Abstract
Introduction
Vitamin D is a fat soluble hormone necessary for calcium homeostasis. Recently studies have demonstrated that vitamin D may be important to the health of the cardiovascular system.
Methods
Adults ≥ 50 years with HF were recruited for assessment of serum 25-Hydroxyvitamin D (25OHD) concentrations. Cardiopulmonary exercise testing was used to assess functional capacity. Proximal muscle strength was evaluated with a Biodex leg press and health status was assessed with the Kansas City Cardiomyopathy Questionnaire (KCCQ). Univariate associations between physical performance and health status measures and 25OHD followed by a linear regression model were used to study associations, adjusting for other potential explanatory variables.
Results
Forty adults age 67.8 ±10.9 yrs (55% women and 57.5% AA) with mean EF 40% were analyzed (NYHA II in 70% and III in 30%). Co-morbidities included 77.5% HTN, 47.5% diabetes. The mean 25OHD concentration was 18.5 ± 9.1 ng/ml, mean peak VO2 14 ± 4 ml/kg/min. In univariate regression analysis, 25OHD was positively associated with peak VO2 (p=0.045). Multivariable regression analysis sustained positive association between 25OHD and peak VO2 (p=.044), after adjusting for age, race, and respiratory exchange ratio (adjusted R2 = 0.32). Association between proximal muscle strength with the 25OHD concentration was not significant. The KCCQ physical limitation domain score was negatively associated with 25OHD (p=0.04) but was not sustained in multivariable analysis.
Conclusion
25OHD may be an important marker or modulator of functional capacity in patients with HF. Randomized controlled trials are needed to assess the effect of vitamin D repletion on functional performance.
Keywords: Vitamin D, Heart Failure, Functional Capacity
Introduction
Heart failure (HF) can be a debilitating syndrome and is well known to cause functional limitations. Decreased cardiopulmonary reserve, abnormalities in muscle structure and function and neuroendocrine derangements all contribute to a decline in physical performance. Symptoms of fatigue and dyspnea with exercise do not consistently correlate with resting hemodynamic parameters.(1) Coats et al. proposed the muscle hypothesis which integrates the skeletal muscle myopathy resulting from hormonal dysregulation and sympathetic-excitation into the HF syndrome. This hypothesis has been important to understanding the physiologic changes, loss of muscle strength and functional limitations in patients with HF.(2) Furthermore, muscle strength has been found to be predictive of adverse outcomes.(3) Despite this, it continues to be unclear how to improve physical performance in patients with HF, beyond standard therapies such as medications and/or aerobic training.
Vitamin D deficiency has recently been found to be prevalent in those with cardiovascular diseases such as coronary disease and HF in a national sample (4) as well as associated with cardiovascular risk, and events, including HF. (5–7) There is evidence that vitamin D is associated with, and, may down-regulate inflammatory mediators,(8, 9) and promote cell growth and differentiation.(10) Vitamin D may be of particular importance in patients with HF since there is evidence that it down-regulates the renin-angiotensin system(11–13) and reduces blood pressure.(14)
In terms of function, studies have reported on the relationship of vitamin D concentrations and physical performance, with worse performance in those with lower 25OHD concentrations.(15–17) The most rapidly growing group of patients with HF are older adults and since older adults are prone to vitamin D deficiency-related syndromes such as osteomalacia and osteoporosis, they may also be the most at risk for cardiovascular related effects of vitamin D deficiency. Recently, we demonstrated that in patients with HF, low concentration of 25OHD is associated with frailty and a shorter 6 minute walk distance.(16) Impaired walking has also been reported in association with low serum 25OHD concentrations in non-HF populations.(17) Identification of the role 25OHD in functional decline and HF progression remains elusive. Whether vitamin D serves simply as a marker of poor health and nutrition, vs. a mediator of both muscle and cardiovascular function remains unclear.
To further evaluate the relationship of 25OHD concentrations with physical performance in HF, we assessed the cross-sectional relationship between 25OHD, functional capacity and muscle strength in older adults with HF.
