Abstract
Leukemia/lymphoma-related factor (LRF) is a transcriptional repressor, which by recruiting histone deacetylases specifically represses p19/ARF expression, thus behaving as an oncogene. Conversely, in mouse embryonic fibroblasts (MEF), LRF inhibition causes aberrant p19ARF up-regulation resulting in proliferative defects and premature senescence. We have recently shown that LRF is controlled by microRNAs. Here we show that LRF acts on MEF proliferation and senescence/apoptosis by repressing miR-28 and miR-505, revealing a regulatory circuit where microRNAs (miRNAs) work both upstream and downstream of LRF. By analyzing miRNA expression profiles of MEF transfected with LRF-specific short interfering RNAs, we found that miR-28 and miR-505 are modulated by LRF. Both miRNAs are predicted to target alternative splicing factor/splicing factor 2 (ASF/SF2), a serine/arginine protein essential for cell viability. In vertebrates, loss or inactivation of ASF/SF2 may result in genomic instability and induce G2 cell cycle arrest and apoptosis. We showed that miR-28 and miR-505 modulate ASF/SF2 by directly binding ASF/SF2 3′-UTR. Decrease in LRF causes a decrease in ASF/SF2, which depends on up-regulation of miR-28 and miR-505. Alteration of each of the members of the LRF/miR-28/miR-505/ASF/SF2 axis affects MEF proliferation and the number of senescent and apoptotic cells. Consistently, the axis is coordinately modulated as cell senescence increases with passages in MEF culture. In conclusion, we show that LRF-dependent miRNAs miR-28 and miR-505 control MEF proliferation and survival by targeting ASF/SF2 and suggest a central role of LRF-related miRNAs, in addition to the role of LRF-dependent p53 control, in cellular homeostasis.
Keywords: Apoptosis, Gene Expression, Microarray, MicroRNA, Transcription Factors, Mouse Embryo Fibroblasts, Regulatory Network, Senescence
Introduction
Leukemia/lymphoma-related factor (LRF)2 is a BTB/POZ domain Krüppel-like zinc finger transcription factor belonging to the POK protein family. POK proteins exert transcriptional repressive activity by recruiting histone deacetylases and subsequent remodeling of chromatin (1, 2). LRF can act as both transcriptional repressor and activator depending on the promoter context (3–6). LRF is known to have pleiotropic functions during development and differentiation, and it has recently been shown to act as an oncogene (7). Transgenic mouse cell lines overexpressing LRF developed aggressive tumors, and aberrant LRF overexpression was observed in a high percentage of human lymphomas. Conversely, LRF loss caused proliferative defects, premature senescence, and unresponsiveness to oncogenic stimuli. The main mode of action of LRF seems to be through the direct binding to p19ARF, a major tumor suppressor. Alterations of LRF therefore affect the p19ARF/MDM2/p53 pathway (7, 8). However, LRF is also overexpressed in solid tumors, such as colon and bladder cancer, which have frequently lost the p14ARF/p53 pathway (8), and a positive relationship between LRF and p14ARF has been observed in several lung cancers (9). These data suggest possible additional target genes through which LRF might exert its activity.
We have recently shown that LRF is regulated by several microRNA (miRNA) families (10).3 miRNAs have emerged as another important class of regulatory factors distinct from TFs in that they modulate gene expression at the post-transcriptional level. miRNAs are 21–25-nucleotide noncoding RNA produced from RNA polymerase II-transcribed precursors and processed by the RNase III family enzymes Drosha and Dicer to yield mature effector molecules (11, 12). Mammalian miRNAs typically imperfectly base pair with the 3′-untranslated region (3′-UTR) of target mRNAs, causing their translational repression. In mammalian cells, several hundred different miRNAs have been identified and implicated in fundamental cellular and developmental functions. Deviations from normal miRNA expression pattern may have a prominent role in various human diseases (13), especially in cancer (14, 15). There is increasing evidence that TFs and miRNAs can work cooperatively, and preliminary investigations showed that the two types of regulators tend to regulate each other; TF-encoding genes have more miRNA binding sites than other classes of protein coding genes. Vice versa, miRNA coding genes have more TFs binding sites in their promoter than, for example, structural protein coding genes (16). The action of miRNAs in concert with other regulatory processes can basically be classified in two types of miRNA-containing circuits (17). In type I circuits, miRNAs and their targets are oppositely regulated by common upstream factors or processes, resulting in fine-tuning regulation or maintenance of protein steady states. Conversely, coordinated actions of miRNAs and TFs can result in reinforcing a gene expression program of differentiated cellular states (type II circuits).
In this study, we show the existence of a new pathway linking the TF LRF to the RNA-binding protein alternative splicing factor/splicing factor 2 (ASF/SF2) via microRNA modulation. ASF/SF2 is a member of the serine/arginine (SR) protein family originally identified by its role in both constitutive and alternative pre-mRNA splicing (18, 19). Changes in the expression of SR proteins can affect the alternative splicing of an undefined number of cellular transcripts, among which are many oncogenes and tumor suppressor isoforms (20–22). Indeed, alteration of ASF/SF2 expression has been shown to induce apoptosis or cellular transformation depending on the cellular context (23–26). Besides splicing, SR proteins also participate in other cellular processes such as mRNA transport, stability, and translation (27–29). ASF/SF2 has also been shown to be critical for maintenance of genome stability (30, 31). Depletion of ASF/SF2 leads to formation of transcriptional R-loops and DNA rearrangements resulting in a G-phase cell cycle arrest and subsequent programmed cell death. Recent data suggest a role of ASF/SF2 in chromatin dynamics and function; this SR protein might act as “chromatin sensor” and might, with HP1, be necessary for a proper cell cycle progression (32). Our data indicate that in MEF, ASF/SF2 expression is modulated by two microRNAs, miR-28 and miR-505, negatively controlled by LRF. Deregulation of the LRF/miR-28/miR-505/ASF/SF2 axis affects the MEF senescence/apoptosis pathway. These findings identify an additional mechanism through which LRF can exert its activity in MEF.
EXPERIMENTAL PROCEDURES
Reagents
Mouse mature miRNAs (miR-28, miR-505, miR-20a, and miR-Ct) the antagomir (d-28, d-505, and d-Ct), and short interfering RNAs (siRNAs) (mmu-si-LRF, hs-si-LRF, and si-ASF) were synthesized by GenePharma (Shanghai, China). All the restriction and modifying enzymes were from New England Biolabs. Gene Silencer was from Gene Therapy Systems (San Diego, CA). TRIzol reagent, DNase I amplification grade, SuperScript II reverse transcriptase, Dulbecco's modified Eagle's medium-high glucose (DMEM-HG), Opti-MEM, fetal bovine serum (FBS) and Click-iT EdU imaging kit were from Invitrogen. PolyFect, miRNeasy mini kit, miScript reverse transcription kit, and miScript SYBR Green PCR kit were from Qiagen, Milan, Italy, LightCycler 480 Probes Master and LightCycler 480 SYBR Green I Master were from Roche Diagnostics (Mannheim, Germany), miRCURYTM LNA Array version 8.1 and miRCURY labeling kit for miRNAs were from Exiqon A/S, Vedbaek, Denmark. Anti-LRF was from Beth Israel Deaconess Medical Center (Boston, MA). The anti-ASF/SF2 was a gift from Adrian Krainer. X-Gal (5-bromo-4-chloro-3-indolyl-d-galacto side) and the anti-α-tubulin were from Sigma-Aldrich. Enhanced chemiluminescence (ECL) was from Amersham Biosciences. pCMV-MCS plasmid, Herculase DNA polymerase and QuikChange II XL site-directed mutagenesis kit were from Stratagene, La Jolla, CA. pGEM Teasy vector, pGL3 basic vector, Dual-Luciferase reporter assay system cell titer 96 AQueous One solution cell proliferation Assay, and CellTiter Caspase-Glo 3/7 assay system were from Promega, Madison, WI. Cell line Nucleofector kit was from Lonza, Walkersville, MD. The mirVana miRNA detection kit was from Ambion, Austin, TX.
