SUMMARY
The vertebrate limb is a classical model for understanding patterning of three-dimensional structures during embryonic development. While decades of research have elucidated the tissue and molecular interactions within the limb bud required for patterning and morphogenesis of the limb, the cellular and molecular events that shape the limb bud itself have remained largely unknown. We show that the mesenchymal cells of the early limb bud are not disorganized within the ectoderm as previously thought, but instead are highly organized and polarized. Using time lapse video microscopy we demonstrate that cells move and divide according to this orientation. The combination of oriented cell divisions and movements drives the proximal-to-distal elongation of the limb bud necessary to set the stage for subsequent patterning and morphogenesis. These cellular events are regulated by the combined activities of the Wnt and FGF pathways. We show that Wnt5a/JNK is necessary for the proper orientation of cell movements and cell division. In contrast FGF/Mapk signalling pathway, emanating from the AER, does not regulate cell orientation in the limb bud but instead establishes a gradient of cell velocity enabling continuous rearrangement of the cells at the distal tip of limb.
RESULTS
Characterization of chick limb elongation
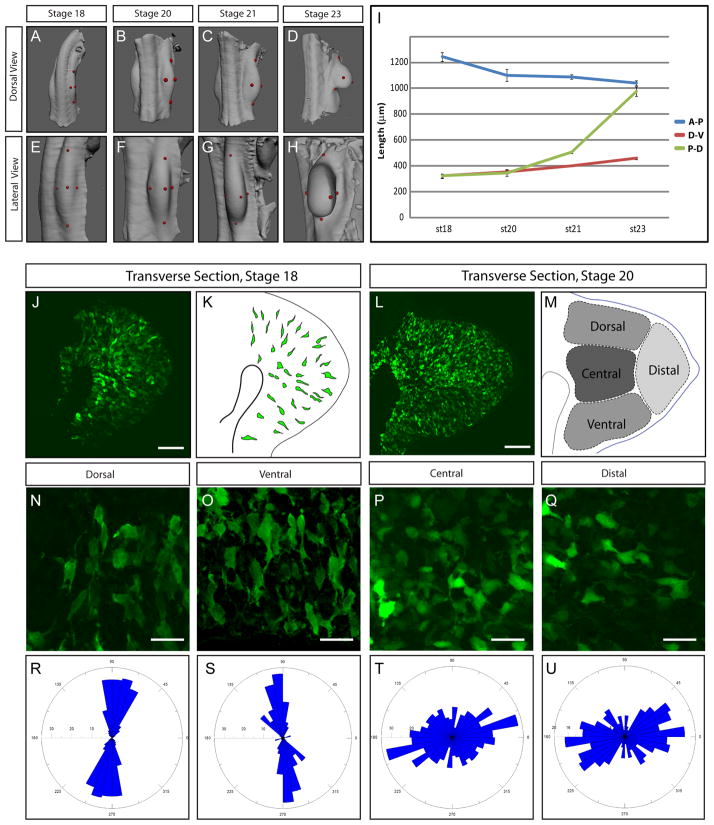
The limb bud forms as a mound of cells slightly elongated along the rostrocaudal axis of the embryo (The limb anteroposterior axis). As it grows, however the early limb bud does not persist as an elongated hemisphere but rather rapidly transforms into a paddle shape with an extended proximodistal axis. Attaining this shape of the progenitor field is critical for producing limb segments and skeletal elements of the correct size and shape. To understand the mechanisms that might be involved in this, we first characterized limb bud elongation at the tissue level. Using Optical Projection Tomography (OPT) we were able to accurately measure all three axis of the limb (Anterior-Posterior (A-P), Dorsal-Ventral (D-V) and Proximal-Distal (P-D)). Axis Measurements were performed on 3D reconstructed limbs of chick embryos at Hambuger and Hamilton (HH) stages 18, 20, 21 and 23 which cover about 24–30 hours of development (Movie S1 and Figure 1A–H). As expected we found that during this time window the P-D axis length increased dramatically (about 3 times). Surprisingly we found that the D-V axis length did not increase much while the A-P axis length actually decreased (Figure 1I). Since cells in the limb mesenchyme have been previously shown to uniformly proliferate at these stages [1][2][3], one would have expected all three axes to increase in length. The fact that the length of the P-D axis is the only one to dramatically increase suggests that differential rates in isotropic proliferation cannot explain limb shape. Cell death has been extensively studied in this context and has been shown to play a role refining the limb shape at later stages of this process. While cell death, known to be present in proximal anterior and posterior part of the limb bud, can explain the decrease in length of the (A-P) axis [3], it cannot account for absence of major growth of the D-V axis. Thus, this analysis strongly suggests that other oriented mechanisms within the limb bud must act to accentuate its growth preferentially along the P-D axis.
Figure 1. Characterization of Limb Bud Elongation at the Tissue and cellular Level.
(A–H) Three dimensional reconstructions of Optical Projection Tomography (OPT) acquisitions at the level of the limb bud of chick embryos at stages HH18 (A, E), 20 (B, F) 21 (C, G) and 23 (D, H) showing dorsal (A, B, C, D) and lateral views (E, F, G, H). The red dots show where the measurements were made, see Movie S1.
(I) Measurements of the length (in μM) of the antero-posterior (A-P, blue line), dorso-ventral (D-V, red line) and proximo-distal (P-D, green line) axes show that the limb bud elongates primarily in the P-D axis. A minimum of n=8 Limbs were analyzed for each time point, Error bars represent standard errors of the mean.
(J) Transverse section of an electroporated chick embryo at stage HH18 revealing the shape of GFP-expressing cells.
(K) Schematic representing the section shown in (D). A few GFP expressing cells have been outlined to show their orientation and elongated shape.
