Abstract
Progression of breast cancer is associated with remodeling of the extracellular matrix, often involving a switch from estrogen dependence to a dependence on EGF receptor (EGFR)/HER-2 and is accompanied by increased expression of the main binding protein for insulin-like growth factors (IGFBP-3). We have examined the effects of IGFBP-3 on EGF responses of breast epithelial cells in the context of changes in the extracellular matrix. On plastic and laminin with MCF-10A normal breast epithelial cells, EGF and IGFBP-3 each increased cell growth and together produced a synergistic response, whereas with T47D breast cancer cells IGFBP-3 alone had no effect, but the ability of EGF to increase cell proliferation was markedly inhibited in the presence of IGFBP-3. In contrast on fibronectin with MCF-10A cells, IGFBP-3 alone inhibited cell growth and blocked EGF-induced proliferation. With the cancer cells, IGFBP-3 alone had no effect but enhanced the EGF-induced increase in cell growth. The insulin-like growth factor-independent effects of IGFBP-3 alone on cell proliferation were completely abrogated in the presence of an EGFR, tyrosine kinase inhibitor, Iressa. Although IGFBP-3 did not affect EGFR phosphorylation [Tyr1068], it was found to modulate receptor internalization and was associated with activation of Rho and subsequent changes in MAPK phosphorylation. The levels of fibronectin and IGFBP-3 within breast tumors may determine their dependence on EGFR and their response to therapies targeting this receptor.
Keywords: Breast Cancer, Carcinogenesis, Cell Surface, Cell Surface Receptor, Cellular Regulation, EGF, Fibronectin, IGFBP-3, Cell Growth
Introduction
Growth factors and their receptors play an essential role in the regulation of normal epithelial cell proliferation, and aberrations in growth factor systems are known to contribute to the development and progression of malignant cell transformation. The insulin-like growth factors (IGF-1 and -II)3 and epidermal growth factor (EGF) are potent cell survival factors and regulators of migration and proliferation. They play an important role in normal mammary gland development and have been heavily implicated as contributing to neoplastic growth in breast and several other cancers (1, 2).
The IGFs are present throughout the body almost entirely in association with six specific, high affinity binding proteins (IGFBP-1–6), which are critical determinants of IGF availability and actions. In addition to acting as the main circulating carrier protein, IGFBP-3 is produced in many tissues where it has multiple effects on cell functions both via its ability to modulate IGF-actions and also due to direct intrinsic actions (3). The production of IGFBP-3 by epithelial cells has been reported to be increased by a number of different agents including p53, TGF-β, vitamin D, and tamoxifen (for review, see Ref. 4), all agents that are associated with growth inhibition and increased apoptosis. In addition we and others have reported that IGFBP-3 can accentuate apoptosis of different cells including prostate, breast, colorectal, and esophageal epithelial cells (for review, see Ref. 4). We then defined a pathway by which IGFBP-3 could elicit these effects on apoptosis of breast epithelial cells: IGFBP-3 bound to β1 integrins and caveolin 1 and increased their association, which culminated in the recruitment of focal adhesion kinase and the subsequent activation of MAPK (5).
Prospective epidemiology initially suggested that a low plasma IGFBP-3/high IGF-I ratio correlates with an increased risk of developing premenopausal breast cancer (6). In concurrence with this, other data have supported an association between low circulating IGFBP-3 combined with elevated IGF-I and both early stage breast cancer and pre-menopausal ductal carcinoma in situ (7, 8). These cumulative reports promoted a general impression that IGFBP-3 has actions that would counterbalance those of IGFs with negative effects on cell growth and survival (9) resulting in proposals for IGFBP-3 to be developed as an anticancer therapeutic (10).
In contrast to these data there have, however, been many other reports that IGFBP-3 can positively stimulate the proliferation (11) and survival (12) of various cells. Since the original reports, subsequent prospective epidemiology has also implicated a positive association between plasma IGFBP-3 and the risk of premenopausal breast cancer (13). In addition there have been several reports that in breast tumors the expression of IGFBP-3 is positively associated with large, highly proliferative tumors and poor prognostic markers (14, 15). Furthermore, we have previously reported that in contrast to its inhibitory effects on breast cancer cells, IGFBP-3 promoted the proliferation and survival of the relatively normal, non-malignant, anchorage-dependent MCF-10A cells (12), which we also showed was dependent upon β1 integrins and subsequent activation of MAPK (5).
We went on to show that although IGFBP-3 could reduce cell attachment and enhance apoptosis of Hs578T breast cancer cells when these were cultured on plastic, collagen, or laminin, when the same cells were cultured on fibronectin, IGFBP-3 had the opposite effects and increased cell attachment and acted as a cell survival factor (16). Cholesterol-stabilized complexes are required for normal integrin signaling, and we showed that disrupting such complexes also reversed the intrinsic action of IGFBP-3 (5). These reports challenge the widely held view that IGFBP-3 normally has inhibitory actions and suggest that its actions may depend not just on cell type but also on cell context.
In breast cancer, stromal cell ECM protein expression is altered or increased with malignant progression. Studies have demonstrated that fibronectin expression in breast cancer is not only greater than in normal breast parenchyma (17) but that expression is associated positively with lymph node metastasis and predicts an increased mortality in these patients (17, 18).
In addition to the IGF-independent effects of IGFBP-3 being intimately linked with integrin receptor signaling, the actions of EGF are also known to be influenced by changes in the extracellular matrix. Fibronectin promotes clustering of α5β1 and α1β1 integrins, which results in activation of EGFR and enhances EGFR coupling to the MAPK pathway via Shc (19, 20).
A number of reports have shown that EGF differentially regulates IGFBP-3 expression depending on cell type (21, 22). Clinically, overexpression of the EGFR is associated with a poor outcome in breast cancer, with an observed reduction in disease-free interval and overall survival, negative estrogen receptor status, and higher metastatic potential (23, 24). In vitro studies demonstrated that T47D cells transfected to overexpress IGFBP-3 resulted initially in growth inhibition but that they became resistant to its inhibitory effects at increasing passages (25), which has also been shown subsequently in an in vivo model (26). Furthermore, in vitro this effect was shown to be associated with both up-regulation of the EGFR as well as increased responsiveness to EGF (26). IGFBP-3 has also been shown to potentiate EGF-induced proliferation in non-malignant mammary epithelial cells (27).
Before interventions utilizing IGFBP-3 as a potential therapeutic and to optimize current strategies targeting EGFR, it is essential to better understand the interaction that exists between IGFBP-3 and EGF. Therefore, we have examined the effects of IGFBP-3 on EGF-mediated growth in both normal and malignant breast epithelial cell lines in the context of differences in ECM. We have also sought to identify the signaling pathways responsible for these effects.
EXPERIMENTAL PROCEDURES
Materials
Recombinant EGF and the Rho kinase inhibitor ROCK, Y-27632, were purchased from Calbiochem. Recombinant, human non-glycosylated IGFBP-3 was a gift from Dr. C. A. Macck (Celtrix Pharmaceuticals, Santa Clara, CA). A 15-amino acid peptide sequence spanning two mid-region serines of IGFBP-3 (amino acids 105–119), which we have termed the serine phosphorylation domain (SPD) peptide, was synthesized at the microchemical facility of the Babraham Institute, Cambridge, UK. We have demonstrated previously that of 17 short peptides corresponding to different regions of IGFBP-3, SPD was the only peptide to mimic the actions of full-length IGFBP-3 on cell death and that SPD was unable to interact with IGF-I (28). Fibronectin, laminin, and the anti-phospho (p) EGF receptor (Tyr1068) antibody were purchased from Sigma. Anti-MHC class 1 antibody was bought from Santa Cruz. Anti-p44/42 MAPK and anti-p-p38 MAPK were obtained from Promega, and anti-p-Akt was purchased from Cell Signaling Technologies. Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and anti-p-HER2 were purchased from Chemicon International, and the anti-EGF receptor, anti-Rho antibody, and Rhotekin Rho binding domain bound to glutathione-agarose were from Upstate Biotechnology. Iressa (EGFR-tyrosine kinase inhibitor, which can also inhibit HER-2) was a kind gift from AstraZeneca (Cheshire, UK). Tissue culture plastics were purchased from Greiner Labortechnik LTD (Stonehouse, UK). The enhanced chemiluminescence reagents were bought from Amersham Biosciences, and the BCA protein assay reagent kit was purchased from Pierce.
