person-based (i.e., unit of analysis was person rather than hip). Hips with radiographic evidence of inflammatory arthritis (i.e., rheumatoid arthritis) at baseline or follow up were excluded, and joints that had undergone replacement were classified as affected with symptomatic hip OA because hip OA is the most common indication for hip replacements2..
The overall lifetime risk for symptomatic hip OA was estimated as a model-predicted prevalence of symptomatic hip OA at age 85 for those who survived to at least this age; in this model, age was the independent variable and symptomatic hip OA the dependent variable. We then estimated lifetime risks stratified by sex, race, highest educational attainment, BMI, and history of hip injury. BMI, educational attainment, and history of hip injury were modeled as time dependent variables, that is, we analyzed participants’ values at each time point. BMI was examined in three separate models: 1) at age 18, 2) at baseline and follow up, and 3) in a summary of BMI over the life course (i.e., BMI at age 18, at baseline, and at follow up). In model 1 and 3, BMI was examined as a two category variable—under-/normal weight or overweight/obese — because there was insufficient sample size to examine overweight and obese separately.
When considering how to model BMI, continuous BMI at baseline and follow-up were compared. Participants’ BMI, on average, increased by only 1.0 unit at follow-up. A potential interaction between age and categorized BMI in association with symptomatic hip OA was evaluated; it was not statistically significant (p=0.114). Because there is no published evidence that a change of 1.0 BMI unit changes risk of OA onset, BMI (categorized) was treated as a time-dependent covariate (that is, the BMI of participants at each observation point was analyzed). We also modeled lifetime risk with BMI at age 18 as a continuous variable. We observed a curvilinear relationship resulting from unstable estimates for the small number of respondents who were overweight/obese at age 18 and had a high lifetime risk.
Lifetime risk is the probability of developing a condition over a lifetime. This lifetime probability is equal to the cumulative incidence of a condition over the cohort’s lifetime. Furthermore, the cumulative incidence of symptomatic hip OA is equal to lifetime prevalence because symptomatic hip OA is a persistent, low-mortality condition. There are at least two strengths to including all cohort members, regardless of OA status at baseline. First, OA symptoms may be intermittent or abate (e.g., responsiveness to treatment of symptoms). By including prevalent and incident cases, we captured a higher proportion of participants who have ever had symptomatic OA. Second, the cumulative aspect of the lifetime risk estimate ensures data from all participants, including those who may subsequently die or leave the cohort for other reasons (e.g., move outside the catchment area), which may reduce selection bias.
Estimates were derived from logistic regression models by using generalized estimating equations (GEE). We used GEE logistic regression, rather than traditional time-to-event survival analysis methods used in other studies of lifetime risk, for several reasons. First, many participants had the condition of interest at baseline, which would exclude them from a time-to-event analysis. Second, there was considerable cohort attrition between baseline and follow-up, typical in cohort studies. Therefore, life table analysis would result in an overestimation of risk because of the extensive censoring among those participants who were absent at follow-up. Finally, while survival analysis methods are indicated when modeling time to event, in studies of onset of slowly evolving conditions, such as OA, a precise measure of the time to event, or date of OA onset, is unknown without frequent follow-up of cohort members. Therefore, without a date of OA onset, lifetime risks were derived using GEE logistic regression. GEE logistic regression models the probability of onset at, or prior to, the current observation time and therefore provides estimates for points along the Kaplan-Meier curve, similar to estimates derived in a more traditional time-to-event analysis. The sample analyzed comprised people aged 45-93 years and therefore the predicted probabilities were interpolated (i.e., based on a set of known data points for younger and older cohort members) rather than projected from a sample of younger participants.
We conducted a preliminary analysis to assess whether the relationship between age and lifetime risk was linear. Continuous age was modeled as untransformed and transformed [i.e., logarithm (age), square root (age1/2), and the addition of a quadratic term (age + age2)] . Untransformed continuous age was used in the remaining analyses because its association with lifetime risk provides a simpler (linear) interpretation, the quadratic age term was not statistically significant at α=0.05, and the p values for the ln(age) and age1/2 terms were similar in significance (p=0.001) to the untransformed age term.
We estimated the probability of developing symptomatic hip OA by age 85. Analyses were conducted in SUDAAN 15, with adjustment for three sources of error resulting from the study design—repeated measures across study participants, multiple participants per household, and a two-stage clustered sampling design.
We conducted a sensitivity analysis to determine the potential bias of cohort attrition on the lifetime risk estimate. First, we conducted backward selection logistic regression (explanatory variables were age [five year categories]; sex; race; educational attainment; BMI at age 18; and BMI at baseline [history of hip injury was not included because of insufficient sample size]) to identify risk factors (at α = 0.10) for onset of symptomatic hip OA between baseline and follow up. Age and race were significantly associated with incident disease. Second, we calculated the proportion of the sample that each combination of the two strata, including missing values, represented. Steps one and two of these analyses were limited to participants who did not have symptomatic hip OA at baseline.