(L) Transverse section of an electroporated embryo at stage 20 showing the shape of GFP expressing cells (in green).
(M) Schematic representing four regions (dorsal, ventral, central and distal) of the section shown in (L).
(N–Q) Enlargements of the dorsal (N), ventral (O), central (P) and distal (Q) limb bud regions of a chick embryo at stage HH21 embryos showing the shape of the GFP-expressing cells.
(R–U) Quantification of the angle between the P-D axis of the limb bud and the longest axis of GFP expressing cell observed in the dorsal (R), ventral (S), central (T)_ and distal (U) regions at stage HH21. Angle of each cell longest axis is shown on a bidirectional Rosette graph which is divided into bins of 5°. The number of cells per bin is indicated on the radial axis. Quantifications were made on a total number of n=1468 cells with a minimum of n=300 cells for each area. Scale Bars in the lower right corner represent 50 μm (J, L) and 20 μm (R–U)
Mesenchymal cells of the limb bud are oriented
The early limb bud is generally conceptualized as an ectodermal bag containing a mound of uniformly distributed and randomly arranged mesenchymal cells. However we reasoned that if there were oriented cellular processes in the limb bud, they should be reflected in the organization of the mesenchymal cells themselves. To this end we electroporated a GFP reporter gene into the early chick limb mesoderm. Since not all the cells incorporate the plasmid DNA carrying the transgene, the shape of the cells becomes easily observable (Figure 1J, L). Strikingly we found that at about stage 18 cells are not disorganized. Mesenchymal cells display an apparent radial orientation, such that they are elongated and bipolar with protrusions in direction of the overlying ectoderm (Figure 1J–K). At later stages (stage 20, Figure 1L and stage 23, not shown) cells still display an orientation but exhibit regional differences (Figure 1L–M). At stage 20, quantifications showed that cells located in the ventral and dorsal sides close to the ectoderm (in about a 100 μm range) are greatly elongated (length/width L/W=4 SEM=0.081 n=304 cells and 3.7 SEM=0.009 n=568 respectively) and are perpendicularly aligned to the ectoderm (Figure 1N, R, O, S,). Cells located distally, close to the AER, appear to be oriented toward the ectoderm but are not as elongated (L/W=1.87 SEM=0.004 n=302, figure 1Q, U) while cells located centrally do not show evidence of organization and do not appear to be elongated (L/W=1.5 SEM=0.002 n=308, Figure 1P, T).
Live imaging reveals oriented cell movements and oriented division in the limb mesenchyme
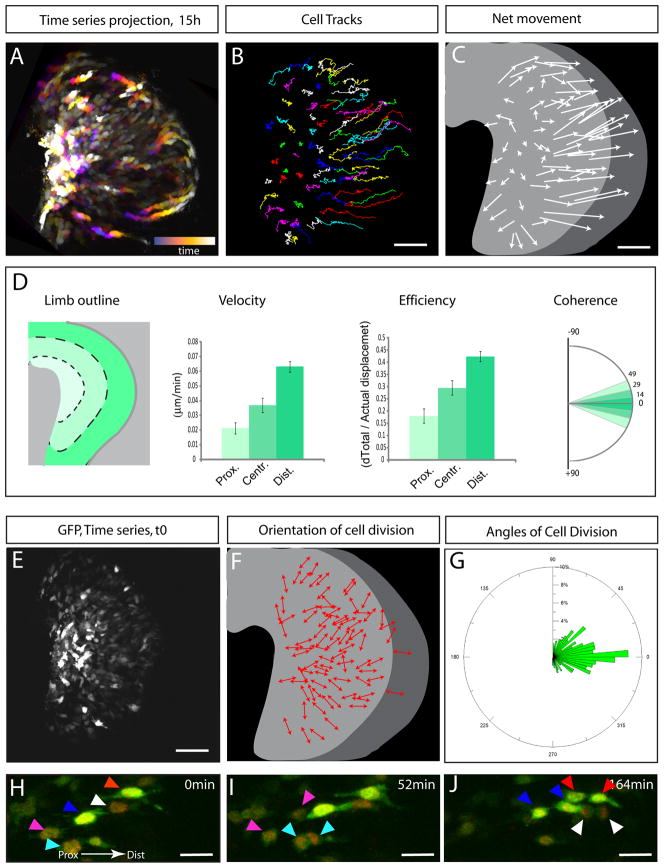
We next utilized time-lapse microscopy to investigate how this organization arises. GFP electroporated chick embryos were transversally sectioned (200 μm thick) using a vibratome and selected sections encompassing GFP labeled limbs were cultured under controlled atmosphere and examined using 2-photon microscopy. Explants were kept in culture on average 12 to 15 hours and an image was taken every 3–5min (Figure 2A and Movie S2). We found that labeled cells move actively within the limb mesenchyme. These movements exhibit several characteristics. First, the movements of cells show a clear directionality and as predicted by the cell shape analysis, cells moved toward the overlying ectoderm (Figure 2B–C). Oriented protrusions described above were found to be persistent and very stable as cells appear to use them to pull themselves toward the ectoderm. Second we noted a gradient in cell velocity, with cells located distally moving faster than cells located more proximally (Figure 2D). Third, we also noted a gradient in the degree of coordination of movements. This was quantified by measuring the degree of coherence (similarity in trajectory of adjacent cells [4]) and efficiency (linearity of trajectory) of cell movement. We found that cells located close to the ectoderm move more coherently and more efficiently (as defined by the ratio of the net movement over the total displacement) than cells located more proximally (Figure 2D). Thus this analysis reveals that cells composing the early limb mesenchyme constantly rearrange through highly organized movements.