Methods
The study was approved by the Institutional Review Board at University Hospitals/Case Medical Center. Participants were recruited from the heart failure and general cardiology practices at both the tertiary care site and satellite clinics. The study is a double blinded, randomized controlled trial of cholecalciferol vs. placebo. Baseline data is presented in this manuscript. Inclusion criteria included age 50 or older with either systolic or preserved systolic function HF, NYHA class II–IV at the time of study enrollment, maximum tolerated doses of HF medications as per the primary cardiologist before enrollment into the study, 25OHD concentration ≤37.5 ng/ml. Potential participants were excluded for primary hyperparathyroidism or hypercalcemia, nephrolithiasis, a diagnosis of osteoporosis, hemo or peritoneal dialysis and/or creatinine of > 2.5mg/dL, current use of daily vitamin D greater than 400 IU, corticosteroids, PTH, androgen or estrogen, current illicit drug user or ≥ 3 alcoholic drinks a day, metastatic or advanced cancer , myocardial infarction in the preceding 6 months. Also excluded were individuals who were on medications which could lower 25OHD concentrations or bioavailability of oral vitamin D administration including: ketoconazole, colestipol, cholestyramine, mineral oil, phenobarbitol, and phenytoin. Screening for inclusion into the study was conducted as a two step process. Initially, volunteers were screened by medical history for the exclusion criteria listed above, and if qualified, a serum 25OHD concentration was collected.
Measures
Cardiopulmonary Stress Testing (CPX)
CPX for functional capacity was performed using a Modified Naughton protocol. (Medical Graphics, St Paul, Mn) Breath by breath on-line gas measurements were obtained at resting baseline and throughout the exercise protocol to measure minute ventilation (VE), tidal volume (VT), respiratory rate, oxygen uptake (VO2), and carbon dioxide production (VCO2).(18) Peak VO2 was defined as the highest VO2 in the last minute of symptom-limited exercise. Ventilatory threshold (VT) defined as the VO2 at which anaerobic metabolism prevails in the periphery was measured by the V-slope method.(19) The Borg scale was used to assess patient effort and patients were encouraged to Rating of Perceived Exertion (RPE)>15 (medium -hard)(20) and a respiratory exchange ratio (RER= VCO2/VO2) > 1.05. RER serves as a measure of effort. On-line ECG monitoring was obtained in addition to standard 12-lead electrocardiograms at rest and at the end of each stage of exercise. Gas exchange collection continued into recovery with 1 minute of active cool down at 1.5 mph, 0% elevation followed by resting while sitting in a chair until a return to baseline VO2 is observed or up to 6 minutes.
Isokinetic Muscle Testing
Muscular strength and power were assessed in the dominant leg using the Biodex System 3 pro isokinetic dynamometer (Biodex, Shirley, NY). The protocol was designed specifically for the older adult and prior to testing each subject was given proper instruction on how to breathe correctly during testing and to avoid valsalva breath- holding during the exercise. Quadriceps strength during knee extension and hamstring strength during knee flexion were measured with the subject in a seated position over a range of motion of ~90° using a speed of 60°s−1. Muscle strength was assessed as peak torque in Newton-meters (Nm) and also peak torque/body weight represented as Nm/kg.
Serum Analysis
Blood was collected and stored at 2–8ºC. 25OHD was measured by chem-illuminescence immunoassay (ARUP Salt Lake City, Utah) with an intra-assay CV of 3 and 6% and a between assay variability of 6 to 11%. Parathyroid Hormone was measured by chemlumuometric technology (Siemens Dimension Vista Systems, Newark, DE by University Hospitals clinical laboratory). Creatinine, BUN, albumin, calcium were measured at University Hospitals clinical laboratory.
Kansas City Cardiomyopathy Questionnaire
The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a 23-item self-administered instrument that gives an overall clinical and summary score but is also divided into four subscales (domains) including: symptoms (frequency, severity and recent change over time), physical limitations, social functioning, and quality of life. The sensitivity of the KCCQ has been shown to be greater than that of the Minnesota Living with HF and the SF-36 questionnaires.(21) The questionnaire takes approximately 6–8 minutes to complete. Scores range from 0–100; higher scores reflect better health status.
Statistical Analyses
Pearson’s correlation coefficient was used to assess the associations between the physical performance measures and 25OHD. A level of ≤ .05 was considered significant. Following the significant associations revealed by Pearson’s correlation coefficient, we used a linear regression model to study these associations after adjusting for other potential explanatory variables. To study the association between 25OHD and peak VO2 (39 subjects successfully completed CPX), we modeled the log (natural) transformed peak VO2 to better meet normality assumptions of the linear regression model. Predictors which reached the 0.1 level of significance in univariate analysis were included in multivariable regression analysis. All potential pair wise interactions were tested and were not significant. All analyses were conducted using SAS software (Version 9.2, SAS Institute Inc, Cary, NC).