Cell Culture
Human breast cancer cells, T47D, and Hs578T were purchased from ECACC (Porton Down, Wiltshire, UK) and grown in a humidified 5% carbon dioxide atmosphere at 37 °C. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM, BioWhittaker, Verviers, Belgium) supplemented with 10% fetal bovine serum (Invitrogen), penicillin (50 IU/ml; Britannia Pharmaceuticals, Redhill, UK), streptomycin (50 μg/ml; Celltech Pharmaceuticals, Slough, UK), and l-glutamine (2 mm; Sigma) growth media (GM). The relatively normal MCF-10A cell line was purchased from the American Type Culture Collection (ATCC Manassas, VA). This is a spontaneously immortalized breast epithelial cell line that maintains a relatively normal phenotype as determined by 1) lack of tumorgenicity in nude mice, 2) three-dimensional growth in collagen, 3) growth control by hormones and growth factors, 4) lack anchorage-independent growth, and 5) formation of domes in confluent cultures (29). The MCF-10A cells were maintained in 1:1 mixture of Ham's F-12 medium and Dulbecco's modified Eagle's medium with 2.5 mm l-glutamine (DMEM:F-12, Invitrogen). This was supplemented with 5% horse serum (Invitrogen), penicillin, and streptomycin (as above), 20 ng/ml EGF (Calbiochem), 100 ng/ml cholera toxin (Sigma), 10 μg/ml insulin (Novo Nordisk, West Sussex, UK), and 0.5 μg/ml hydrocortisone (Sigma). Experiments were performed in phenol red- and serum-free DMEM and Hams Nutrient Mix F-12 supplemented with penicillin and streptomycin (as above), sodium bicarbonate (0.12%; Sigma), bovine serum albumin (BSA) (0.2 mg/ml; Sigma), and transferrin (0.01 mg/ml; Sigma) (SFM).
Western Immunoblotting
MCF-10A, T47D, and Hs578T cells (0.3–0.5 × 106 cells) were grown in GM on either plastic or fibronectin (0.25 μg/ml) to 80% confluency in T25 flasks and then washed twice with phosphate-buffered saline (PBS) followed by the addition of SFM for 24 h. For assessment of EGFR phosphorylation, MCF-10A cells were co-incubated with Iressa (1 μm) and EGF (0–25 ng/ml) for 4 h. EGFR and HER2 phosphorylation was also monitored in response to IGFBP-3 (100 ng/ml) for 10 min and 4 and 8 h and in response to a preincubation with IGFBP-3 (100 ng/ml) for 24 h followed by EGF (5 ng/ml MCF-10A; 2 ng/ml T47D) spiked into the media for 15 min. For assessment of activated p-38 MAPK, p-Akt, and p44/42 MAPK, all cell lines were treated with EGF (5 or 25 ng/ml) and IGFBP-3 (100 ng/ml), spiked into the media either alone or in combination for 5, 15, 30, and 120 min. p44/42 MAPK was also monitored in MCF-10A cells treated with IGFBP-3 (100 ng/ml) and Iressa (1 μm), spiked into the media for 15 min. Cells were then lysed on ice for 10 min (1 ml; 10 mm Tris-HCl, 5 mm EDTA, 50 mm NaCl, 30 mm sodium pyrophosphate, 50 mm sodium fluoride, 100 μm sodium orthovanadate, 1% Triton, 1 mm phenylmethylsulfonyl fluoride; pH 7.6). Lysates were then centrifuged at 14,000 × g for 15 min at 4 °C. The protein content of each sample was determined using a BCA Protein Assay Reagent kit, and equivalent amounts of protein were loaded onto gels. Proteins were separated by SDS-polyacrylamide gel electrophoresis and then transferred onto a nitrocellulose membrane followed by immunoblotting. Nonspecific binding sites on the nitrocellulose membranes were blocked overnight with 5% milk in Tris-buffered saline, 2% Tween for probing with anti-GAPDH, anti-EGF receptor, anti-p-Akt, and anti-Rho (all at 1:1000) or blocked with 3% BSA for probing with anti-p-MAP kinase (1:5000) or anti-p-p38 (1:1000) or blocked with 5% BSA for probing with anti-p-EGF receptor (1:750) and anti-p-HER2 (1:2000). After the removal of excess unbound antibody, an anti-mouse antibody (1:10,000 for GAPDH, 1:2,000 for Rho), anti-sheep antibody (1:2000 for EGF receptor), or anti-rabbit antibody (1:10,000 for p-MAPK and p-p38, 1:5,000 for HER2, 1:2,500 for p-Akt, and 1:1,500 for p-EGF receptor) conjugated to peroxidase was added for 1 h. Binding of the peroxidase was visualized by enhanced chemiluminescence according to the manufacturers' instructions. Optical density measurements were determined using a scanning densitometer (Bio-Rad) and analyzed using Totalab (Nonlinear Dynamics Ltd, Newcastle upon Tyne, UK).
Growth Assays
Cell Counting; Trypan Blue Dye Exclusion
All cells were collected after trypsinization, and the resulting cell suspension was loaded onto a hemocytometer (1:1) with the dye trypan blue, which is excluded by viable cells. Cells were counted from which total cell number and the percentage of dead cells relative to control were calculated. In all experiments basal cell death was very low and was not altered with any treatment.
Tritiated Thymidine Incorporation
MCF-10A and T47D cells were seeded on either plastic, laminin (0.25 μg/ml), or fibronectin (0.25 μg/ml) at 5 × 104 and 2 × 104 cells/well, respectively, in 24-well plates in GM. Before being switched to SFM for a further 24 h the cells were washed twice with PBS. Cells were dosed with EGF at 5 ng/ml (Hs578T) and 5–25 ng/ml (MCF-10A and T47D), IGFBP-3 (100 ng/ml) with and without Y-27632 (5 μm), and SPD peptide (10 ng/ml) for 48 h. The cells were incubated with 0.1 μCi of [3H]thymidine/well for the final 4 h of the dosing time period. After the removal of the supernatant, cells were then washed with 500 μl of 5% trichloroacetic acid (Merck) at 4 °C for 10 min followed by incubation with sodium hydroxide (400 μl of 1 m NaOH, Fisher) for 1 h at room temperature. The resulting suspension was placed into individual scintillation vials, and 3 ml of scintillation fluid were added. Samples were analyzed using a Beckman Scintillation Counter LS6500. Data were recorded as disintegrations/min.
Cell Surface Protein Biotinylation and Purification Assay
T75 cm2 (X8) flasks were seeded (2–3.5 × 106/flask) in GM for 24 h and then replaced with SFM for a further 48 h. Cells were treated (1 h) with or without EGF (5 ng/ml MCF-10A; 2 ng/ml T47D), IGFBP-3 (100 ng/ml), or EGF and IGFBP-3 in combination. The medium was removed, and the cells were washed (×2) with 8 ml of ice-cold PBS (8 ml). After removal of PBS, the biotin solution (10 ml) was added (for 5 min; 4 °C on an orbital shaker) and then replaced with ice-cold PBS (10 ml) and 500 μl of Quenching Solution (500 μl). The cells were then lysed and processed to assess the internalization of the EGF receptor according to the manufacturers' instructions using the Pinpoint Cell Surface Protein Isolation kit (Pierce). The resulting samples were then analyzed for EGFR and for GAPDH as an internal loading control and MHC class 1 (Santa Cruz, 1:500 primary and 1:5000 anti-rabbit secondary) as an external loading control by Western immunoblotting.
Rho Activation Assays
Activation assays were performed as described by Pellegrin et al. (30). In brief, MCF-10A cells were seeded at 0.3 × 106 per T25 culture flask for 24 h after which the GM was replaced with SFM for 24 h. Cells were treated with IGFBP-3 (100 ng/ml) or with sphingosine 1-phosphate (1 μm), used as a positive control, for 1–5 min. After treatment, the supernatant was removed, and the cells were then washed twice with Tris-buffered saline (50 mm Tris base, 140 mm NaCl; pH 7.6). Before cell lysis (50 mm Tris HCl, pH 7.2, 1% Triton X-100, 0.1% SDS, 500 mm NaCl, 10 mm MgCl2, and protease and phosphatase inhibitor cocktails, 1:100). Samples were then centrifuged at 14,000 × g at 4 °C for 10 min. Of the cell lysates, equal volumes were incubated with 20–30 μg of Rhotekin RBD protein agarose beads (GST-TRB) and rotated at 4 °C for 45 min, and equal volumes were also removed for assessment of total Rho as a loading control. The samples were washed 4 times with 600 μl of Tris buffer (50 mm Tris HCl, pH 7.2, 1% Triton X-100, 150 mm NaCl, 10 mm MgCl2, and protease and phosphatase inhibitor cocktails, 1:100) and centrifuged at 5000 rpm for 20 s at 4 °C, and the was supernatant removed. The samples were eluted with 50 μl of Laemmli sample buffer containing 40 mm DTT and boiled for 10 min. Samples were spun at 5000 rpm for 20 s, and the supernatant was then loaded onto the gel and SDS-PAGE and Western blotting was performed for Rho (as detailed above).
