Abstract
The serotonin transporter (SERT) is a member of the SLC6 family of solute carriers. SERT plays a crucial role in synaptic neurotransmission by retrieving released serotonin. The intracellular carboxyl terminus of various neurotransmitter transporters has been shown to be important for the correct delivery of SLC6 family members to the cell surface. Here we studied the importance of the C terminus in trafficking and folding of human SERT. Serial truncations followed by mutagenesis identified sequence spots (PG601,602, RII607–609) within the C terminus relevant for export of SERT from the endoplasmic reticulum (ER). RI607,608 is homologous to the RL-motif that in other SLC6 family members provides a docking site for the COPII component Sec24D. The primary defect resulting from mutation at PG601,602 and RI607,608 was impaired folding, because mutated transporters failed to bind the inhibitor [3H]imipramine. In contrast, when retained in the ER (e.g. by dominant negative Sar1) the wild type transporter bound [3H]imipramine with an affinity comparable to that of the surface-expressed transporter. SERT-RI607,608AA and SERT-RII607–609AAA were partially rescued by treatment of cells with the nonspecific chemical chaperone DMSO or the specific pharmacochaperone ibogaine (which binds to the inward facing conformation of SERT) but not by other classes of ligands (inhibitors, substrates, amphetamines). These observations (i) demonstrate an hitherto unappreciated role of the C terminus in the folding of SERT, (ii) indicates that the folding trajectory proceeds via an inward facing intermediate, and (iii) suggest a model where the RI-motif plays a crucial role in preventing premature Sec24-recruitment and export of incorrectly folded transporters.
Keywords: Endoplasmic Reticulum (ER), Intracellular Trafficking, Membrane Proteins, Neurotransmitter Transport, Neurotransmitters, Pharmacochaperone, Serotonin, Serotonin Transporter
Introduction
Neurotransmitters are responsible for terminating signal transmission between neurons and between neurons and effector cells, by depleting the extracellular milieu from their cognate neurotransmitter. In addition, rapid repetitive use of the synapse is contingent on the action of neurotransmitter because the retrieval of the neurotransmitter allows for continuous refilling of synaptic vesicles (1). The human serotonin transporter (hSERT)2 belongs to the SLC6 (Solute Carrier 6) family of Na+/Cl−-dependent plasma membrane transporters and is responsible for reuptake of serotonin (5-HT) from the synaptic cleft (2). Among others the SLC6 family includes transporters for dopamine (DAT), norepinephrine (NET), γ-aminobutyric acid (GAT1–4), and glycine (GLYT1 and GLYT2) (3). All members of this family have 12 transmembrane-spanning segments (TM) with their N and C terminus on the intracellular side. Crystallization of a bacterial homolog (LeutAa (4)) revealed the general topology of the transporters: the transmembrane domains form two bundles (TM 1–5 and TM 6–10) with TM 11 and 12 forming a dimerization interface. This dimerization interface has also been identified in SERT (5); dimerization is thought to be a prerequisite for trafficking of transporters from the ER to the cell surface (6–7). The structural information provided by the bacterial homolog does not allow for conjectures on the arrangement of the intracellular termini of eukaryotic transporters. However, this is an important issue because: (i) the N terminus integrates input signals provided by regulatory modifications such as phosphorylation (8–9) and ubiquitination (10). (ii) The C terminus contains sequence elements that allow for docking of components of the ER export machinery and of the exocyst (11–12). It thus supports routing of the transporter from the ER through the ERGIC (13) to its destination in the presynaptic specialization (14). (iii) There is circumstantial evidence to suggest that the conformation of the N terminus affects the transport cycle and translocation of substrate through the hydrophobic core (9, 15–16). Thus the state of the N terminus must be relayed to the central cavity and vice versa. N and C termini are in close vicinity (5); thus it is likely that there is also a communication between the C terminus and the hydrophobic core of the transporter.
Here we addressed the role of the C terminus of SERT. There are obvious differences between the C terminus of SERT and that of its closest relatives, the monoamine transporters NET and DAT: masking or mutating the very C-terminal amino acids of NET (17) and DAT (18) results in their intracellular retention. In contrast, SERT tolerates the addition of large tags to both its N-and C terminus (5). The C terminus of SERT is shorter and its last 22 amino acids diverge from those of NET and DAT: a conserved aspartate residue (9 amino acids downstream from the RL-motif required for Sec24D binding) has, for instance, been identified as an additional contact site with Sec24D (19). This aspartate is present in all mammalian SLC6 family members but SERT and the taurine transporter (where it is replaced by proline, a non-conservative substitution). Consistent with earlier data (20), we found that SERT tolerated deletion of up to 16 amino acids in its C terminus. Further deletions impaired cell surface expression and antagonist binding. We scanned through the region surrounding the Sec24 binding site and identified two spots that are required for correct folding of the protein. One of these sites, RI607,608, coincided with the Sec24 binding motif. The corresponding mutant SERT- RI607,608AA was partially rescued by pharmacochaperoning with ibogaine but not with any other SERT ligand. Because ibogaine binds to the inward facing conformation the observations suggest that folding of SERT proceeds via an intermediate that is related to the inward facing conformation.
EXPERIMENTAL PROCEDURES
Materials
Standard chemical reagents were purchased from Sigma-Aldrich. [3H]Imipramine, [3H]5-HT, and [3H]β-CIT were purchased from PerkinElmer. Bovine serum albumin (BSA) and Complete protease inhibitor mixture were from Roche (Mannheim, Germany), SDS from BioMol GmbH, Tris, and scintillation mixture (Rotiszint® eco plus) from Carl Roth GmbH + Co. Rabbit polyclonal GFP antibody (ab290) and anti-calnexin antibody (ab13504) were from Abcam Plc (Cambridge, UK). Protein A-Sepharose and anti-rabbit IgG1-anitbody linked to horseradish peroxidase were from Amersham Biosciences. ER Tracker Blue-White DPX was from Molecular Probes (Leiden, The Netherlands), the protein deglycosylation kit was from New England Biolabs, Inc.
DNA Constructs and Cloning
Primers used for cloning and mutagenesis were from Operon Biotechnologies (Cologne, Germany) and are described in supplemental Table S1: SERT ΔC3, ΔC7, and ΔC12 were amplified by PCR and cloned into peYFP-vector (Clontech, Mountainview, CA) using HindIII and KpnI, SERTΔC30 via HindIII and EcoRV. YFP-SERTΔC15, YFP-SERTΔC16, and YFP-SERTΔC17 were cloned by inserting a stop codon into the open-reading frame, using QuikChange II XL Site-directed Mutagenesis kits (Stratagene Europe). All mutations and truncations were confirmed by sequencing.