Study Support
The study was supported by an American Heart Association Scientist Development Grant and the KL2 at Case Western Reserve University. The authors are solely responsible for the design and conduct of the study, all study analyses and drafting and editing of the paper.
Results
Two hundred and seventeen patients with symptomatic HF, including systolic dysfunction and preserved systolic function, were screened for enrollment. The majority of patients screen – failed (29%) due to taking disqualifying medications including >400 IU of vitamin D a day. In addition, 12% had a creatinine >2.5 mg/dL, 12% had a history of kidney stones or osteoporosis, 8% were NYHA class 1, and 10% were unable to walk on a treadmill or ride a bicycle due to mobility problems. Forty patients (mean age 67.8±10.9, 55% women and 57.5% AA; 67.5% non-ischemic) were enrolled in the study as shown in Table 1. The mean EF was 40 ±14%, 70% were NYHA class II and 30% were NYHA class III. Comorbidities included 77.5% with hypertension and 47.5 % with diabetes. Mean serum 25OHD concentration was 18.5±9.1 ng/ml. 25OHD varied by race with AA having significantly lower 25OHD concentration than Caucasians (16 ng/ml vs. 22 ng/ml; p=0.04). 25OHD concentration did not vary by age (<65, vs. ≥ 65) BMI (< 30 vs. ≥ 30) or sex.
Table I.
Characteristics of the Study Participants (n=40)
| Age(yrs), mean (SD) | 67.8 ± 10.9 |
| Sex, n (%) | |
| Males | 18 (45) |
| Females | 22 (55) |
| Race, n (%) | |
| African American | 23 (57.5) |
| White | 17 (42.5) |
| BMI (kg/m2), mean (SD) | 33.5 ± 7.6 |
| Cause of HF, n (%) | |
| Ischemic | 13 (32.5) |
| Non-Ischemic | 27 (67.5) |
| NYHA Class, n (%) | |
| II | 28 (70) |
| III | 12 (30) |
| Years with HF, mean (SD) | 7.1 ± 6.4 |
| Ejection Fraction, n (%) | 39.5 ± 13.8 |
| Diabetes n (%) | 19 (47.5) |
| Stroke n (%) | 5 (12.5) |
| Pulmonary Disease n (%) | 19 (47.5) |
| Hyperlipidemia n (%) | 35 (87.5) |
| Peripheral Vascular Disease n (%) | 2 (5) |
| Hypertension n (%) | 31 (77.5) |
| Stroke n (%) | 5 (12.5) |
| Current Smokers n (%) | 6 (15) |
| Serum Tests | |
| Calcium (mg/dL) | 9.2 ± .4 |
| Albumin (g/dL) | 4.0 ± .3 |
| Phosphorus (mg/dL) | 3.5 ± .5 |
| Parathyroid hormone (pg/mL) | 61.8 ± 40.6 |
| Serum BUN (mg/dL) | 24 ± 11.2 |
| Serum Creatinine (mg/dL) | 1.2 ± .4 |
| Serum 25OHD (ng/mL) | 18.5 ± 9.1 |
| Medications* | |
| ACE Inhibitor, n (%) | 25 (62.5) |
| Enalapril EQ dose (mg) | 30.8 |
| Angiotensin Inhibitor Blocker, n (%) | 11 (27.5) |
| Valsartan EQ dose (mg) | 210.2 |
| Beta Blocker, n (%) | 34 (85) |
| Metoprolol EQ dose (mg) | 133.8 |
| Loop Diuretic, n (%) | 30 (75) |
| Furosemide EQ dose (mg) | 54.72 |
| Aldosterone Antagonist, n (%) | 10 (25) |
| Spironolactone dose (mg) | 19.8 |
Doses are given in mean daily dose and converted to a standard medication.
The mean peak VO2 was 14±4 ml/kg/min with a mean RER of 0.96 (range 0.76–1.12). The mean VE/VCO2 was 34.4 ± 7.6 indicating a mildly impaired ventilatory efficiency within the group. Peak torque adjusted for body weight in healthy populations usually reflects better strength in men than women. In this cohort of patients there was no statistical difference in peak torque between men and women. The KCCQ scores showed the lowest score at 65.4 ± 26 for the physical limitation domain to the highest of 75.8 ± 21.2 for the total symptom score. (Table II)
Table II.
