Abstract
In normal adult retinas, NGF receptor TrkA is expressed in retinal ganglion cells (RGC), whereas glia express p75NTR. During retinal injury, endogenous NGF, TrkA, and p75NTR are up-regulated. Paradoxically, neither endogenous NGF nor exogenous administration of wild type NGF can protect degenerating RGCs, even when administered at high frequency. Here we elucidate the relative contribution of NGF and each of its receptors to RGC degeneration in vivo. During retinal degeneration due to glaucoma or optic nerve transection, treatment with a mutant NGF that only activates TrkA, or with a biological response modifier that prevents endogenous NGF and pro-NGF from binding to p75NTR affords significant neuroprotection. Treatment of normal eyes with an NGF mutant-selective p75NTR agonist causes progressive RGC death, and in injured eyes it accelerates RGC death. The mechanism of p75NTR action during retinal degeneration due to glaucoma is paracrine, by increasing production of neurotoxic proteins TNF-α and α2-macroglobulin. Antagonists of p75NTR inhibit TNF-α and α2-macroglobulin up-regulation during disease, and afford neuroprotection. These data reveal a balance of neuroprotective and neurotoxic mechanisms in normal and diseased retinas, and validate each neurotrophin receptor as a pharmacological target for neuroprotection.
Keywords: Axon, Drug Action, Neurodegeneration, Neurotrophic Factor, Receptors, Retina, RGC, Axotomy, Glaucoma, Optic Nerve
Introduction
Neuropathic diseases of the retina that involve the death of retinal ganglion cells (RGCs)4 are irreversible. This is because RGCs are neurons whose fibers and axons make up the optic nerve (ON) and relay visual input from the retina to the cerebral cortex.
Commonly used animal models of neuropathy that cause RGC death include ON axotomy and glaucoma. ON axotomy is an acute model of trauma where the optic nerve is completely severed, causing rapid death of the RGCs (∼90% within 2 weeks). Glaucoma is a chronic and progressive optic nerve neuropathy often concomitant with elevated intraocular pressure (IOP) (1). The etiology of RGC death in glaucoma remains unknown.
One mechanism is the deprivation of survival signals that neurotrophins provide by acting through the TrkA and TrkB receptors expressed in RGCs (2, 3). Indeed, activation of TrkA (4) or TrkB (5) directly activate pro-survival signals during glaucoma and rescues RGCs from death during ON axotomy or glaucoma. However, it seems paradoxical that whereas TrkA activity is protective, neither endogenous nerve growth factor (NGF) (up-regulated in glaucoma (6)) nor exogenous NGF applied as a drug afford effective RGC neuroprotection during ON axotomy or glaucoma (4, 7).
A second mechanism of RGC death in glaucoma is the increased production of tumor necrosis factor-α (TNF-α) (8–10) and α2-macroglobulin (α2M) (11). These neurotoxic factors are produced by activated microglia (12), which express the neurotrophin receptor p75NTR (7). Indeed, the p75NTR receptor has been implicated in the acute release of TNF-α during acute toxicity leading to RGC death within a few hours after intravitreal injection of glutamate (13) or after activation by pro-NGF (14). However, in the normal retina p75NTR and many of its ligands are present (the mature and the precursor neurotrophins). Thus it is not clear what homeostatic balance keeps the normal adult retina from degenerating. In addition, the actual role of p75NTR in retinal diseased states is not known.
The retina offers the peculiar advantage that whereas p75 is found almost exclusively on glia/Muller cells, TrkA receptors are almost exclusively expressed in RGCs (7). This lends to a situation where a ligand such as NGF can bind to each receptor in each cell population, triggering signals that can be beneficial or deleterious. This is especially important to understand during disease states in which neurotrophic approaches have been attempted to prevent neuronal death.
Here, the roles of p75NTR and TrkA in normal adult retina and in retinal disease states are studied using models of chronic (glaucoma) or acute (optic nerve axotomy) neurodegeneration. We also studied the receptor mechanisms that regulate homeostatic balance of baseline versus pathological levels of the neuroprotective and neurotoxic proteins NGF, TNF-α, and α2M.
The data indicate that ligand activation of p75NTR in glia activates neurotoxic pathways that negate the protective effect of TrkA activation in RGCs, whereas ligand activation of TrkA negates the neurotoxic action of p75NTR. The mechanism of p75NTR neurotoxicity in glaucoma is through the chronic up-regulation of TNF-α and α2M neurotoxic proteins. These data reveal a balance of protective and deleterious neurotrophic effects in normal and diseased retinas, and validate each neurotrophin receptor as a pharmacological target.
MATERIALS AND METHODS
All animal procedures adhered to the IACUC guidelines for use of animals in research, and to protocols approved by McGill University Animal Welfare Committees.
Animals
Wistar rats (female, 250–300 g; Charles River) were kept in a 12-h dark-light cycle with food and water ad libitum. All animal manipulations were performed between 9 a.m. and 12 p.m. Deep anesthesia was used during cauterization (induction of glaucoma), ON transection, fluorogold labeling, intraocular injection procedures, optical coherence tomography measurements, and euthanasia (ketamine, xylazine, and acepromazine injected intraperitoneally: 50/5/1 mg/kg). For measuring IOP, light anesthesia was used (a gas mixture of oxygen, 2% isofluorane mixture, at a rate of 2.5 liter/min).
Glaucoma Model and Intraocular Pressure
The episcleral vein cauterization model of rat (15, 16) is validated in comparative studies (17, 18). We have described the methods previously (4, 6, 11). IOP was measured using a Tonopen XL applanation tonometer (19, 20) immediately after episcleral vein cauterization surgery and every week until the end point of each experiment. In the glaucoma model, ∼1.7-fold elevated IOP was maintained for as long as 4 months (data not shown).
Optic Nerve Transection
We have described the ON axotomy model in the rat (5). The ON was exposed and was completely transected 0.5–1.0 mm posterior to the eyeball with the use of microtweezers, sparing vessels. Normal blood circulation in the retina was ascertained.
Intravitreal Injections
The experimental eyes were injected with test or control vehicle, whereas the contralateral eyes served as naive normal controls (4, 6, 11).