Cell Culture and Transfection
HEK293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with high glucose (4.5 g/liter) and l-glutamine (584 mg/liter), supplemented with 10% fetal calf serum (FCS) and gentamicin (50 μg/ml). Transfections were either done using the CaPO4 precipitation method (for co-localization studies by fluorescence microscopy, uptake and binding assays) or by using Lipofectamine PlusTM 2000 Reagent (Invitrogen) (for co-immunoprecipitation of calnexin and SERT mutants).
Fluorescence Microscopy
HEK293 cells (3 × 105 cells) were seeded on poly-d-lysine (PDL)-coated 15 mm coverslips and were transfected 24 h later by the CaPO4 precipitation method. Forty-eight hours after transfection, the cells were analyzed by confocal microscopy. The cells were kept in a Krebs-HEPES buffer (10 mm HEPES, 120 mm NaCl, 3 mm KCl, 2 mm CaCl2, 2 mm MgCl2, and 2 mm glucose monohydrate), and images were acquired with a Zeiss Axiovert LSM510 confocal laser-scanning microscope. Images were analyzed with a Zeiss LSM Image Browser (version 3: Zeiss AG, Oberkochen, Germany). Membranes of the endoplasmic reticulum were stained with the fluorescent ER Tracker Blue-White DPX as previously described (11); the plasma membrane was visualized by incubating the cells in trypan blue as outlined earlier (21).
Uptake Assay
Uptake of [3H]5-HT was determined as described previously (21). In brief, 24 h after transfection, HEK 293 cells were detached and seeded in 48-well plates (6 × 105/well) precoated with PDL. The next day, the medium was removed, and cells were washed with 1 ml of Krebs-HEPES buffer. Cells were incubated with the indicated concentration of 5-HT ranging from 0.2 μm to 30 μm; the specific activity of [3H]5-HT was varied between 30 cpm/fmol (0.2 μm) to 200 cpm/pmol (30 μm) by addition of unlabeled 5-HT. The incubation lasted for 1 min and was followed by a rapid rinse with ice-cold Krebs-HEPES buffer. Nonspecific uptake was determined by preincubation of the cell with 10 μm paroxetine for 5 min. Cells were lysed by 1% SDS, the lysate was transferred to scintillation vials, and the radioactivity was determined by liquid scintillation counting.
Binding Assay
All steps of membrane preparation were done on ice: 48 h after transfection, the medium was removed, and the cell layer was washed three times with cold PBS. The cells were mechanically detached in PBS and harvested by centrifugation. The cell pellet was resuspended in 0.5 ml of hypotonic buffer (25 mm HEPES, 2 mm MgCl2, and 1 mm EDTA, pH 7.3) in the presence of a mixture of protease inhibitors, frozen in liquid nitrogen, followed by rapid thawing and sonication (three times for 10 s). Membranes were collected by centrifugation at 40,000 × g for 15 min. These were resuspended in the same buffer at a protein concentration of ∼5 mg/ml and frozen in liquid nitrogen. The protein concentration was measured by Coomassie Brilliant Blue binding. Citalopram, imipramine, paroxetine, and protein were diluted in binding assay buffer (20 mm Tris-HCl, pH 7.5, 1 mm EDTA, 2 mm MgCl2, 120 mm NaCl, and 3 mm KCl). Membranes (16–20 μg/assay) were incubated with different concentration of [3H]β-CIT or [3H]imipramine at 22 °C for 60 min ([3H]β-CIT became commercially unavailable for a certain period; hence we resorted to using [3H]imipramine). Nonspecific binding was determined in parallel in presence of 3 μm paroxetine. The binding was terminated by filtration onto GF/B glass microfiber filters presoaked in 0.5% polyethyleneimine. The filters were dissolved in scintillation mixture, and the radioactivity was counted.
Co-immunoprecipitation and Immunoblotting
Forty-eight hours after transfection, HEK 293 cells (2.5 × 106/condition) were washed three times with ice-cold PBS buffer, harvested, lysed in 0.5 ml of RIPA buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium dexoxycholate, and 0.1% SDS), and incubated at 4 °C for 60 min with gentle rotation. The lysate was collected by centrifugation at 50,000 g for 30 min at 4 °C. Equal amounts of protein (∼1 mg/sample) were incubated overnight in the absence or presence of anti-GFP antibody (4 μl) or of anti-calnexin antibody (2.5 μl). Subsequently, pre-equilibrated protein A-Sepharose (6 mg of protein A-Sepharose/sample) was added and incubated at 4 °C for 5 h with gentle rotation. The protein A-Sepharose beads were collected by centrifugation and then washed three times with RIPA buffer (without SDS). Bound proteins were eluted by denaturation in 0.1 ml of loading buffer containing 40 mm dithiothreitol and 1% mercaptoethanol at 45 °C for 10 min. Aliquots (15 μl) were loaded onto SDS-polyacrylamide gels. After the proteins had been resolved by denaturing electrophoresis, they were transferred to nitrocellulose membranes. Immunoreactive bands were detected by appropriate antibodies (directed against GFP or calnexin) and a horseradish peroxidase-conjugated second antibody using enhanced chemiluminescence. Immunoprecipitates generated in a similar manner were incubated in the absence and presence of endoglycosidase H and PNGase F using the NEB assay kit according to the protocol of the manufacturer.
RESULTS
Truncations of the Carboxyl Terminus Cause ER Retention of Apparently Incorrectly Folded SERT
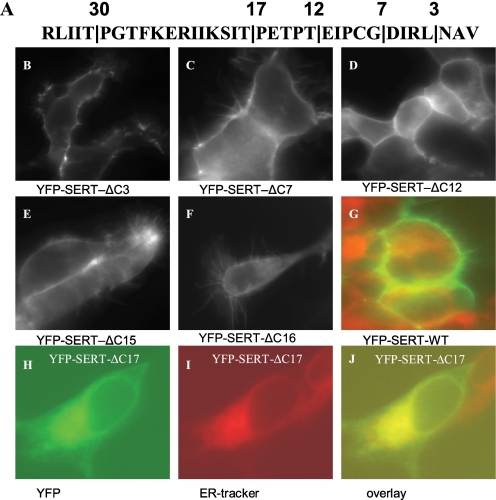
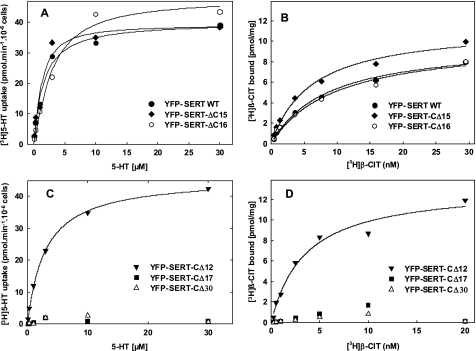
The sequence of SERT differs substantially from that of its closest relatives in its last 35 amino acids (Table 1). Hence, the carboxyl terminus of SERT was serially truncated to define the portion of the C terminus that was dispensable for cell surface localization of the transporter and to identify the region that contained candidate motifs for interaction with the trafficking machinery. It is evident from Fig. 1 that truncation of the C terminus by up to 16 amino acids did not impair the capacity of the resulting mutated transporters to reach the cell surface. Accordingly, [3H]5-HT uptake (shown for SERT-ΔC15 and SERT-ΔC16 in Fig. 2A) and binding of the inhibitor [3H]β-CIT (Fig. 2B) by the mutated transporters was similar to that of wild-type SERT (average KD, Bmax as well as Km and Vmax values are given in supplemental Table S2). In contrast further truncation (shown for SERT-ΔC17 (Fig. 1, H–J) resulted in transporters that were trapped within the cell and co-localized with a fluorescent marker of the endoplasmic reticulum. The same holds true for further truncation, i.e. SERT-ΔC30 (data not shown). In intact cells, plasma membrane transporters can only mediate uptake of substrate if they reach the cell surface. It is therefore not surprising that these mutants failed to mediate uptake of substrate (Fig. 2C). However, the SERT-ΔC17 and SERT-ΔC30 also failed to bind the inhibitory radioligand [3H]5-HT (Fig. 2D).