Functional and Health Status Testing
| Cardiopulmonary Stress Test* mean (SD) | ||
| Peak VO2 (mL/kg/min) | 14 ± 4 | |
| Men | 14.8 ± 4.7 | |
| Women | 13.4 ± 3.4 | |
| Percent Predicted VO2 | 68.6 ± 18.9 | |
| Peak Exercise VE/VCO2 | 34.4 ± 7.6 | |
| Respiratory Exchange Ratio | .96 ± 0.1 | |
| Ventilatory Threshold** (mL/kg/min) | 13.8 ± 3.9 | |
| Exercise Time (min) | 8.5 ± 8.2 | |
| Isokinetic Muscle Testing at 60°s−1 | ||
| Peak Torque§(Nm/kg) | Extension | Flexion |
| Men (n=18) | 38.3±14.6 | 17.1±6.7 |
| Women (n= 21) | 35.1±12.5 | 16.1±6.1 |
| Kansas City Cardiomyopathy Questionnaire | ||
| Overall Summary Score | 68.9 ± 22.9 | |
| Subscales | ||
| Physical Limitation Score | 65.4 ± 26 | |
| Symptom Score | 75.8 ± 21.2 | |
| Quality of Life Score | 66 ± 27.4 | |
| Social Limitation Score | 67.6 ± 28.4 | |
Thirty-nine participants had successful CPX testing
Twenty-two participants attained their VT
adjusted for body weight
In univariate analysis predictors of peak VO2 [log(natural) transformed] assessed were age, race, serum 25OHD, RER, HF etiology, EF, BMI, and sex. There was statistically significant positive association of 25OHD with peak VO2 (p=0.045). (Figure I) Predictors which reached the 0.1 level of significance in univariate analysis were included in multivariable regression analysis. Serum 25OHD maintained an independent relationship with peak VO2. (Table III) (Adjusted R2=0.32) This model explained 32% of the variability in peak VO2. Based on this regression model, for every 5 unit (ng/ml) increase in serum 25OHD, the VO2 increased by 4.6%.
Figure 1.
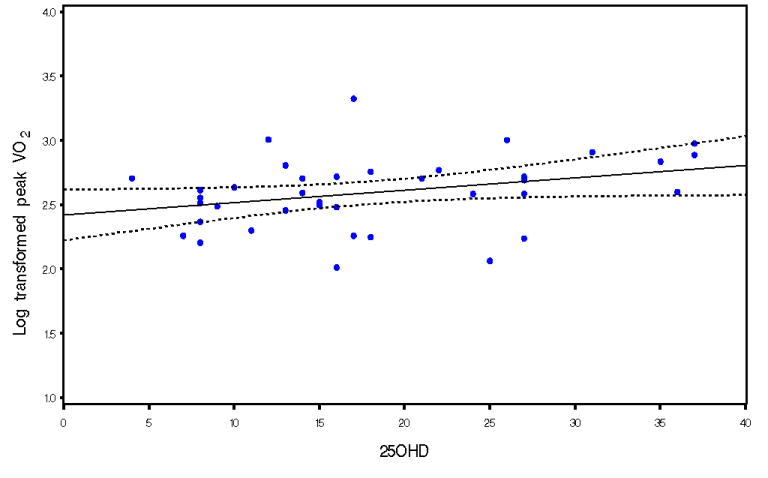
Univariate relationship between log transformed peak VO2 and 25OHD
Table III.
Univariate and Multivariable Analysis for the log(natural) transformed Peak VO2 as the Dependant Variable
| Univariate Regression Analysis | Multivariable Regression Analysis* | |||
|---|---|---|---|---|
| Variable | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value |
| Age | 0.993 (0.985,1.001) | 0.08 | 0.995 (0.987,1.004) | 0.26 |
| Race** | 1.169 (0.978,1.40) | 0.08 | 1.107 (0.924,1.325) | 0.26 |
| 25OHD | 1.009 (1.000,1.019) | 0.045 | 1.009 (1.000,1.018) | 0.044 |
| RER | 3.258 (1.413,7.514) | 0.007 | 2.614 (1.077,6.348) | 0.035 |
| HF Etiology† | 0.822 (0.662, 0.991) | 0.04 | 0.891 (0.747, 1.061) | 0.188 |
| EF | 0.997 (0.991,1.004) | 0.40 | R2=0.41; Adjusted R2=0.32 | |
| BMI | 0.994 (0.982,1.005) | 0.31 | ||
| Sex§ | 0.918 (0.765,1.101) | 0.35 | ||
Variables which reached p≤0.1 in univariate analysis were included in the multivariable analysis.