Fluorogold Retrograde Labeling
RGCs were retrogradely labeled with a 4% Fluorogold solution (Fluorochrome, Englewood, CO) applied bilaterally to the superior culliculous (4, 11), with minor modifications as described (5). In ON, axotomy retrograde labeling was performed 7 days before surgical transection. In glaucoma, retrograde labeling was performed at day 35 after ocular hypertension (e.g. 7 days before the experimental end point). The RGCs were labeled throughout the whole retina, both in glaucoma and normal eyes. The RGC numbers reported are consistent with those reported by other groups (7).
Quantification of Retinal Structures Using Fourier Domain Optical Coherence Tomography (FD-OCT)
FD-OCT is a non-invasive method that allows time kinetic studies in the same animal, acquisition of retinal images with axial tissue resolution nominally better than 4 μm, and repeatability of the measurements from B-scans better than 1 μm. Details of the method have been published (5). During retinal scanning, 3 volumes were acquired in different sectors of the retina containing the ON head and retinal blood vessels as landmarks. In post processing, six B-scans were randomly selected from each volume. The retinal thickness measurements were performed with ImageJ software. In each B-scan the thickness of the nerve fiber layer-ganglion cell layer-inner plexiform layer (NGI) was measured at four adjacent points at a distance 1.5 mm from the ON head.
Drug Treatments
Drug treatments were done with the experimenters blinded to treatment code. In each rat we used one experimental eye (right eye, OD) with or without treatment, and the naive contralateral eyes (left eye, OS) were always normal and untreated and were standardized to 100% RGCs.
Nerve Growth Factor and NGF Mutants
All NGF forms are recombinant. Wild type NGF (binds TrkA and p75NTR) serves as an internal control. NGF-KKE mutant (herein termed NGF-C) is a selective TrkA agonist (21, 22). The NGF-Delta 9/13 mutant (herein termed NGF-A) is a selective p75NTR agonist (23).
Anti-NGF Antibody
Anti-NGF monoclonal antibody, NGF30 mAb, binds to NGF (Kd 600 nm, measured in Biacore studies, data not shown) and blocks NGF-p75NTR binding without affecting NGF-TrkA binding and activation (24). Here, we also show that NGF30 mAb blocks pro-NGF binding to p75NTR, as expected because binding epitope is also present in pro-NGF.
p75NTR Small Molecule Antagonists
LM-24 was reported (25). Synthesis of THX-B (1,3-diisopropyl-1-[2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-purin-7-yl)-acetyl]-urea) was by coupling theophylline-7-acetic acid to N,N′-diisopropyl carbodiimide in dimethyl formamide for 4 h at 60 °C. After cooling the solvent was evaporated in vacuum. The residue was dissolved in a minimal amount of chloroform and purified by flash chromatography on Silica Gel 60A with chloroform:methanol (98/2) as eluant. The fractions with the product were pooled and evaporated, yielding 44% of the compound as a white powder; Rf = 0.68 (chloroform:methanol (9/1), Rf = 0.23 (ethyl acetate). The empirical formula (F.W. 365) was confirmed by LC/MS. The main molecular peak was found at 365 with a second peak at 387 for the sodium salt (m.p. = 117–20 °C). The 1H NMR spectra (300 MHz) (data not shown) are consistent with the expected structure. The design, synthesis, and properties of THX-B analogs will be described elsewhere.
Drug Regimen in Vivo
In all cases, the vehicle and drugs were coded and the experimenters were masked to the treatment (double-blinded studies). Codes were broken upon deposit of a summary of the data. All intravitreal injections delivered were 3 μl. For proteins, 1.0 μg was injected (wild type NGF stock (12 μm), NGF mutants stock (12 μm), mAb NGF30 stock (2 μm), and mouse IgG control stock (2 μm)). For small molecules a total dose of 3.0 μg was injected (LM-24 (3.0 mm stock), THX-B (3.0 mm stock)). PBS was the vehicle control.
For studies in the glaucoma animal model the injections were performed at days 14 and 21 after cauterization. The end point was at day 42 of high IOP. Thus, in this paradigm there is pre-existing damage for 14 days before treatment (measured at ∼8% RGC death (4, 6, 11)); and the treatments require long lived efficacy in the constant presence of retinal stress due to high IOP.
For studies in the ON axotomy animal model the injections were performed immediately after ON transection (whereas the rat was still under general anesthesia). For testing a higher frequency of wild type NGF, this reagent was administered immediately after ON transection and also at 7 days after optic nerve transection (2× treatment). The end point was 7 or 14 days after ON sectioning, as indicated.
Anti-NGF Antibody Binding to Pro-NGF
Anti-NGF monoclonal antibody NGF30 was tested in ELISA as described (24). In brief, in 96-well plates 50 ng/well of mouse NGF, mouse pro-NGF (Alomone Labs, Israel), or control human NGF were immobilized. Human NGF is a control because NGF30 mAb does not bind to it. After blocking with binding buffer (phosphate-buffered saline containing 0.5% bovine serum albumin), anti-NGF mAb NGF30 or control IgG (50 nm) were added as primary reagents. After further washings and blocking, horseradish peroxidase (HRP)-conjugated goat anti-rat Ab (Sigma) was added as a secondary reagent and then the wells were washed 3 times in binding buffer and once in PBS. Peroxidase activity was determined colorimetrically using the TMB One Solution (Promega) substrate. Assays were repeated 3 times, with replicates of 4 wells in each assay.
Quantification of Selective NGF-p75NTR Antagonism by Competitive ELISA
Competitive ELISAs are published (24) and are as described above for ELISAs of NGF30 mAb. Murine NGF (50 ng) was immobilized onto polystyrene 96-well microtest plates. Primary reagents were added in binding buffer: recombinant chimeric p75-Fc or recombinant chimeric TrkA-Fc (R&D), or anti-NGF mAb NGF30 (24); each at 50 nm. The competitors THX-B or LM24 were added at the indicated concentrations for 45 min. After washing, HRP-conjugated secondary reagents (Sigma) were added for 30 min (goat anti-human Fc for p75-Fc and TrkA-Fc; and goat anti-rat Ab for NGF30 mAb). Assays were repeated >4 times, with replicates of 4 wells for each assay. Wells that received vehicle, inactive compounds, or no competitor served as controls and were standardized as 100% binding. Wells with no NGF immobilized but with all other reagents added were treated as background (<15% of maximal signals) and were standardized to 0% binding.