TABLE 1.
Sequence alignment of the C-terminal region of selected SLC6 family transporters
hSERT and rSERT are human and rat SERT, respectively; hGAT1 and rGAT1 are the human and rat GAT1, respectively; hTaurT is the human taurine transporter. The Sec24-binding RI-motif is colored gray as are an invariant glycine and proline. The residue in the position +9 from the RI-motif is bold-faced (proline in the serotonin and taurine transporters, an acidic residue in all others including those not shown).
FIGURE 1.
Subcellular localization of C-terminally truncated versions of SERT. HEK293 cells (0.7 × 106 cells) were transiently transfected with plasmids (5 μg) encoding YFP-tagged wild type SERT and with truncated versions of the transporter as indicated. The sites of truncation are indicated in the carboxyl terminus of hSERT (A). Transfected cells were seeded onto poly-d-lysine-coated coverslips 24 h after transfection. Confocal images were acquired after an additional 24 h by confocal microscopy with a 488 nm excitation wavelength; the emission was captured between 505 and 530 nm. Cells expressing wild-type SERT (G), YFP-SERT-ΔC17 (H, I, and J), were preincubated with an ER-specific dye (fluorescent ER Tracker Blue-White DPX); images were also acquired using excitation wavelength of 485 nm and an emission wavelength window of 475–525 nm to visualize ER Tracker Blue-White DPX. Images were overlaid using the MetaMorph software.
FIGURE 2.
Truncation of the C terminus by more than 16 amino acids abrogates transport of 5-HT and binding of the inhibitor [3H]β-CIT by SERT. HEK293 cells (4 × 106 cells) were transiently transfected with plasmids (20 μg) encoding YFP-tagged wild-type SERT or the truncated versions SERT-CΔ15, SERT-CΔ16 (A and B), SERT-CΔ12, SERT-CΔ17, and SERT-CΔ30 (C and D). The uptake of [3H]5-HT was determined after 48 h as outlined under “Experimental Procedures” (A and C). Membranes (15–30 μg/assay) prepared from the transfected cells 48h after transfection were incubated with the indicated concentrations of [3H]β-CIT, and the binding reaction was carried out as outlined under “Experimental Procedures” (B and D). Data are from a single experiment done in duplicate, which is representative for at least two additional experiments (see also supplemental Table S2).
Scanning the C Terminus of SERTs for Motifs That Are Required for Expression of Functional Transporter
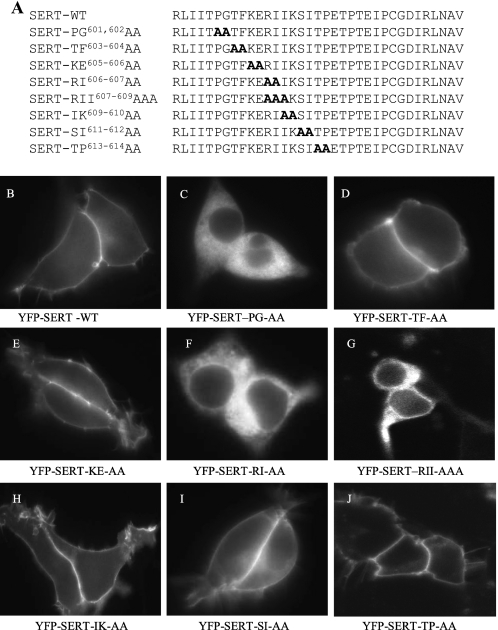
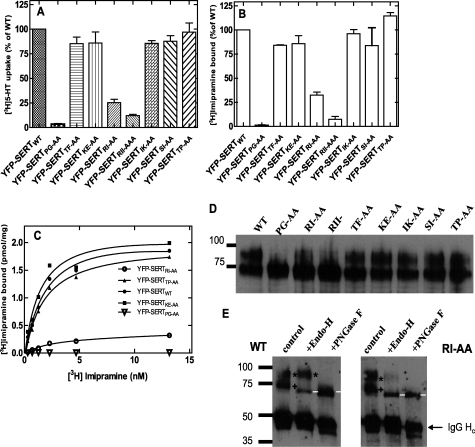
The findings summarized above suggested that residues N terminal to the last 16 amino acids were required for ER export of SERT and/or possibly for folding of the protein. We scanned the region between Pro601 and Pro614 (which covers the amino acids between the Δ17 and Δ30 truncation, Fig. 3A) by pairwise substitutions with alanines. RI607,608 is the homologous position to the RL-motif required for binding of Sec24D to GAT1 (12) and to GlyT1 (19); in SERT it is followed by a second isoleucine, which is unique to SERT (all other SLC6 family members have a hydrophilic residue or alanine in this position). Accordingly, we also created a triple mutation SERT-RII607–609AAA. We also created a mutation, where RI607,608 was replaced by two serine residues rather than alanine. This mutant was phenotypically indistinguishable from SERT-RI607,608AA (data not shown). The mutated transporters were heterologously expressed in HEK293 cells and examined by confocal microscopy for subcellular localization of the transporter. Apart from SERT-PG601,602AA (Fig. 3C), SERT-RI607,608AA (Fig. 3F), and SERT-RII607–609AAA (Fig. 3G), all other mutated versions were predominantly visualized at the cell surface. Interestingly, SERT-TP613–614AA was also inserted into the cell membrane (Fig. 3J), although this mutation affects Pro614, i.e. the first amino acid deleted in the Δ17 truncation. We compared substrate uptake by cells transiently expressing mutated transporters with that of cells that expressed wild-type SERT (Fig. 4A). This recapitulated the findings obtained by fluorescence microscopy: most substitutions did not significantly affect uptake (see also supplemental Table S2). This suggested that the transporters reached the cell surface, and their function was not affected. In contrast, uptake of [3H]5-HT was essentially undetectable in cells expressing SERT-PG601,602AA and was greatly reduced in cells expressing SERT-RI607,608AA and SERT-RII607–609AAA. In GAT1, mutation of the RL-motif results in intracellular retention of the transporter, which eventually reaches the cell surface by bulk flow rather than by concentrative COPII-dependent export (12). It was therefore not surprising that the corresponding mutation also impaired cell surface delivery of SERT. The PG-motif may also play a similar role in ER-export. However, when membranes were prepared from cells expressing SERT-PG601,602AA, SERT-RI607,608AA, or SERT-RII607–609AAA and used for binding assays, binding of the tricyclic antidepressant [3H]imipramine was greatly reduced in SERT-RI607,608AA (Fig. 4B, open circle in Fig. 4C) or SERT-RII607–609AAA (Fig. 4B, open square in Fig. 4C) and essentially undetectable in SERT-PG601,602AA (Fig. 4B, open triangle in Fig. 4C). The other mutants were found at levels comparable to wild type SERT (Fig. 4B) and their affinity was similar (shown for in Fig. 4C for SERT-TP613–614AA and SERT-KE605–606AA; summarized for all other mutants in supplemental Table S2).