Radioimmunoassay
The basal levels of IGF-I and IGF-II produced by the MCF-10A, Hs578T, and T47D cells, as measured by radioimmunoassays described previously (31), were less than the level of detection limit (1 ng/ml) on both plastic and fibronectin for the duration of the described experiments.
Statistical Analysis
The data were analyzed with the Microsoft Excel version 5.0a software package using analysis of variance followed by least significant difference post hoc test. A statistically significant difference was considered to be present at p < 0.05.
RESULTS
In all experiments we found the same trends using either cell counting or tritiated thymidine incorporation. Therefore, we have only included data from one technique to illustrate each point. In addition, in all experiments basal cell death and the proportion of floating cells were very low, and these did not change with treatment.
IGFBP-3 Has Differential Effects on EGF-induced Cell Growth
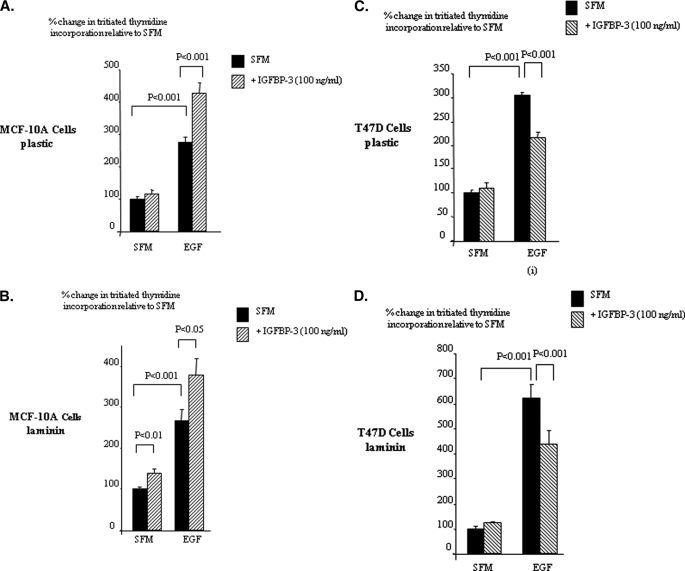
With the MCF-10A cells on plastic, EGF increased DNA synthesis (p < 0.001), and this was increased further with the combination of EGF and IGFBP-3 (p < 0.001) (Fig. 1A). We have shown previously that the intrinsic effects of IGFBP-3 on Hs578T breast cancer cells were identical when these cells were plated onto plastic or laminin (16), one of the major components of the basement membrane. We, therefore, assessed if the enhancement of EGF-induced growth of MCF10A cells by IGFBP-3 was also observed in the presence of laminin and found the profile to be essentially the same as on plastic with a synergistic increase in cell growth (p < 0.05) (Fig. 1B).
FIGURE 1.
IGFBP-3 modulation of EGF-induced growth of breast epithelial cells. MCF-10A (A, plastic; B, laminin) and T47D (C, plastic; D, laminin) were dosed with EGF (0–25 ng/ml), IGFBP-3 (100 ng/ml), or a combination of each for a further 48 h. Cell proliferation was assessed using tritiated thymidine incorporation. Graphs represent the mean of three experiments that were each performed in triplicate.
With T47D breast cancer cells on plastic, EGF caused a marked increase in DNA synthesis compared with controls, which was significantly abrogated in the presence of IGFBP-3 (p < 0.001) (Fig. 1C). We similarly assessed if the inhibition of EGF-induced by IGFBP-3 was also observed in the presence of laminin and observed the same trends as on plastic with the EGF-induced increase in DNA synthesis being abrogated in the presence of IGFBP-3 (p < 0.001) (Fig. 1D).
We saw the same trends for both cell lines using cell counting (data not shown). We also observed the same results with Hs578T breast cancer cells; IGFBP-3 alone had no effect, but the increase in total cell number induced by EGF was similarly blocked in the presence of IGFBP-3 (data not shown). These findings indicate that IGFBP-3 on both plastic and laminin markedly potentiated the mitogenic response to EGF in the non-malignant breast epithelial cells but, in contrast, inhibited the response in breast cancer cells.
Fibronectin Reverses the Actions of IGFBP-3 on EGF-induced Cell Growth
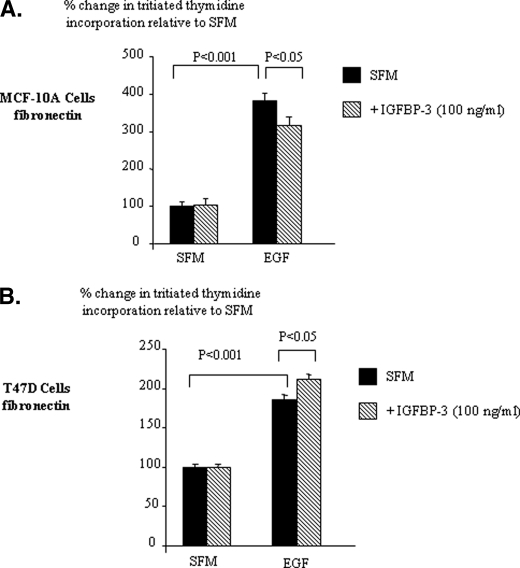
We have shown previously that the ability of IGFBP-3 to modulate cell attachment and apoptosis was reversed in the presence of fibronectin (16). Therefore, we next assessed if the effects of IGFBP-3 on EGF-induced cell growth were similarly affected. With the MCF-10A cells, EGF increased DNA synthesis (p < 0.001), which was inhibited in the presence of IGFBP-3 (p < 0.05) (Fig. 2A). Essentially IGFBP-3 negated the potentiating effect of fibronectin on the EGF response.
FIGURE 2.
IGFBP-3 modulation of EGF-induced growth of breast epithelial cells on fibronectin. MCF-10A (A) and T47D (B) cells were dosed with EGF (0–25 ng/ml), IGFBP-3 (100 ng/ml), or a combination of each for a further 48 h. Cell proliferation was assessed using tritiated thymidine incorporation. Graphs represent the mean of three experiments that were each performed in triplicate.
With the T47D cells on fibronectin, the ability of EGF to increase (p < 0.001) DNA synthesis was increased further in the presence of IGFBP-3 (p < 0.05) (Fig. 2B). We observed the same trends for both cell lines using cell counting (data not shown). We also observed similar trends to those we saw with T47D cells with Hs578T breast cancer cells (data not shown). These results indicate that the effects of IGFBP-3 on EGF-induced growth of non-malignant breast epithelial cells and breast cancer cells are both reversed when the cells are cultured on fibronectin.
The Differential Effects of IGFBP-3 on EGF-induced Cell Growth Are IGF-independent
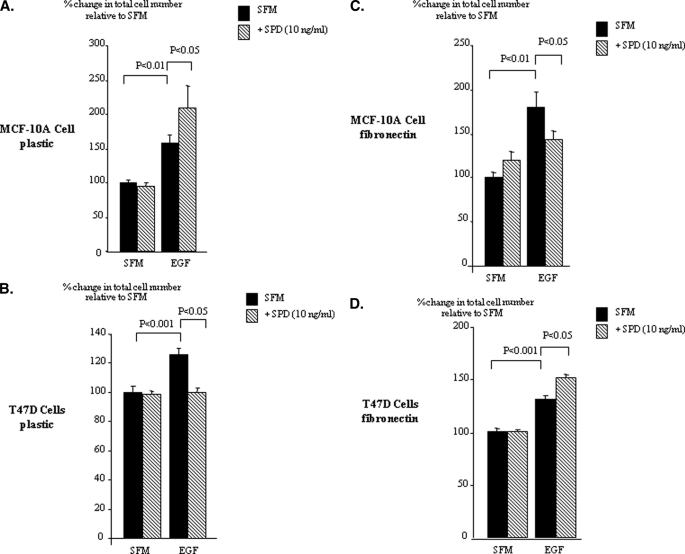
Unlike Hs578T cells, both the MCF-10A and T47D cells are IGF-responsive. Therefore, we confirmed that the effects of IGFBP-3 on EGF-induced growth in these cell lines were IGF-independent by using SPD (defined under “Experimental Procedures”). To observe a growth effect of SPD alone in the MCF-10A cells, as we have described previously (16), these cells require a period of 48 h in SFM before dosing. In the experimental paradigm we use to assess modulation of EGF-induced growth, the cells are exposed to SFM for just 24 h before dosing. Therefore, under these conditions in the MCF-10A cells we would not expect to observe an effect of SPD alone. With the MCF-10A cells cultured on plastic, SPD alone had no effect. EGF caused a significant increase in cell growth (p < 0.01), and in combination with SPD there was an enhanced increase in total cell number (p < 0.05) (Fig. 3A).