Figure 2. Cells of the limb exhibit oriented cell movements and oriented cell division.
(A) Projection of a 15 hours time series view (corresponding to the time-lapse movie S2) of a limb explant of a GFP electroporated chick embryo. The projection is color coded; early times series are displayed in blue and late time series are progressively displayed in orange then white as the time lapse experiment progresses as indicated by the bar in the lower right corner. (see Movie S2)
(B) Cell tracks from time lapse experiment presented in
(A) (the colors were randomly chosen). Cells were manually tracked.
(C) Schematic representing for each cell tracked in (B) the net movement (as shown by the length of each arrow) and the direction (as shown by the arrowheads)
(D) Quantification of cell velocity (in μm.min−1, second panel,), efficiency (i.e., ratio between the distance between t0 and tf and and the distance of covered by the whole track, third panel) and coherence (i.e., standard deviation in angle of two cell directions within a range of 50 μm, fourth panel) in three arbitrarily subdivided areas (represented by shades of green) along the P-D axis (as schematized in the first panel).
(E) View of the first time series (t=0) from the experiment presented in (A).
(F) Schematic representing the orientation of each cell (red arrows) that divided during the course of the time lapse experiment in (A). (See movie S2)
(G) Quantification of the angle between the P-D axis of the limb bud and the axis of cell division. Angle of each cell division is shown on a unidirectional Rosette graph which is divided in bins of 5°. The relative percentage of cell division per bin is indicated on the radial axis. Quantifications were made on a total number of n=331 cell divisions, 5 embryos.
(H–I) Time series at t=0 (H), t=52 min (I) and t=164 min (J) of a time lapse experiment (Movie S3) showing preferential P-D cell division at the distal end of the limb bud. The colored arrowheads points at co-electroporated cells with both a GFP (in green) and H2bRFP (in red) construct and their progeny (same arrowhead color). Scale Bars in the lower right corner represent 50 μm (B, C, E) and 20 μm (H–J)
As cells moved toward the ectodermal layer we noted that they tend to divide such that daughter cells separate along the direction of their movements (i.e. in direction of the ectoderm, Movie S2–S3, Figure 2E–F, H–J). We quantified this result and found that overall, mesenchymal cells divide preferentially along the proximal- distal axis (Figure 2G). It is important to note that in dorsal and ventral part of the limb, cells divide with a greater angle in relation to the proximal-distal axis (i.e. closer to the D-V axis). Interestingly in these regions we noted that in many cases over the course of the time lapse experiment, daughter cells that are pushed proximally re-intercalate via the oriented movements described above. Thus the oriented movements allow the intercalation of newly generated daughter cells perpendicular to the ectoderm ensuring the elongation of the limb bud along its Proximal-Distal axis. Altogether these data show that the limb bud elongates via the combination of oriented cell division and oriented movements.
Wnt5a regulates cell organization and cell movement in the mouse limb bud
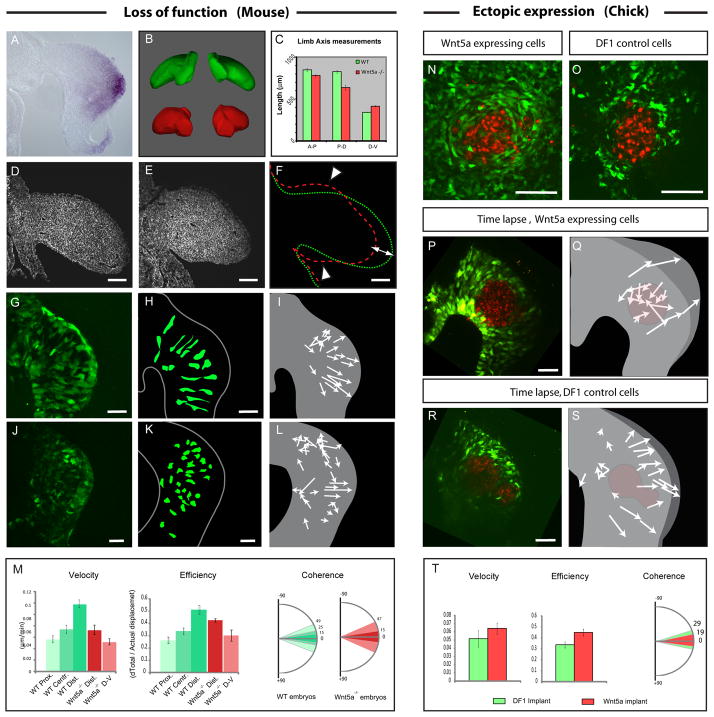
The Wnt/PCP pathway has been shown to regulate both oriented movement and cell division in developing embryos (for review see [5]). Thus, this pathway was an obvious candidate for regulating the oriented events taking place in limb development. Interestingly, it is known that Wnt5a is expressed in a proximal to distal gradient within mesenchyme as well as in the distal ectoderm from as early as E9.5 in the mouse and as early as stage 18 in the chick limb bud ([6][7][8] and Figure 3A). Moreover mice mutant for Wnt5a exhibit strong limb morphogenesis defects with shortened and malformed skeletal elements [8]. This phenotype, which does not involve specification or patterning defects, had been proposed to be due to a decrease in proliferation. However, in light of our cellular data, we decided to reexamine limbs of Wnt5a mutant embryos. We first quantified limb axis proportions in WT and Wnt5a−/− mouse E10.5 embryos using optical projection tomography (Figure 3A–B). We found that mutant limbs exhibit a 10% decrease in volume (not shown), a result consistent with the previously described decrease in proliferation [8]. However we found that the relative proportions of the Wnt5a mutant limbs were also affected. Limbs of Wnt5a−/− embryos exhibit a slight decrease in the length of A-P axis, an much greater decrease in their P-D axis and more surprisingly an increase in the length of the D-V axis when compared to WT littermates (Movie S4, Figure 3B–C and D–F). The observation that the D-V axis increases in length in the mutant compared to control limbs, cannot be explained by a decrease in cell proliferation. Thus these quantifications indicate that limb elongation is impaired in Wnt5a−/−; moreover concomitant changes in the relative proportion of D-V and P-D axes suggested that this could be due to defects in oriented cellular events rather than to a defect in cell proliferation.