FIGURE 3.
Scanning of the region between Pro601 and Pro614 to identify spots required for export to the cell surface. A, representation of the (pairwise) substitution of amino acids by alanine. B, confocal imaging of the mutated transporters. Transient expression and confocal microscopy were done as outlined in the legend to Fig. 1.
FIGURE 4.
Mutations of SERT in the position PG601,602, RI607,608, and RII607–609 blunt 5-HT uptake and binding of [3H]imipramine. HEK293 cells were transiently transfected with plasmids encoding the indicated mutants. A, cellular uptake of [3H]5-HT was determined at 3 μm [3H]5-HT; assay conditions were otherwise as outlined in the legend to Fig. 2. All transfections and determinations were done in parallel. To account for interassay variation in transfection efficiency, data were normalized to uptake determined in wild type SERT expressing cells; this 100% uptake corresponded to 27.5 ± 1.9 pmol/106 cells/min. B, binding of [3H]imipramine (3.6 nm) to membranes prepared from cells transiently expressing different versions of SERT; this 100% binding corresponded to 1.34 ± 0.03 pmol/mg. Data in panels A and B are means from five independent experiments carried out in duplicate; error bars indicate S.E. C, binding of [3H]imipramine to membranes (15–30 μg/assay) prepared from cells transiently expressing wild-type SERT, SERT-PG601,602AA, SERT-KE605–606AA, SERT-RI607,608AA, and SERT-TP613–614AA. Data are from a representative experiment done in duplicate which is representative for at least two additional experiments (see also supplemental Table S2). D, membranes were prepared from transiently transfected cells; the YFP-tagged versions of SERT were visualized with an anti-GFP antibody. E, wild-type SERT and SERT-RI607,608AA were enriched by immunoprecipitation, denatured, and subjected to deglycosylation by incubation (for 1 h at 37 °C) in the absence (control) and presence of endoglycosidase H (Endo H) or PNGase F. * denotes the mature glycosylated upper band, which is resistant to Endo H; + is the lower band, which is cleaved by Endo H to generate a smaller product (marked with white line). Note that the immunoglobulin heavy chain is also visible (IgG Hc) and that this is deglycosylated by PNGase F. Numbers indicate the position of molecular mass markers (in kDa).
We also determined the expression level of the transporters in transiently transfected cells. Two forms were resolved by denaturing gel electrophoresis (Fig. 4D). In all mutants but SERT-PG601,602AA, -RI607,608AA and -RII607–609AAA, the level of the upper band was comparable to that observed with wild-type SERT. In the three affected mutants the upper band was reduced with levels of SERT-RI607,608AA > SERT-RII607–609AAA ≫ SERT-PG601,602AA. This order was consistent with the reduction in uptake (Fig. 4A) and in binding (Fig. 4B). We surmised that the upper band corresponded to the mature fully glycosylated form and the lower band to the core glycosylated (ER-resident) form. This conjecture was verified by immunoprecipitating wild type SERT and a representative mutated version (SERT-RI607,608AA) and subjecting these proteins to deglycosylation by endoglycosidase H and PNGase F (Fig. 4E). Endoglycosidase H cannot cleave complex type glycans that are attached while the protein traffics through the Golgi. Accordingly, the upper band (marked by an asterisk in Fig. 4E) was not affected by endoglycosidase H treatment. In contrast, endoglycosidase H reduced the mobility often lower band (marked by a cross in Fig. 4E) to an extent that was consistent with removal of the core glycan (product marked with a white line in Fig. 4E). As expected PNGase F deglycosylated both forms. Hence both wild-type and mutant SERT migrated as a single species (Fig. 4E).
ER-trapped SERT Still Binds [3H]Imipramine
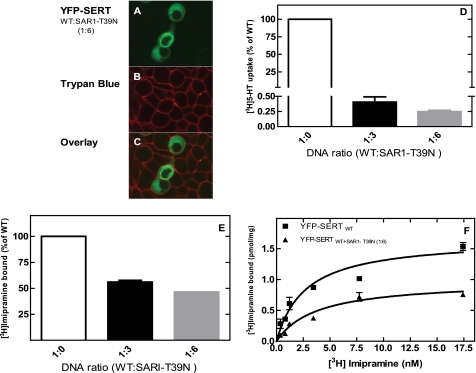
There are mechanisms that preclude the premature activation of membrane proteins: for instance rhodopsin is stabilized in the inactive conformation by the high cholesterol content of the membranes, while trafficking in the secretory pathway (22). SERT is also exquisitely sensitive to the cholesterol content of membranes (23–24). It is therefore conceivable that SERT is inactive when retained in the ER, and this suffices to explain reduced capacity of SERT-PG601,602AA, SERT-RI607,608AA, and of SERT-RII607–609AAA to bind [3H]imipramine. This possibility was explored by co-expressing wild-type SERT with a dominant-negative version of SAR1a (SAR1a-T39N) in HEK293 cells (12). As expected, co-expression of Sar1a-T39N resulted in intracellular retention of SERT (Fig. 5A) and reduced cellular uptake to less than 1% of that observed in cells transfected with a control vector (Fig. 5B). Nevertheless membranes prepared from SAR1a-overexpressing cells contained abundant amounts SERT that bound [3H]imipramine (Fig. 5C). The affinity of the ER-trapped SERT was comparable to that of transporter residing in the plasma membrane (Fig. 5D; KD 2.7 ± 0.6 nm and 3.7 ± 1.0 nm for SERT and SERT+ SAR1a-T39N, respectively). Retention of SERT was also achieved by overexpression of Sec24D-VN, a mutated version that disrupts ER-export of SLC6 family members in a dominant-negative manner (12). This approach gave similar results (not shown).