FIGURE 3.
SPD modulation of EGF-induced growth of breast epithelial cells. MCF-10A (A and C) and T47D (B and D) cells were dosed with EGF (0–25 ng/ml), SPD (10 ng/ml), or a combination of each for a further 48 h on either plastic (A and B) or fibronectin (0.25 μg/ml) (C and D). Cell proliferation was assessed by cell counting. Graphs represent the mean of three experiments that were each performed in triplicate.
On plastic with the T47D cells, SPD alone had no effect on cell growth, whereas EGF significantly increased cell proliferation (p < 0.001). The ability of EGF to enhance cell growth was significantly reduced in the presence of SPD (p < 0.05) (Fig. 3B). With MCF-10A cells grown on fibronectin, SPD alone had no effect. EGF increased cell growth (p < 0.01), which was significantly reduced in the presence of SPD (p < 0.05) (Fig. 3C). On fibronectin with T47D cells, SPD alone had no effect. However, the ability of EGF to significantly increase cell proliferation (p < 0.001) was further enhanced in the presence of SPD (p < 0.05) (Fig. 3D).
The Effects of IGFBP-3 on Cell Growth Involve the EGF Receptor
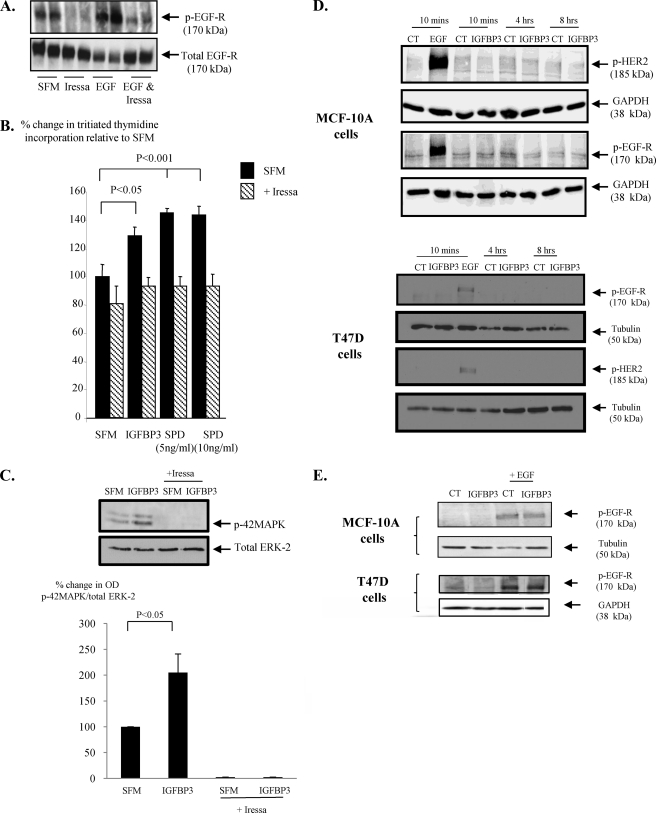
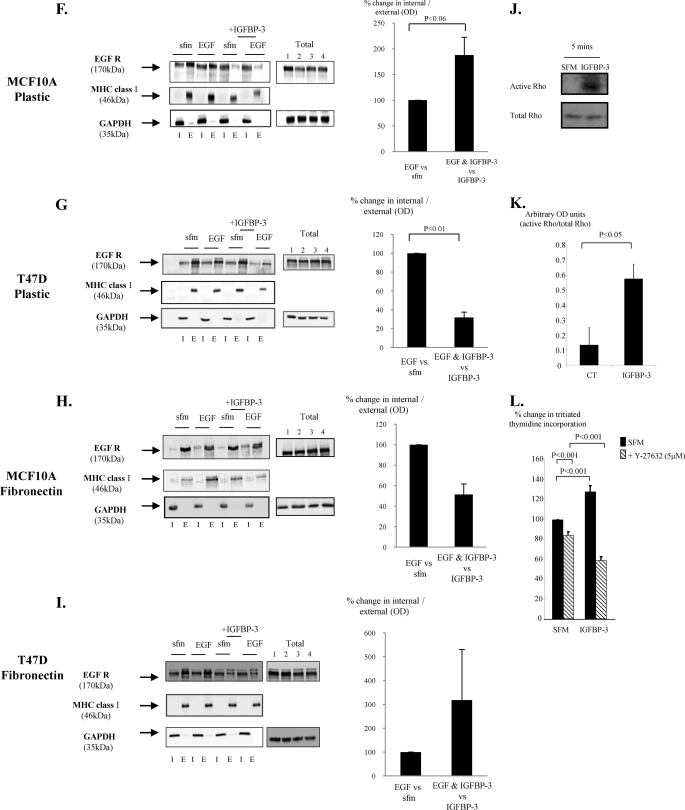
Having established that IGFBP-3 was able to differentially modulate EGF-induced growth in non-malignant breast versus breast cancer cells, we next examined a potential mechanism by which IGFBP3 could be eliciting these effects. To confirm that IGFBP-3 required the EGFR to exert its effects on EGF-induced growth, we used Iressa, which is a tyrosine kinase inhibitor and can block the phosphorylation and/or the internalization of the EGFR. We confirmed an effective dose of Iressa on MCF-10A cells by examining the phosphorylation status of EGFR. The doses of Iressa used were not cytotoxic and did not affect cell adhesion as there was no effect on either the levels of cell death or on the levels of floating cells (data not shown). As treatments only, therefore, affected cell growth and as we had established that the same trends were observed for cell counts and thymidine incorporation, we only report data for the latter. After a co-incubation, we found Iressa alone blocked basal activation of the EGFR, and the EGF-induced EGFR phosphorylation was markedly abrogated (Fig. 4A). We have shown previously in the relatively normal MCF-10A breast epithelial cells that both IGFBP-3 and SPD act as mitogens in an IGF-independent manner (12). Fig. 4B shows that IGFBP-3 and SPD after 48 h in SFM each promoted cell growth (p < 0.05), which was completely blocked in the presence of Iressa. Another EGFR inhibitor, AG1478, also completely blocked the ability of IGFBP-3 or SPD to induce cell growth in the MCF-10A cells (data not shown). In addition, co-incubation of Iressa or AG1478 with IGFBP-3 or SPD had no additive negative effect on MCF-10A cells grown on fibronectin (data not shown). These results suggest that IGFBP-3 works via the EGFR. We have also previously shown that IGFBP-3/SPD-induced cell proliferation in the non-malignant MCF-10A cells is MAPK-dependent (5), and in Fig. 4C we show that that IGFBP-3 can no longer activate MAPK (p < 0.05) in the presence of Iressa. First, assessing EGFR/HER2 phosphorylation, we found that IGFBP-3 alone had no effect in either MCF-10A or T47D cells (Fig. 4D). In addition we showed that in both cell lines EGF-induced activation of the EGFR was also unaffected in the presence of IGFBP-3 (Fig. 4E). We also performed these experiments at 5 and 15 min on fibronectin and obtained the same results (data not shown). It has, however, become apparent that in addition to the initial activation of the receptor, its cellular localization also has important effects on consequent cell signaling. Upon activation, the receptor is internalized to endosomes where adaptor proteins recruit scaffolding proteins that facilitate the assembly of signaling complexes. The internalization of EGFR to endosomes is often required for full activation of MAPK and a proliferative response (32–34). We, therefore, also examined whether IGFBP-3/SPD affects EGFR internalization. Indeed Fig. 4, F–I, show that the combination of IGFBP-3 and EGF in the MCF-10A cells and T47D cells, respectively, did result in alterations in EGFR internalization. With the non-malignant MCF-10A cells, EGF-induced receptor internalization was increased by 87.6% (p < 0.06) but decreased by 68.2% (p < 0.01) in the malignant T47D cells. Furthermore, we demonstrated that these effects in the non-malignant and malignant cell lines were reversed when the cells were grown on fibronectin such that EGF-induced receptor internalization was reduced with MCF-10A cells but enhanced in the T47D cells. Rho is known to play a key role in endocytic trafficking (35), and it has been shown previously that internalization of EGFR can be Rho-dependent (36, 37). With MCF-10A cells, we found that IGFBP-3 activated Rho after 2 min, which was maximal at 5 min. In contrast, the activation of Rho by a positive control, sphingosine 1-phosphate at 1 min had disappeared by 2 min (data not shown). Fig. 4, J and K, confirm that IGFBP-3 significantly (p < 0.05) activated Rho after treatment for 5 min, and Fig. 4L confirms that Rho was involved in the proliferative effects of IGFBP-3, as blocking Rho-associated kinase (ROCK) actually reversed the effects of IGFBP-3 such that IGFBP-3 then significantly (p < 0.001) inhibited cell growth.