Third, using the proportions estimated in the previous step, we selected a random sample from each of the five groups of nonparticipants at follow up (i.e., had a household interview only, declined participation, lost to follow up, moved from study area, physically or mentally unable to participate). Fourth, we estimated the overall lifetime risk: Randomly selected persons were recoded as having OA at follow up and remaining members of each group were recoded as unaffected. We conducted steps three and four 10 times to determine the range of the simulated lifetime risk estimates.
Results
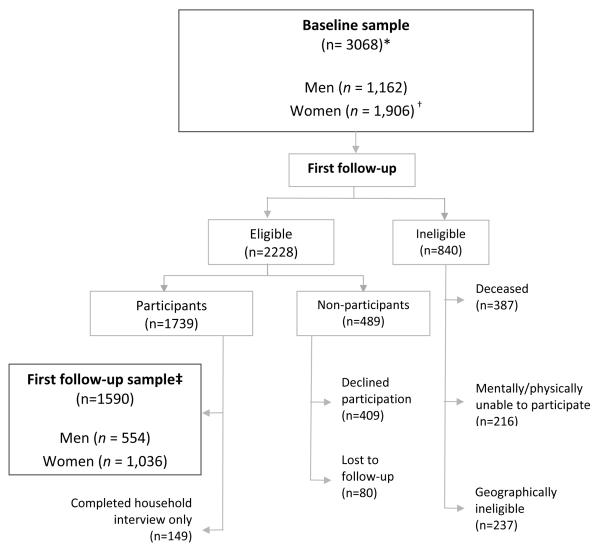
The average age of the 2,756 eligible participants at baseline was 63 years (range = 45–93 years). The baseline sample excluded 321 women who were ineligible for having x-rays at baseline because they were aged <50 years (Fig. 1). The weighted sample comprised 18% blacks, 53% women, and 55% with an annual of income of <$20,000 (Table 1). Sixty-five percent of the participants were married or in common-law marriages, and 58% had at least a high school education. Only 11% of participants reported being overweight or obese at age 18, but 67% were overweight or obese at baseline. Forty-one percent reported hip or groin symptoms, and 13% had symptomatic OA in at least one hip at baseline when symptoms were defined as either the presence of pain, aching, or stiffness or groin pain.
Fig. 1.
Study sample at baseline and first follow up
* Baseline response rate=3068/5138=60%; clinic cooperation rate=3068/3690=83%.
† Women aged < 50 years (i.e., reproductive age) did not have pelvic radiographs (n=312).
† First follow-up sample comprised those who completed clinic examination and household interview (response rate=1590/2228=83%; clinic cooperation rate=1590/ 1739=91%). All women had hip radiographs at first follow up because they were aged ≥ 50 years.
Table I.
Selected sociodemographic and clinical characteristics of Johnston County Osteoarthritis Project cohort at baseline—hip analyses (n = 2,756*)
| Variable | Percentage†,‡,§ |
|---|---|
| Age (years) | |
| 45–59 | 42 |
| 60–74 | 44 |
| ≥75 | 14 |
| Women | 53 |
| Black | 18 |
| Marital status | |
| Married/common-law | 65 |
| Widowed | 23 |
| Household income <$20,000 per year | 55 |
| Education | |
| Less than high school | 42 |
| Completed high school | 32 |
| More than high school | 26 |
| Body mass index at age 18∥ | |
| Under or normal (<25) | 89 |
| Overweight or obese (≥25) | 11 |
| Body mass index∥ | |
| Under or normal (<25) | 32 |
| Overweight (25 to <30) | 42 |
| Obese (≥30) | 25 |
| Symptoms¶ | |
| Pain, aching, or stiffness in at least one hip | 37 |
| Pain in groin | 16 |
| Pain, aching, or stiffness in at least one hip or pain in groin | 41 |
| Symptomatic OA in at least one hip** | 12 |
| Total joint replacement in at least one hip†† | <1 |
| History of hip injury | |
| In either hip | 6 |
| In radiographically affected hip | 1 |
OA = osteoarthritis.
Excludes 312 women of reproductive age who were ineligible for hip radiographs at baseline but eligible for hip radiographs at first follow up. The characteristics of the entire cohort (n = 3,068) at baseline is described elsewhere4.
Weighted to Johnston County population distribution in the 1990 United States Census.
Some percentages were rounded and may not equal 100.