FIGURE 5.
Trapping of wild-type SERT in the endoplasmic reticulum abolishes uptake but does not affect binding of [3H]imipramine. HEK 293 cells (0.7 × 106 cells) were transfected with a combination of plasmids encoding YFP-SERT (1 μg) + empty control plasmid or of the dominant negative version of SAR1a (SAR1 T39N). A, the cellular distribution of YFP-tagged SERT was visualized by confocal microscopy as outlined in the legend to Fig. 1. B, cell surface was visualized by staining with trypan blue (excitation wavelength: 543 nm and long path filter: 585 nm). C, overlay of the YFP and trypan blue staining. D, uptake of [3H]5-HT was determined at 2 μm serotonin as outlined under “Experimental Procedures.” Data were normalized to uptake determined in wild-type SERT-expressing cells; this 100% uptake corresponded to 17.8 ± 1.3 pmol/106 cells/min. E and F, binding of [3H]imipramine to membranes (15–30 μg/assay) prepared from cells transiently expressing wild-type SERT in the absence and presence of the indicated ratios of plasmid encoding SAR1a-T39N. The reaction contained 2.5 nm [3H]imipramine (E) or the indicated concentrations of radioligand (F) and was carried out as outlined under “Experimental Procedures.” In E, binding determined in the absence of co-expressed SAR1a-T39N was set 100% to normalize for interassay variation in transient transfections; this 100% value corresponds to 0.35 ± 0.12 pmol/mg. Data are means from four independent experiments carried out in duplicate; error bars indicate S.E.
Recovery of SERT-PG601,602AA, SERT-RI607,608AA, and of SERT-RII607–609AAA in Complex with Calnexin
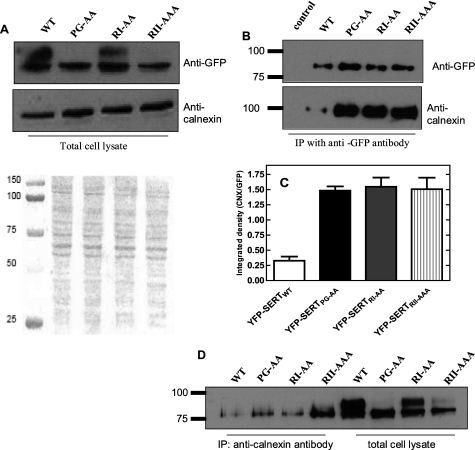
We used two approaches to obtain more direct evidence for defective folding. (i) Bacterial expression of SERT and of mutated versions tagged on its C terminus with GFP. This approach relies on the assumption that the C-terminal GFP can only undergo correct folding if the preceding polypeptide chain adopts a stable conformation (25). However, this strategy failed, because expression of GFP-tagged wild type SERT did not give rise to fluorescent bacteria, while the positive (the GFP-tagged E. coli dipeptide transporter YdgR) and negative controls (an untagged version of YdgR) gave the expected results (data are summarized in supplemental Fig. S1). (ii) We exploited the ER-resident chaperone calnexin as folding sensor (26). Lysates were prepared from cells expressing wild-type SERT and the three mutants suspected of defective folding. These lysates were used as a starting material for immunoprecipitation. When applied onto a denaturing polyacrylamide gel with a low monomer concentration, it was possible to resolve two species (Fig. 6A): the band migrating with a larger apparent mass was diffuse in appearance and hence consistent with extensive glycosylation. The lower sharp band presumably represented the core glycosylated species. This assignment is consistent with the observation that the diffuse band was prominent in wild type SERT, present to a lesser extent in SERT-RI607,608AA (which does reach the cell surface to some extent) and essentially undetectable in SERT-RII607–609AAA and SERT-PG601,602AA (Fig. 6A). Endogenous calnexin levels were reasonably similar, regardless of the SERT version expressed (Fig. 6A). Wild type SERT and the mutants were recovered from the lysate by immunoprecipitation using the antibody directed against GFP (Fig. 6B). It is evident from Fig. 6B and the quantification in Fig. 6C that substantial amounts of calnexin were co-immunprecipitated with the three mutated versions of SERT. In contrast, only trace amounts of calnexin were present in the material immunoprecipitated from wild-type SERT containing lysates (left hand lane in the lower blot Fig. 6B), although these immunoprecipitates contained abundant amount of SERT (see left hand lane in the upper blot of Fig. 6B). Comparable results were observed, if the immunoprecipitation was done with calnexin and the level of co-immunoprecipitated SERT was assessed by blotting for GFP (Fig. 6D). It is worth noting that, as expected, only the lower (core glycoysylated) band was recovered in complex with calnexin (Fig. 6D).
FIGURE 6.
Complex formation of SERT-PG601,602AA, SERT-RI607,608AA, and SERT-RII607–609AAA with calnexin. HEK293 cells (2.5 × 106 cells) were transiently transfected with plasmids driving the expression of wild-type SERT, SERT-PG601,602AA, SERT-RI607,608AA, and SERT-RII607–609AAA. Forty-eight hours after transfection, detergent lysates were prepared from cells subjected to immunoprecipitation with an antibody directed against GFP or calnexin as outlined under “Experimental Procedures.” A, lysate were blotted for GFP and calnexin. The Ponceaus S-stained nitrocellulose is shown to document equivalent loading. B, aliquots of the immunoprecipitate by anti-GFP (corresponding to 2.5 × 105 cells) were applied onto a SDS-polyacrylamide gel (10% monomer concentration in the resolving gel) and blotted for the YFP-tag of SERT and calnexin. C, integrated density was quantified using ImageJ 1.43 and used to calculate the ratio of calnexin (CNX over SERT (GFP)) immunoreactivity. D, calnexin was immunoprecipitated from cell lysates, and the levels of associated SERT was visualized by blotting with the anti-GFP antibody. For comparison, the level of SERT in the lysate was also visualized. Note that the immunoprecipitate only contains the lower band. Data are from a representative experiment that was reproduced three more times in independent transfections.