FIGURE 4.
The effects of IGFBP-3 on cell growth involve the EGF receptor. A, MCF-10A cells were dosed with EGF (10 ng/ml) or Iressa (1 μm) or a combination of the two for 4 h. Cells were then lysed and processed for p-EGFR followed by total EGFR to show comparable levels of protein loaded. The blot is representative of experiments repeated three times (arbitrary optical density units for A: CT = 0.31, Iressa = 0, EGF = 0.97; EGF + Iressa = 0.19). CT, control. B, MCF-10A cells were dosed with IGFBP-3 (100 ng/ml) or SPD (5 and 10 ng/ml) in the presence or absence of Iressa (1 μm) for 24 h. Cell proliferation was assessed using tritiated thymidine incorporation. The graph represents the mean of three experiments that were each performed in triplicate. C, MCF-10A cells were dosed with IGFBP-3 (100 ng/ml) with or without Iressa (1 μm) for 15 min. Cells were lysed as in A and immunoblotted for p-P42/44MAPK followed by ERK-2 as a loading control. The graph shows the percentage change in OD of three representative blots. D, MCF10A and T47D cells were dosed with EGF (10 min) or IGFBP-3 (10 min to 8 h), E, MCF10A and T47D cells were dosed with EGF in the presence or absence of IGFBP-3. D and E were processed and assessed for HER2 and/or EGFR phosphorylation as in A. F–I, MCF-10A and T47D cells were treated as in E for 1 h on either plastic (A and B, n = 3 for each) or on fibronectin (C and D, n = 2 for each) and then lysed and processed to assess the internalization of the EGF receptor (as described under “Experimental Procedures”). Representative blots showing changes in levels of EGFR inside the cell (I) or on the cell surface (E) are shown for each experiment. We also show total levels of EGFR (where 1 = SFM, 2 = EGF, 3 = IGFBP-3, 4 = EGF+IGFBP-3) together with GAPDH as a loading control. We used MHC class 1 as a loading control for the external fraction and GAPDH for the internal component. The graphs show the percentage change in ODs between internal EGFR compared with external EGFR corrected for their respective controls. J and K, MCF-10A cells were dosed with IGFBP-3 (100 ng/ml; 5 min) and processed for Rho activation as described under “Experimental Procedures.” The graph represents the mean of three separate experiments. L, MCF-10A cells were treated with IGFBP-3 (100 ng/ml) with and without Y-27632 (5 μm) for 24 h and assessed for tritiated thymidine incorporation. The graph represents the mean of three experiments that were each performed in triplicate.
IGFBP-3 Affects EGF-induced MAPK Phosphorylation
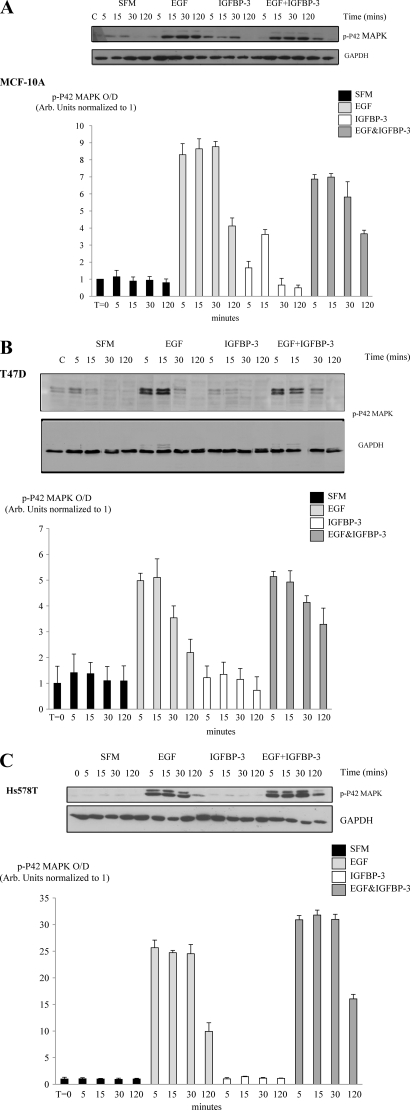
We have shown previously in MCF10A cells that the promotion of cell growth by IGFBP-3 is MAPK-dependent (5). In the MCF-10A cells, EGF alone significantly activated p44/42 MAPK at all time points (p < 0.05). IGFBP-3 alone also activated p44/42 MAPK but only significantly at 15 min (p < 0.001). In combination with EGF, IGFBP-3 significantly enhanced p44/42 MAPK phosphorylation at all but the longest time point (p < 0.01) (Fig. 5A).
FIGURE 5.
IGFBP-3 affects EGF-induced activation of p44/42 MAPK in breast epithelial cells. Cells were dosed EGF (0–25 ng/ml) or IGFBP-3 (100 ng/ml) or in combination for 5, 15, 30, and 120 min, lysed, and probed for p44/42 MAPK and also GAPDH to show equal loading of protein. Representative Western immunoblots for p44/42 MAPK and GAPDH are shown for MCF-10A cells (A), T47D cells (B), and Hs578T cells (C). The bottom panel in each figure shows the mean densitometry values of three separate experiments upon which statistical analysis was performed and included in the results.
EGF also activated Akt but at 5 and 15 min only (p < 0.05) and p38 MAPK at all but the longest time point (p < 0.05). IGFBP-3 alone, however, had no effect on p-38 MAPK or Akt activation. The EGF-induced activation of p-38 MAPK or Akt were also unaffected in the presence of IGFBP-3 (data not shown).
In the T47D cells EGF alone also significantly increased p44/42 MAPK phosphorylation (p < 0.001). The activation status of p44/42 MAPK was unaffected in the presence of IGFBP-3 alone. In contrast to the MCF-10A cells, the ability of EGF to activate p44/42 MAPK was markedly inhibited in combination with IGFBP-3 (p < 0.05) and had returned to base line by 30 min (Fig. 5B). EGF also activated Akt at all time points but had no effect on p-38 MAPK phosphorylation. p38-MAPK or Akt were unaffected by IGFBP-3 alone, and the EGF-induced activation of Akt was unaffected by IGFBP-3 (data not shown).
In the Hs578T cells, EGF alone significantly increased p44/42 MAPK phosphorylation at all time points (p < 0.05), whereas IGFBP-3 alone was without effect. As in the T47D cells, the ability of EGF to activate p44/42 MAPK was inhibited by IGFBP-3 but only significantly at 5 min (p < 0.001) (Fig. 5C). EGF did not affect Akt activation but significantly increased p-38 MAPK phosphorylation at 5 and 15 min (p < 0.01); the activation status of these were unaffected by IGFBP-3 or the combination (data not shown).
IGFBP-3 Affects EGF-induced MAPK Phosphorylation on Fibronectin
In the MCF-10A cells on fibronectin, EGF alone significantly activated p44/42 MAPK at all time points (p < 0.001 respectively), whereas IGFBP-3 alone activated p44/42 MAPK but only significantly at 15 min (p < 0.001). These findings were very similar to the responses observed when the cells were cultured on plastic. In combination with EGF, however, IGFBP-3 significantly inhibited p44/42 MAPK phosphorylation at all but the longest time point (p < 0.05) (Fig. 6A). EGF also activated Akt at all but the longest time point (p < 0.05) and p38 MAPK at all time points (p < 0.05), whereas IGFBP-3 alone had no effect on either p-38 MAPK or Akt activation. In addition, EGF-induced activation of p-38 MAPK or Akt was unaffected in the presence of IGFBP-3 (data not shown).