The denominator of percentages do not include participants with the following missing baseline data: marital status (n = 1); income (n = 545); education (n=7); body mass index (BMI) at age 18 (n = 130); BMI (n = 92); history of hip injury (n = 7); pain, aching, and/or stiffness in at least one hip (n = 61); hip replacement status (n=61); groin pain (n = 28); radiographs for both hips (n = 49); symptoms and radiographs for both hips (n = 40).
BMI at age 18 was calculated from self-reported height and weight. Baseline BMI was calculated from height and weight measurements taken at baseline clinical examination.
Independent of hip radiographic OA status.
Symptomatic was defined as either “pain, aching, or stiffness in at least one hip joint” or “pain in groin” in the radiographically affected hip. This includes people with total hip replacements in at least one hip.
Observed in radiographs.
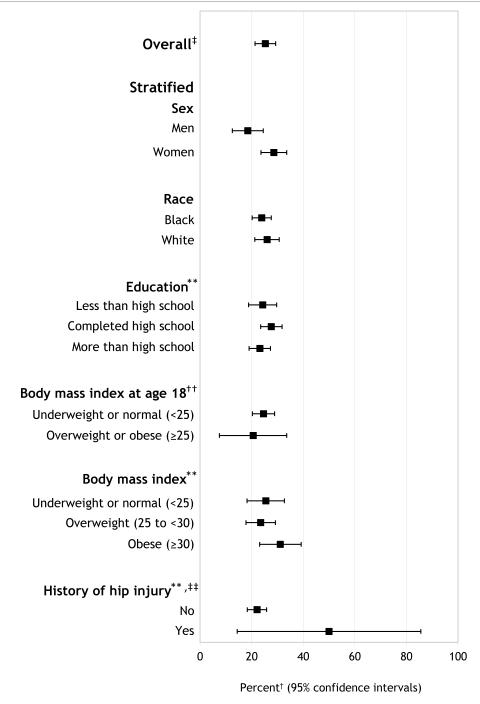
The overall lifetime risk for symptomatic hip OA was 24.2% (data not presented) when the definition of symptoms was limited to presence of pain, aching, or stiffness. The lifetime risk increased minimally to 25.3% when defined as the presence of either pain, aching, or stiffness or groin pain (Table 2). The overall lifetime risk estimated in the sensitivity analysis (i.e., simulation of estimate as if there was no loss to follow up) was 29.4 % (range = 29.1% to 29.9%) (data not presented).
Table II.
Lifetime risk of symptomatic* hip osteoarthritis in the Johnston County Osteoarthritis Project cohort
| Proportion† (95% confidence interval) |
|
|---|---|
| Stratified | |
| Sex | |
| Men | 18.5 (12.5–24.5) |
| Women | 28.6 (23.6–33.6) |
| Race | |
| Black | 23.9 (20.2–27.6) |
| White | 26.0 (21.2–30.7) |
| Education‡ | |
| Less than high school | 24.3 (18.8 –29.7) |
| Completed high school | 27.6 (23.5 – 31.8) |
| More than high school | 23.2 (20.9 – 29.7) |
| Body mass index at age 18 years** | |
| Underweight or normal (<25) | 24.6 (20.3 – 28.9) |
| Overweight or obese (≥25) | 20.6 (7.5– 33.6) |
| Body mass index‡,** | |
| Underweight or normal (<25) | 25.5 (18.2 –32.7) |
| Overweight (25 to <30) | 23.5 (17.8–29.2) |
| Obese (≥30) | 31.1 (23.1–39.2) |
| History of hip injury‡,†† | |
| No | 22.1 (18.3–25.8) |
| Yes | 50.0 (14.4–85.6) |
| Overall‡‡ | 25.3 (21.3 – 29.3) |
Symptomatic was defined as either “pain, aching, or stiffness in at least one hip joint” or “pain in groin” in the radiographically affected hip.
Weighted to Johnston County population distribution in the1990 United States Census.
Education, body mass index (BMI), and history of hip injury were time dependent (i.e., participants’ measurements at baseline and follow up were analyzed).
BMI at age 18 was calculated from self-reported height and weight. Baseline BMI was calculated from height and weight measurements at baseline clinical examination.
History of hip injury in the symptomatic and radiographically affected joint
Stratified lifetime risk estimates may not sum to overall lifetime risk estimate because of missing data for stratification variables (Table 1).
Women were 10% more likely than men to develop symptomatic hip OA (28.6% [95% CI = 23.6–33.6] versus 18.5% [95% CI = 12.5–24.5]) (Table 2). There were no differences in lifetime risk by race or education levels. The lifetime risk for participants who reported a hip injury in the symptomatic and radiographic affected hip was 50.0% (95% CI = 14.4–85.6) compared with 22.1% (95% CI=18.3-25.8) among those reporting no injury.