Quantification of RGCs Survival
Quantification of labeled RGCs was performed as reported previously (4, 7, 11). At the experimental end point, both eyes were enucleated and retinas were flat-mounted on a glass slide with the vitreous side up. Pictures for each retina were taken using a fluorescence microscope (Carl Zeiss Meditec, Jena, Germany), with 12 pictures/retina at ×20 magnification. For each quadrant there were 3 pictures (at a radial distance of 1, 2, and 3 mm from the optic nerve). Microglia and macrophages were excluded according to their morphology as previously reported (26). Fluorogold retrograde labeling measures RGC retrograde transport. Because transport deficits precede glaucomatous RGC death (20, 27), quantification of labeled RGCs is a valid measure of RGC death.
In all cases, manual RGC counting was performed by two independent persons masked to the protocol. Also, automated quantitative counting were done with “Metamorph” software (Molecular Devices) using the module “Count Nuclei” to identify cells as unique objects (28). The selected parameters for retinal ganglion cells were 8–15 μm for the width range. Both manual and automated counts were generally in accordance, and deviations among them were <5% per picture. Comparisons of the results from manual and automated counting methods showed insignificant deviations, and therefore results from the three counts (2 manual, 1 automated) were averaged.
Statistical Analysis of RGC Survival
In each rat standardization of the % RGC survival was calculated as the ratio of the experimental eye versus contralateral normal control eye (RGCexperimental/RGCcontralateral × 100%; OD/OS). The % RGCs survival for each experimental group (untreated, PBS, NGF-wt, NGF mutants) were averaged ± S.E.; the number (n) of ratios are indicated in each graph and legend. For IOP data, FD-OCT data, and RGCs counting data, the mean ± S.E. are shown. After quantification of Western blot data, the mean ± S.D. are shown. Data analysis was performed using GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA). Comparison between the RGCs survival rate used one-way analysis of variance with Dunnett's multiple comparison test. For IOP and FD-OCT results, the data were analyzed by repeated measures of analysis of variance with SPSS 13.0 software followed by post hoc tests (Tukey HSD) for comparisons among the groups.
Quantification of TNF-α and α2-Macroglobulin Expression
Both retinas of each animal were detergent solubilized, and studied in independent gels standardized to loading control. The ratio of the glaucoma eye ± treatment versus the normal contralateral eye was calculated, and results were averaged ± S.E., n = 3 animals per group. In glaucoma, all drug treatments were done at day 14 of hypertension. In one group the retinas were harvested at day 17 of glaucoma (3 days after intraocular injection) and in the other group the retinas were harvested at day 19 of glaucoma (5 days after intraocular injection). Normal eyes treated with NGF-A were collected in the indicated days. After SDS-PAGE and Western transfer, membranes were immunoblotted with rabbit polyclonal antibodies against TNF-α (Preprotech) or α2M (Santa Cruz) at a 1:3000 dilution. Goat anti-rabbit secondary antibodies conjugated to horseradish peroxidase (Sigma) were used at a 1:10,000 dilution. Loading was controlled with antibodies to β-actin (Sigma). For digital quantification, membranes were scanned and analyzed using ImageJ software.
Bioassays ex Vivo: Pro-NTF Killing Assay
Cell lines B104 (expressing ∼50,000 p75NTR/cell) and nnr5 (expressing ∼150,000 p75NTR/cell) do not express detectable TrkA, and do not respond to NGF (29, 30). Cells (5,000 cells/well) were cultured in 96-well plates (Falcon, Lincoln Park, NJ) in normal culture medium (RPMI1640, Hepes, glutamine, 5% serum). Wells were supplemented with NGF, BDNF, pro-NGF, or pro-BDNF (Alomone Labs) (50 nm final) in the presence or absence of THX-B (20 μm final). Wells containing all culture conditions but no cells were used as blanks. The growth/survival profile of the cells was quantified using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma) 72 h after plating, by reading optical density at 595 nm with the blanks subtracted. In our experience, only ∼50% of these assays result in significant cell death, likely because of the instability of the pro-neurotrophins in solution (31). Assays where pro-neurotrophins did not cause cell death are not reported, because there is no death to antagonize.
RESULTS
Two in vivo models of RGC degeneration were used. Ocular hypertension is a model of glaucoma that causes chronic and progressive RGC death. ON axotomy is a model of traumatic injury that causes acute and rapid RGC death. The animal models used have been reported previously (4, 7, 11).
Wild Type NGF Fails to Protect RGCs
Pharmacological treatment with exogenous wild type NGF immediately after ON axotomy has been reported to not alter the rate of RGCs death quantified after 7 or 14 days (7). We replicated those results (data not shown). However, to exclude potential pharmacokinetic issues affecting NGF efficacy, we also tested a higher frequency of treatment.
Wild type NGF was applied twice, once immediately after axotomy and a second time 7 days post-axotomy. Quantification of RGCs was done at day 14 post-axotomy. This paradigm revealed a marginal and statistically not significant improvement with 16% RGCs surviving versus 10% RGCs in untreated axotomy (Fig. 1A). This result further supports previous reports that wild type NGF does not significantly alter the pattern of RGC death in ON axotomy.
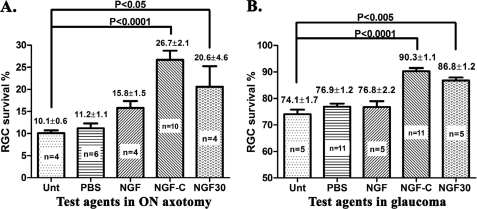
FIGURE 1.
Endogenous NGF-TrkA interactions are protective for RGCs during retinal degeneration. Quantification of surviving RGCs: A, at 14 days post-ON axotomy; B, at 42 days of chronic glaucoma. The density of RGCs in the untreated contralateral normal eye is 100%. In ON axotomy intravitreal injections of the indicated agents were done once immediately after ON axotomy, except for NGF, which was dosed immediately and at day 7 post-axotomy (2 times treatment). In glaucoma intravitreal injections were done at days 14 and 21 of glaucoma, with constant high IOP measured throughout the test.
Treatment with wild type NGF at days 14 and 21 of glaucoma results in 77% RGCs surviving at the 42-day end point. This is not statistically different from untreated glaucoma or PBS-treated glaucoma control groups that, respectively, have 74 and 77% labeled RGCs after 42 days. Both the untreated glaucoma eyes and NGF-treated glaucoma eyes experienced a significant RGC loss versus normal contralateral eyes (p ≤ 0.0001) (Fig. 1B). Similar data have been reported elsewhere (4). These data confirm previous reports that intravitreal delivery of wild type NGF is not effective in either model of RGC degeneration.