Cells and Culture Conditions
Wild-type MEF were isolated from 13.5-day mouse embryos. Briefly, embryos were mechanically fragmented and then incubated with trypsin (0.25% in PBS, pH 7.5) and continuously stirred at 37 °C for 15–20 min. After 10-min centrifugation at 290 × g, pellets were resuspended in DMEM-HG without FBS and centrifuged for 10 min at 290 × g. After three washings, the cell suspensions were distributed in culture dishes containing DMEM-HG +10% FBS. Cells were trypsinized at confluence (passage 1). The propagation protocol 6T3 (6 × 105 cells/100-mm diameter dish transferred every 3 days) was followed. HEK 293T and HeLa cells were grown in DMEM + 10% FBS. All cells were grown at 37 °C in a humidified atmosphere containing 6% CO2.
Plasmids
A 1500-bp fragment of the 3′-UTR of mouse Sfrs1 var1 (accession number NM_173374) was obtained by PCR amplifying mouse genomic DNA with the following primers: forward (5′-GCAGATCTCGCTCTCGTAC-3′) and reverse (5′-ATCCTCCCTATCCTATCCAC-3′) (Temperature annealing 55 °C). To generate the ASF-UTR construct, the amplified fragment was cloned downstream of EGFP ORF within the pEGFP-C1 plasmid. A stop codon was inserted between the EGFP and the target fragment so that the transcribed mRNA was a hybrid molecule but the translated protein was EGFP. The mutated versions, mut-28 and mut-505, were generated utilizing the ASF-UTR plasmid as template and modifying the miR-28 or the miR-505 seed binding site using the QuikChange II XL site-directed mutagenesis kit. The mutagenic primers used were: miR-28 (position 1045 mRNA Sfrs1var1) Sfrs1var1 forward (5′-GTAATATCCCCTTA*CTA*G*TAACATCTACATTC-3′) and reverse (5′-GAATGTAGATGTTAC*T*AGT*AAGGGGATATTAC-3′); miR-505 (position 1013 mRNA Sfrs1 var1) forward (5′-AATGGGCTAAAC*TC*TA*GAATTGCATTCTTGTG-3′) and reverse (5′-CACAAGAATGCAATTC*TAG*AG*TTTAGCCCATT-3′), where the asterisks indicate the mutated bases. Lpp1 mouse promoter (2700 bp upstream the transcription start site +1) was amplified with the primer pair forward (5′-CCTGTGCTCACTATGGAAG-3′) and reverse (5′-AAAGTGAAACTTCAGCAGCAG-3′) and cloned in the pGEM T-easy vector, sequenced, and subcloned in the pGL3 basic vector, generating the pLpp-pro plasmid. Promoter deletion mutants were obtained by digestion, and subsequent religation, of the pLpp-pro plasmid with the following enzymes: KpnI (pLpp1), KpnI-HindIII (pLpp2), and Pst1 (pLpp3). All the generated constructs were sequenced by Eurofins MWG/Operon, Ebersberg, Germany. The pcDNA3.1-His-mouse Pokemon construct (pLRF, the LRF-expressing plasmid) was a gift from PierPaolo Pandolfi.
miRNA Microarray
To identify miRNAs controlled by LRF, the miRNA expression profiles of P2 MEF transfected with siRNA-LRF and MEF at passage 2 (P2MEF) transfected with siRNA-Ct were performed as follows. Total RNA (2 μg) was labeled and manually hybridized to Exiqon miRCURY LNA Array 8.0 following the manufacturer's protocol. Differential labeling of total RNA samples with dyes spectrally equivalent to Cy3TM and Cy5TM fluorophores allowed comparison of miRNA expression profiles of MEF with WT LRF level (internal reference) and MEF silenced for LRF. The labeling method allows selective labeling of miRNA in the total RNA sample. The hybridized microarrays were scanned with a GenePix 4000B instrument, and data were acquired and analyzed with GenePix Pro software. Data were normalized with print-tip Loess method by the CARMAweb application developed at the Institute for Genomics and Bioinformatics of Graz University of Technology (33).
Expression Analysis of miRNAs
Total RNA was extracted from 0.5/1 × 106 cells with miRNeasy mini kit following the manufacturer's recommendation. Mature miR-28, miR-505, miR-139-5p, miR-135, miR677, and miR-20a were quantified using the miScript system according to the manufacturer's instructions. Oligonucleotides (5′-AAGGAGCTCACAGTCTATTGAG-3′, 5′-AGTCAACACTTGCTGGTTTTCT-3′, 5′-TCTACAGTGCACGTGTCTCCAG-3′, 5′-TACAGTTTCTACATTGCATTTAG-3′, 5′-TATGGCTTTTTATTCCTATGTGA-3′, 5′-TTCAGTGATGATTAGCTTCTGA-3′, and 5′-CGCAAGGATACACGCAAATTC-3′) were used as forward primers, respectively, for miR-28, miR-505, miR-139-5p, miR-20, miR-135, miR-677, and U6 in the real-time amplification mixtures. All reactions were performed in triplicate, and data were analyzed as described (see “Real-time PCR Analysis”) using U6 as internal control. The relative expression ± S.E. of three independent experiments in triplicate is shown.
Real-time PCR Analysis
Total RNA was extracted from 5 × 105 cells using the RNeasy mini kit. After DNase treatment, 1 μg of total RNA was retrotranscribed using SuperScript II reverse transcriptase following the manufacturer's instructions. Real-time PCR (qRT-PCR) was carried out with LightCycler 480 (Roche Applied Science). TaqMan probes and oligonucleotides used were as follows: for Lrf, forward (5′-AACTACGACCTGAAGAACCACATG-3′), reverse (5′-AGATGGTCGGAGCGCACA-3′), and probe (5′-CTGCGGCCATACCAGTGCGATAGC-3′); for p19ARF, forward (5′-CATGGGTCGCAGGTTCTTG-3′), reverse (5′-GCTCGCTGTCCTGGGTCTC-3′), and probe (5′-CACTGTGAGGATTCAGCGCGCG-3′). Sfrs1 var1 and var2, ATP11c, and Lpp1 transcripts were quantified using LightCycler 480 SYBR Green I Master and the following primers: Sfrs1 var1, forward (5′-TTTAGATCTCACGAGGGAGAA-3′), reverse (5′-CGTGGTGATCCTCTGCTTC-3′); Sfrs1 var2, forward (5′-AAAGCTTAGATCTTTCAATGG-3′), reverse (5′-CGTGGTGATCCTCTGCTTC-3′); ATP11c, forward (5′-GGAATTCATTGAATGCTGCATAG-3′), reverse (5′-CTTTTAGCTCCTTTCACCAAAGC-3′); and Lpp1 forward (5′-CCAGCCGCTTCTACAATGTC-3′), reverse (5′-TCTCCAGGATCCACACTGCA-3′). All reactions were performed in triplicate. Relative quantification of gene expression was calculated as described (34). Transcript values were normalized with those obtained from the amplification of GAPDH (internal control) with the following primers: forward (5′-GCCTTCCGTGTTCCTACCC-3′), reverse (5′-TGCCTGCTTCACCACCTTC-3′) for SYBR Green analysis and probe (5′-CCTGGAGAAACCTGCCAAGTATGATGACATC-3′) when TaqMan quantification was performed.
RNase Protection Assay
The RNase protection assay was performed using the mirVana miRNA detection kit following the manufacturer's protocol. Antisense RNA oligonucleotide probes (U6, 5′-GAAUUUGCGUGUCAUCCUUGCGAAAACCAGAG-3′; miR-505, 5′-AGAAAACCAGCAAGUGUUGACGCCAGAG-3′; and miR-28, 5′-CUCAAUAGACUGUGAGCUCCUUGGACAC-3′) were end-labeled using T4 polynucleotide kinase (PNK), kinase buffer and [γ-32P]ATP (6000 Ci/mmol). Then, 2–3 μg of total RNA from each sample was incubated with the probes at 42 °C for 2 h. Samples were run on a 15% polyacrylamide gel. Bands were quantified using a PhosphorImager.