Figure 3. Wn5a regulates limb bud elongation and cell orientation in the mouse.
(A) Wnt5a expression detected by in situ hybridization showing higher expression in the distal mesenchyme of the early limb bud.
(B) Wild Type (WT) (green) and Wnt5a−/− mutant limb buds (red) reconstructed and virtually dissected from Optical Projection Tomography acquisitions of E10.5 Mouse embryo. Wnt5a−/− limbs exhibit elongation defects and appear roundish as compared to control. (See Movie S4)
(C) Measurements (in μM) of the A-P (left bars), D-V (central bars) and P-D (right bars) axes lengths in WT (green bars) and Wnt5a−/− (red bars) embryos.
(D–E) Transverse sections of WT (D) and Wnt5a−/− (E) mouse embryos at E10.5 stained with Phalloidin (in white).
(F) Outline of the WT (in green) and Wnt5a−/− (in red) limb buds shown in (D) and (E), respectively, showing the effect of the loss of Wnt5a on the relative proportions of the limb bud (i.e., A-P axis, white arrowheads and P-D axis (white arrow)).
(G, J) Transverse sections of WT GFP X+/− (G) and Wnt5a−/−; XGFP+/− (J) mouse embryos at E9.25 showing the shape of GFP expressing cells.
(H, K) Schematics representing outlines of GFP expressing cells from sections shown in (G) and (J).
(I, L) Schematics showing the net movement (arrows lengths) and the direction (arrowheads) of cells during time lapse experiments performed in WT GFP X+/− (I) and Wnt5a−/−; XGFP+/− (L) mouse embryos at E9.25 (Movie S5).
(M) Quantification of cell velocity (first panel), efficiency, (second panel) and coherence (third panel) in the proximal, central and distal regions of WT mouse limb bud (represented by shades of green, as schematized in the first panel of Figure 2D) and in the most distal and dorsal/ventral parts of Wnt5a −/− mouse limb buds ( dark red and light red respectively).
(N–O) Transverse section of chick limb buds electroporated with a GFP construct (in green) and implanted with control (O) or cWnt5a-expressing (N) DF1 cells stained with DiI (in red).
(P, R) First time series (t=0) from time lapse experiments (Movie S6) showing control DF1 cells (R) or cWnt5a-expressing cells (P, in red) implanted in limb buds previously electroporated with a GFP construct (in green).
(Q, S) Schematics showing the net movement (arrows lengths) and direction (arrowheads) of GFP-expressing cells from (P) and (R). GFP-expressing cells move toward the source of Wnt5a in (Q), and normally, towards the ectoderm in (S). The position of control DF1 cells (S) and Wnt5a-expressing cells (Q) is indicated in red. (see Movie S6).
(T) Quantification of cell velocity (first panel), efficiency, (second panel) and coherence (third panel) of GFP labeled cells surrounding implanted DF1 cells (in green) or Wnt5a expressing cells (in red). Scale Bars in the lower right corner represent 50 μm.
To determine whether cell organization was disrupted in limb of Wnt5a−/− embryos we took advantage of a mouse strain carrying a ubiquitously expressed GFP transgene inserted on the X chromosome [9]. Because the X chromosome is randomly inactivated, female heterozygote embryos expressed GFP in a mosaic manner allowing one to distinguish cell shape and to follow cell behavior [10]. We found that in WT Wnt5a+/+; EGFP X+/− E9.25 embryos mesenchymal cells of the limb are elongated toward the ectoderm as in chick embryos (Figure 3G–H and Figure S1). However in mutant Wnt5a−/−; EGFP X+/− embryos, cells are not elongated and do not display any oriented protrusions (Figure 3J–K). This result was also confirmed using Scanning Electron Microscopy (Figure S1). We next tracked the behavior of these cells using live imaging 2-photon microscopy as described above. We found that as in the chick, in WT; EGFP X+/− embryos cells display oriented movements and move toward the overlying distal ectoderm (Movie S9 and Figure 3G–I). Again similar to the chick, cell movements exhibit a proximodistal gradient in velocity, efficiency and coherence (Figure 3M). In Wnt5a−/−; EGFP X+/− embryos, cells located in the vicinity of the AER move toward the ectoderm with a comparable coherence as observed in WT embryos, they however move at reduced velocity and reduced efficiency compared to control cells (Figure 1M). In contrast cells located in the dorsal and ventral domains of the limb bud exhibit disorganized movements which exhibit a lower velocity, efficiency and coherence than in WT limbs (Movie S5 and Figure 3J–M).
Conversely we tested whether Wnt5a is able to change the orientation of cells of the limb when expressed ectopically. We found that when DF1 cells expressing Wnt5a were implanted in GFP electroporated limbs of chick embryos, GFP labeled cells around the graft were elongated and intermingled with the cells expressing the ectopic Wnt5a (Figure 3N). Live imaging showed that limb cells located around the source of Wnt5a rapidly change their orientation and intercalate in between Wnt5a expressing cells through highly coherent and efficient movements (Movie S6, Figure 3P–Q, T). This behavior was not observed when control DF1 Cells were implanted. Cells did not show any sign of reorientation and moved normally toward the ectoderm (Movie S6, Figure 3R, S). Thus taken together these results indicate that Wnt5a is able to orient cells of the limb mesenchyme and that endogenous Wnt5a at least in part is responsible for the observed oriented movements in the limb mesenchyme.