Rescue of SERT-RI607,608AA and SERT-RII607–609AAA but Not of SERT-PG601,602AA by Chemical- and Pharmacochaperones
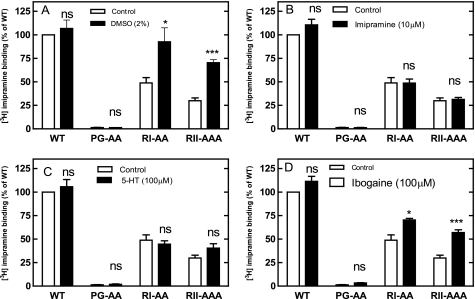
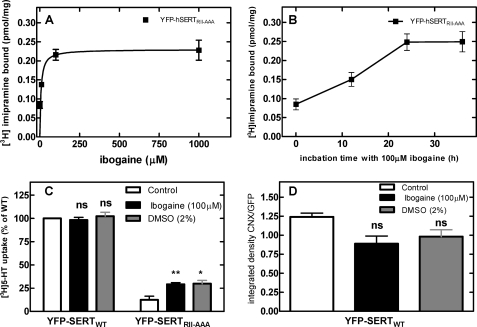
In many instances folding deficiencies can be corrected by chemical and pharmacological chaperones. These are small molecules that assist in folding of a protein either in a nonspecific manner (e.g. DMSO) or by virtue of a specific interaction with their cognate target (27). We therefore tested if the nonspecific chemical chaperone DMSO rescued SERT mutants by incubating transiently transfected cells for 24 h in the presence of 2% DMSO (Fig. 7A). This resulted in a substantial increase in the level of SERT-RI607,608AA and SERT-RII607–609AAA that bound [3H]imipramine. In contrast, the level of wild-type SERT was not increased by DMSO treatment. We searched for specific pharmacochaperones by examining the effect of the inhibitor imipramine (which binds to the outward facing conformation), the substrate serotonin (which induces an occluded state) and ibogaine (which preferentially binds to the inward facing conformation; see Ref. 28). Neither imipramine (Fig. 7B) nor serotonin (Fig. 7C) nor para-chloroamphetamine (not shown) nor incubation at low temperature (not shown) affected the functional (i.e. binding competent) level of any of the SERT versions tested, but ibogaine effectively increased the amount of active SERT-RI607,608AA and SERT-RII607–609AAA (Fig. 7D). SERT-PG601,602 did not respond to any of these ligands. Similarly, the levels of wild-type SERT were not enhanced by any manipulation. Half-maximal stimulation was seen at 6.7 ± 3.5 μm ibogaine (Fig. 8A), i.e. in the range of affinity (6.3 ± 1.3 μm) previously determined (28). The maximum effect was achieved within a 24 h incubation (Fig. 8B). Incubation of cells expressing SERT-RII607–609AAA with ibogaine or DMSO also increased the level of substrate uptake indicating that the pharmacochaperoned mutant transporter eventually reached the cell surface (Fig. 8C). The pharmacochaperoning effect of ibogaine or of DMSO ought to result in a decline in association of SERT-RII607–609AAA with the folding sensor calnexin. Immunoprecipitates of SERT-RII607–609AAA retrieved from ibogaine- and DMSO-treated cells contained on average lower amounts of calnexin (Fig. 8D). However, this difference was not statistically significant. We ascribe this failure to the low sensitivity of the method employed, which cannot reliably detect a decline of complexes by some 25%.
FIGURE 7.
Rescue of the mutant by different chemical and pharmacochaperones. HEK293 cells were transfected with cDNAs encoding wild-type SERT, SERT-PG601,602AA, SERT-RI607,608AA, and SERT-RII607–609AAA. Twenty-four hours after transfection 2% DMSO (A), 100 μm 5-HT (B), 10 μm imipramine (C), or 100 μm ibogaine (D). After an incubation for another 24 h, membranes were prepared; the binding was carried out with these membranes (100 μg/assay) in the presence of 1 nm [3H]imipramine as outlined in the legend to Fig. 5. Binding to membranes prepared from untreated control cells expressing wild-type SERT was set to 100% to normalize for interassay variation in transient transfections; this 100% value corresponds to 0.32 ± 0.11 pmol/mg. Data are means from three independent experiments carried out in duplicate; error bars indicate S.E. Note that all assays were done in parallel such that the control conditions are the same in each panel; the separation in individual panels is to render the data more accessible. Data are means from five independent experiments carried out in duplicate; error bars represent S.E. Differences were tested for statistical significance by paired t test (*, p < 0.05; ***, p < 0.001).
FIGURE 8.
Ibogaine-induced change in [3H]imipramine binding by (A and B), substrate uptake by (C), and calnexin association with SERT-RII607–609AAA (D). Transiently transfected cells were treated 24 h after transfection for another 24 h with the indicated concentrations of ibogaine (A) or for the indicated incubation times with 100 μm ibogaine (B). Membranes were prepared from the cells and binding of 2.5 nm [3H]imipramine binding was determined as outlined for Fig. 7. C, pretreatment schedule was similar as in A; the incubation was done in the presence of 100 μm ibogaine and 2% DMSO. Uptake was determined in the presence of 3 μm [3H]5-HT. Data are means from 3 (A and B) and 4 (C) independent experiments carried out in duplicate; error bars represent S.E. Differences were tested for statistical significance by ANOVA (*, p < 0.05; **, p < 0.01). D, transiently transfected HEK 293 cells expressing SERT-RII607–609AAA were treated with ibogaine and DMSO as in A. The cells were subsequently lysed, SERT was recovered by immunoprecipitation, and the level of SERT and of calnexin were determined by immunoblotting as outlined in the legend to Fig. 6, B and C. Shown are the ratios of calnexin (CNX) immunoreactivity over SERT (GFP) immunoreactivity. The differences were not statistically significant (Kruskal-Wallis test, means ± S.E.; n = 5).
DISCUSSION
Delivery of SLC6 family members to the cell surface is contingent on motifs in the carboxyl terminus (11–14, 19, 28). The C terminus of SERT has not yet been scrutinized for its role in trafficking but it was noted previously that truncations lead to transporters that fail to translocate substrate and to reach the cell surface (20). It was also noted that substitutions of alanines for amino acids N-terminal to the last 16 residues reduced activity, but mechanistic details were not provided (20). Results from our truncation experiments are consistent with these experiments and showed, in addition, that the retained proteins were inactive.
By following up on these earlier observations, we discovered that the C terminus was required to assist the folding process in the endoplasmic reticulum. Specifically, there are two spots; PG601,602 and RI607,608 that are indispensable for producing a functional transporter. R607 was also identified as a critical residue by Larsen and coworkers while PG601,602 was not examined (20). Our experiments provide several arguments to support this interpretation. (i) Serial truncation and the pertinent point mutations did not only result in loss of surface expression, but also in binding. (ii) Forced retention of SERT in the ER does not abolish binding. Thus loss of inhibitor binding cannot be attributed to possible alternative explanations, i.e. a lipid composition in the ER that is not conducive to a binding competent state or the presence of an inhibitory protein that precludes premature activation of the transporter. (iii) The fact that abundant amounts of the pertinent mutants (SERT-PG601,602AA, SERT-RI607,608AA) were recovered in complex with calnexin proves that these proteins did not achieve a folded state. It is worth noting that when heterologously expressed in HEK293 (and other) cells, wild-type SERT is not prone to associate with calnexin (29) indicating that these cells contain the machinery to efficiently support its folding. (iv) The folding deficiency of SERT-RI607,608AA was remedied by chemical chaperoning with DMSO and by pharmacochaperoning with ibogaine.
