Abstract
Transmitter molecules bind to synaptic acetylcholine receptor channels (AChRs) to promote a global channel-opening conformational change. Although the detailed mechanism that links ligand binding and channel gating is uncertain, the energy changes caused by mutations appear to be more symmetrical between subunits in the transmembrane domain compared with the extracellular domain. The only covalent connection between these domains is the pre-M1 linker, a stretch of five amino acids that joins strand β10 with the M1 helix. In each subunit, this linker has a central Arg (Arg3′), which only in the non-α-subunits is flanked by positively charged residues. Previous studies showed that mutations of Arg3′ in the α-subunit alter the gating equilibrium constant and reduce channel expression. We recorded single-channel currents and estimated the gating rate and equilibrium constants of adult mouse AChRs with mutations at the pre-M1 linker and the nearby residue Glu45 in non-α-subunits. In all subunits, mutations of Arg3′ had similar effects as in the α-subunit. In the ϵ-subunit, mutations of the flanking residues and Glu45 had only small effects, and there was no energy coupling between ϵGlu45 and ϵArg3′. The non-α-subunit Arg3′ residues had Φ-values that were similar to those for the α-subunit. The results suggest that there is a general symmetry between the AChR subunits during gating isomerization in this linker and that the central Arg is involved in expression more so than gating. The energy transfer through the AChR during gating appears to mainly involve Glu45, but only in the α-subunits.
Keywords: Allosteric Regulation, Cholinergic Receptor, Nicotinic Acetylcholine Receptors, Protein Domains, Receptor Structure-Function
Introduction
Acetylcholine receptors (AChRs)2 are ligand-gated ion channels that mediate fast chemical synaptic transmission (1). The binding of two acetylcholine molecules to the extracellular domain (ECD) triggers a rapid, global, and reversible conformational change that increases the affinity of the two agonist-binding sites and opens a cation conduction pathway through the transmembrane domain (TMD). Defects in AChRs originating from inherited mutations that alter gating kinetic properties or expression cause myasthenic disorders (2). Electrophysiology studies have identified many mutations that produce abnormal AChR gating, but a detailed understanding of the mechanism by which the agonist affinity change and channel opening/closing are linked is not yet available.
The adult neuromuscular AChR consists of five homologous subunits (two α-subunits and one each of the β-, δ-, and ϵ-subunits) folded symmetrically around the central axis of the pore (3). Its structure is modular, as the extracellular N-terminal half of each subunit is a β-barrel and the transmembrane C-terminal half is a four-α-helix bundle (M1–M4). The five sets of β-barrels form the ECD, and the five sets of α-helices form the TMD. Several structures have revealed important features that are relevant to understanding the mechanism of energy transfer through the protein in the gating isomerization, including the Torpedo AChR (4), two prokaryotic pentameric ligand-gated ion channels crystallized in either a non-conducting (ELIC) (5) or a presumably conducting conformation (GLIC) (6, 7), an ECD fragment of the mouse AChR α-subunit (8), and the ECD homolog, the acetylcholine-binding protein (9). A comparison of the ELIC and GLIC x-ray structures suggests that the pore-lining M2 helices tilt tangentially and radially as part of the channel-opening process (6, 7). Despite this structural information, we are still unsure of the molecular events that constitute the affinity change at the binding sites and the conductance change at the pore or the intermediate events that couple structural changes in these two widely separated domains (10).
The interface between the ECD and TMD is a complex region that has been studied in several members of the Cys loop receptor channel family (11–18). This region has many charged residues and hence the potential for many non-bonded interactions. The only covalent link between the ECD and TMD is a stretch of five residues that join strand β10 with the M1 helix, known as the “pre-M1” linker (Fig. 1). This linker has a central positively charged Arg that is conserved among all pentameric ligand-gated receptor channels (12). Here, we will call this position Arg3′ to mark it as the third position in this linker. Lee and Sine (12) proposed that the perturbation of a salt bridge between αArg3′ and loop 2 residue αGlu45 is the principal event that links the ECD and the TMD in gating, but other results suggest that αArg3′ plays a smaller role in gating but is important for receptor expression (14, 17).
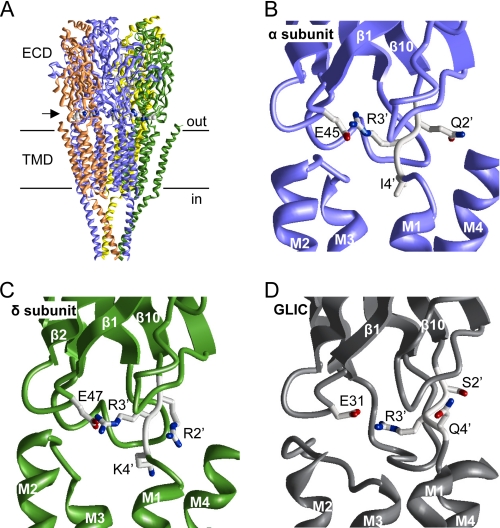
FIGURE 1.