Wnt5a regulates oriented cell division in the limb bud
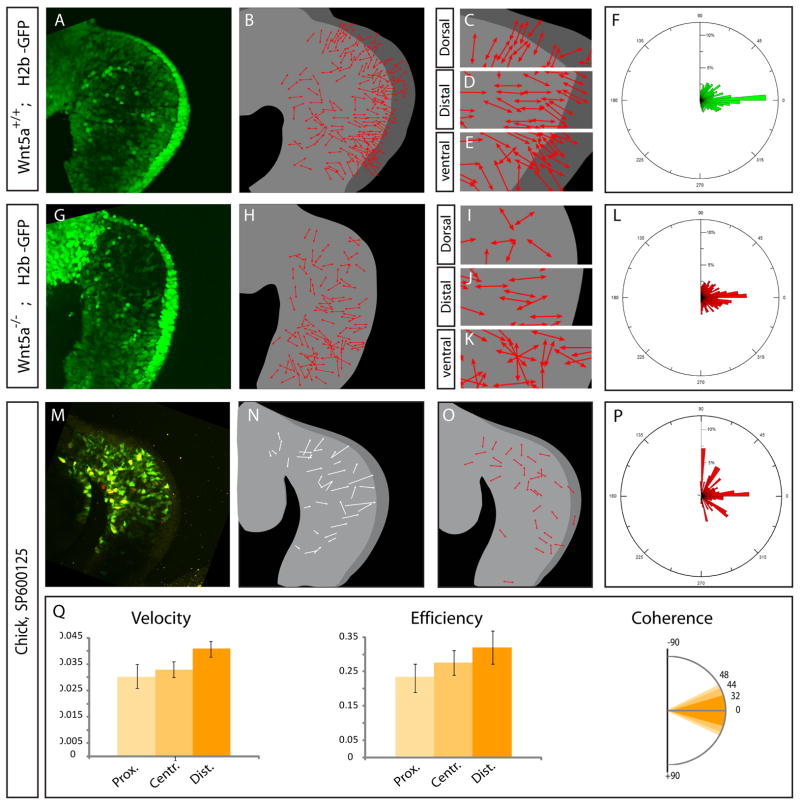
We next examined orientation of cell division in the Wnt5a mutant limb buds. To this end, we performed time lapse experiment on mouse embryos ubiquitously expressing a transgene coding for a fusion between the human Histone H2b and the GFP proteins [11]. Similar to chick embryos, we observed that in WT H2bGFP E9.5 mouse embryos, cells divide in direction of the overlying ectoderm (Movie S7, Figure 4A–E). Quantification of the angle of cell divisions demonstrated a strong bias along the P-D axis (n=1071 cell divisions, Figure 4F). In Wnt5a−/−; H2bGFP embryo, cells located distally divide in the direction of the overlying ectoderm, as observed in WT embryos (Figure 4G–K). However cell divisions occurring in dorsal and ventral domains of the limb bud are disorganized. As a result the overall distribution of angle of cell divisions revealed a significantly weaker P-D bias (n=791 cell divisions (χ2=93.943 d.o.f=35 p<0.00001), Movie S7 and Figure 4L). Thus Wnt5a regulates at least partially the orientation of cell division within limb mesenchymal cells. Altogether these data demonstrate that Wnt5a contributes to the control of limb bud elongation by regulating oriented movements and oriented cell division in the limb mesenchyme. The preferential loss of oriented cellular processes in the dorsal and ventral domains of the Wnt5a mutant limb buds is consistent with the increase in the D-V axis in these mutants, however the fact that there still is some P-D elongation in the absence of Wnt5a suggests there may be some redundancy in the system.
Figure 4. Wnt5a/JNK regulates orientation of cell division.
(A, G) First time series (t=0) of time lapse experiments (Movie S7) on limb buds of WT/H2bGFP (A) or Wnt5a−/−; H2bGFP (G) mouse embryos at E9.5.
(B, H) Schematics representing the orientation of cell division (red arrows) from time lapse experiments in (A) and (G), respectively.
(C–E, I–K) Enlargements of the dorsal (C, I), distal (D, J) and ventral (E, K) areas showing regional differences in the orientation of cell divisions.
(F, L, P) Quantifications of the angle between the P-D axis and the axis of cell divisions identified from the time lapse experiments performed in WT H2bGFP (F) and Wnt5a−/−; H2bGFP (L) mouse embryos at E9.5, and chick limb buds treated with SP600125 (P), respectively as shown in (A, G and M). The rosette graph is divided in bins of 5°, the relative proportion in percentage of cell division per bin is indicated on the radial axis.
(M) First time series (t=0) from a time lapse experiment (see Movie S7) of chick limb bud explants electroporated with GFP (in green) and H2bRFP (in red) constructs and cultured in presence of the JNK inhibitor SP600125.
(N) Schematic showing the net movement (arrows lengths) and the direction (arrowheads) of cells shown in (M).
(O) Schematic representation of the direction of cell division (red arrows) shown in (M)
(Q) Quantification of cell velocity ( first panel), efficiency, (second panel) and coherence (third panel) in the proximal, central and distal regions (represented by shades of orange, as schematized in the first panel of Figure 2D) of chick limb treated with SP600125.