FIGURE 6.
IGFBP-3 affects EGF-induced activation of p44/42 MAPK in breast epithelial cells on fibronectin. Cells were dosed EGF (0–25 ng/ml) or IGFBP-3 (100 ng/ml) or in combination for 5, 15, 30, and 120 min, lysed, and probed for p44/42 MAPK and also GAPDH to show equal loading of protein. Representative Western immunoblots for p44/42 MAPK and GAPDH are shown for MCF-10A cells (A), T47D cells (B), and Hs578T cells (C). The bottom panel in each figure shows the mean densitometry values of three separate experiments upon which statistical analysis was performed and included in the results.
In the T47D cells on fibronectin, EGF alone also significantly increased p44/42 MAPK phosphorylation at all time points (p < 0.001), whereas IGFBP-3 alone was without effect. This response to EGF alone time-dependently returned to base line, but this did not occur in the presence of IGFBP-3; the ability of EGF to activate p44/42 MAPK was sustained for longer in combination with IGFBP-3 (Fig. 6B). EGF had no effect on Akt or p-38 activation at any time point, and these were also unaffected by IGFBP-3 or the combination (data not shown).
With the Hs578T cells on fibronectin, EGF alone significantly increased p44/42 MAPK phosphorylation at all time points (p < 0.001), but the activation status of p44/42 MAPK was unaffected in the presence of IGFBP-3 alone. As in the T47D cells, the ability of EGF to activate p44/42 MAPK was enhanced in combination with IGFBP-3 and at every time point (p < 0.001) (Fig. 6C). EGF also increased Akt activation (p < 0.01) at all time points and increased p-38 phosphorylation at all but the longest time point (p < 0.001). p38-MAPK or Akt were unaffected in the presence of IGFBP-3 alone or the combination of EGF and IGFBP-3 (data not shown). Comparing the cells, we found that the -fold changes in MAPK activation were relatively comparable on both matrices, which correlated with their similar growth responses to EGF (data not shown).
DISCUSSION
Epithelial cells receive important signals from soluble growth factors and from insoluble matrix proteins. Tumor invasion and metastasis involves complex changes in the normal cell-cell and cell-matrix interactions (17). Laminin and collagen 1V are the major components of the basement membrane upon which normal breast epithelial cells reside (38), but in breast tumors, where normal tissue architecture is disrupted, the cells are exposed to less laminin in comparison to extremely high levels of fibronectin (39). Fibronectin in breast tumors has been correlated with lymph node status and an increased mortality risk (17). This markedly different ECM observed in breast cancers would be expected to greatly alter the response to growth factors. EGF actions on cell proliferation, migration, and survival involve anchorage-dependent signals mediated via integrin receptors (40). A number of specific integrin receptors have been shown to physically associate with EGFR (41, 42) and with HER-2 (43). Integrins can induce EGFR phosphorylation in the absence EGFR ligands (44) and can modulate EGF and heregulin-induced signaling (45).
In the normal breast, estrogen acts in concert with local growth factors to regulate epithelial cell function. As breast tumors progress they can acquire independence from estrogen and, hence, resistance to endocrine therapies, which represents a major clinical problem. This acquired resistance appears to involve adaptive increases in activity of local growth factor systems, particularly EGF and IGF (46). With progression to estrogen independence, there is a concomitant increase in expression of IGFBP-3 (3, 4). It has previously been reported that IGFBP-3 potentiated EGF-promoted growth of non-malignant breast epithelial cells (27). We have now examined the effects of IGFBP-3 on EGF-promoted growth in both non-malignant and malignant breast epithelial cells in the context of different ECM, as would occur with tumor progression. We confirmed that IGFBP-3 potentiated the EGF-promoted growth of non-malignant breast epithelial cells and found that the opposite occurred in breast cancer cells where IGFBP-3 inhibited EGF-induced growth. We have also shown that a fragment of IGFBP-3 that does not bind to IGFs has the same actions, indicating that these actions of IGFBP-3 are independent of IGF binding. These effects were the same when the cells were cultured on plastic or on laminin-coated plates; however, when the same cells were cultured on fibronectin-coated plates the effect of IGFBP-3 on EGF responses were reversed. On fibronectin, IGFBP-3 inhibited EGF-promoted growth of non-malignant cells but enhanced EGF-promoted growth of breast cancer cells. These results are entirely consistent with our previous work, which indicated that IGFBP-3 could reduce cell adhesion and accentuate apoptotic responses of breast cancer cells on laminin and collagen, but these effects were reversed on fibronectin (16). The ability of fibronectin to switch the actions of IGFBP-3 may account for the apparent ambiguity in the literature, relating to whether the presence of IGFBP-3 will have a positive or negative effect on breast cancer cells and adds to the evidence that integrins are critically involved in IGFBP-3 actions. Thrombospondin, which can activate integrin-associated protein in addition to integrin receptors (47), can switch the inhibitory effects of IGFBP-3 on cell attachment (16). Furthermore, the effects of IGFBP-3 on apoptosis and attachment are blocked in the presence of an RGD-containing fibronectin fragment (16).
The enhanced response to EGF when the cells were cultured on fibronectin is consistent with the reports that fibronectin promotes clustering of α5β1 and α1β1 integrins and activation of EGFR and enhances EGFR coupling to the MAPK pathway via Shc (19, 20). Epithelial cells express a large repertoire of integrin receptors that changes with malignant transformation and the consequent ECM remodeling. It has been reported that more than half of unstimulated EGFRs are concentrated in caveolae (48), within which numerous signaling molecules aggregate. Caveolin-1 is scaffolding protein-enriched within caveolae and has been shown to associate with integrins (20) and IGFBP-3 (5, 49). The particular matrix with which the cells interact will affect associations/disassociations between EGFR/HER-2 and integrin receptors, with consequences on subsequent downstream signaling. Through its interaction with integrin receptors and their ancillary proteins, IGFBP-3 may modify these associations; this may account for the differential effects of IGFBP-3 on EGF-induced growth between normal and cancer cells and also the matrix-dependent differences within each cell type.
As we have shown previously, IGFBP-3 and SPD promoted basal growth of the normal MCF10A cells in serum-free media without the addition of EGF in a MAPK-dependent manner (5). We found, however, that this induction of cell growth by IGFBP-3 and SPD was completely abrogated by EGFR inhibitors, Iressa and AG1064, as was the ability of IGFBP-3 to activate MAPK. This implies that IGFBP-3 intrinsically modulated EGF receptor activity, potentially via integrin receptors. We found that IGFBP-3 did not increase the basal phosphorylation of EGFR, which was also noted by Martin et al. (27). However, in contrast to this report (27), we did not see modulation of EGF-induced activation of EGFR in the presence of IGFBP-3. Another report also showed an effect of IGFBP-3 on EGF actions in an endometrial cancer cell line, and like us, they also observed no effect on EGF receptor phosphorylation (50). However, we only assessed the phosphorylation status at Tyr1068, which had previously been reported to be affected, and it would be interesting to address if other sites of the EGFR were differentially phosphorylated, although no effect on EGF receptor phosphorylation in endometrial cancer cells was observed when using a general phosphotyrosine antibody (50). We did make the novel observation that IGFBP-3 affected internalization of the EGF receptor and subsequent activation of MAPK. The regulation of EGFR trafficking has been reported to involve Rho (36, 37). We showed that EGF-induced growth of the MCF-10A cells was associated with an increase in EGFR internalization, both of which were enhanced in the presence of IGFBP-3, but the growth and EGFR internalization were inhibited in the T47D cells. We also found that these effects were reversed in each cell line when grown on fibronectin. In preliminary experiments we observed that IGFBP-3 acutely increased the active component of Rho and a Rho inhibitor blocked its proliferative effect on MCF-10A cells. In future studies the role of Rho in mediating IGFBP-3 effects should be confirmed using specific molecular manipulations. It has been shown that EGFR internalization to endosomes facilitates association with the adaptor protein p14, which then localizes the scaffold protein MP1 (MEK1 partner), which in turn potentiates activation of the ERK signaling cascade (33). In MCF-10A cells, where IGFBP-3 promotes EGFR internalization, more EGFR would be available to associate with p14 and MP1, intensifying MAPK signaling and the proliferative response as we observed. In the T47D cells, however, the internalization of EGFR was reduced in the presence of IGFBP-3, and EGF-induced MAPK signaling and growth was inhibited by IGFBP-3. It would be interesting to investigate these movements and associations with dual-staining confocal microscopy. Although EGF was able to activate p-38 MAPK, p44/42 MAPK, and p-Akt, we found that only EGF-induced p44/42 MAPK was modulated by IGFBP-3 in all three cell lines. This specific effect on one signaling pathway may be due to altered interactions with p14 and MP1. growth. It has been reported previously in the MCF-10A cells that p-38 MAPK was also involved in IGFBP-3 actions (27). Others have found that intrinsic signaling by IGFBP-3 can involve p-Akt (51), and potentiation of IGF activity by IGFBP-3 has also been shown to be via p-Akt (52). Together these reports suggest that IGFBP-3 can use different pathways to elicit its effects depending upon the context of different growth factors, different cell types, or the cells extracellular matrix.