Little is known about the role of the C terminus in the folding of SLC6 family members, but it is well appreciated that the C terminus supports folding of G protein-coupled receptors (GPCR) (30–33). Truncation made in the proline-rich part of A1-receptor C terminus precludes folding of the protein (31). This is reminiscent of the phenotype of SERT-CΔ17, which severs the following proline-rich segment. We note that the alanine substitution of TP613,614 did not phenocopy the effect of the truncation on folding and ER-export. This discrepancy can be rationalized by assuming that, in the point mutant, the replacement of Pro614 can be compensated for by the presence of the other C-terminal residues. More recently, a hydrophobic tetrad (ILLV) has been shown to be essential for folding of an SLC2 transporter family member, the Na+-K+-2Cl−-cotransporter-1 (NKCC1) (34). Mechanistically, the mutations, in both GPCR and in NKCC1, have been proposed to affect the capacity of the C terminus to recruit cytoplasmic chaperones and to interact with residues in intracellular loops. The latter interaction is thought to be crucial to assist in stabilizing the assembly of the hydrophobic helical core.
Pharmacochaperoning has been extensively studied in certain mutated GPCRs, because ligand-assisted folding may remedy the disease resulting from the mutation (35). To the best of our knowledge, there has been no report that investigated pharmacochaperoning of SLC6 transporters. Pharmacochaperoning of SERT has the following salient features. (i) Expression of wild type SERT is neither enhanced by the (universal) chemical chaperone DMSO nor by typical ligands regardless of their conformational preference. This is consistent with the interpretation that the folding machinery present in HEK293 cells efficiently supports the maturation of SERT. (ii) Defective folding of SERT-RI607,608AA (and its relative SERTRII607–609AAA) can be partially remedied but only by DMSO or ibogaine, which binds to the inward-facing conformation (28). In contrast, folding of SERT-RI607,608AA was not rescued by imipramine (which binds to the outward-facing conformation) the substrate serotonin (which prefers the occluded state) or para-chloro-amphetamine (which induces transporter-mediated efflux (36)). We stress that all compounds selected can readily permeate into cells Thus, their inability to act as pharmacochaperones cannot be attributed to a diffusion barrier that shielded their target, i.e. the mutant SERT residing in the ER. (iii) The observation that ibogaine was the only compound that acted as a pharmacochaperone suggests that the folding trajectory proceeds via the inward facing conformation. This is also consistent with the ionic gradient that exists over the membrane of the endoplasmic reticulum: the lumen of the ER corresponds to the extracellular milieu but is devoid of Na+. These conditions favor accumulation of the inward facing conformation (37). Accordingly, folding intermediates of SERT will eventually pass through this state. It is therefore readily rationalized why ibogaine is the only compound capable of pharmacochaperoning a folding-defective transporter mutant.
The second amino acid mutated in SERT-PG601,602AA mutant is Gly602, which corresponds to Gly585 in DAT. Mutation to alanine resulted in a complete retention of the resulting DAT- G585A in the ER (38). In their work, Miranda et al. did not directly determine whether DAT-G585A was functional (i.e. bound inhibitory radioligands) but proposed that it was correctly folded because it formed complexes with and retained wild-type DAT in the ER. It may be argued that SERT-PG601,602AA was correctly folded but did not bind [3H]imipramine because it is in the inward facing conformation. We consider this unlikely. Mutated versions of SERT, which are trapped in the inward facing conformation and thus have very low affinity for inhibitory radioligands, are nevertheless readily exported to the cell surface (16, 21). We therefore propose an alternative explanation for the phenotype of DAT-G602A, namely that the protein is poorly folded and co-aggregates with wild type. This interpretation is supported by the finding that the C-terminally mutated Na+-K+-2Cl−-cotransporter-1 (NKCC1) is trapped in large aggregates of non-functional protein rather than dimers (34).
Folding of transmembrane proteins is thought to be assisted by chaperones (e.g. GRP78, calnexin) within the ER lumen. Based on our observations, we propose that folding of SERT is also assisted by a chaperone that binds to its intracellular C-terminal portion. In fact, there is precedent in support for this conjecture: folding of CFTR (the cystic fibrosis transmembrane conductance regulator), for instance, is assisted by several chaperones that bind to the cytoplasmic surface of CFTR (39). Similarly, the DnaJ/Hsp 40 chaperone DRIP78 binds to C terminus of the D1-dopamine receptor (30). In our hypothetical model we propose that the spots relevant for the interaction with this putative chaperone include the (conserved) 607RI608 (the 601PG602) motif. Both motifs are conserved in all SLC6 family members. The 607RI608-motif is the conserved motif for interaction with Sec24 (12). Our model posits that the putative chaperone binds to this region and thus samples the folding state of the transporter. This would lead to an attractive proof reading mechanism: binding of Sec24 is contingent on prior release of the chaperone, which precludes premature recruitment of Sec24 and export of a partially unfolded transporter. This model is currently being explored.
This work was supported by the Austrian Science Fund/FWF (SFB3506, to H. H. S.; SFB3507, to P. C. S.-B., and SFB3510, to M. F.) and a stipend from the Egyptian Ministry of Higher Education and State for Scientific Research (to A. E.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Fig. S1.
- SERT
- serotonin transporter
- DAT
- dopamine transporter
- endoglycosidase H
- endo-β-N-acetylglucosaminidase H
- NET
- norepinephrine transporter
- GAT1
- γ-aminobutyric acid transporter
- GLYT
- glycine transporter
- ER
- endoplasmic reticulum
- HEK293
- human embryonic kidney
- 5-HT
- 5-hydroxytryptamine (serotonin)
- DMSO
- dimethyl sulfoxide
- YFP
- yellow fluorescent protein
- PDL
- poly-d-lysine
- PNGase-F
- peptide-N4-(acetyl-β-glucosaminyl)-asparagine amidase.