JNK acts downstream of Wnt5a in oriented cell processes
The non canonical Wnt/Planar Cell Polarity (PCP) pathway has been shown to lead to changes in gene expression through activation of cJun N-Terminal Kinase (Jnk) and Wnt5a has been shown to induce Jnk activation [12] [13]. We therefore tested whether direct modulation of Jnk might have a stronger effect than Wnt5a itself. We thus examined orientation of cell division following inhibition of Jnk activity. We cultured Chick embryo explants co-electroporated with GFP and H2bRFP fusion protein in presence of the Jnk inhibitor SP600125 and live imaged cell behavior (Figure 4M, O). We quantified the angle of cell divisions (n=191 cell divisions) and found that in presence of SP600125, the planes of cell division were totally disorganized (Figure 4P). Time lapse experiments also showed disorganized cell movements. While SP600125 treated cells generally move toward the distal end of the limb bud, cells generate protrusions in many directions and exhibit much lower coherence and efficiency than controls (Figure 4Q). Thus Jnk activity is required to orient cell division and cell movement in the limb mesenchyme.
FGF signalling is required for cell movement but does not drive cell orientation
The FGF signalling pathway plays major roles during limb development. Moreover, FGF family members are expressed distally in the limb bud, and previous studies have suggested that FGF activity can act as a chemoattractant in the limb [14]. We therefore decided to re-investigate its functions in light of the cellular events we described above. While at later stages FGF signalling is found in a narrow distal stripe, FGF8 is initially expressed in a broad domain in the distal ectoderm (Figure 5A) and only later becomes restricted to the AER. Thus in principle, FGF activity could contribute to the oriented processes along the dorsal and ventral margins of the early limb bud as well as at the distal tip. Moreover, as previously described, we found that ERK/MAPK, a downstream effector of FGF signalling, displays a graded phosphorylation with high intensity at the distal tip and low intensity at the proximal end, reflecting the extent of the FGF pathway in the mesenchyme (Figure 5B–C) [15]. This is strikingly similar to the gradient in cell movement that we observed in the limb (Figure 5C,).
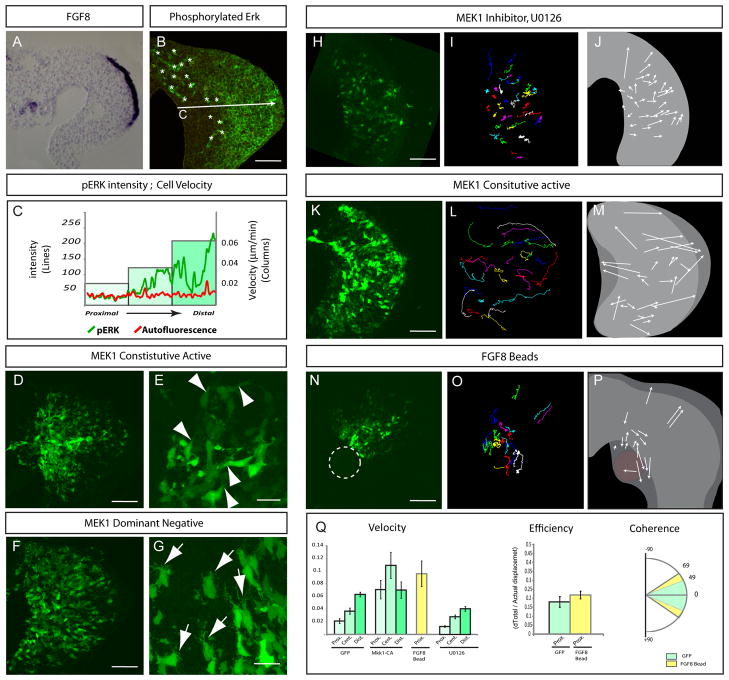
Figure 5. FGF/MAPK signalling promotes cell movement.
(A) Transverse section showing FGF8 expression detected by in situ hybridization in the distal ectoderm of a chick limb bud.
(B) Transverse section of a limb bud stained with a phosphorylated-ERK/MAPK antibody.
(C) Intensity profile of pERK (green line) and non specific red autofluorescence (red line) along the Proximal-Distal axis of the limb as shown in (B) correlated with the velocity of cells from the proximal, central and distal regions of the limb bud ( Columns) as shown in Figure 2D.
(D) Transverse sections of chick embryos limb buds co-electroporated with constitutively active Mek (CA-MEK) and GFP constructs.
(E) High magnification showing lamellipodia of electroporated, GFP expressing cells (white arrowheads).
(F) Transverse sections of chick limb buds electroporated with both dominant negative MEK1 and GFP constructs.
(G) High magnification showing filopodia protruding from cells electroporated with dominant negative MEK1 and GFP (white arrows).
(H, K, N) First time series (t=0) of time lapse experiments (Movie S8) on chick limb buds electroporated with Constitutive active MEK1/GFP (K), GFP only (H, N) but cultured in presence of the MEK1 inhibitor U1026 (H), or GFP only and cultured with a bead soaked in FGF8 (N) (see movie S8).
(I, L, O) Cell tracks from a time lapse experiment shown in (H), (K) and (N), respectively.
(J, M, P) Schematics representing the net movement (arrows lengths) and direction (arrowheads) of cells tracked (I), (L), and (O), respectively.
(Q) Left graph: quantifications of the velocity (in μm.min−1, left panel) of cells electroporated with GFP-only (left bars), constitutive active MEK1 (central left bars), in presence of FGF8 beads (central right bars, yellow) or U0126 (right bars).within the proximal, central and distal regions (shades of green, see Fig. 2D) of the limb bud. Centre graph: Quantification of the efficiency of proximal cells of the limb electroporated with GFP only or in presence of a FGF8 bead (yellow). Right graph: quantification of the coherence of the movement of cells located within the proximal region of the limb bud electroporated with GFP (in green) or exposed to FGF8 beads (in red). Scale Bars in the lower right corner represent 50 μm.