Structure of the pre-M1 linker. A, the cryo-EM structure of the Torpedo AChR (4). The horizontal lines mark approximately the membrane. The arrow points to the location of the pre-M1 linker. The Arg3′ side chains of all subunits are space-filled. B and C, close-up view of the pre-M1 linker of the α- and δ-subunits in the Torpedo AChR. The side chains of a Glu in loop 2 and pre-M1 linker positions 2′, 3′, and 4′ are shown as sticks, where carbons, oxygens, and nitrogens are colored gray, red, and blue, respectively. The backbones of the ECD, TMD, and loops are displayed as smooth ribbons, flat ribbons, and rods, respectively. D, close-up view of the pre-M1 linker of GLIC (7). Structures were displayed using MVM (ZMM Software Inc.).
The affinity change at the binding sites involves mainly α-subunit residues, but the opening and closing of the gate near the M2 equator involve the rearrangements of atoms in all five subunits. One goal of our experiments was to assay the symmetry of the gating energy changes at the pre-M1 linker, a location that is about halfway between the binding sites and the gate. In general, previous results indicate that large gating energy changes in the ECD are predominantly in the α-subunit but become more evenly spread among all subunits in the TMD (12, 19–25). Although the mechanism of this spreading of the gating energy changes is not clear, an intersubunit energy transfer has been found between αTyr127 and ϵAsn39 or δAsn41 (19).
So far, only one mutation at one pre-M1 linker position has been studied in all subunits using single-channel kinetic analysis. A Gln substitution at the fourth linker residue modestly increases diliganded gating (by ∼3-fold) in the α-subunit but is without effect in the non-α-subunits (26). Here, we report the effects of mutations of multiple pre-M1 residues in non-α-subunits, estimated from the single-channel rate and equilibrium constants of the adult mouse AChR gating isomerization.
EXPERIMENTAL PROCEDURES
Mutagenesis and Expression
A detailed description of our methods is described by Jha et al. (27). Mutant AChR cDNAs were made by QuikChangeTM site-directed mutagenesis (Stratagene) and confirmed by sequencing. We made 26 mutants of the ϵ-subunit, three double-mutant combinations of the ϵ-subunit, two mutants of the β-subunit, and two mutants of the δ-subunit. HEK293 cells were transiently transfected by the calcium phosphate precipitation method. HEK cells were incubated with 2.5–5 μg of mouse WT or mutant cDNAs in a 35-mm culture dish at a subunit ratio of 2:1:1:1 (α/β/δ/ϵ). After ∼16 h of incubation at 37 °C, the transfected cells were washed with HEK culture medium. Electrophysiology recordings were performed 20–40 h post-transfection.
Single-channel Recordings and Kinetic Analysis
Recordings were carried out in the cell-attached patch configuration at room temperature (23 °C). The pipette and bath solutions were both Dulbecco's PBS (137 mm NaCl, 0.9 mm CaCl2, 2.7 mm KCl, 1.5 mm KH2PO4, 0.5 mm MgCl2, and 8.1 mm Na2HPO4 at pH 7.2). Pipettes were pulled from borosilicate capillaries to a resistance of ∼10 megohms and coated with SYLGARD (Dow Corning Corp., Midland, MI). The pipette solution contained 0.5 mm acetylcholine, 20 mm choline, or 5 mm carbamylcholine. These agonist concentrations are approximately five times the corresponding equilibrium dissociation constants (Kd); thus, almost all currents arose from diliganded AChRs. Because the mutations were far from the binding site, we assumed that they did not change Kd. The high concentration of agonist caused partial channel block, which decreases both the apparent single-channel current amplitude and the apparent closing rate constant. Although none of the mutations changed the degree of channel block, nonetheless we measured the closing rate constant using low concentrations of agonist (30 μm acetylcholine, 200 μm choline, and 200 μm carbamylcholine), at which channel block is insignificant. The diliganded gating equilibrium constant (E2) was calculated as the ratio of the opening/closing rate constant. Choline was used to measure the diliganded opening rate constant for AChR mutants where E2 was larger than or equal to the WT; acetylcholine was used for mutants where E2 was less than the WT; and carbamylcholine was used to measure mutants where E2 was approximately equal to the WT. It has been shown for many non-binding site mutations that different agonists support the same Φ-values and fold-changes in E2 (21).