Lifetime risk was similar across levels of BMI at baseline and at follow up (Table 2). Similarly, the lifetime risk for participants reporting being under- or normal weight (26.4%) at age 18 was similar to participants who reported being overweight or obese (21.7%, respectively) (Table 2). We examined BMI across three points (age 18, baseline, and follow up). There was sufficient sample size to examine BMI trajectories among only those who reported being under or normal weight at age 18. We found no statistically significant differences across the estimates.
Discussion
The overall lifetime risk for symptomatic hip OA was 25.3%, suggesting that one in four Johnston County residents who live to age 85 are at risk of developing symptomatic hip OA. Although it was not a statistically significant difference, the lifetime risk was higher for women (28.6%) than men (18.5%), which is consistent with previous prevalence and incidence studies of symptomatic hip OA16.
We found similar lifetime risks for blacks and whites, and the race-specific prevalence of symptomatic hip OA in the JoCo Project cohort also was the same for blacks and whites8. The race-specific prevalence of radiographic hip OA has been compared in at least four other studies. Two African studies found a lower prevalence among blacks17, 18, whereas two US studies—a national, population-based National Health and Nutrition Examination Survey I (NHANES I) survey19 and a survey of senior citizen centers in Brooklyn, New York18—indicated a comparable prevalence among blacks and whites. Hip replacements are a well recognized and effective procedure for reducing pain and improving physical function among people with debilitating hip OA. Some studies have found evidence of greater unmet need for hip replacements among blacks compared with whites20. Our analysis did not account for differences in symptom severity, an indication for hip replacement. However, the similar race-specific risk estimates suggest an equal need for hip replacements for blacks and whites.
Lifetime risk also was similar across education levels. Education was used as an indicator of socioeconomic status because self-reported income data were missing for a high proportion (20%) of the baseline study sample, which is consistent with many epidemiologic studies21 . At least one previous study has found an association between education and prevalent hip OA22, but education is not a recognized risk factor for incident disease23.
Although lifetime risk was higher for participants with a self-reported hip injury (50.0% [95% CI = 14.4–85.6]) than those without (22.1% [95% CI = 18.3–25.8]), the difference was not statistically significant. Hip injury and onset of hip OA have been linked in previous studies19, 24. The lack of association in this study may have resulted from the small number of people who reported a hip injury in the radiographically affected hip at baseline.
The association between BMI and total hip replacement is strong 25-27, but the evidence for association using other definitions of hip OA is equivocal. A meta-analysis of studies examining the association between BMI and hip OA indicated moderate evidence of a relationship (summary odds ratio=2) between BMI and hip OA when all studies were considered (i.e., studies including clinical and radiographic definitions) but no relationship when limited to studies examining radiographic disease only28. Four longitudinal studies have reported that obesity at age 18 predicts a moderate to strongly increased risk for symptomatic hip OA and hip joint replacements in later life25, 26, 29, 30. Obesity at age 18 and at the time of hip replacement was independently associated with an increased risk for total hip replacement among women in the Nurses Cohort Study25. However, another study reported that obesity in early life was associated with an increased risk for hip replacement, but weight gain in the fourth and fifth decades of life did not predict later risk for hip replacement31. We found similar risks in all BMI analyses which is consistent with evidence from studies examining radiographic hip OA; to date, too few JoCo study participants have undergone hip replacement procedures to reliably estimate an association between BMI and hip replacement. The majority of participants reported being under- or normal weight at age 18. Our analysis of BMI across three time points found that no differences across varying life course BMI trajectories; however, because a small proportion of respondents reported being overweight or obese at age 18, there was only sufficient power to estimate disease risk among respondents who reported being under or normal weight at age 18. In our study, BMI at age 18 was self-reported and is likely subject to recall bias32. Although there was a substantial difference in BMI at age 18 and baseline, the prevalence of overweight or obesity among all, including younger, adults has increased substantially in recent decades 33, 34 ; it is plausible that BMI in this cohort was substantially lower among participants at age 18.
At least seven different definitions of hip OA have been used across epidemiologic studies to classify hip OA, including K-L grades, minimal joint space width (JSW) and Croft’s grade35. K-L grades are the most common measure35. Two potential limitations of K-L classification are the emphasis on osteophytes35 and potentially problematic intra- and inter- rater reliability when assessing radiological features relative to a published atlas35,36. Relative to other measures, K-L grades show lower incidence and similar or lower prevalence of radiographic hip OA36, 37; a strong association between K-L grades and hip pain among women and people aged ≥65 years (comparable or better than JSW)38; moderate to high inter-rater and intra-rater agreement; similar or higher predictive validity for total hip replacements compared with JSW and Croft’s grade37,38; and moderate to strong predictive validity for progression of hip OA, especially among people with hip pain at baseline37, 39.