A TrkA-selective NGF Mutant Provides Neuroprotection ON Axotomy and in Glaucoma
In ON axotomy treatment with a mutant NGF selective agonist of TrkA, but which does not bind to p75 (termed NGF-C), affords neuroprotection. A one time treatment with NGF-C was significantly neuroprotective, with 27% RGCs at day 14 post-axotomy (p ≤ 0.0001 versus untreated or PBS-treated axotomy control groups that have 11% RGCs) (Fig. 1A). These are consistent with previous reports using other selective TrkA agonists (4, 7).
In glaucoma, treatment with NGF-C at days 14 and 21 of disease results in 90% of the RGCs surviving at the day 42 end point (p ≤ 0.0001 versus untreated glaucoma or PBS-treated glaucoma control groups that have 74 and 77% surviving RGCs respectively) (Fig. 1B). These data demonstrate that, whereas wild type NGF is not effective, an NGF mutant selective agonist of TrkA affords significant neuroprotection in both models of RGC degeneration.
Restricting the Interactions of Endogenous NGF and Pro-NGF with p75NTR as a Strategy for Neuroprotection during Glaucoma and ON Axotomy
Expression of endogenous (wild type) NGF is up-regulated during injury. Paradoxically, this protein appears to be ineffective in protecting neurons, perhaps because pro-NGF is also up-regulated. To study the potential role of endogenous NGF and pro-NGF, we targeted these proteins with a biological response modifier.
An anti-NGF monoclonal antibody termed mAb NGF30 specifically blocks the ability of NGF to functionally bind to p75NTR, but leaves NGF-TrkA binding and function intact (24). Here, we also show that NGF30 mAb binds to pro-NGF in a manner that is indistinguishable from NGF (Table 1). This is expected because the epitope is present in both proteins.
TABLE 1.
mAb NGF30 binds to pro-NGF and to NGF
ELISA binding studies demonstrate direct binding to immobilized NGF or pro-NGF, and no binding to BDNF or pro-BDNF relative to control irrelevant IgG. Data are from colorimetric readouts with blanks subtracted, O.D. ± S.E. of 3 independent assays, each assay in quadruplicate.
| Immobilized protein | OD ± S.E. (× 103) |
|
|---|---|---|
| NGF30 | IgG | |
| BSA | 30 ± 8 | 30 ± 11 |
| NGF | 373 ± 39a | 35 ± 9 |
| Pro-NGF | 406 ± 24a | 34 ± 8 |
| BDNF | 38 ± 12 | 30 ± 8 |
| Pro-BDNF | 21 ± 3 | 24 ± 7 |
a p < 0.01 versus negative controls.
Treatment with mAb NGF30 immediately after axotomy affords 21% RGC survival at the 14-day post-axotomy end point (p ≤ 0.05 versus untreated axotomy groups) (Fig. 1A). Treatment of glaucomatous eyes with mAb NGF30 at days 14 and 21 affords 87% RGC survival at the day 42 glaucoma end point (p ≤ 0.005 versus untreated glaucoma or PBS-treated glaucoma groups) (Fig. 1B). As control, no neuroprotection is seen after treatment with an irrelevant IgG antibody (data not shown).
Together, these data indicate that in the injured retina there are adequate levels of endogenous mature NGF, which are bioavailable to protect neurons through TrkA; and that preventing NGF and pro-NGF from interacting with p75NTR is sufficient to achieve neuroprotection. This is remarkable given that other ligands of p75NTR are present in retina (e.g. pro-BDNF, mature BDNF, etc.) (32–34).
An NGF Mutant Selective p75NTR Agonist Is Degenerative to RGCs
The data above suggested that the failure of wild type NGF to protect injured retinas may be due to NGF or pro-NGF activation of p75NTR. This would be true for NGF that is endogenously produced or for NGF exogenously applied as a therapeutic. To study the role of ligand-dependent activity of p75NTR in RGC death, we used a mutant NGF, termed NGF-A, that is a selective p75NTR agonist but does not activate TrkA (23).
In normal eyes a single intravitreal injection of NGF-A causes progressive and significant RGC death: 15% RGCs are lost after 21 days, and 22% RGCs are lost after 28 days compared with the untreated normal contralateral eyes (p ≤ 0.0001) (Fig. 2A). These data demonstrate that in the normal adult retina, exacerbated p75NTR activity causes RGC death.
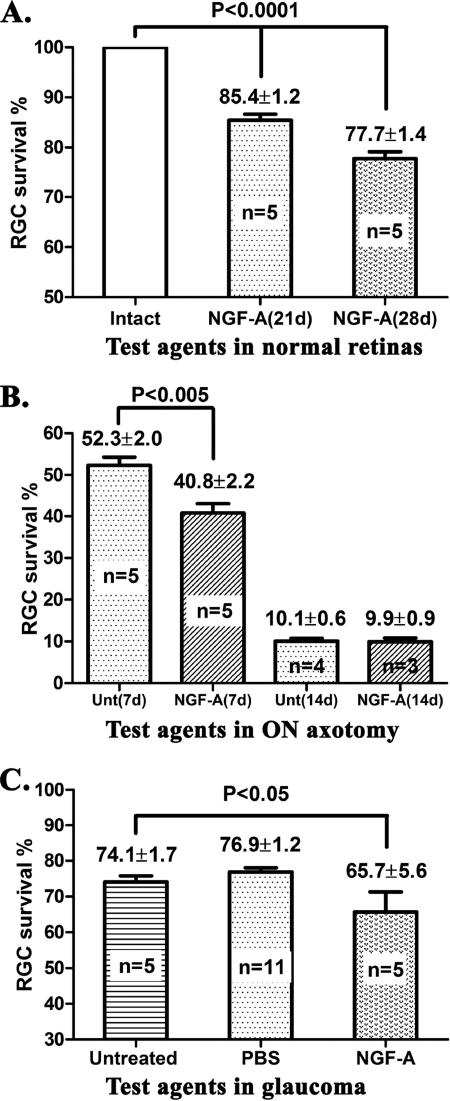
FIGURE 2.