The ability of IGFBP-3 to modulate EGF-induced growth in an intrinsic manner adds to the accumulating evidence suggesting that IGFBPs play an important role in the regulation of cell growth, death, adhesion (for review, see Ref. 4), and migration (53) independently of their interactions with IGFs. In addition to the six high affinity IGFBPs, there are a larger group of proteins that share more limited homology but clearly form more distant relatives of a superfamily of related proteins (54) that includes another group of proteins referred to as CCN proteins, which include NOV, mac25, CTGF, and CYR61. The CCN proteins are, like IGFBPs, all cysteine-rich modular proteins with many pleiotropic actions on cell functions similar to the intrinsic actions of IGFBP-3 (55). No specific cell surface receptors have been described for CCN proteins, but it is now recognized that they generally act via integrin receptors with which they interact through non-classical recognition sequences (56). Although IGFBP-3 also does not possess a classical integrin recognition sequence, it has been reported to associate with the β1 integrin (5, 49) and to bind with high affinity to many other proteins that are recognized to interact directly with integrins, including fibronectin (57), fibrin and fibrinogen (58), plasminogen (59), ADAM12 (60), caveolin-1, and the transferrin receptor (49, 61). In support of this, a recent report has demonstrated that IGFBP-3 potentiation of EGF-induced growth in the non-malignant MCF10A cells involves activation of sphingosine kinase 2, which resulted in sphingosine-1 phosphate production acting on sphingosine 1-phosphate 1/3 receptors (62). Evidence shows that S1P-mediated signaling via S1P1 receptors can utilize Rho to activate integrin αvβ3 (63). Collectively these reports are consistent with our data indicating another way in which IGFBP-3 can modulate integrin function. It is possible that IGFBP-3 interacts with integrin receptors via one of these intermediates or directly via a non-classical integrin recognition sequence. It is clear that integrins play a pivotal role in both the actions of EGF and the intrinsic effects of IGFBP-3. In addition to these similarities between IGFBPs and CCN proteins, it has recently been reported that CYR61 (CCN1) (64) mediates many of the effects of heregulin (a member of the EGF-like growth factor family) on breast cancer cells. Heregulin up-regulated CCN1, which then promoted cell growth and survival via αvβ3 integrin receptor-mediated activation of MAPK (65). These interactions are very analogous to those that we have found between IGFBP-3 and EGF. It has been reported that EGF regulates IGFBP-3 production (21, 22), and we have described in this study and previously that that the intrinsic effects of IGFBP-3 on cell growth also involves integrin receptor activation and phosphorylation of MAPK culminating in cell proliferation (5).
This study has demonstrated that IGFBP-3 can inhibit EGF-induced growth of breast cancer cells, but with the same cells on fibronectin, the actions of IGFBP-3 switch to promoting EGF-induced proliferation. As breast tumors progress, a number of changes occur including up-regulation of EGFR/HER-2 (23), IGFBP-3(14, 15), and fibronectin (17) production. This would then culminate in an environment that is optimal for promoting growth, resulting in a more aggressive tumor. Our findings may also have important implications in relation to agents that target EGFR, such that in an early tumor environment it may be anticipated that the presence of IGFBP-3 would reduce EGF-induced growth and thereby the efficacy of drugs targeting EGFR. However, in a more advanced tumor, the presence of IGFBP-3 could potentially enhance EGF-induced proliferation. Tumor growth would be more EGF-dependent, and this could markedly increase the efficacy of such interventions. These and other studies may eventually provide us with better markers of drug efficacy and allow more specific patient selection.
Acknowledgments
We acknowledge Dr. Harry Mellor, Dr. Alexandra Gampel, Dr. Carla Burrows, and Jane Carter.
This work was supported by The Bristol University Cancer Research Fund.
- IGF
- insulin-like growth factor
- IGFBP
- IGF binding protein
- EGFR
- EGF receptor
- ECM
- extracellular matrix
- SPD
- serine phosphorylation domain
- GM
- growth media
- SFM
- serum-free medium.
REFERENCES
- 1.Pollak M. N. (2004) Novartis Found. Symp. 262, 84–98; discussion 98–107, 265–268, Review [PubMed] [Google Scholar]
- 2.Hynes N. E., Stern D. F. (1994) Biochim. Biophys. Acta 1198, 165–184 [DOI] [PubMed] [Google Scholar]
- 3.Firth S. M., Baxter R. C. (2002) Endocr. Rev. 23, 824–854 [DOI] [PubMed] [Google Scholar]
- 4.Perks C., Holly J. (2003) Horm. Metab. Res. 35, 828–835 [DOI] [PubMed] [Google Scholar]
- 5.Burrows C., Holly J. M., Laurence N. J., Vernon E. G., Carter J. V., Clark M. A., McIntosh J., McCaig C., Winters Z. E., Perks C. M. (2006) Endocrinology 147, 3484–3500 [DOI] [PubMed] [Google Scholar]
- 6.Hankinson S. E., Willett W. C., Colditz G. A., Hunter D. J., Michaud D. S., Deroo B., Rosner B., Speizer F. E., Pollak M. (1998) Lancet 351, 1393–1396 [DOI] [PubMed] [Google Scholar]
- 7.Bruning P. F., Van Doorn J., Bonfrèr J. M., Van Noord P. A., Korse C. M., Linders T. C., Hart A. A. (1995) Int. J. Cancer 62, 266–270 [DOI] [PubMed] [Google Scholar]
- 8.Bohlke K., Cramer D. W., Trichopoulos D., Mantzoros C. S. (1998) Epidemiology 9, 570–573 [PubMed] [Google Scholar]
- 9.Baxter R. C. (2001) Mol. Pathol. 54, 145–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H. Y., Moon H., Chun K. H., Chang Y. S., Hassan K., Ji L., Lotan R., Khuri F. R., Hong W. K. (2004) J. Natl. Cancer Inst. 96, 1536–1548 [DOI] [PubMed] [Google Scholar]
- 11.Kansra S., Ewton D. Z., Wang J., Friedman E. (2000) Int. J. Cancer 87, 373–378 [DOI] [PubMed] [Google Scholar]
- 12.McCaig C., Fowler C. A., Laurence N. J., Lai T., Savage P. B., Holly J. M., Perks C. M. (2002) Br. J. Cancer 86, 1963–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renehan A. G., Zwahlen M., Minder C., O'Dwyer S. T., Shalet S. M., Egger M. (2004) Lancet 363, 1346–1353 [DOI] [PubMed] [Google Scholar]
- 14.Rocha R. L., Hilsenbeck S. G., Jackson J. G., Lee A. V., Figueroa J. A., Yee D. (1996) J. Natl. Cancer Inst. 88, 601–606 [DOI] [PubMed] [Google Scholar]
- 15.Vestey S. B., Perks C. M., Sen C., Calder C. J., Holly J. M., Winters Z. E. (2005) Breast Cancer Res. 7, R119–R129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCaig C., Perks C. M., Holly J. M. (2002) J. Cell Sci. 115, 4293–4303 [DOI] [PubMed] [Google Scholar]