RT-PCR Analysis of Alternatively Spliced Isoforms
50 ng of cDNA prepared from transfected MEF as described in the previous paragraph was used as template for RT-PCR together with the following primers: Bcl-x, forward (5′-AGTTTGAACTGCGGTACCGGCG-3′), reverse (5′-TCTGATATGCTGTCCCTGGG-3′), Ich-1, forward (5′-ATGCTAACTGTCCAAGTCTA-3′), reverse (5′-GTCTCATCTTCATCAACTCC-3′). The ratios of Ich-1S to Ich-1L and of Bcl-xS to Bcl-xL were measured using the Optiquant program.
EGFP Reporter Assay
HEK 293T cells were seeded at a density of 1.5 × 105 cells/well in 24-well dishes. Next, 24 h later, cells were transfected with 125 ng of ASF-UTR, mut-28, or mut-505. PolyFect was used as transfectant, according to the manufacturer's recommendations. 24 h after transfection, cells were trypsinized and centrifuged, and the cell pellet was resuspended in PBS; 36 h after transfection, fluorescence was quantitated by cytofluorometry (FACSCalibur, BD Biosciences). Approximately 104 cells/sample were analyzed.
Transactivation Experiments
HEK 293T cells were transfected as described in the EGFP reporter assay. In each transfection, 200 ng of pLpp-pro, the pLpp1, pLpp2, or pLpp3 plasmids was co-transfected with 80 ng of pLRF or pCMV as control plasmid in the presence of 100 ng of Renilla-expressing plasmid as internal standard. Luciferase assays were performed 48 h after transfection using the Dual-Luciferase reporter assay system. Firefly luciferase activity was normalized to Renilla activity for each transfected well. Three independent experiments assayed in triplicate were performed.
Gain- and Loss-of-Function Experiments
Exponentially growing P2MEF were transfected with specified miRNAs or siRNA or with miR-Ct. Briefly, 15 μl of Opti-MEM and 21 μl of transfection buffer plus 80 nm miRNA were mixed with a solution of Gene Silencer (5 μl) plus Opti-MEM (25 μl). After a 10-min incubation, Opti-MEM was added up to 500 μl. Then, the transfection mixture was added to 1.5 × 105 cells seeded 24 h before transfection in 30-mm diameter dishes. After 4 h, the medium was replaced with DMEM-HG +10% FBS. With this protocol, 90% of P2MEF were transfected (data not shown). The antagomirs d-28, d-505, and d-Ct were transfected using the same procedure. 6 h after transfection, 2 × 104 cells were seeded in a 30-mm diameter dish and used for senescent and apoptotic cell determination. For quantification of LRF, p19Arf, ASF/SF2, miR-28, and miR-505 expression, cells were collected 48 h after transfection.
Western Blot Analysis
Samples of 5 × 105 cells were lysed (20 mm Tris-HCl, pH 8.0, 20 mm NaCl, 10% glycerol, 1% Nonidet P-40, 10 mm EDTA, 2 mm PMSF, 2.5 μg/ml leupeptin, 2.5 μg/ml pepstatin). Proteins (10–30 μg/lane) were separated on 12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Immunoblots were probed with the following primary antibodies: anti-LRF (1:200), anti-ASF/SF2 (1:4,000), and anti-tubulin (1:40,000). Signals were revealed after incubation with recommended secondary antibody coupled to peroxidase using ECL. Scanned images were quantified using the Optiquant software.
Senescence-associated β-Galactosidase Assay
Samples of 2 × 104 transfected cells were seeded in 30-mm diameter dishes, and 48 h after transfection, dishes were washed once with PBS and fixed for 3–5 min at room temperature with PBS containing 2% formaldehyde, 0.2% glutaraldehyde. Cells were then washed three times with PBS and incubated at 37 °C with fresh senescence-associated (SA)-β-galactosidase (β-gal) staining solution containing 1 mg/ml X-Gal (stock = 20 mg/ml in dimethyl sulfoxide (DMSO)), 5 mm potassium ferrocyanide, 5 mm potassium ferricyanide, 2 mm MgCl2 in PBS, pH 6.0. After 24 h, cells were washed in PBS and stained with 20 μg/ml Hoechst 33342 for 10 min. Dishes were scored with a Leica ILDM inverted microscope to count SA-β-gal+ cells and Hoechst-positive nuclei. The ratios SA-β-gal + cells/Hoechst-positive nuclei of 25 consecutive fields (∼500 cells) were used to determine the frequency of the event.
Cell Proliferation Assay
6 h after transfection, 7 × 103 cells were seeded in 96-well plates in duplicate and grown for 72 h. Cell density was measured by cell titer 96 AQueous solution cell proliferation assay according to the manufacturer's recommendations. The proliferation rate was also determined using the Click-iT EdU imaging kit according to the manufacturer's recommendations.
Apoptotic Assay
6 h after transfection, 104 cells were seeded in Luminometer Plates (96-well); 48 h after transfection, apoptotic cells were determined using the Caspase-Glo3/7 assay system according to the manufacturer's recommendations.
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA). Statistical differences were determined by unpaired t test, with values of p < 0.05 considered statistically significant. Each experimental point in the graph represents the mean ± S.E. of at least three independent experiments.
RESULTS
LRF Down-regulation Modulates miRNAs in MEF
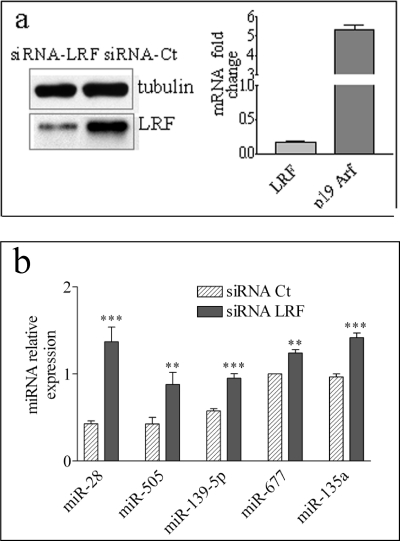
To explore the possibility that miRNAs might constitute part of the LRF regulatory network, LRF was silenced in MEF at passage 2 (P2MEF) by siRNA-LRF, and the miRNA global expression profiles were compared with those of cells transfected with a control siRNA (siRNA-Ct). LRF down-regulation (Fig. 1a, left panel), besides increasing p19ARF mRNA levels as expected (7) (Fig. 1a, right panel), alters the expression of a limited number of miRNAs, whereas for the majority, the expression profile did not change (not shown). Because LRF is a transcriptional repressor, we focused our attention on the significantly up-regulated miRNAs as they might be under its direct transcriptional control. Among these, miR-139-5p, miR-677, miR-135a, miR-505, and miR-28 were selected as they showed the greatest increase (>3 S.D.), and their expression changes were validated by qRT-PCR (Fig. 1b).
FIGURE 1.
LRF down-regulation modifies the global miRNA profiles in MEF. a, P2MEF cells were transfected with siRNA-LRF and with a scrambled siRNA (siRNA-Ct). To verify the effect of transfection, LRF protein level was determined by Western blot (left panel). As further control, qRT-PCR was performed to measure mRNA level of LRF and of its direct target p19ARF (right panel). RNAs extracted from transfected cells were manually hybridized to Exiqon miRCURY LNA Array version 8.1. b, five miRNAs showing a positive Z-score >3 S.D. were further validated by qRT-PCR analysis. U6 was used for data normalization. The values reported in the qRT-PCR analysis were the mean of three independent transfection experiments. **, p < 0.01; ***, p < 0.001.