NGF-p75 interactions are deleterious to RGC survival in normal eyes and during retinal degeneration. Quantification of surviving RGCs: A, normal eyes at 21 or 28 days post-treatment; B, at 7 or 14 days post-ON axotomy; C, at 42 days of chronic glaucoma. Intravitreal injections of the indicated agents were done immediately after ON axotomy, and at days 14 and 21 of glaucoma. The density of RGCs in the untreated contralateral normal eye is 100%.
In ON axotomy, a single intravitreal injection of NGF-A accelerated RGC death. At the day 7 post-axotomy end point, 41% of RGCs survive in the NGF-A-treated group, whereas the untreated axotomy group has 52% RGC survival (Fig. 2B). This is a significant increase in RGC death (p ≤ 0.005) in this acute model of neurodegeneration. In ON axotomy, the deleterious effects of NGF-A can only be measured after 1 week, because at 2 weeks RGC death is already nearly maximal in all groups (Fig. 2B).
In glaucoma, a single intravitreal injection of NGF-A at day 14 accelerated RGC death, and only 66% RGCs survive at the day 42 end point (p ≤ 0.05 versus untreated glaucoma or PBS-treated glaucoma groups) (Fig. 2C). Hence, exacerbated ligand-dependent p75NTR activity can accelerate RGC death in a chronic model of neurodegeneration.
Together, data from Figs. 1 and 2 suggest that ligands able to activate TrkA (NGF-C mutant, or NGF30-NGF complexes) are protective even in the presence of pro-apoptotic p75NTR ligands such as pro-BDNF and BDNF. In contrast, ligands able to activate p75NTR (NGF-A or endogenous pro-NTFs) are deleterious to RGCs even in the presence of TrkA ligands such as endogenous NGF.
Antagonists of p75NTR
To further study p75NTR as a pharmacological target, we tested antagonists of this receptor in ON axotomy and glaucoma. Previous studies have shown that the peptidic p75NTR antagonist protected RGCs in ON axotomy (7) but the same p75NTR antagonist did not protect RGCs in a chronic model of glaucoma (4). Therefore, we tested less peptidic and more stable p75NTR antagonists.
The p75NTR antagonist THX-B was developed in our laboratory, and the p75NTR antagonist LM-24 was published by others (25). The chemical structures of THX-B and LM-24 are shown in Fig. 3A.
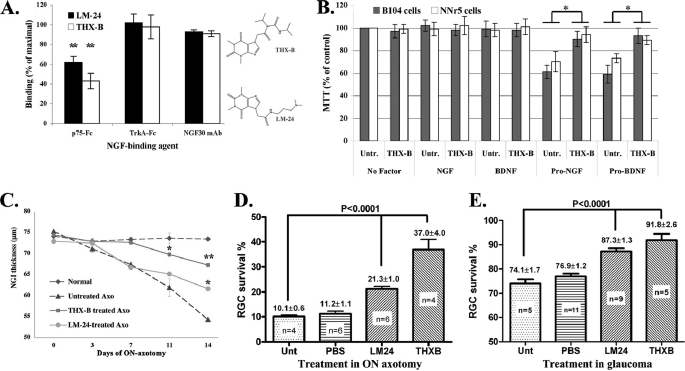
FIGURE 3.
THX-B and LM-24 inhibit NGF-p75 binding selectively, and during degeneration they protect retinal structure and delay RGC death. A, wild type NGF was immobilized on ELISA plates and binding of the indicated primary reagents (p75-Fc (n = 5), TrkA-Fc (n = 4), or anti-NGF mAb NGF30 (n = 3) were tested at 50 nm final concentrations. Binding was revealed with HRP-labeled anti-Fc secondary reagents. Untreated wells are standardized to 100%. LM24 or THX-B (each at 30 μm) inhibit the binding of NGF·p75 selectively, and do not inhibit NGF·TrkA or NGF·NGF30 binding. Data are shown as % inhibition ± S.E., of the indicated number (n) of assays, each assay in triplicate; **, p < 0.01. Shown are the chemical structures of THX-B and LM-24. B, B104 (gray bars) and nnr5 cells (white bars) were cultured in serum-containing media, supplemented with the indicated growth factor (50 nm) ± THX-B (20 μm). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide data are standardized to untreated control ± S.D., n = 4 replicates. *, p < 0.05. Similar data were obtained in two independent experiments where pro-NTFs had a deleterious effect. C, the thickness of the RGC and nerve fiber layer (containing RGC soma, fibers and axons) was measured by FD-OCT, as described (5). Each time point is the average of 3 individual rats measured over time ± S.E., in each rat the left eye is the normal untreated control. *, p < 0.05; **, p < 0.01. D and E, the p75 antagonists THX-B and LM24 significantly prevent the death of RGCs in ON axotomy (end point day 14) and glaucoma (end point day 42). Data are % RGC survival ± S.E., of the indicated (n) number of rats. The RGC density in the untreated contralateral normal eye is 100%.
Inhibition of NGF-p75 binding by THX-B and LM-24 was tested in competitive ELISA, and selectivity was evaluated by lack of effect upon NGF-TrkA or NGF-NGF30 interactions (Fig. 3A). THX-B and LM-24 antagonized NGF-p75NTR interactions selectively, and did not interfere with TrkA or mAb NGF30 binding to NGF (Fig. 3A).
The use of mAb NGF30 is an ideal control, because the mAb is known to recognize a p75NTR binding domain of NGF. These data suggest that the compounds bind to p75, and prevent the ability of p75 to bind to NGF.
Similar data were obtained in ELISAs where pro-NGF was immobilized (data not shown). As further evidence, the effect of THX-B on the pro-apoptotic function of pro-NGF or pro-BDNF was tested in functional 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays measuring cell metabolism (Fig. 3B). Pro-NGF and pro-BDNF significantly (p < 0.05) reduce the viability of B104 (∼50,000 p75NTR per cell) or nnr5 cells (∼200,000 p75NTR per cell). Addition of THX-B in these conditions prevents the loss of cell viability, indicating antagonism of pro-NTF-p75NTR.
Antagonists of p75NTR Prevent Loss of Retinal Structure after ON Axotomy
The relative in vivo efficacy for THX-B and LM-24 was tested using the ON axotomy model. A quantitative non-invasive structural end point, FD-OCT, was performed in the same animal over time to measure the integrity of the neuronal layers. We quantified the axons and fibers of the RGCs that contribute to the thickness of the nerve fiber layer, the ganglion cell layer, and the inner plexiform layer, herein termed NGI. Maintenance of the NGI structure correlates with RGC health (5).