- 17.Ioachim E., Charchanti A., Briasoulis E., Karavasilis V., Tsanou H., Arvanitis D. L., Agnantis N. J., Pavlidis N. (2002) Eur. J. Cancer 38, 2362–2370 [DOI] [PubMed] [Google Scholar]
- 18.Gorczyca W., Holm R., Nesland J. M. (1993) Anticancer Res. 13, 851–858 [PubMed] [Google Scholar]
- 19.Wary K. K., Mainiero F., Isakoff S. J., Marcantonio E. E., Giancotti F. G. (1996) Cell 87, 733–743 [DOI] [PubMed] [Google Scholar]
- 20.Wary K. K., Mariotti A., Zurzolo C., Giancotti F. G. (1998) Cell 94, 625–634 [DOI] [PubMed] [Google Scholar]
- 21.Edmondson S. R., Murashita M. M., Russo V. C., Wraight C. J., Werther G. A. (1999) J. Cell. Physiol. 179, 201–207 [DOI] [PubMed] [Google Scholar]
- 22.Takaoka M., Harada H., Andl C. D., Oyama K., Naomoto Y., Dempsey K. L., Klein-Szanto A. J., El-Deiry W. S., Grimberg A., Nakagawa H. (2004) Cancer Res. 64, 7711–7723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sainsbury J. R., Malcolm A. J., Appleton D. R., Farndon J. R., Harris A. L. (1985) J. Clin. Pathol 38, 1225–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsutsui S., Ohno S., Murakami S., Hachitanda Y., Oda S. (2002) Breast Cancer Res. Treat. 71, 67–75 [DOI] [PubMed] [Google Scholar]
- 25.Firth S. M., Fanayan S., Benn D., Baxter R. C. (1998) Biochem. Biophys. Res. Commun. 246, 325–329 [DOI] [PubMed] [Google Scholar]
- 26.Butt A. J., Martin J. L., Dickson K. A., McDougall F., Firth S. M., Baxter R. C. (2004) J. Clin. Endocrinol. Metab. 89, 1950–1956 [DOI] [PubMed] [Google Scholar]
- 27.Martin J. L., Weenink S. M., Baxter R. C. (2003) J. Biol. Chem. 278, 2969–2976 [DOI] [PubMed] [Google Scholar]
- 28.Hollowood A. D., Stewart C. E., Perks C. M., Pell J. M., Lai T., Alderson D., Holly J. M. (2002) J. Cell. Biochem. 86, 583–589 [DOI] [PubMed] [Google Scholar]
- 29.Soule H. D., Maloney T. M., Wolman S. R., Peterson W. D., Jr., Brenz R., McGrath C. M., Russo J., Pauley R. J., Jones R. F., Brooks S. C. (1990) Cancer Res. 50, 6075–6086 [PubMed] [Google Scholar]
- 30.Pellegrin S., Mellor H. (2008) Curr. Protoc. Cell Biol. Chapter 14, Unit 14.8 [DOI] [PubMed] [Google Scholar]
- 31.Davies S. C., Holly J. M., Coulson V. J., Cotterill A. M., Abdulla A. F., Whittaker P. G., Chard T., Wass J. A. (1991) Clin. Endocrinol. (Oxf) 34, 501–506 [DOI] [PubMed] [Google Scholar]
- 32.Hoeller D., Volarevic S., Dikic I. (2005) Curr. Opin Cell Biol. 17, 107–111 [DOI] [PubMed] [Google Scholar]
- 33.Teis D., Wunderlich W., Huber L. A. (2002) Dev. Cell 3, 803–814 [DOI] [PubMed] [Google Scholar]
- 34.Miaczynska M., Pelkmans L., Zerial M. (2004) Curr. Opin. Cell Biol. 16, 400–406 [DOI] [PubMed] [Google Scholar]
- 35.Qualmann B., Mellor H. (2003) Biochem. J. 371, 233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gampel A., Parker P. J., Mellor H. (1999) Curr. Biol. 9, 955–958 [DOI] [PubMed] [Google Scholar]
- 37.Gampel A., Mellor H. (2002) Biochem. J. 366, 393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Hernandez A., Amenta P. S. (1983) Lab. Invest. 48, 656–677 [PubMed] [Google Scholar]
- 39.Ioachim E., Kamina S., Kontostolis M., Agnantis N. J. (1997) Virchows Arch. 431, 311–316 [DOI] [PubMed] [Google Scholar]
- 40.Cabodi S., Moro L., Bergatto E., Boeri Erba E., Di Stefano P., Turco E., Tarone G., Defilippi P. (2004) Biochem. Soc. Trans. 32, 438–442 [DOI] [PubMed] [Google Scholar]
- 41.Yu X., Miyamoto S., Mekada E. (2000) J. Cell Sci. 113, 2139–2147 [DOI] [PubMed] [Google Scholar]
- 42.Mariotti A., Kedeshian P. A., Dans M., Curatola A. M., Gagnoux-Palacios L., Giancotti F. G. (2001) J. Cell Biol. 155, 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falcioni R., Antonini A., Nisticò P., Di Stefano S., Crescenzi M., Natali P. G., Sacchi A. (1997) Exp. Cell Res. 236, 76–85 [DOI] [PubMed] [Google Scholar]
- 44.Moro L., Venturino M., Bozzo C., Silengo L., Altruda F., Beguinot L., Tarone G., Defilippi P. (1998) EMBO J. 17, 6622–6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuwada S. K., Li X. (2000) Mol. Biol. Cell 11, 2485–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicholson R. I., Hutcheson I. R., Hiscox S. E., Knowlden J. M., Giles M., Barrow D., Gee J. M. (2005) Endocr. Relat. Cancer 12, S29–S36 [DOI] [PubMed] [Google Scholar]
- 47.Li Z., Calzada M. J., Sipes J. M., Cashel J. A., Krutzsch H. C., Annis D. S., Mosher D. F., Roberts D. D. (2002) J. Cell Biol. 157, 509–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mineo C., Gill G. N., Anderson R. G. (1999) J. Biol. Chem. 274, 30636–30643 [DOI] [PubMed] [Google Scholar]
- 49.Singh B., Charkowicz D., Mascarenhas D. (2004) J. Biol. Chem. 279, 477–487 [DOI] [PubMed] [Google Scholar]
- 50.Mochizuki T., Sakai K., Iwashita M. (2006) Growth Horm. IGF Res. 16, 202–210 [DOI] [PubMed] [Google Scholar]
- 51.Ricort J. M., Binoux M. (2004) Biochem. Biophys. Res. Commun. 314, 1044–1049 [DOI] [PubMed] [Google Scholar]
- 52.Conover C. A., Bale L. K., Durham S. K., Powell D. R. (2000) Endocrinology 141, 3098–3103 [DOI] [PubMed] [Google Scholar]
- 53.Abrass C. K., Berfield A. K., Andress D. L. (1997) Am. J. Physiol. 273, F899–F906 [DOI] [PubMed] [Google Scholar]
- 54.Hwa V., Oh Y., Rosenfeld R. G. (1999) Endocr. Rev. 20, 761–787 [DOI] [PubMed] [Google Scholar]
- 55.Perbal B. (2001) Bull Cancer 88, 645–649 [PubMed] [Google Scholar]
- 56.Lau L. F., Lam S. C. (1999) Exp. Cell Res. 248, 44–57 [DOI] [PubMed] [Google Scholar]
- 57.Gui Y., Murphy L. J. (2001) J. Clin. Endocrinol. Metab. 86, 2104–2110 [DOI] [PubMed] [Google Scholar]
- 58.Campbell P. G., Durham S. K., Hayes J. D., Suwanichkul A., Powell D. R. (1999) J. Biol. Chem. 274, 30215–30221 [DOI] [PubMed] [Google Scholar]
- 59.Oesterreicher S., Blum W. F., Schmidt B., Braulke T., Kübler B. (2005) J. Biol. Chem. 280, 9994–10000 [DOI] [PubMed] [Google Scholar]
- 60.Loechel F., Fox J. W., Murphy G., Albrechtsen R., Wewer U. M. (2000) Biochem. Biophys. Res. Commun. 278, 511–515 [DOI] [PubMed] [Google Scholar]
- 61.Lee K. W., Liu B., Ma L., Li H., Bang P., Koeffler H. P., Cohen P. (2004) J. Biol. Chem. 279, 469–476 [DOI] [PubMed] [Google Scholar]
- 62.Martin J. L., Lin M. Z., McGowan E. M., Baxter R. C. (2009) J. Biol. Chem. 284, 25542–25552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L., Lee J. F., Lin C. Y., Lee M. J. (2008) Histochem. Cell Biol. 129, 579–588 [DOI] [PubMed] [Google Scholar]
- 64.Menéndez J. A., Mehmi I., Griggs D. W., Lupu R. (2003) Endocr. Relat. Cancer 10, 141–152 [DOI] [PubMed] [Google Scholar]
- 65.Vellon L., Menendez J. A., Lupu R. (2005) Oncogene 24, 3759–3773 [DOI] [PubMed] [Google Scholar]