LRF Represses the Promoter of the miR-28 Host Gene LPP
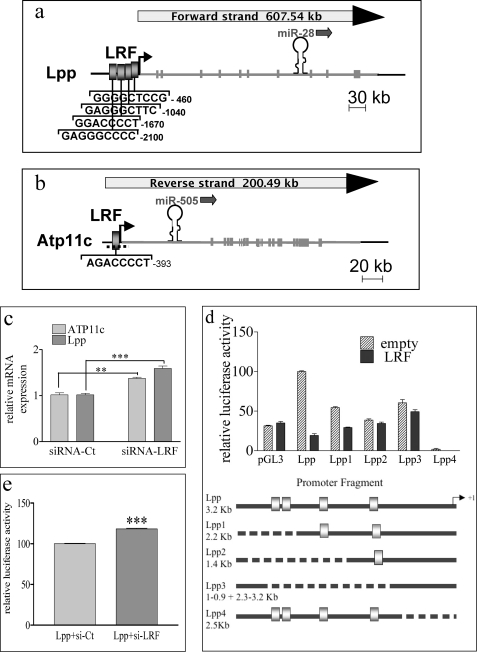
The most pronounced increase in miRNA abundance after LRF down-regulation was observed for miR-28 and miR-505 (Fig. 1b and supplemental Fig. S1). Both miRNAs have no identified homologs in any vertebrate genomes and are intragenic; miR-28 is located in the eighth intron of the Lpp gene on chromosome 16 (Fig. 2a), whereas miR-505 transcript overlaps the first intron of the Atp11c gene on chromosome X (Fig. 2b). LRF down-regulation induced a significant increase in both Lpp and Atp11c transcripts (Fig. 2c), as expected if their expression is transcriptionally controlled by LRF. Degenerated consensus sequences for LRF binding have already been identified by two groups (7, 35). Both degenerated consensus sequences, characterized by a high content of C/G bases, were searched for in the Lpp and Atp11c promoters using PatSearch. A schematic representation of the sites identified in the two promoters is presented in Fig. 2, a and b. Transactivation assays were performed with Lpp promoter constructs fused to luciferase reporter gene in HEK293 recipient cells, which do not appreciably express LRF. Overexpression of LRF in HEK293 cells efficiently represses the Lpp promoter (Fig. 2d). Conversely, MEF transfection with an siRNA against human LRF increases the Lpp promoter activity. The 20% increase was in keeping with the low LRF level in this cell line (Fig. 2e). Progressive deletions of the putative LRF binding sites strongly impair the repressive effect of LRF on the Lpp promoter with the exception of the binding site closest to the transcription starting site (Fig. 2d). These data suggest that all three distal binding sites are required for full LRF activity and indicate that LRF may control miR-28 transcription by directly binding to the Lpp promoter, where it functions as transcriptional inhibitor. The same approach could not be used for the Atp11c gene due to the presence of a large CpG island spanning the Atp11c transcription start site and including the LRF putative binding site (Fig. 2b). Although at present we have no evidence that miR-505 is transcriptionally controlled by LRF, this miRNA was included in the study as its regulation is strongly affected by LRF down-regulation.
FIGURE 2.
LRF reduction affects the expression of Lpp1 and Atp11c genes. a and b, schematic representation of miRNA localization and putative LRF binding sites in Lpp (a) and ATP11c (b) genes. Gray blocks, LRF binding sites. The dashed line shows the localization of the CpG island spanning from −700 to +500 of the ATP11c gene (b). MEF were transfected with siRNA-LRF and siRNA-Ct. c, after 48 h, total RNA was extracted, and qRT-PCR was performed to quantify ATP11c and Lpp transcripts. d, trans-repression assays in HEK293 cells transfected with LRF-expressing vector along with Lpp promoter luciferase-based vectors in the presence of Renilla vector as internal control. Top, promoter activities were measured 48 h after transfection using the Dual-Luciferase reporter assay system. Bottom, schematic representation of the WT and deleted Lpp promoters cloned in the luciferase reporter vector. e, HEK293 cells were transfected with siRNA-LRF or siRNA-Ct and the Lpp promoter-luciferase vector in the presence of Renilla vector as internal control. All the experiments were performed in triplicate. **, p < 0.01; ***, p < 0.001.
Both miR-28 and miR-505 Target the Splicing Factor ASF/FS2
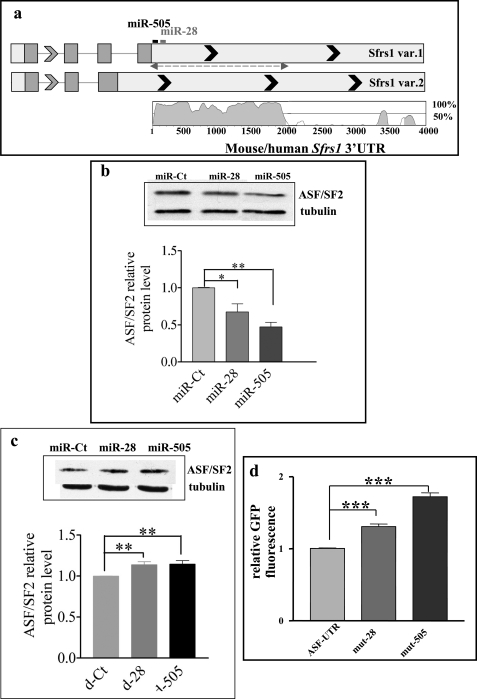
Because LRF affects senescence and apoptosis in MEF (8, 10, 36), we used TargetScan and miRanda algorithms to seek putative target genes of miR-28 and miR-505, involved in the senescence/apoptosis pathway. We found that the two miRNAs share a common target, ASF/SF2; the seed match positions of these miRNAs are schematically reported in Fig. 3a. ASF/SF2, codified by the Sfrs1 gene, is a prototypical serine- and arginine-rich protein with important roles in splicing and other aspects of mRNA metabolism (27, 37, 38). ASF/SF2 is essential for cell viability (19, 39). In vertebrates, loss or inactivation of ASF/SF2 results in genomic instability and induction of G cell cycle arrest and apoptosis (30, 31). Vice versa, a slight up-regulation of ASF/SF2 has an antiapoptotic effect in serum-starved MEF, and it is sufficient to transform immortal rodent fibroblasts, suggesting that ASF/SF2 may behave as an oncogene (24). These observations indicate that modulations of ASF/SF2 expression might affect MEF cell growth, apoptosis, or senescence, making this protein a valid miRNA target to study.
FIGURE 3.
miR-28 and miR-505 down-regulate ASF/SF2 by directly binding its 3′-UTR. a, schematic representation of the splicing variants of mouse Sfrs1 gene. Putative binding sites for miR-28 and miR-505 are depicted. Top, the dashed double arrow shows the cloned part of the 3′-UTR. Bottom, phylogenetic conservation of the genomic region containing the 3′-UTR of Sfrs1 variant 1. Vista was used to generate pairwise alignment between the genomic sequence from mouse and human. b, P2MEF were transfected with 80 nm miR-Ct, miR-28, and miR-505. Then, 48 h after transfection, proteins were extracted, and Western blot of ASF/SF2 and tubulin as internal control was performed. Top, an example of the Western blot. Bottom, densitometric quantification of three experiments performed as described above. Band intensity was normalized to that of tubulin. c, P2MEF were transfected with 80 nm decoy-Ct (d-Ct), d-28, and d-505. Bottom, proteins were extracted and analyzed by Western blot as described in b. Top, an example of the Western blot. d, relative GFP fluorescence in HK293 cells transfected with ASF-UTR vector (EGFP-C1 vector containing the GFP gene fused to the WT ASF/SF2 3′-UTR), mut-28, and mut-505 (containing the ASF/SF2 3′-UTR mutated in the seed match for miR-28 and miR-505, respectively). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To verify whether ASF/SF2 protein translation is controlled by miR-28 and/or miR-505, the expression of ASF/SF2 was analyzed in P2MEF transfected with either miRNA mimics (synthetic RNA duplexes in which the 3′-5′ strand is identical to the native miRNA) (gain of function) or decoy miRNAs (antisense 2′-O-methylated RNA oligonucleotides fully complementary to target miRNAs) (loss of function). A monoclonal antibody directed against the N-terminal portion of the splicing factor was used to quantify ASF/SF2; although the antibody should detect both isoforms (248 and 201 amino acids long) generated by alternative splicing of the Sfrs1 transcript (40) and differing in the C-terminal region (Fig. 3a), only the higher molecular weight form was detected. This result, in keeping with literature (41), suggests that the isoform 2 transcript, although detectable by qRT-PCR, is not efficiently translated.