In vivo treatment with THX-B and LM-24 (3-μg dose immediately after ON axotomy) delayed the loss of retinal structure. THX-B provides significant preservation of the NGI thickness at days 11 and 14, p < 0.01 compared versus untreated. LM-24 provides limited preservation of NGI thickness and only at day 14, p < 0.05 compared versus untreated (Fig. 3C).
Antagonists of p75NTR Prevent RGC Degeneration in Axotomy and Glaucoma
Quantification of surviving RGC (fluorogold labeled) after 14 days of ON axotomy shows that treatment with THX-B results in 37% RGC survival, and treatment with LM-24 results in 21% RGC survival. This difference is significant, p < 0.0001, compared versus the control axotomy group that has 10% RGC survival after 14 days (Fig. 3D).
In glaucoma, treatment with THX-B or LM-24 was done at days 14 and 21 of glaucoma, and quantification of the surviving RGC was at day 42 of glaucoma. Experimental eyes experienced constant high IOP throughout (data not shown). THX-B treatment results in 92% RGC survival, and LM24 treatment results in 87% RGC survival. This difference is significant; p < 0.0001, compared versus the control glaucoma groups where 74–77% RGCs survive (Fig. 3E).
THX-B has significantly better efficacy than LM24 in protecting NGI thickness, most significantly early after injury (p < 0.05 comparing THX-B and LM-24 at day 11 of ON axotomy) (Fig. 3C). Moreover, THX-B has significantly better efficacy than LM24 in rescuing RGCs from death after ON axotomy (p < 0.05 comparing THX-B and LM-24 at the end point) (Fig. 3D). On the other hand, it is noteworthy that in vitro both compounds inhibit NGF-p75NTR and pro-NGF-p75NTR interactions to a similar degree (Fig. 3A).
Paracrine Mechanism of RGC Death Up-regulated by p75NTR Agonists
In retina p75NTR is expressed in glia, and these cells are known to secrete neurotoxic factors such as TNF-α and α2M. Thus, p75NTR-mediated effects upon RGCs appear to be non-cell autologous. This hypothesis was tested.
Injection of a selective p75NTR agonist NGF-A in normal eyes induced the up-regulation of TNF-α and α2M, compared versus normal uninjected contralateral eyes (Fig. 4, A and B). At 3 or 5 days post-injection of NGF-A, TNF-α and α2M increased progressively to levels comparable with those seen after 19 days of glaucoma, where TNF-α is up-regulated ∼3.1-fold (p ≤ 0.0001 versus normal retina), and α2M is up-regulated ∼2.9-fold (p ≤ 0.0001 versus normal retina).
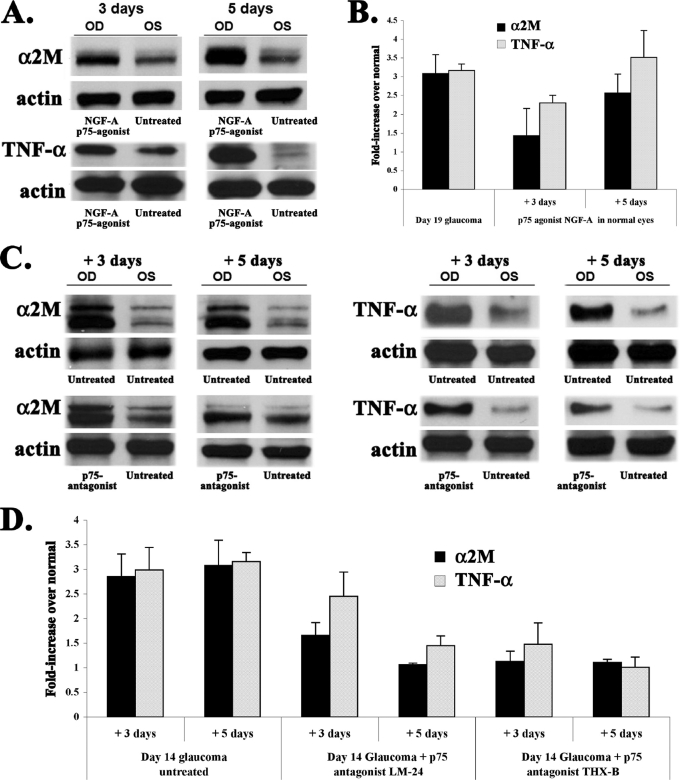
FIGURE 4.
Production of neurotoxic proteins α2M and TNF-α are exacerbated by a p75 agonist and inhibited by p75 antagonists. A, normal eyes were untreated (OS, left eye control) or injected intravitreally with p75 agonist NGF-A (OD, right eye). Three or 5 days after injection, retinal levels of α2M and TNF-α were quantified by standardized Western blotting versus actin loading control. Representative data from three assays using retinas processed independently are presented. B, quantification of the fold-change in α2M and TNF-α, compared versus control normal retinas. Each time point is the average from 3 independent assays ± S.E. The NGF-A p75 agonist causes time-dependent increases similar to those seen in glaucoma (shown here as a reference). C, day 14 glaucomatous eyes (OD, right eyes) were untreated or injected intravitreally with p75 antagonist THX-B. Naive contralateral eyes (OS, left eyes) were untreated controls. Three or 5 days after injection (e.g. glaucoma days 17 or 19) detergent extracts from the retinas were studied by Western blotting for α2M and TNF-α, standardized versus actin loading control. Representative data from three assays using retinas processed independently. D, quantification of the fold-change in α2M and TNF-α, compared versus control normal retinas. Each time point is the average from 3 independent assays ± S.E. p75 antagonists LM-24 and THX-B cause a time-dependent inhibition of α2M and TNF-α that are up-regulated during glaucoma.
A Paracrine Neurotoxic Mechanism of RGC Death in Glaucoma Is Inhibited by p75NTR Antagonists
p75NTR antagonists were injected intravitreally after 14 days of glaucoma, and the levels of TNF-α and α2M were quantified 3 (e.g. day 17 of glaucoma) or 5 days later (e.g. day 19 of glaucoma) (Fig. 4C).
The levels of both neurotoxic factors are reduced 3 days after injection of the p75NTR antagonists, and are nearly normalized 5 days after injection of p75NTR antagonists (quantification in Fig. 4D). This is remarkable given that the glaucomatous eyes endured constant high IOP (measured IOP data not shown).