To investigate its role in the movement of limb cells, we interfered in several ways with the FGF/Mapk pathway. First we electroporated the limb mesoderm with dominant negative and constitutively active forms of MEK1 [16] which acts upstream of Erk/MAPK. In MEK1 dominant negative electroporated embryos, cells are not as elongated as in controls and exhibit a great number of very thin cellular protrusions resembling filopodia (Figure 5F–G). Importantly cells are not misoriented suggesting that the MAPK pathway does not regulate orientation. Conversely cells expressing the constitutively active form of MEK1 are elongated and exhibit thick cellular extensions resembling lamellipodia (Figure 5D–E). Lamellipodia are characteristic of motile cells and are believed to be the motor pulling cells forward during movement, while filopodia are characteristic of sensory functions. Thus, this result suggested a role for the FGF pathway in modulating motility of limb cells. Live imaging revealed that when MEK1 was inhibited using a specific inhibitor U0126, cells moved at much lower velocity than control cells but in the appropriate orientation (Movie S8, Figure 5H–J, Q). A similar effect was observed using either the FGFR1 inhibitor SU5402 or the dominant negative form of MEK1 (Figure S2). Moreover we did not observe any defects in the orientation of cell division when limb buds were treated with U0126 as compared to control limb buds (data not shown). However when the constitutively active MEK1 construct was electroporated, cells displayed an exaggerated motile behavior with a much higher velocity than control cells. The original gradient of velocity observed in control GFP electroporation was lost when cells expressed MEK1 constitutively active form (Movie S8, Figure 5K–M, Q). These data indicate that the FGF/MAPK pathway acts to promote cell movements.
In order to further understand the cellular events driven by FGF signalling, we exposed proximal cells of the limb, that are normally not exposed to FGF signalling, to an ectopic source of FGF8. In such limbs, proximal cells displayed higher velocity than in control limb buds (Movie S8, Figure 5N–Q). Interestingly as these proximal cells move, they very rapidly invade the source of FGF8 provoking elongation of the mesenchyme toward the proximal end of the limb bud. A Similar effect was observed when beads soaked in FGF8 or when DF1 cells expressing FGF8 were applied (Movie S8, Figure 5N–P and Figure S2). These results are consistent with FGF8 acting as a chemoattractant for limb mesenchymal cells, as previously suggested [14]. Strikingly however, in spite of the localized source of FGF, the movements induced by FGF8 display no obvious constant orientation. Cells extend a high number of protrusions but in no particular direction and frequently change direction as they are moving. Moreover cells show very poor coherence and poor efficiency revealing a low degree of organization in their movement (Figure 5Q). Thus FGF8 is not able to orient mesenchymal cells of the limb. This is in net contrast with results obtained when Wnt5a expressing cells are implanted in the limb bud. In this situation cells display very organized movements exhibiting high coherence and high efficiency as they move toward the source of Wnt5a (Figure 3P–Q). These results suggest that FGF8 unlike Wnt5a does not actually chemoattract surrounding cells by inducing oriented movements, but instead acts to increase the velocity of random movements of these cells. As the velocity of these random movements appears to be biased perhaps proportional to the concentration of FGF8, cells eventually move closer to the source of FGF8 through mass action. Altogether these results demonstrate that FGF signalling is dispensable for cell orientation in the limb but is required to induce a gradient of cell movement that is necessary to drive limb bud elongation.
DISCUSSION
Taken together, our data suggest that orientation of cell division and cell movements are sufficient to explain the oriented growth of the limb bud. As this manuscript was in preparation, Boehm et al. published a study directly testing the alternative proliferation rate model [17]. The authors combine biological measurements with computational modeling and conclude that such a model is not realistic. They therefore propose that the limb elongates through oriented cell events, such as oriented cell division and oriented cell movements. This study is in agreement with our present finding and supports our model.
In a second related study published as this manuscript was in preparation, Wyngaarden et al. investigated tissue movement at the onset of limb bud formation. They found that the limb bud initiates its formation along the anteroposterior axis by recruiting lateral plate mesoderm cells via rostral to caudal movement of tissue and cell divisions [18]. Similar to the morphogenic processes elucidated here, these movements involve oriented cellular properties regulated, at least in part, by Wnt5a.
We examined subsequent steps of limb morphogenesis, and show that Wnt5a and JNK activity are required to drive proper limb morphogenesis, acting to orient cellular processes including mitosis and directional cell movements. In cell culture Wnt5a has been shown to bind to Ror2 (an orphan tyrosine kinase receptor) and to activate Jnk [12]. Consistent with our in vivo results, Wnt5a/Ror2 activity induces polarized cell migration and reorientation of the microtubule organizing center in a Jnk dependant manner in vitro. Moreover, mice mutant for Ror2 and double mutants for Ror1 and Ror2 exhibit phenotypes very similar to Wnt5a mutant mice [19][20].