Gain-of-function experiments showed that ASF/SF2 protein is significantly reduced in MEF transfected with either miR-28 or miR-505 as compared with cells transfected with a control miRNA (miR-Ct, Fig. 3b). The same results were obtained by transfecting the miRNA mimic in HeLa cells (supplemental Fig. S2).
In agreement with gain-of-function analysis, loss-of-function experiments showed that the transfection of d-28 and d-505 significantly increases ASF/SF2 protein as compared with transfection with a control decoy (Fig. 3c, d-Ct). To confirm that miR-28 and miR-505 are direct regulators of ASF/SF2, the 3′-UTR variant 1 of mouse Sfrs1 in particular, a 1800-bp fragment of the 3′-UTR, spanning the two miRNA binding sites and representing the phylogenetically conserved part of Sfrs1 3′-UTR (Fig. 3a), was cloned downstream of the GFP coding sequence (ASF-UTR). We compared the ASF-UTR GFP activity in HEK293 recipient cells with equivalent constructs where either the miR-28 (mut-28) or the miR-505 (mut-505) seed sites were mutated. Inactivation of either miR-28 or miR-505 binding sites in the ASF-UTR significantly increases GFP expression, indicating a direct binding of these miRNAs on ASF/SF2 3′-UTR (Fig. 3d).
LRF Modulates ASF/SF2 via miR-28 and miR-505 Regulation
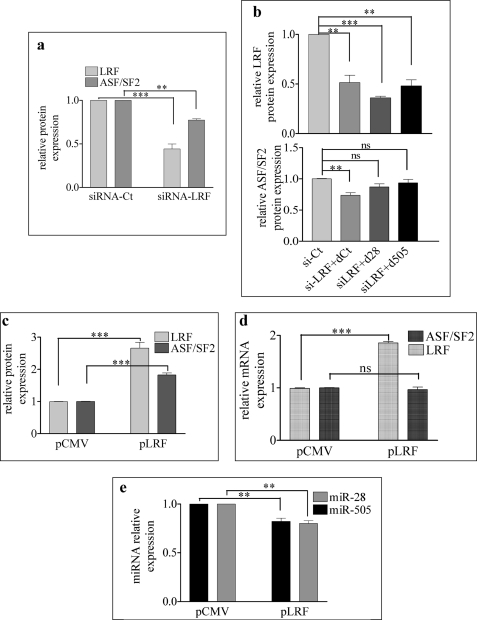
As both miR-28 and miR-505 regulate ASF/SF2 levels and in turn are controlled by (miR-28) or at least are responsive to (miR-505) LRF, LRF down-regulation would be expected to decrease ASF/SF2 levels. Indeed, we found that a 50% decrease of LRF is accompanied by a 20% decrease of ASF/SF2 (Fig. 4a). Moreover, the ASF/SF2 reduction induced by siRNA-LRF is partially counterbalanced by the co-transfection with either d-28 or d-505 (Fig. 4b).
FIGURE 4.
LRF modulates ASF/SF2 via miR-28 and miR-505 regulation. a, MEF were transfected with siRNA-LRF and siRNA-Ct. After 48 h, LRF and ASF/SF2 protein levels were evaluated. b, MEF were co-transfected with si-Ct, siRNA-LRF + d-Ct, siRNA-LRF + d-28, or siRNA-LRF + d-505. Top and bottom, after 48 h, proteins were extracted and LRF (top) and ASF/SF2 (bottom) were quantified. Data are the mean of three experiments. MEF were transfected with control plasmid (pCMV) and LRF-expressing vector (p-LRF) using Amaxa nucleoporator device. ns, not significant. c–e, after 48 h, LRF and ASF/SF2 protein levels (c), LRF and ASF/SF2 isoform 1 mRNA levels (d), and miRNA levels (e) were evaluated. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To further confirm the connection between LRF, miR-28/miR-505, and ASF/SF2 expression, we transfected MEF with a plasmid-expressing LRF. As MEF are refractory to plasmid transfection by standard methods (lipofection), the Amaxa nucleoporator device was utilized to obtain a satisfactory efficiency of transfection (∼40% cells were transfected). As expected, the overexpression of LRF increased ASF/SF2 protein but not mRNA (Fig. 4, c and d) and down-regulated the expression of miR-505 and miR-28 (Fig. 4e). All these findings indicate that miR-28, miR-505, and ASF/SF2 might be part of the LRF regulatory network.
ASF/SF2 Does Not Affect the Expression Level of miR-28 and miR-505
Mir-28 and miR-505 are intronic miRNA (42, 43). Several data suggest that Drosha processing does not impair splicing. Drosha processing takes place after the transcript is tied to the splicing commitment but before the intron is excised. Therefore, variation of splicing factors should not affect miRNA maturations. However, we decided to verify whether the ASF/SF2 modulation might alter the expression of miR-28 and miR-505. ASF/SF2 was down-regulated by transfecting MEF with two siRNAs that decrease both isoforms of the splicing factor (supplemental Fig. S3, b and c). No significant variations of the miRNA levels nor of LRF protein level (data not shown) were observed in MEF transfected with ASF/SF2-specific siRNA as compared with MEF transfected with siRNA-Ct (supplemental Fig. S3d).
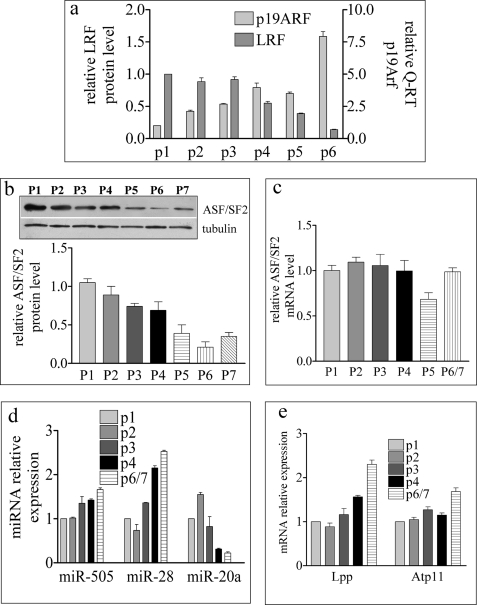
The LRF/miR-28/miR-505/ASF/SF2 Axis Is Coordinately Modulated during Culture-induced Senescence
MEF become senescent after few passages in culture (44–46), and we have recently shown that LRF expression progressively diminishes along with passage (46). This prompted us to investigate whether the proposed LRF/miR-28/miR-505/ASF/SF2 axis takes place during culture-induced senescence. Indeed, the continuous decrease of LRF with increasing passages not only correlates with p19ARF transcriptional increase (10, 47, 48) (Fig. 5a) but also correlates with a progressive reduction of ASF/SF2 (Fig. 5b). It is of note that the progressive decrease of ASF/SF2 protein is not a consequence of ASF/SF2 mRNA reduction (Fig. 5c) in keeping with the hypothesis that the down-regulation of this factor is at the post-transcriptional level.
FIGURE 5.
LRF/miR-28/miR-505/ASF/SF2 axis is modulated during serial passages of MEF in culture. a, densitometric quantification of Western blot and qRT-PCR quantification, respectively, of LRF and p19ARF in MEF passages. b and c, densitometric quantification of Western blot (b) and qRT-PCR quantification (c) of ASF/SF2 in MEF passages. b, top, an example of the Western blot analysis for ASF/SF2. d and e, qRT-PCR quantification of miRNA (d) and Lpp and Atp11c transcripts during MEF passages (e).
Moreover, miR-28 expression, and to a lesser extent miR-505 expression (Fig. 5d), as well as that of the respective host genes Lpp and Atp11c (Fig. 5e), increase during culture-induced senescence. A general increase in microRNAs is not a characteristic of senescent MEF because miR-20a, a regulator of LRF (10), was down-regulated with passages (Fig. 5d). The finding that LRF down-regulation during senescence correlates with miR-28 and 505 transcriptional increase and ASF/SF2 post-transcriptional decrease supports a model whereby LRF may also regulate senescence through the miR-28/miR-505/ASF/SF2 axis.