These results demonstrate that in normal eyes, ligand-dependent activation of p75NTR induces the elevation of neurotoxic factors that cause RGC death. Moreover, they demonstrate that in glaucoma RGC death is caused by the p75NTR-dependent up-regulation of TNF-α and α2M production.
This increase in neurotoxins is fully reversible by p75 antagonists. The endogenous ligand that activates p75NTR-dependent events is competed by LM24 and THX-B, and is likely to at least include NGF.
DISCUSSION
We studied the function of the TrkA and p75NTR receptors and endogenous NGF in normal retinas and retinas stressed chronically (glaucoma) or acutely (ON axotomy). We used pharmacological probes that target both receptors (wild type NGF, no effect), a selective agonist of TrkA (NGF mutant NGF-C, neuroprotective), a selective agonist of p75NTR (NGF mutant NGF-A, neurodegenerative), a biological response modifier that blocks endogenous NGF and pro-NGF from binding to p75NTR (anti-NGF mAb NGF30, neuroprotective), and small molecule antagonists of p75NTR (THX-B and LM24, neuroprotective).
In diseased states, expression of endogenous NGF is up-regulated, but this is not sufficient to protect RGCs. The use of exogenous wild type NGF did not protect RGCs in diseased states either, even with repeated dosing. Why is endogenous and exogenous NGF incapable of supporting RGC survival in diseased states?
We postulated that NGF (and/or pro-NGF) binding to up-regulated p75NTR in glia may be deleterious to neurons through up-regulation of two proteins that are known to be neurotoxic: TNF-α and α2M. Although TNF-α kills neurons directly via TNF-α receptors expressed in RGCs (9), the neurotoxic mechanism of α2M is multifactorial (11) and includes regulation of growth factor activity or stability, binding to LRP-1 receptors, and other activities.
The action of p75NTR is ligand-responsive. The p75NTR selective ligand NGF-A induces RGC death in the normal retina; and accelerates RGC death in diseased eyes. Furthermore, a biological response modifier that blocks endogenous NGF and pro-NGF (mAb NGF30) affords protection to the RGCs during disease.
These data demonstrate that endogenous NGF is bioavailable and capable of neuroprotection, provided its engagement to p75NTR is blocked. In further support of the hypothesis, selective agonists of TrkA (e.g. NGF-C) protect RGCs in chronic and acute neurodegeneration. In our experimental paradigms the data are not conclusive as to whether the TrkA agonism is more important than the p75NTR antagonism.
Given that mAb NGF30 only blocks NGF-p75NTR and pro-NGF-p75NTR, but there remain other endogenous ligands of p75NTR active in the eye (e.g. BDNF and pro-BDNF), it seems clear that the TrkA agonism is important for neuroprotection. Also, the fact that p75NTR antagonists (LM24 and THXB) were effective also supports the parallel importance of p75NTR antagonism for promoting survival.
On the other hand, agonism of p75NTR is deleterious to RGCs: it can kill RGCs in the normal eye, and exacerbates disease in ON axotomy and glaucoma. Because p75NTR is not expressed or is expressed poorly in RGCs (7, 13), the evidence points to a non-cell autologous mechanism that implicates TNF-α and α2M neurotoxicity. This is consistent with previous reports implicating p75NTR in TNF-α production during glutamate toxicity, which is a very fast and acute form of injury affecting retina within 4 h (13).
The glaucoma and ON axotomy models of neurodegeneration are intrinsically different. Glaucoma is a slow, chronic and progressive disorder, and elevated IOP creates an intraocular crisis that exerts stress on each layer of the retina and the optic nerve head and fibers. In contrast, optic nerve transection is an acute disorder, where the problem is extraocular because it damages RGC axons and only in late stages affects the RGC cell body. Despite their differences, both axotomized and glaucomatous retinas up-regulate NGF, TrkA, and p75NTR (6, 7, 35, 36). Based on the literature and the pharmacological results reported here, we propose three strategies in glaucoma and related forms of neuropathy or axonopathy.
One strategy is based on the selective agonism of TrkA, to convey neuroprotection. A second strategy is antagonism of p75NTR to prevent neurotoxicity. A third strategy is based on the modification of endogenous NGF with the use of biological response modifiers; this would convey neuroprotection and perhaps also reduce neurotoxicity. Future work will test different kinetics and doses of these agents, with the expectation that they can be applied to other models of neurodegeneration.
Acknowledgments
We thank Arielle Cantor, Wang Hui, and Sophia Biyao Xiao for counting RGCs. Sieun Lee helped with image reconstruction. We are thankful for the use of the Biacore 3000 SPR instrument in the Department of Biochemistry, Medical College of Wisconsin, and the help of Drs. Nancy Dahms and Richard Bohnsack in obtaining the SPR data. Neurotrophins were a gift from Alomone Laboratories (Israel).
This work was supported, in whole or in part, by National Institutes of Health Grant NS24380 from the United States Public Health Service (to K. E. N.), Canadian Institutes of Health Research Grant MOP 192060 (to H. U. S.), and a grant from the Canadian Institutes of Health Research/Natural Sciences and Engineering Research of Canada Collaborative Health Research Projects (to M. V. S.).
- RGC
- retinal ganglion cell
- ON
- optic nerve
- IOP
- intraocular pressure
- α2M
- α2-macroglobulin
- FD-OCT
- Fourier domain optical coherence tomography
- NGI
- nerve fiber layer-ganglion cell layer-inner plexiform layer.