We provide a model-predicted prevalence of OA by age 85 for those who achieve this age or older. This can be reasonably interpreted as the lifetime risk of OA for people who live to at least 85 years. This differs from a definition that estimates the risk of disease for the remaining lifetimes of people who live to varying ages 5,6,7. However, because age 85 is a reasonable expected lifespan for individuals in the US, this estimate represents an informative, helpful, and relevant quantity which would be meaningful to most individuals, as they see themselves potentially living to that age. Our results are mortality-adjusted in the sense that we assume that for the portion of the sample that has died, they would have had OA in the same proportion as those who lived and are estimated by the model to have OA by age 85.
Our lifetime risk estimates were likely underestimated for five reasons. First, the sensitivity analysis found an estimate of 29.4%. This slightly higher lifetime risk may indicate an association between disease status and nonparticipation at first follow up; physical limitations caused by the onset of symptomatic hip OA between baseline and follow up was one reason for nonparticipation at first follow up. Second, the JoCo OA Project sample comprised men aged ≥45 years and women aged ≥50 years (pelvic radiographs were not obtained for women of reproductive age). The onset of hip OA is very uncommon among people aged 45 years or younger40, 41. Nevertheless, there may have been cases of symptomatic hip OA in the younger Johnston County population that were not captured in this study.
Third, interviewers determined participants’ history of hip pain through oral questioning at the household interview. Birrell et al. reported that schematics are slightly more sensitive than verbal query in detecting hip pain12. Therefore, a small proportion of participants in our study were potentially misclassified as not having hip symptoms. As well, we defined symptoms of hip OA as pain in the hip or groin. However, symptoms of hip OA can manifest in other parts of the broad hip region, including the low back. Other sites in the hip region were not included in the analysis because only hip and groin pain were measured at both baseline and at follow up.
Fourth, OA symptoms may be intermittent 42.. We derived the lifetime risk estimate using symptom status at both baseline and at follow up to increase the likelihood of capturing experience of hip pain, thus reducing misclassification of symptomatic hip OA. Last, a maximum of 11 years of follow-up data were available. We believe that lifetime risk will be higher with increased observation time, as previous studies of lifetime risk have reported higher probabilities with increasing observation time6, 43, 44.
While estimating prevalence and incidence among people aged ≥85 years can be problematic because of decreased survival (i.e., small sample sizes at older ages), the lifetime risk statistic is a cumulative measure and uses pooled information from across age groups. Therefore, disease risk at age 85 can be estimated with increased precision. GEE repeated-measures modeling was used to reduce selection bias and to increase statistical power, as data for all cohort members were analyzed, regardless of follow-up status. The proportion of the sample participating in the first follow up (among those who were eligible) was 71%, and 90% of this group completed the x-ray evaluation (Figure 1)4, 10.
We have estimated the lifetime risk of symptomatic hip OA to be one in four and previously reported the lifetime risk of symptomatic knee OA to be nearly one in two. The higher occurrence of symptomatic knee OA compared with hip OA is consistent with higher frequency of knee OA observed using other measures of disease burden (e.g., prevalence and incidence). Various statistical methods have been used to derive lifetime risk estimates for other chronic conditions and there is considerable variability in the characteristics of the samples (e.g., age, race/ethnicity) and sampling frames (e.g., clinic- versus population based). We believe that this substantial heterogeneity precludes comparisons of lifetime risk estimates across conditions.
We recommend caution in generalizing our results to the US population. In 1990, the distribution of age and sex in the baseline Johnston County population was comparable to the US population4, 45, 46, but the Johnston County population had a higher proportion of black (18% versus 12%), rural (76% versus 25%), less educated (35% versus 25% had not completed high school), and lower-income residents (median income of $25,169 versus $30,056). The differences in race and education may be unimportant because although Johnston County had a higher proportion of blacks and people with less education, we found that lifetime risks were similar by race and educational attainment. The proportion of overweight or obese participants aged ≥45 years in the United States and Johnston County was similar (66% in baseline JoCo OA study sample [1990–1997] versus 63.0% in the United States in 1988–1994 NHANES 47).
The lifetime risk statistic is considered an accessible statistic for describing risk to lay audiences. It is familiar to the general public because it has been used to convey the person-level risk of other chronic conditions, such as breast cancer48. The JoCo OA Project is the only longitudinal, population-based study of OA in the United States that includes blacks and whites of both sexes who are middle aged and older. The uniqueness of this sample has enabled us to generate estimates from a sociodemographically diverse sample. The high lifetime risk for symptomatic hip OA observed in our study further illustrates the substantial public health burden of arthritis across a range of diverse groups.