Reference group: African American
Reference group: ischemic
Reference group: males
From the KCCQ, serum 25OHD concentrations were correlated with the physical limitation score (r=0.32, p=0.04). Univariate regression analysis showed that the BMI, 25OHD, sex, race, NYHA class, and etiology of HF (ischemic or non-ischemic) were associated with the KCCQ physical limitation score at a 0.1 level of significance and were therefore included in the multivariable regression model. In multivariable regression modeling only race (AA having lower scores) and the NYHA class (lower class with higher scores) remained significant (p=0.01; p<.001; adjusted R2 = 0.40). This model explained 40% of the variance in the KCCQ physical limitation score.
Measure of isokinetic muscle strength (peak torque adjusted for body weight in flexion) showed no association with serum 25OHD concentration. Older age trended toward significance at p=0.08, with older age relating to lower peak torque. Univariate analysis of race, sex, EF and 25OHD concentration were not significantly associated with peak torque.
Discussion
In a small group of older HF patients, we found that serum 25OHD concentrations ≤37.5ng/ml were associated with peak VO2 as a measure of functional capacity but not with isokinetic muscle strength. In addition, the KCCQ physical limitation domain was associated with 25OHD concentrations only in univariate analysis. This relationship was lost with multivariable modeling. We have previously demonstrated an association between 25OHD concentrations with the 6-minute walk distance and frailty in a cohort of patients with HF.(16) This study expands that observation to include peak VO2, the gold-standard measure of functional capacity. The relationship between VO2 and 25OHD level was shown previously in HF patients who were NYHA class III or IV and referred for cardiac transplantation.(22) This observed association expands what was seen previously in evaluating an older more heterogeneous population in sex and race, as well as HF severity.
The participants in this study had either systolic or preserved systolic HF with functional limitations measured by NYHA classification. We choose to be inclusive of both types of heart failure since both are equally disabling and have abnormal peak VO2 (23), albeit systolic failure incurs a higher risk of mortality.(24) Including HF-PSF is important in studies of older adults since at least 50% of older patients will have preserved systolic function.(25) In addition, the interaction that vitamin D may have on the renin-angiotensin system(RAAS) (13, 26) would not be specific to the ejection fraction, in that both types of HF have RAAS activation.
In the patient with HF, 25OHD concentrations may be directly impacted by their exposure to sunlight. Patients with low VO2 often have low activity levels and therefore may spend less time out of doors exposed to UVB light. From this study there is know way to know if having HF and low physical activity/sun exposure is lowering the 25OHD level and uninvolved in the pathophysiology of HF, or if 25OHD concentrations are directly involved in worsening the functional capacity. Lab work in vitamin D has demonstrated direct myocardial effects of vitamin D including regulation of extracellular matrix protein turnover, calcium flux and myocardial contractility, and antiproliferative and hypertrophic effects. (27) Although, much of this work has been done with 1,25(OH)2D.
Our findings of lower concentration of 25OHD in the African American patients in this cohort are consistent with the National Health and Nutrition Examination Survey (NHANES). The NHANES report comparing 1988 to 2004 has noted that although concentrations of 25OHD are remaining constant in the general population overall, African Americans are an at-risk subgroup in which serum 25OHD concentrations have become more deficient over time.(28) The predominance of African American participants in our cohort is important when examining the association of vitamin D with heart disease. Vitamin D deficiency has been implicated as having influence over the hypertensive patient, increasing risk for adverse cardiovascular outcome.(7) Further, vitamin D deficiency may confer more risk for disease and cardiovascular mortality in African Americans than other racial groups.(29, 30) This disparity indicates a considerable and reversible public health challenge which requires further evaluation in a diverse patient population.