REFERENCES
- 1.Quigley H. A. (2005) Eye 19, 1241–1248 [DOI] [PubMed] [Google Scholar]
- 2.Pease M. E., McKinnon S. J., Quigley H. A., Kerrigan-Baumrind L. A., Zack D. J. (2000) Invest. Ophthalmol. Vis. Sci. 41, 764–774 [PubMed] [Google Scholar]
- 3.Saragovi H. U., Hamel E., Di Polo A. (2009) Curr. Alzheimer Res. (2009) 6, 419–423 [DOI] [PubMed] [Google Scholar]
- 4.Shi Z., Birman E., Saragovi H. U. (2007) Dev. Neurobiol. 67, 884–894 [DOI] [PubMed] [Google Scholar]
- 5.Bai Y., Xu J., Brahimi F., Zhuo Y., Sarunic M. V., Saragovi H. U. (2010) Invest. Ophthalmol. Vis. Sci. 51, 4722–4731 [DOI] [PubMed] [Google Scholar]
- 6.Rudzinski M., Wong T. P., Saragovi H. U. (2004) J. Neurobiol. 58, 341–354 [DOI] [PubMed] [Google Scholar]
- 7.Lebrun-Julien F., Morquette B., Douillette A., Saragovi H. U., Di Polo A. (2009) Mol. Cell Neurosci. 40, 410–420 [DOI] [PubMed] [Google Scholar]
- 8.Tezel G., Wax M. B. (2004) Curr. Opin. Ophthalmol. 15, 80–84 [DOI] [PubMed] [Google Scholar]
- 9.Nakazawa T., Nakazawa C., Matsubara A., Noda K., Hisatomi T., She H., Michaud N., Hafezi-Moghadam A., Miller J. W., Benowitz L. I. (2006) J. Neurosci. 26, 12633–12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wax M. B., Tezel G. (2002) Mol. Neurobiol. 26, 45–55 [DOI] [PubMed] [Google Scholar]
- 11.Shi Z., Rudzinski M., Meerovitch K., Lebrun-Julien F., Birman E., Di Polo A., Saragovi H. U. (2008) J. Biol. Chem. 283, 29156–29165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai Y., Shi Z., Zhuo Y., Liu J., Malakhov A., Ko E., Burgess K., Schaefer H., Esteban P. F., Tessarollo L., Saragovi H. U. (2010) Invest. Ophthalmol. Vis. Sci., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebrun-Julien F., Duplan L., Pernet V., Osswald I., Sapieha P., Bourgeois P., Dickson K., Bowie D., Barker P. A., Di Polo A. (2009) J. Neurosci. 29, 5536–5545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebrun-Julien F., Bertrand M. J., De Backer O., Stellwagen D., Morales C. R., Di Polo A., Barker P. A. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 3817–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Valenzuela E., Shareef S., Walsh J., Sharma S. C. (1995) Exp. Eye Res. 61, 33–44 [DOI] [PubMed] [Google Scholar]
- 16.Laquis S., Chaudhary P., Sharma S. C. (1998) Brain Res. 784, 100–104 [DOI] [PubMed] [Google Scholar]
- 17.Naskar R., Wissing M., Thanos S. (2002) Invest. Ophthalmol. Vis. Sci. 43, 2962–2968 [PubMed] [Google Scholar]
- 18.Urcola J. H., Hernández M., Vecino E. (2006) Exp. Eye Res. 83, 429–437 [DOI] [PubMed] [Google Scholar]
- 19.Danias J., Kontiola A. I., Filippopoulos T., Mittag T. (2003) Invest. Ophthalmol. Vis. Sci. 44, 1138–1141 [DOI] [PubMed] [Google Scholar]
- 20.Buckingham B. P., Inman D. M., Lambert W., Oglesby E., Calkins D. J., Steele M. R., Vetter M. L., Marsh-Armstrong N., Horner P. J. (2008) J. Neurosci. 28, 2735–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahapatra S., Mehta H., Woo S. B., Neet K. E. (2009) J. Biol. Chem. 284, 33600–33613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rydén M., Hempstead B., Ibáñez C. F. (1997) J. Biol. Chem. 272, 16322–16328 [DOI] [PubMed] [Google Scholar]
- 23.Hughes A. L., Messineo-Jones D., Lad S. P., Neet K. E. (2001) J. Neurosci. Res. 63, 10–19 [DOI] [PubMed] [Google Scholar]
- 24.Saragovi H. U., Zheng W., Maliartchouk S., DiGugliemo G. M., Mawal Y. R., Kamen A., Woo S. B., Cuello A. C., Debeir T., Neet K. E. (1998) J. Biol. Chem. 273, 34933–34940 [DOI] [PubMed] [Google Scholar]
- 25.Massa S. M., Xie Y., Yang T., Harrington A. W., Kim M. L., Yoon S. O., Kraemer R., Moore L. A., Hempstead B. L., Longo F. M. (2006) J. Neurosci. 26, 5288–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thanos S. (1991) Neurosci. Lett. 127, 108–112 [DOI] [PubMed] [Google Scholar]
- 27.Soto I., Oglesby E., Buckingham B. P., Son J. L., Roberson E. D., Steele M. R., Inman D. M., Vetter M. L., Horner P. J., Marsh-Armstrong N. (2008) J. Neurosci. 28, 548–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scotter E. L., Narayan P., Glass M., Dragunow M. (2008) J. Neurosci. Methods 171, 174–179 [DOI] [PubMed] [Google Scholar]
- 29.Maliartchouk S., Debeir T., Beglova N., Cuello A. C., Gehring K., Saragovi H. U. (2000) J. Biol. Chem. 275, 9946–9956 [DOI] [PubMed] [Google Scholar]
- 30.Maliartchouk S., Saragovi H. U. (1997) J. Neurosci. 17, 6031–6037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masoudi R., Ioannou M. S., Coughlin M. D., Pagadala P., Neet K. E., Clewes O., Allen S. J., Dawbarn D., Fahnestock M. (2009) J. Biol. Chem. 284, 18424–18433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansen P., Giehl K., Nyengaard J. R., Teng K., Lioubinski O., Sjoegaard S. S., Breiderhoff T., Gotthardt M., Lin F., Eilers A., Petersen C. M., Lewin G. R., Hempstead B. L., Willnow T. E., Nykjaer A. (2007) Nat. Neurosci. 10, 1449–1457 [DOI] [PubMed] [Google Scholar]
- 33.Lee R., Kermani P., Teng K. K., Hempstead B. L. (2001) Science 294, 1945–1948 [DOI] [PubMed] [Google Scholar]
- 34.Srinivasan B., Roque C. H., Hempstead B. L., Al-Ubaidi M. R., Roque R. S. (2004) J. Biol. Chem. 279, 41839–41845 [DOI] [PubMed] [Google Scholar]
- 35.Cui Q., Tang L. S., Hu B., So K. F., Yip H. K. (2002) Invest. Ophthalmol. Vis. Sci. 43, 1954–1964 [PubMed] [Google Scholar]
- 36.Hu B., Yip H. K., So K. F. (1998) Glia 24, 187–197 [PubMed] [Google Scholar]