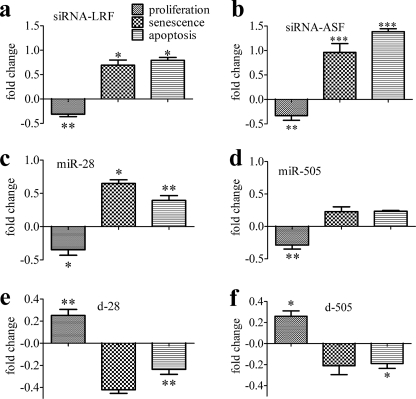
Biological Consequences of Deregulating the LRF/miR-28/miR-505/ASF/SF2 Axis
To gain more insight into the role of LRF/miR-28/miR-505/ASF/SF2 axis in senescence, each component was experimentally deregulated. LRF down-regulation with a specific siRNA not only reduced proliferation and EdU incorporation and enhanced the percentages of SA-βgal+ cells, as already shown (10), but also increased apoptosis (Fig. 6a and supplemental Fig. S4).
FIGURE 6.
Biological consequences of LRF/miR-28/miR-505/ASF/SF2 axis dysregulation. a–f, P2MEF were transfected with siRNA-Ct, siRNA-LRF (a), siRNA-ASF (b), miR-28 (c), miR-505 (d), d-Ct and d-28 (e), and d-505 (f). After 48 h, cells were trypsinized and seeded for different assays as described under ”Experimental Procedures.“ Graphs show the fold changes (negative and positive) in the number of proliferating, senescent, and apoptotic cells. Each value was obtained by dividing the percentage of cells transfected with si-LRF (a), si-ASF/SF2 (b), miR-28 (c), and miR-505 (d) versus cells transfected with si-Ct or by dividing the percentage of cells transfected with d-28 (e) and d-505 (f) versus cells transfected with d-Ct. Fold changes in a–d were calculated with respect to siRNA-Ct, whereas fold changes in e and f were calculated with respect to d-Ct. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
The overexpression of miR-28, and to a lesser extent that of miR-505, which reduces ASF/SF2 protein level, also inhibited cell proliferation and EdU incorporation and increased the percentages of both SA-βgal+ and apoptotic cells (Fig. 6, c and d, and supplemental Fig. S4). Conversely, antago-miRNA transfection stimulated MEF proliferation and decreased the number of senescent and apoptotic cells (Fig. 6, e and f).
P2MEF silenced for ASF/SF2 showed reduced cell proliferation as compared with MEF transfected with siRNA-Ct, as shown by cell counts and by EdU incorporation (Fig. 6b and supplemental Fig. S4); moreover, the reduction of cell proliferation was paralleled by a doubling of SA-βgal+ cells (Fig. 6b). As ASF/SF2 reduction has been reported to induce apoptosis, (30, 31), the number of apoptotic cells in ASF/SF2-silenced MEF was determined, and a significant increase in apoptotic cells was also found (Fig. 6b).
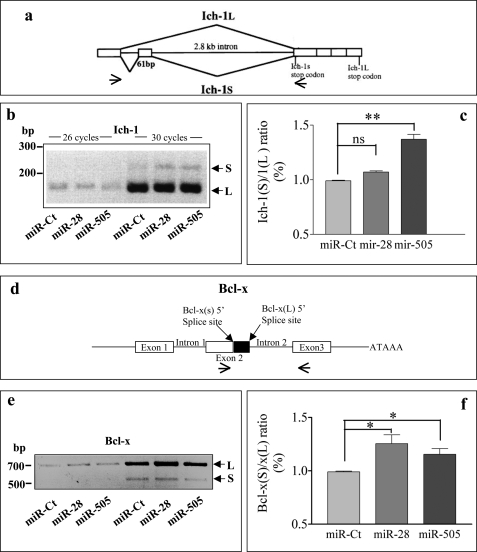
Modulation by miR-28 and miR-505 Can Affect ASF/SF2 Alternative Splicing Activity
As both miR-28 and miR-505 regulate ASF/SF2 levels, which in turn are known to affect the alternative splicing of several pro- and antiapoptotic genes, we decided to analyze the expression of some ASF/SF2 targets after miR-28 or miR-505 overexpression. We tested two ASF/SF2 targets whose alternative splicing results in the formation of products playing opposite roles in apoptosis: Ich-1 and Bcl-x (25, 26). In HeLa, the overexpression of ASF/SF2 promotes exon skipping and the consequent decrease of the ratio between the short form (Ich-1S antiapoptotic) and the long form (Ich-1L proapoptotic) of Ich-1, suggesting a proapoptotic role of ASF/SF2 in this cell line (26). Vice versa, in HEK 293 cells, ASF/SF2 overexpression induces the expression of the antiapoptotic Bcl-xL isoforms (25). In MEF, overexpression of miR-505 and miR-28 modifies the ratio between the isoforms of both genes (Fig. 7), although changes induced by miR-28 on Ich-1 are not significant. The increase we observed in the Ich-1S/1L and the Bcl-xS/xL ratio are in agreement with the effect of the ASF/SF2 decrease reported in literature (25, 26). Intriguingly, in the case of Ich-1, the antiapoptotic isoform is induced by miRNA overexpression (especially miR-505). In conclusion, the low modulation observed for Ich-1 and Bcl-x genes, along with the contradictory biological effects they have in MEF, suggest that apoptosis induction by miRNA overexpression is not related to the modulation of the two ASF/SF2 targets.
FIGURE 7.
The regulation of Ich-1 and Bcl-x alternative splicing by miR-28 and miR-505 overexpression. a and d, schematic representation of the alternative splicing of Ich-1 (a) and Bcl-x (d) genes. P2MEF were transfected with siRNA-Ct, miR-28, and miR-505. After 48 h, total RNA was extracted, and cDNA was prepared. Alternatively spliced Ich-1 (b) and Bcl-x (e) products were detected by RT-PCR with murine-specific primers. Primer positions are indicated by small arrows in the lower part of panels a and d. c and f, densitometric quantification of three experiments performed as described in b and e. Values expressed as S/L ratio and obtained using Optiquant program. *, p < 0.05; **, p < 0.01. ns, not significant. S and L, short and long isoforms.
DISCUSSION
We have recently reported that the transcriptional repressor LRF is under miRNA control in MEF (10) and that its modulation by the miR-20 family addresses cells toward senescence. We have also found that culture-induced senescence is characterized by down-regulation of LRF and up-regulation of specific miRNAs, some of which, such as miR-290, are causatively correlated to senescence (46).
Transcription of miRNAs, in common with all genes transcribed by RNA polymerase II, can be modulated by transcription factors (49–51). We therefore determined the effect of the transcriptional repressor LRF on the miRNA expression profile of MEF. Of the eight miRNAs whose expression negatively correlated with LRF levels, the greatest changes were found for miR-28 and miR-505. In this study, we show that LRF is able to modulate the promoter activity of the Lpp gene, which hosts miR-28 (Fig. 2d), suggesting a direct control of miR-28 expression by LRF. Moreover, the increase of miR-28 expression (and its host gene, Fig. 5, d and e) observed during culture-induced senescence correlates with LRF down-regulation, supporting a physiological role of LRF as transcriptional suppressor of this miRNA.
Unfortunately, a direct interaction of LRF with the promoter of the miR-505 host gene Atp11c, containing LRF putative binding sites, could not be assessed due to sequence constraints; however, the finding that the transcription of the miR-505/Atp11c gene is enhanced in all circumstances where LRF levels decreased suggests that this gene might be modulated by LRF. It is of note that miR-505/Atp11c does not increase as much as miR-28/Lpp (Fig. 5, d and e) during culture-induced senescence, suggesting that other factors may be involved in the regulation of miR-505/Atp11c during senescence. Interestingly, two E2F1 binding sites were identified in the promoter of Atp11c, suggesting that miR-505 might be under E2F1-positive transcriptional control. Because E2F1 decreases during senescence (46, 52), we might hypothesize that the limited accumulation miR-505/Atp11c might result from the synchronous decrease of an activator (E2F1) and a repressor (LRF) of the Atp11c promoter.