REFERENCES
- 1.Nelson N. (1998) J. Neurochem. 71, 1785–1803 [DOI] [PubMed] [Google Scholar]
- 2.Rudnick G. (2006) J. Membr. Biol. 213, 101–110 [DOI] [PubMed] [Google Scholar]
- 3.Masson J., Sagné C., Hamon M., El Mestikawy S. (1999) Pharmacol. Rev. 51, 439–464 [PubMed] [Google Scholar]
- 4.Singh S. K. (2008) Channels 2, 380–389 [DOI] [PubMed] [Google Scholar]
- 5.Just H., Sitte H. H., Schmid J. A., Freissmuth M., Kudlacek O. (2004) J. Biol. Chem. 279, 6650–6657 [DOI] [PubMed] [Google Scholar]
- 6.Scholze P., Freissmuth M., Sitte H. H. (2002) J. Biol. Chem. 277, 43682–43690 [DOI] [PubMed] [Google Scholar]
- 7.Moss F. J., Imoukhuede P. I., Scott K., Hu J., Jankowsky J. L., Quick M. W., Lester H. A. (2009) J. Gen. Physiol. 134, 489–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fog J. U., Khoshbouei H., Holy M., Owens W. A., Vaegter C. B., Sen N., Nikandrova Y., Bowton E., McMahon D. G., Colbran R. J., Daws L. C., Sitte H. H., Javitch J. A., Galli A., Gether U. (2006) Neuron 51, 417–429 [DOI] [PubMed] [Google Scholar]
- 9.Binda F., Dipace C., Bowton E., Robertson S. D., Lute B. J., Fog J. U., Zhang M., Sen N., Colbran R. J., Gnegy M. E., Gether U., Javitch J. A., Erreger K., Galli A. (2008) Mol. Pharmacol. 74, 1101–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miranda M., Dionne K. R., Sorkina T., Sorkin A. (2007) Mol. Biol. Cell 18, 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farhan H., Korkhov V. M., Paulitschke V., Dorostkar M. M., Scholze P., Kudlacek O., Freissmuth M., Sitte H. H. (2004) J. Biol. Chem. 279, 28553–28563 [DOI] [PubMed] [Google Scholar]
- 12.Farhan H., Reiterer V., Korkhov V. M., Schmid J. A., Freissmuth M., Sitte H. H. (2007) J. Biol. Chem. 282, 7679–7689 [DOI] [PubMed] [Google Scholar]
- 13.Farhan H., Reiterer V., Kriz A., Hauri H. P., Pavelka M., Sitte H. H., Freissmuth M. (2008) J. Cell Science 121, 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiterer V., Maier S., Sitte H. H., Kriz A., Rüegg M. A., Hauri H. P., Freissmuth M., Farhan H. (2008) J. Neuroscience 28, 12453–12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guptaroy B., Zhang M., Bowton E., Binda F., Shi L., Weinstein H., Galli A., Javitch J. A., Neubig R. R., Gnegy M. E. (2009) Mol. Pharmacol. 75, 514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sucic S., Dallinger S., Zdrazil B., Weissensteiner R., Jørgensen T. N., Holy M., Kudlacek O., Seidel S., Cha J. H., Gether U., Newman A. H., Ecker G. F., Freissmuth M., Sitte H. H. (2010) J. Biol. Chem. 285, 10924–10938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauman P. A., Blakely R. D. (2002) Arch. Biochem. Biophys. 404, 80–91 [DOI] [PubMed] [Google Scholar]
- 18.Torres G. E., Yao W. D., Mohn A. R., Quan H., Kim K. M., Levey A. I., Staudinger J., Caron M. G. (2001) Neuron 30, 121–134 [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Sánchez E., Díez-Guerra F. J., Cubelos B., Giménez C., Zafra F. (2008) Biochem. J. 409, 669–681 [DOI] [PubMed] [Google Scholar]
- 20.Larsen M. B., Fjorback A. W., Wiborg O. (2006) Biochemistry 45, 1331–1337 [DOI] [PubMed] [Google Scholar]
- 21.Korkhov V. M., Holy M., Freissmuth M., Sitte H. H. (2006) J. Biol. Chem. 281, 13439–13448 [DOI] [PubMed] [Google Scholar]
- 22.Albert A. D., Boesze-Battaglia K. (2005) Prog. Lipid. Res. 44, 99–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnani F., Tate C. G., Wynne S., Williams C., Haase J. (2004) J. Biol. Chem. 279, 38770–38778 [DOI] [PubMed] [Google Scholar]
- 24.Tate C. G., Haase J., Baker C., Boorsma M., Magnani F., Vallis Y., Williams D. C. (2003) Biochim. Biophys. Acta 1610, 141–153 [DOI] [PubMed] [Google Scholar]
- 25.Waldo G. S., Standish B. M., Berendzen J., Terwilliger T. C. (1999) Nat. Biotechnol. 17, 691–695 [DOI] [PubMed] [Google Scholar]
- 26.Ou W. J., Cameron P. H., Thomas D. Y., Bergeron J. J. (1993) Nature 364, 771–776 [DOI] [PubMed] [Google Scholar]
- 27.Welch W. J., Brown C. R. (1996) Cell Stress Chaperones 1, 109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs M. T., Zhang Y. W., Campbell S. D., Rudnick G. (2007) J. Biol. Chem. 282, 29441–29447 [DOI] [PubMed] [Google Scholar]
- 29.Korkhov V. M., Milan-Lobo L., Zuber B., Farhan H., Schmid J. A., Freissmuth M., Sitte H. H. (2008) J. Mol. Biol. 378, 337–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bermak J. C., Li M., Bullock C., Zhou Q. Y. (2001) Nat. Cell Biol. 3, 492–498 [DOI] [PubMed] [Google Scholar]
- 31.Pankevych H., Korkhov V., Freissmuth M., Nanoff C. (2003) J. Biol. Chem. 278, 30283–30293 [DOI] [PubMed] [Google Scholar]
- 32.Carrel D., Hamon M., Darmon M. (2006) J. Cell Sci. 119, 4276–4284 [DOI] [PubMed] [Google Scholar]
- 33.Duvernay M. T., Dong C., Zhang X., Zhou F., Nichols C. D., Wu G. (2009) Mol. Pharmacol. 75, 751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nezu A., Parvin M. N., Turner R. J. (2009) J. Biol. Chem. 284, 6869–6876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conn P. M., Ulloa-Aguirre A., Ito J., Janovick J. A. (2007) Pharmacol. Rev. 59, 225–250 [DOI] [PubMed] [Google Scholar]
- 36.Sitte H. H., Freissmuth M. (2010) J. Neurochem. 112, 340–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sitte H. H., Hiptmair B., Zwach J., Pifl C., Singer E. A., Scholze P. (2001) Mol. Pharmacol. 59, 1129–1137 [DOI] [PubMed] [Google Scholar]
- 38.Miranda M., Sorkina T., Grammatopoulos T. N., Zawada W. M., Sorkin A. (2004) J. Biol. Chem. 279, 30760–30770 [DOI] [PubMed] [Google Scholar]
- 39.Farinha C. M., Nogueira P., Mendes F., Penque D., Amaral M. D. (2002) Biochem. J. 366, 797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]