The deficiency of vitamin D prompts the question of repletion. Improving function with vitamin D repletion was recently studied in a 20 week randomized placebo controlled trial of 105 patients with systolic HF, mean age 78.9 years, and selected for low 25OHD concentrations.(31) The mean baseline 25OHD concentration in the treatment group increased from 8.21 ng/ml to 17.4 ng/ml with 2 oral doses of 100,000 IU of ergocalciferol (vitamin D2) given at baseline and again at 10 weeks. The study failed to show a change in physical performance outcome measure of the 6-minute walk test. The 25OHD concentration achieved in this study is unlikely an adequate change in the 25OHD concentration to see a response in physical performance. Similarly, a lack of improvement in VO2 was seen in a study of 123 adults with HF (83% men, race not reported) who were supplemented with 2000 IU cholecalciferol.(32) The mean concentration of 25OHD achieved was 26 ng/ml, at this concentration there was a reduction in parathyroid hormone (PTH) and inflammatory mediators indicating a physiologic effect but this concentration may be too low to impact a change in functional capacity which is noted by the authors. A drop in the concentration of PTH in and of itself may be beneficial to reduce cardiovascular risk, disease and related mortality.(33, 34) Although, there was no relationship between PTH and reported outcomes in this study.
An adequate 25OHD concentration for improved physical function is between 16 ng/ml and 38 ng/ml.(15) For reducing cardiovascular risk there is evidence that the optimum level is above 40ng/ml.(35) Studies of other disease states including bone mineral density have found adequate 25OHD concentration to be approximately 30ng/ml for physiologic benefit.(36) In addition in a review of physical performance in older adults with vitamin D supplementation found that studies which included calcium supplementation had a positive effect over studies which gave vitamin D alone.(37)
The measure of health status, the KCCQ, did not show a significant relationship with the 25OHD concentration except for the physical limitation subscale in univariate analysis. Clearly the most powerful predictor of the physical limitation score is the functional class which is clinically appropriate and expected.(38) Race was also a significant predictor in modeling. African American patients are also known to have worse physical function scores when health status is measured.(39, 40) This finding requires further evaluation in older AA patients with HF.
There was no relationship with isokinetic muscle strength and 25OHD concentrations in HF patients. This was unexpected in that vitamin D deficiency has known skeletal muscle effects with severe vitamin D deficiency characterized by proximal muscle weakness and pain. Particularly older adults who are deficient in vitamin D have muscle weakness, decreased muscle mass, and are prone to falls.(15, 26, 41) Poor physical performance represented by slowed walk time, decreased grip strength, and measures of frailty has been correlated with vitamin D deficiency in both HF and non-HF populations.(17, 26, 37) Due to the limits of cross-sectional study design, measures of muscle strength with vitamin D repletion in functionally limited patients is still warranted since the relationship between skeletal muscle and vitamin D is so well established.
Study Limitations
This study is limited by the cross-sectional design and therefore causality cannot be determined. Nonetheless, the results are hypothesis generating and clinical trials of vitamin D therapy in HF should assess the response of functional capacity in the older adult. This study did not demonstrate a relationship between health status or muscle strength and the serum concentration of 25OHD. This may be due to the small sample size and includes a large variance making statistical significance less likely.
Clinical Implications
The results of this study add to a growing body of clinical evidence that vitamin D may have influence over the health of the cardiovascular system. This pilot study begins to draw together the better known effect of vitamin D on the musculoskeletal system with emerging evidence of the relationship of vitamin D to the cardiovascular system. This study itself may not change clinical practice in patients with HF, but it is provocative and provides a basis for further research. Should these associations be directly related to causation, the repletion of Vitamin D in the elderly population with HF could have a broad public health impact. Furthermore, recommendations to maintain adequate vitamin D status are important to multiple physiologic processes and perhaps to decrease the risk of cancer. Testing of vitamin D status and repletion should be considered as part of standard clinical practice. (42)
Conclusion
Patients with HF may have an increase vulnerability to vitamin D deficiency due to the disease process which effects cardiopulmonary reserve and skeletal muscle function. Although there was not evidence from this cross-sectional analysis that there is an association with isokinetic muscle strength and 25OHD concentrations, the association with peak VO2 may indicate a more complicated relationship with the cardiovascular system.