It is known that miRNAs target more genes and possibly multiple pathways, so it is not always easy to identify which target/s is/are responsible for the biological effects. In our case, it was intriguing to find that both miR-28 and miR-505 have one common predicted target, ASF/SF2. ASF/SF2 is the prototypical member of the SR protein family, a group of splicing factors whose SR name reflects the presence of a characteristic serine/arginine-rich domain (53, 54). SR proteins both are essential for constitutive splicing and are also able to participate in alternative splicing (55). It is known that ASF/SF2 is up-regulated in many cancers and transforms immortal rodent fibroblasts by activating the mammalian target of rapamycin (mTOR) pathway (56, 57). Conversely, silencing of ASF/SF2 induces cell cycle arrest and apoptosis. The mechanism by which SF2/ASF depletion induces G/M arrest and apoptosis appears to be due to the formation of an RNA/DNA hybrid, the so-called R-loop structures with consequent double strand breaks and DNA rearrangements (58). With gain-of-function and loss-of-function experiments, we demonstrated that ASF/SF2 is under the control of both miR-28 and miR-505, an effect exerted by direct binding to the 3′-UTR of ASF/SF2. Moreover, down-regulation of ASF/SF2 with specific siRNAs inhibits cell cycle and increases senescence and apoptosis. However, si-ASF biological effects were greater than those observed with miR-28, miR-505, or LRF-specific siRNA. Although we do not have a definite explanation for this, one may speculate that ASF/SF2 is one of the final effectors of senescence/apoptosis, and as such, its direct down-regulation is more efficient than that obtained by inhibition of its upstream regulators.
All these data attribute to LRF a role as antiapoptotic gene in MEF; LRF silencing, as well as miR-28 overexpression, induces apoptosis, suggesting that the oncogenic function of LRF is exerted by inhibiting both senescence and apoptosis, the two main tumor suppressor mechanisms. Recently, the role of LRF in suppressing apoptosis has been shown in erythropoietic cells; LRF acts downstream of GATA1 by suppressing Bim, a major apoptosis inducer in hematopoietic cells, thus ensuring continued production of red blood cells (59).
Another interesting finding of this study is that miR-28, but not miR-505, is causatively involved in senescence as its overexpression increases the number of SA-βgal+ MEF. These results could imply that LRF exerts its antisenescence activity not only by repressing p19ARF/p53 (7, 10, 58) but also by inhibiting miR-28. The role of miR-505 in MEF senescence appears to be more complex than that of miR-28. The overexpression of miR-505 while significantly reducing MEF proliferation, indicating that it may control genes of the cell cycle, has a limited effect on both senescence and apoptosis. In line with these data might be the observed increase of the antiapoptotic isoforms of Ich-1 as a consequence of miR-505 overexpression (Fig. 7a). This is in line with our previous results showing that miR-505 was a LRF-responsive miRNA rather than a senescence-responsive miRNA (46).
It is of note that miR-28 induces more senescence/apoptosis than miR-505, although the latter is more efficient in down-regulating ASF/SF2. We have previously shown that the miR-20 family directs MEF toward senescence by down-regulating multiple targets (LRF, E2F1, and other unknown targets (10)), whereas other miRNA families such as miR-100 or miR-125b, which target LRF, do not have this property.4 These findings indicate that the final biological outcome of a miRNA derives from the combinatorial effect of multiple targets (16, 51). In line with this concept, we can postulate that miR-28 modulates genes other than ASF/SF2, which contribute to induction of senescence or/and apoptosis.
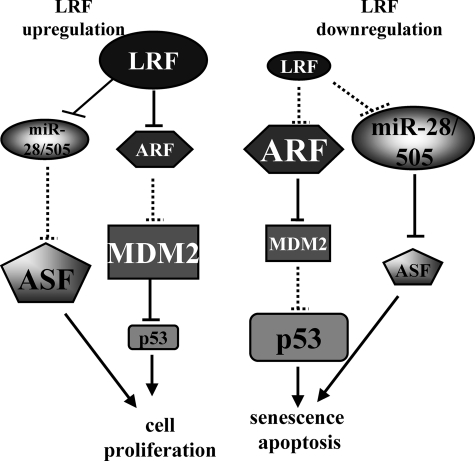
In conclusion, our work has several implications. (a) It extends the class of miRNA transcriptional regulators to LRF, which belongs to the POK protein family; (b) it proposes miR-28 as a novel tumor suppressor candidate as it directs cells toward apoptosis/senescence by down-regulating the oncogene ASF/SF2; and (c) it highlights that the oncogenic potential of LRF may be multiple as it is not limited to the repression of senescence via the p19ARF/(p14arf)/p53 pathway but also of senescence and apoptosis via modulation of miR-28-miR-505/ASF/SF2 axis (Fig. 8).
FIGURE 8.
Proposed roles of the LRF/miR-28/miR-505/ASF/SF2 axis (modified from Ref. 8). The modulation of miR-28 and miR-505 induced by LRF up- or down-regulation affects ASF/SF2 level and reinforces the role of LRF in MEF proliferation, senescence, and apoptosis. Dashed lines indicate loss of repression. Smaller forms indicate protein/miRNA decrease.
Supplementary Material
Acknowledgments
We thank Dr. A. Krainer for the anti-ASF/SF2 antibody; Dr. P. P. Pandolfi for the pcDNA3.1-His-mouse Pokemon construct; and Dr. M. Minks and Dr. F. Cremisi for critical reading of this manuscript.
This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC) (Project 4753) and Istituto Superiore di Sanità (ISS) (Project 527/A/3A/4).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
L. Verduci, M. Simili, M. Rizzo, A. Mercatanti, M. Evangelista, L. Mariani, G. Rainaldi, and L. Pitto, unpublished results.
G. Rainaldi, unpublished results.
- LRF
- leukemia/lymphoma-related factor
- ASF/SF2
- alternative splicing factor/splicing factor 2
- Edu
- 5-ethynyl-2′-deoxyuridine
- miRNA
- microRNA
- MEF
- mouse embryonic fibroblast(s)
- DMEM-HG
- DMEM-high glucose
- qRT
- quantitative RT
- SA
- senescence-associated
- SR protein
- serine/arginine protein
- TF
- transcription factor
- var
- variant.