The FGF signalling pathway has been shown to be important to drive cell proliferation, cell survival, and specification of limb mesenchymal cells. Our study indicates that an additional role of FGF activity is to promote the velocity of cell movements within the limb bud, thereby promoting its elongation. Consistent with this, conditional inactivation of FGFR1 in the limb mesoderm disrupts the relative proportions of the limb bud and consequently of the skeletal elements [21]. In these mutants, the length P-D axis is reduced while the D-V and A-P axes are expanded. Strikingly, the same result was obtained by applying FGF4 beads to the developing limb [14]. Here we have shown that the effect of FGF/MAPK signalling emanating from the AER is different than the effect induced by Wnt5a in the limb bud. While Wnt5a induces directional movement of cells, FGF8 acts to induce rapid albeit disorganized movements. However like Wnt5a, FGF activity ultimately results in distal elongation. These observations suggest that FGF8 acts by inducing random movements but with a higher velocity as cells move close to the source. As this manuscript was in preparation a study from Benazeraf et al. proposed that the FGF pathway drives the tail bud elongation in the chick embryo by promoting random cell movements [22]. They suggest that FGF creates a gradient of cell motility and that the tail bud elongates by mass action of random cell movement at the posterior end of the embryo. While our data indicate a similar mode of FGF action, cells in the limb bud additionally undergo oriented processes of cell division and directional movements under the influence of Wnt5a. Our study indicates that it is the combined action of non canonical Wnt and FGF and activity that integrates orientation and movement, consequently driving the limb bud elongation, and thereby establishing a progenitor field of the proper dimensions for the subsequent patterning and morphogenesis of the limb anatomy.
HIGHLIGHTS.
Cells of the early limb bud are highly organized and polarized.
Wnt5a/JNK activity proximodistally orients cell movements and cell division.
FGF/Mapk signaling establishes a distally-biased gradient of cell motility.
Together Wnt5a and FGF integrate orientation and movement, driving limb elongation.
Supplementary Material
Acknowledgments
We thank Drs. Carla Kim, Andy McMahon, Elaine Fuchs, Rudolf Jaenisch and Andras Nagy for sharing mice strains. We would like to thank Andrew Russell for help with statistical tests. J.G. is a fellow of the Human Frontier Science Program (HFSP). C.V is supported by grants UO1-HL080731, PO1-AI05490 and RO1-EB006432. This work was supported by a grant R01-HD045499 from NIH to C.J.T.
References
- 1.HORNBRUCH A, WOLPERT L. Cell Division in the Early Growth and Morphogenesis of the Chick Limb. Nature. 1970;226:764–766. doi: 10.1038/226764a0. [DOI] [PubMed] [Google Scholar]
- 2.Ede DA, Law JT. Computer simulation of vertebrate limb morphogenesis. Nature. 1969;221:244–248. doi: 10.1038/221244a0. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Terán MA, Hinchliffe JR, Ros MA. Birth and death of cells in limb development: a mapping study. Dev Dyn. 2006;235:2521–2537. doi: 10.1002/dvdy.20916. [DOI] [PubMed] [Google Scholar]
- 4.Ulrich F, et al. Wnt11 Functions in Gastrulation by Controlling Cell Cohesion through Rab5c and E-Cadherin. Developmental Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Tada M, Kai M. Noncanonical Wnt/PCP signaling during vertebrate gastrulation. Zebrafish. 2009;6:29–40. doi: 10.1089/zeb.2008.0566. [DOI] [PubMed] [Google Scholar]
- 6.Gavin BJ, McMahon JA, McMahon AP. Expression of multiple novel Wnt-1/int-1-related genes during fetal and adult mouse development. Genes Dev. 1990;4:2319–2332. doi: 10.1101/gad.4.12b.2319. [DOI] [PubMed] [Google Scholar]
- 7.Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 9.Hadjantonakis AK, Cox LL, Tam PP, Nagy A. An X-linked GFP transgene reveals unexpected paternal X-chromosome activity in trophoblastic giant cells of the mouse placenta. Genesis. 2001;29:133–140. doi: 10.1002/gene.1016. [DOI] [PubMed] [Google Scholar]
- 10.Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A. PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development. 2009;136:2039–2048. doi: 10.1242/dev.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oishi I, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes to Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 13.Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12:779–792. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Muneoka K. Cell migration and chick limb development: chemotactic action of FGF-4 and the AER. Dev Biol. 1999;211:335–347. doi: 10.1006/dbio.1999.9317. [DOI] [PubMed] [Google Scholar]
- 15.Corson LB, Yamanaka Y, Lai KV, Rossant J. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development. 2003;130:4527–4537. doi: 10.1242/dev.00669. [DOI] [PubMed] [Google Scholar]
- 16.Delfini M, Dubrulle J, Malapert P, Chal J, Pourquié O. Control of the segmentation process by graded MAPK/ERK activation in the chick embryo. Proc Natl Acad Sci USA. 2005;102:11343–11348. doi: 10.1073/pnas.0502933102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boehm B, Westerberg H, Lesnicar-Pucko G, Raja S, Rautschka M, Cotterell J, Swoger J, Sharpe J. The role of spatially-controlled cell proliferation in limb bud morphogenesis. PloS Biology. doi: 10.1371/journal.pbio.1000420. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyngaarden LA, Vogeli KM, Ciruna BG, Wells M, Hadjantonakis A, Hopyan S. Oriented cell motility and division underlie early limb bud morphogenesis. [Accessed June 23, 2010];Development. 2010 doi: 10.1242/dev.046987. Available at: http://www.ncbi.nlm.nih.gov.ezp-prod1.hul.harvard.edu/pubmed/20554720. [DOI] [PMC free article] [PubMed]
- 19.Takeuchi S, et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5:71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 20.Nomi M, et al. Loss of mRor1 Enhances the Heart and Skeletal Abnormalities in mRor2-Deficient Mice: Redundant and Pleiotropic Functions of mRor1 and mRor2 Receptor Tyrosine Kinases. Mol Cell Biol. 2001;21:8329–8335. doi: 10.1128/MCB.21.24.8329-8335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verheyden JM, Lewandoski M, Deng C, Harfe BD, Sun X. Conditional inactivation of Fgfr1 in mouse defines its role in limb bud establishment, outgrowth and digit patterning. Development. 2005;132:4235–4245. doi: 10.1242/dev.02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benazeraf B, Francois P, Baker RE, Denans N, Little CD, Pourquie O. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature. 2010;466:248–252. doi: 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.