Figure 2.
Lifetime risk of symptomatic* hip OA in the JoCo OA.
* Symptomatic was defined as either “pain, aching, or stiffness in at least one hip joint” or “pain in groin” in the radiographically affected hip.
† Weighted to Johnston County population distribution in the 1990 United States Census.
‡ Stratified lifetime risk estimates may not sum to overall lifetime risk estimate because of missing data for stratification variables (Table 1).
** Education, BMI, and history of hip injury were time dependent (i.e., participants’ measurements at baseline and follow-up were analyzed).
†† BMI at age 18 was calculated from self-reported height and weight. Baseline BMI was calculated from height and weight measurements at baseline clinical examination.
‡‡ History of hip injury in the symptomatic and radiographically affected joint.
Acknowledgements
We would like to thank the following: the dedicated staff of the Johnston County Osteoarthritis Project, including Janice Woodard, Linda Miles, Edwin Hartman, MD, Erik Myers and Fang Fang; Miriam Cisternas for her invaluable technical assistance; and the project participants who made this study possible.
Funding source: Cooperative agreements S043 and S3486 from the Centers for Disease Control and Prevention through the Association of Schools of Public Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant numbers: 5-P60-AR-30701 and 5-P60-AR49465.
Footnotes
Conflict of interest: The authors report no conflicts of interest.
DisclaimerThe findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions - Louise Murphy: Conception and design (reported study); Analysis and interpretation of the data; Drafting of the article; Critical revision of the article for important intellectual content; Final approval of the article; Statistical expertise; Responsibility for the integrity of the work as a whole, from inception to finished article alx2@cdc.gov
Charles G Helmick: Conception and design (Study topic) ; Analysis and interpretation of the data; Critical revision of the article for important intellectual content; Administrative, technical, or logistic support; Final approval of the article
Todd Schwartz: Conception and design (statistical methods of reported study); Analysis and interpretation of the data; Critical revision of the article for important intellectual content; Final approval of the article; Administrative, technical, or logistic support; Statistical expertise
Jordan B Renner: Critical revision of the article for important intellectual content; Final approval of the article; Collection and assembly of data; Administrative, technical, or logistic support
Gail Tudor: Conception and design (statistical methods of reported study); Critical revision of the article for important intellectual content; Final approval of the article; Statistical expertise
Gary Koch: Conception and design (statistical methods of reported study); Analysis and interpretation of the data; Critical revision of the article for important intellectual content; Final approval of the article; Statistical expertise
Anca Dragomir: Critical revision of the article for important intellectual content; Final approval of the article; Collection and assembly of data; Administrative, technical, or logistic support
William Kalsbeek: Conception and design (Johnston County Osteoarthritis Project sampling design); Critical revision of the article for important intellectual content; Final approval of the article; Statistical expertise
Gheorghe Luta: Conception and design (statistical methods of reported study); Critical revision of the article for important intellectual content; Final approval of the article; Statistical expertise; Administrative, technical, or logistic support
Joanne Jordan: Conception and design (Johnston County Osteoarthritis Project); Analysis and interpretation of data; Critical revision of article for important intellectual content; Final approval of the article; Provision of study material or patients; Collection and assembly of data; obtaining of funding; Administrative, technical, or logistic support.
References
- 1.Salaffi F, Carotti M, Stancati A, Grassi W. Health-related quality of life in older adults with symptomatic hip and knee osteoarthritis: a comparison with matched healthy controls. Aging clinical and experimental research. 2005 Aug;17(4):255–263. doi: 10.1007/BF03324607. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. The Journal of bone and joint surgery. 2001 Nov;83-A(11):1622–1629. doi: 10.2106/00004623-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Agency for Healthcare Research and Quality HCUP Nationwide Inpatient Sample (NIS) 2007 National statistics - principal procedure only. Outcomes by 81.51 Total Hip Replacement 2009. 2009 http://hcupnet.ahrq.gov/
- 4.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis and rheumatism. 2008 Sep 15;59(9):1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feuer EJ, Wun LM, Boring CC, Flanders WD, Timmel MJ, Tong T. The lifetime risk of developing breast cancer. Journal of the National Cancer Institute. 1993 Jun 2;85(11):892–897. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Wilson PW, Larson MG, et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. The American journal of cardiology. 2004 Jul 1;94(1):20–24. doi: 10.1016/j.amjcard.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. Jama. 2003 Oct 8;290(14):1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 8.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. The Journal of rheumatology. 2009 Apr;36(4):809–815. doi: 10.3899/jrheum.080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan JM, Linder GF, Renner JB, Fryer JG. The impact of arthritis in rural populations. Arthritis Care Res. 1995 Dec;8(4):242–250. doi: 10.1002/art.1790080407. [DOI] [PubMed] [Google Scholar]
- 10.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. The Journal of rheumatology. 2007 Jan;34(1):172–180. [PubMed] [Google Scholar]
- 11.Kellgren JL, J Radiological assessment of osteoarthrosis. Annals of the rheumatic diseases. 1957:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birrell F, Lunt M, Macfarlane GJ, Silman AJ. Defining hip pain for population studies. Annals of the rheumatic diseases. 2005 Jan;64(1):95–98. doi: 10.1136/ard.2003.018788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson BH, Bilgrad R. Use of the National Death Index in cancer studies. Journal of the National Cancer Institute. 1986 Oct;77(4):877–881. [PubMed] [Google Scholar]
- 14.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. American journal of epidemiology. 1994 Dec 1;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 15.Research Triangle Institute SUDAAN user manual release 8.0. pp. 543–624.