Supplementary Material
Acknowledgments
Dr. Boxer and this work are supported by the KL2RR024990 and in part by the American Heart Association Scientist Development Grant 0635055N. The project described was supported by the Clinical and Translational Science Collaborative (CTSC) and utilized the REDCap database. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson JR, Rayos G, Yeoh TK, Gothard P, Bak K. Dissociation between exertional symptoms and circulatory function in patients with heart failure. Circulation. 1995;92(1):47–53. doi: 10.1161/01.cir.92.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Coats AJ. The “muscle hypothesis” of chronic heart failure. J Mol Cell Cardiol. 1996;28(11):2255–62. doi: 10.1006/jmcc.1996.0218. [DOI] [PubMed] [Google Scholar]
- 3.Hulsmann M, Quittan M, Berger R, et al. Muscle strength as a predictor of long-term survival in severe congestive heart failure. Eur J Heart Fail. 2004;6(1):101–7. doi: 10.1016/j.ejheart.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004) Am J Cardiol. 2008;102(11):1540–4. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 7.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller RR, Hicks GE, Shardell MD, et al. Association of serum vitamin D levels with inflammatory response following hip fracture: the Baltimore Hip Studies. J Gerontol A Biol Sci Med Sci. 2007;62(12):1402–6. doi: 10.1093/gerona/62.12.1402. [DOI] [PubMed] [Google Scholar]
- 9.Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R. Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab. 2003;88(10):4623–32. doi: 10.1210/jc.2003-030358. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 11.Kong J, Qiao G, Zhang Z, Liu SQ, Li YC. Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int. 2008;74(12):1577–81. doi: 10.1038/ki.2008.452. [DOI] [PubMed] [Google Scholar]
- 12.Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 2003;88(2):327–31. doi: 10.1002/jcb.10343. [DOI] [PubMed] [Google Scholar]
- 13.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86(4):1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 15.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80(3):752–8. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 16.Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008;56(3):454–61. doi: 10.1111/j.1532-5415.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 17.Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92(6):2058–65. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 18.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982;65(6):1213–23. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 19.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60(6):2020–7. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 20.Borg G. Borg's Perceived Exertion and Pain Scale. Champaign, Illinois: Human Kinetics; [Google Scholar]
- 21.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 22.Shane E, Mancini D, Aaronson K, et al. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med. 1997;103(3):197–207. doi: 10.1016/s0002-9343(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 23.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. Jama. 2002;288(17):2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 24.Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Krumholz HM. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41(9):1510–8. doi: 10.1016/s0735-1097(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 25.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–72. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 27.Pilz S, Tomaschitz A, Drechsler C, Dekker JM, Marz W. Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res. doi: 10.1002/mnfr.200900474. [DOI] [PubMed] [Google Scholar]
- 28.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med. 2010;8(1):11–8. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P. 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol. 2009;20(12):2631–9. doi: 10.1681/ASN.2009030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin d supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2010;3(2):195–201. doi: 10.1161/CIRCHEARTFAILURE.109.907899. [DOI] [PubMed] [Google Scholar]
- 32.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 33.Hagstrom E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119(21):2765–71. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 34.Pilz S, Tomaschitz A, Drechsler C, et al. Parathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiography. Eur Heart J. 31(13):1591–8. doi: 10.1093/eurheartj/ehq109. [DOI] [PubMed] [Google Scholar]
- 35.May H, Anderson JL, Lappe DL, Horne BD, Bair TL, Muhlestein JB. Stratifying Cardiovascular Risk by Vitamin D Levels: What are the Optimal CutOffs. J Am Coll Cardiol. 2010;55:A59.E563. [Google Scholar]
- 36.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 37.Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J Am Geriatr Soc. 2003;51(9):1219–26. doi: 10.1046/j.1532-5415.2003.51405.x. [DOI] [PubMed] [Google Scholar]
- 38.Heidenreich PA, Spertus JA, Jones PG, et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47(4):752–6. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Kaul P, Lytle BL, Spertus JA, DeLong ER, Peterson ED. Influence of racial disparities in procedure use on functional status outcomes among patients with coronary artery disease. Circulation. 2005;111(10):1284–90. doi: 10.1161/01.CIR.0000157731.66268.E1. [DOI] [PubMed] [Google Scholar]
- 40.Spertus J, Safley D, Garg M, Jones P, Peterson ED. The influence of race on health status outcomes one year after an acute coronary syndrome. J Am Coll Cardiol. 2005;46(10):1838–44. doi: 10.1016/j.jacc.2005.05.092. [DOI] [PubMed] [Google Scholar]
- 41.Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62(4):440–6. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Souberbielle JC, Body JJ, Lappe JM, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev. 2010 doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