REFERENCES
- 1.Melnick A., Carlile G., Ahmad K. F., Kiang C. L., Corcoran C., Bardwell V., Prive G. G., Licht J. D. (2002) Mol. Cell Biol. 22, 1804–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin R. J., Nagy L., Inoue S., Shao W., Miller W. H., Jr., Evans R. M. (1998) Nature 391, 811–814 [DOI] [PubMed] [Google Scholar]
- 3.Pendergrast P. S., Wang C., Hernandez N., Huang S. (2002) Mol. Biol. Cell 13, 915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee D. K., Suh D., Edenberg H. J., Hur M. W. (2002) J. Biol. Chem. 277, 26761–26768 [DOI] [PubMed] [Google Scholar]
- 5.Jeon B. N., Yoo J. Y., Choi W. I., Lee C. E., Yoon H. G., Hur M. W. (2008) J. Biol. Chem. 283, 33199–33210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi W. I., Jeon B. N., Park H., Yoo J. Y., Kim Y. S., Koh D. I., Kim M. H., Kim Y. R., Lee C. E., Kim K. S., Osborne T. F., Hur M. W. (2008) J. Biol. Chem. 283, 29341–29354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda T., Hobbs R. M., Merghoub T., Guernah I., Zelent A., Cordon-Cardo C., Teruya-Feldstein J., Pandolfi P. P. (2005) Nature 433, 278–285 [DOI] [PubMed] [Google Scholar]
- 8.Maeda T., Hobbs R. M., Pandolfi P. P. (2005) Cancer Res. 65, 8575–8578 [DOI] [PubMed] [Google Scholar]
- 9.Apostolopoulou K., Pateras I. S., Evangelou K., Tsantoulis P. K., Liontos M., Kittas C., Tiniakos D. G., Kotsinas A., Cordon-Cardo C., Gorgoulis V. G. (2007) J. Pathol. 213, 294–302 [DOI] [PubMed] [Google Scholar]
- 10.Poliseno L., Pitto L., Simili M., Mariani L., Riccardi L., Ciucci A., Rizzo M., Evangelista M., Mercatanti A., Pandolfi P. P., Rainaldi G. (2008) PLoS One 3, e2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambros V. (2004) Nature 431, 350–355 [DOI] [PubMed] [Google Scholar]
- 12.Kim V. N. (2005) Nat. Rev. Mol. Cell Biol. 6, 376–385 [DOI] [PubMed] [Google Scholar]
- 13.Bushati N., Cohen S. M. (2007) Annu. Rev. Cell Dev. Biol. 23, 175–205 [DOI] [PubMed] [Google Scholar]
- 14.Calin G. A., Croce C. M. (2006) Nat. Rev. Cancer 6, 857–866 [DOI] [PubMed] [Google Scholar]
- 15.Garzon R., Fabbri M., Cimmino A., Calin G. A., Croce C. M. (2006) Trends Mol. Med. 12, 580–587 [DOI] [PubMed] [Google Scholar]
- 16.Yu X., Lin J., Zack D. J., Mendell J. T., Qian J. (2008) Nucleic Acids Res. 36, 6494–6503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsang J., Zhu J., van Oudenaarden A. (2007) Mol. Cell 26, 753–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krainer A. R., Conway G. C., Kozak D. (1990) Genes Dev. 4, 1158–1171 [DOI] [PubMed] [Google Scholar]
- 19.Krainer A. R., Conway G. C., Kozak D. (1990) Cell 62, 35–42 [DOI] [PubMed] [Google Scholar]
- 20.Paronetto M. P., Cappellari M., Busà R., Pedrotti S., Vitali R., Comstock C., Hyslop T., Knudsen K. E., Sette C. (2010) Cancer Res. 70, 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A., Baker S. J., Lee C. M., Reddy E. P. (2003) Mol. Cell Biol. 23, 6631–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge K., DuHadaway J., Du W., Herlyn M., Rodeck U., Prendergast G. C. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 9689–9694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zerbe L. K., Pino I., Pio R., Cosper P. F., Dwyer-Nield L. D., Meyer A. M., Port J. D., Montuenga L. M., Malkinson A. M. (2004) Mol. Carcinog. 41, 187–196 [DOI] [PubMed] [Google Scholar]
- 24.Karni R., de Stanchina E., Lowe S. W., Sinha R., Mu D., Krainer A. R. (2007) Nat. Struct. Mol. Biol. 14, 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paronetto M. P., Achsel T., Massiello A., Chalfant C. E., Sette C. (2007) J. Cell Biol. 176, 929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Z. H., Zhang W. J., Rao Y., Wu J. Y. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 9155–9160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanford J. R., Gray N. K., Beckmann K., Cáceres J. F. (2004) Genes Dev. 18, 755–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemaire R., Prasad J., Kashima T., Gustafson J., Manley J. L., Lafyatis R. (2002) Genes Dev. 16, 594–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y., Steitz J. A. (2005) Mol. Cell 17, 613–615 [DOI] [PubMed] [Google Scholar]
- 30.Li X., Wang J., Manley J. L. (2005) Genes Dev. 19, 2705–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X., Manley J. L. (2005) Cell 122, 365–378 [DOI] [PubMed] [Google Scholar]
- 32.Loomis R. J., Naoe Y., Parker J. B., Savic V., Bozovsky M. R., Macfarlan T., Manley J. L., Chakravarti D. (2009) Mol. Cell 33, 450–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rainer J., Sanchez-Cabo F., Stocker G., Sturn A., Trajanoski Z. (2006) Nucleic Acids Res. 34, W498–W503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaffl M. W. (2001) Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pessler F., Hernandez N. (2003) J. Biol. Chem. 278, 29327–29335 [DOI] [PubMed] [Google Scholar]
- 36.Barna M., Hawe N., Niswander L., Pandolfi P. P. (2000) Nat. Genet. 25, 166–172 [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z., Krainer A. R. (2004) Mol. Cell 16, 597–607 [DOI] [PubMed] [Google Scholar]
- 38.Huang Y., Gattoni R., Stévenin J., Steitz J. A. (2003) Mol. Cell 11, 837–843 [DOI] [PubMed] [Google Scholar]
- 39.Wang J., Takagaki Y., Manley J. L. (1996) Genes Dev. 10, 2588–2599 [DOI] [PubMed] [Google Scholar]
- 40.Tacke R., Boned A., Goridis C. (1992) Nucleic Acids Res. 20, 5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanamura A., Cáceres J. F., Mayeda A., Franza B. R., Jr., Krainer A. R. (1998) Rna 4, 430–444 [PMC free article] [PubMed] [Google Scholar]
- 42.Morlando M., Ballarino M., Gromak N., Pagano F., Bozzoni I., Proudfoot N. J. (2008) Nat. Struct. Mol. Biol. 15, 902–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim V. N., Han J., Siomi M. C. (2009) Nat. Rev. Mol. Cell Biol. 10, 126–139 [DOI] [PubMed] [Google Scholar]
- 44.Zhang H. (2007) J. Cell Physiol. 210, 567–574 [DOI] [PubMed] [Google Scholar]
- 45.Chen J. H., Hales C. N., Ozanne S. E. (2007) Nucleic Acids Res. 35, 7417–7428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitto L., Rizzo M., Simili M., Colligiani D., Evangelista M., Mercatanti A., Mariani L., Cremisi F., Rainaldi G. (2009) Physiol. Genomics 39, 210–218 [DOI] [PubMed] [Google Scholar]
- 47.Zindy F., Eischen C. M., Randle D. H., Kamijo T., Cleveland J. L., Sherr C. J., Roussel M. F. (1998) Genes Dev. 12, 2424–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishnamurthy J., Torrice C., Ramsey M. R., Kovalev G. I., Al-Regaiey K., Su L., Sharpless N. E. (2004) J. Clin. Invest. 114, 1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brosh R., Shalgi R., Liran A., Landan G., Korotayev K., Nguyen G. H., Enerly E., Johnsen H., Buganim Y., Solomon H., Goldstein I., Madar S., Goldfinger N., Børresen-Dale A. L., Ginsberg D., Harris C. C., Pilpel Y., Oren M., Rotter V. (2008) Mol. Syst. Biol. 4, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang T. C., Wentzel E. A., Kent O. A., Ramachandran K., Mullendore M., Lee K. H., Feldmann G., Yamakuchi M., Ferlito M., Lowenstein C. J., Arking D. E., Beer M. A., Maitra A., Mendell J. T. (2007) Mol. Cell 26, 745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shalgi R., Lieber D., Oren M., Pilpel Y. (2007) PLoS Comput. Biol. 3, e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dimri G. P., Hara E., Campisi J. (1994) J. Biol. Chem. 269, 16180–16186 [PubMed] [Google Scholar]
- 53.Manley J. L., Tacke R. (1996) Genes Dev. 10, 1569–1579 [DOI] [PubMed] [Google Scholar]
- 54.Fu X. D. (1993) Nature 365, 82–85 [DOI] [PubMed] [Google Scholar]
- 55.Graveley B. R. (2000) Rna 6, 1197–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karni R., Hippo Y., Lowe S. W., Krainer A. R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15323–15327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michlewski G., Sanford J. R., Cáceres J. F. (2008) Mol. Cell 30, 179–189 [DOI] [PubMed] [Google Scholar]
- 58.Bagga S., Bracht J., Hunter S., Massirer K., Holtz J., Eachus R., Pasquinelli A. E. (2005) Cell 122, 553–563 [DOI] [PubMed] [Google Scholar]
- 59.Maeda T., Ito K., Merghoub T., Poliseno L., Hobbs R. M., Wang G., Dong L., Maeda M., Dore L. C., Zelent A., Luzzatto L., Teruya-Feldstein J., Weiss M. J., Pandolfi P. P. (2009) Dev. Cell 17, 527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.