- 16.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2005 Sep;13(9):769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Ali-Gombe A, Croft PR, Silman AJ. Osteoarthritis of the hip and acetabular dysplasia in Nigerian men. The Journal of rheumatology. 1996 Mar;23(3):512–515. [PubMed] [Google Scholar]
- 18.Solomon L, Beighton P, Lawrence JS. Osteoarthrosis in a rural South African Negro population. Annals of the rheumatic diseases. 1976 Jun;35(3):274–278. doi: 10.1136/ard.35.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tepper S, Hochberg MC. Factors associated with hip osteoarthritis: data from the First National Health and Nutrition Examination Survey (NHANES-I) American journal of epidemiology. 1993 May 15;137(10):1081–1088. doi: 10.1093/oxfordjournals.aje.a116611. [DOI] [PubMed] [Google Scholar]
- 20.Emejuaiwe N, Jones AC, Ibrahim SA, Kwoh CK. Disparities in joint replacement utilization: a quality of care issue. Clinical and experimental rheumatology. 2007 Nov-Dec;25(6 Suppl 47):44–49. [PubMed] [Google Scholar]
- 21.Chen JT, Kaddour A, Krieger N. Implications of missing income data. Public Health Rep. 2008 May-Jun;123(3):260. doi: 10.1177/003335490812300303. author reply 260-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Prevalence and burden of osteoarthritis: results from a population survey in Norway. The Journal of rheumatology. 2008 Apr;35(4):677–684. [PubMed] [Google Scholar]
- 23.Bierma-Zeinstra SM, Koes BW. Risk factors and prognostic factors of hip and knee osteoarthritis. Nature clinical practice. 2007 Feb;3(2):78–85. doi: 10.1038/ncprheum0423. [DOI] [PubMed] [Google Scholar]
- 24.Cooper C, Inskip H, Croft P, et al. Individual risk factors for hip osteoarthritis: obesity, hip injury, and physical activity. American journal of epidemiology. 1998 Mar 15;147(6):516–522. doi: 10.1093/oxfordjournals.aje.a009482. [DOI] [PubMed] [Google Scholar]
- 25.Karlson EW, Mandl LA, Aweh GN, Sangha O, Liang MH, Grodstein F. Total hip replacement due to osteoarthritis: the importance of age, obesity, and other modifiable risk factors. The American journal of medicine. 2003 Feb 1;114(2):93–98. doi: 10.1016/s0002-9343(02)01447-x. [DOI] [PubMed] [Google Scholar]
- 26.Wendelboe AM, Hegmann KT, Biggs JJ, et al. Relationships between body mass indices and surgical replacements of knee and hip joints. American journal of preventive medicine. 2003 Nov;25(4):290–295. doi: 10.1016/s0749-3797(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 27.Lohmander LS, de Verdier M Gerhardsson, Rollof J, Nilsson PM, Engstrom G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Annals of the rheumatic diseases. 2009 Apr;68(4):490–496. doi: 10.1136/ard.2008.089748. [DOI] [PubMed] [Google Scholar]
- 28.Lievense AM, Bierma-Zeinstra SM, Verhagen AP, van Baar ME, Verhaar JA, Koes BW. Influence of obesity on the development of osteoarthritis of the hip: a systematic review. Rheumatology (Oxford) 2002 Oct;41(10):1155–1162. doi: 10.1093/rheumatology/41.10.1155. [DOI] [PubMed] [Google Scholar]
- 29.Flugsrud GB, Nordsletten L, Espehaug B, Havelin LI, Engeland A, Meyer HE. The impact of body mass index on later total hip arthroplasty for primary osteoarthritis: a cohort study in 1.2 million persons. Arthritis and rheumatism. 2006 Mar;54(3):802–807. doi: 10.1002/art.21659. [DOI] [PubMed] [Google Scholar]
- 30.Flugsrud GB, Nordsletten L, Espehaug B, Havelin LI, Meyer HE. Risk factors for total hip replacement due to primary osteoarthritis: a cohort study in 50,034 persons. Arthritis and rheumatism. 2002 Mar;46(3):675–682. doi: 10.1002/art.10115. [DOI] [PubMed] [Google Scholar]
- 31.Flugsrud GB, Nordsletten L, Espehaug B, Havelin LI, Meyer HE. Weight change and the risk of total hip replacement. Epidemiology (Cambridge, Mass. 2003 Sep;14(5):578–584. doi: 10.1097/01.ede.0000081800.83206.92. [DOI] [PubMed] [Google Scholar]
- 32.Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by U.S. adults. Epidemiology (Cambridge, Mass. 1995 Jan;6(1):61–66. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Demerath EW, Li J, Sun SS, et al. Fifty-year trends in serial body mass index during adolescence in girls: the Fels Longitudinal Study. Am J Clin Nutr. 2004 Aug;80(2):441–446. doi: 10.1093/ajcn/80.2.441. [DOI] [PubMed] [Google Scholar]
- 34.Knapik JJ, Sharp MA, Darakjy S, Jones SB, Hauret KG, Jones BH. Temporal changes in the physical fitness of US Army recruits. Sports Med. 2006;36(7):613–634. doi: 10.2165/00007256-200636070-00005. [DOI] [PubMed] [Google Scholar]
- 35.Hart DJ, Spector TD. Radiographic criteria for epidemiologic studies of osteoarthritis. J Rheumatol Suppl. 1995 Feb;43:46–48. [PubMed] [Google Scholar]
- 36.Ingvarsson T, Hagglund G, Lindberg H, Lohmander LS. Assessment of primary hip osteoarthritis: comparison of radiographic methods using colon radiographs. Annals of the rheumatic diseases. 2000 Aug;59(8):650–653. doi: 10.1136/ard.59.8.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arden NK, Lane NE, Parimi N, et al. Defining incident radiographic hip osteoarthritis for epidemiologic studies in women. Arthritis and rheumatism. 2009 Apr;60(4):1052–1059. doi: 10.1002/art.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reijman M, Hazes JM, Pols HA, Bernsen RM, Koes BW, Bierma-Zeinstra SM. Validity and reliability of three definitions of hip osteoarthritis: cross sectional and longitudinal approach. Annals of the rheumatic diseases. 2004 Nov;63(11):1427–1433. doi: 10.1136/ard.2003.016477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reijman M, Hazes JM, Pols HA, Bernsen RM, Koes BW, Bierma-Zeinstra SM. Role of radiography in predicting progression of osteoarthritis of the hip: prospective cohort study. Bmj. 2005 May 21;330(7501):1183. doi: 10.1136/bmj.38442.457488.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingvarsson T, Hagglund G, Lohmander LS. Prevalence of hip osteoarthritis in Iceland. Annals of the rheumatic diseases. 1999 Apr;58(4):201–207. doi: 10.1136/ard.58.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis and rheumatism. 1995 Aug;38(8):1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 42.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. The Journal of rheumatology. 2000 Jun;27(6):1513–1517. [PubMed] [Google Scholar]
- 43.Beiser A, D’Agostino RB, Sr., Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Statistics in medicine. 2000 Jun 15-30;19(11-12):1495–1522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 44.Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. Jama. 2002 Feb 27;287(8):1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 45.U. S. Census Bureau. DP-1 . Johnston County; North Carolina: General Population and Housing Characteristics: 1990. http://factfinder.census.gov/servlet/QTTable?_bm=n&_lang=en&qr_name=DEC_1990_STF1_DP1&ds_name=DEC_1990_STF1_&geo_id=05000US37101. [Google Scholar]
- 46.U. S. Census Bureau. DP-1 . United States: General Population and Housing Characteristics: 1990. http://factfinder.census.gov/servlet/QTTable?_bm=n&_lang=en&qr_name=DEC_1990_STF1_DP1&ds_name=DEC_1990_STF1_&geo_id=05000US37101. [Google Scholar]
- 47.Centers for Disease Control and Prevention . National health and Nutrition Examination Survey III (NHANES III) (1988-1994) National Center for Health Statistics; 2007. [Google Scholar]
- 48.Fackelmann K. Refiguring the odds: what’s a woman’s real chance of suffering breast cancer? Science News. 1993;Vol 144:76–77. [Google Scholar]