Cells were held at a pipette potential of +70 mV, which corresponds to a membrane potential of approximately −100 mV. Errors in the rate constants associated with the errors in the membrane voltage are small (in WT AChRs, an ∼70-mV depolarization is necessary to decrease E2 by e-fold) (28). Currents were filtered at 20 kHz and digitized at a sampling frequency of 50 kHz. Kinetic analyses were done using QUB software. Currents were idealized using the SKM (segmental K-Means) algorithm filtered at 12 kHz with a C ↔ O (closed ↔ open) model with starting rate constants of 100 s−1. The diliganded opening (f2) and closing (b2) rate constants were estimated from idealized interval durations using a maximum interval likelihood algorithm after incorporating a dead time of 25 μs (29).
The diliganded rate constants were measured multiple times (n = two to five patches) and then averaged. Φ was estimated as the slope of the linear fit to the log-log rate-equilibrium free energy relationship (R/E analysis). The range energy (kcal/mol) = −0.59 ln(E2max/E2min), where the superscripts are the E2 values for the side chains generating the largest and smallest E2 values, respectively. The coupling free energy was calculated as ΔΔG (kcal/mol) = −0.59 ln((Edouble mutant)/(Emutant 1*Emutant 2)).
RESULTS
ϵ-Subunit Linker
Table 1 shows a sequence alignment of the pre-M1 linker and that Arg3′ is completely conserved. In the α-subunit linker, this is the only positively charged residue, but in the non-α-subunits, it is flanked by two additional basic amino acids.
TABLE 1.
Sequence alignment of the pre-M1 linker
The conserved central Arg at position 3′ is shown in boldface: αArg209, βArg220, δArg223, and ϵArg218 in mouse AChR numbering.
| Protein | Positions 1–5′ |
|---|---|
| Mouse AChR | |
| α-subunit | MQRLP |
| α2-subunit | IRRLP |
| α3-subunit | IRRLP |
| α4-subunit | IRRLP |
| α5-subunit | IKRLP |
| α6-subunit | IRRLP |
| α7-subunit | MRRRT |
| β-subunit | IRRKP |
| β2-subunit | IRRKP |
| β3-subunit | LRRLP |
| β4-subunit | IKRKP |
| δ-subunit | IRRKP |
| ϵ-subunit | IRRKP |
| γ-subunit | IQRKP |
| TorpedoAChR α-subunit | MQRIP |
| ELIC | AVRNP |
| GLIC | ISRQY |
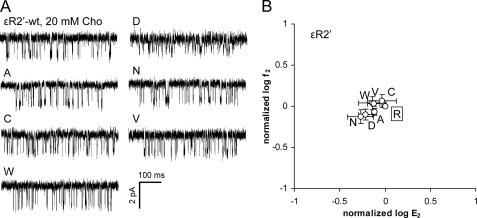
We estimated residue range energy (see “Experimental Procedures”) and Φ-values from AChRs with a mutation of one of the three positively charged amino acids in the ϵ-linker (ϵArg2′, ϵArg3′, ϵLys4′). None of the side chain substitutions at ϵArg2′ (Ala, Cys, Asp, Asn, Trp, or Val) changed the diliganded gating equilibrium constant (E2) by >2-fold (Fig. 2 and Table 2). This set of substitutions was selected to include residues of different size, charge, and hydrophobicity. All mutations caused a slight reduction in E2, with the largest being for Asn (1.8-fold, which corresponds to a range energy of 0.3 kcal/mol). We conclude that like its homolog in the α-subunit, the ϵ2′-side chain is nearly isoenergetic between the ground state conformations, which suggests that this amino acid does not move with respect to its local environment during the gating isomerization.
FIGURE 2.
Mutations of ϵArg2′ (ϵArg217) have little effect on the gating equilibrium constant. The small change in the diliganded gating equilibrium constant (E2, normalized by the WT value) indicates that this position is nearly isoenergetic between the ground state conformations. A, example clusters elicited by 20 mm choline (Cho). B, R/E analysis. Each point represents the average of three patches with its S.D. (Table 2). The WT is boxed.
TABLE 2.
Kinetic parameters for ϵ-subunit pre-M1 mutants
The investigated ϵ-subunit pre-M1 positions studied were ϵArg2′, ϵArg3′, and ϵLys4′, which in the mouse AChR are numbered ϵArg217, ϵArg218, and ϵLys219, respectively. The numbers are the mean ± S.D. ACh, acetylcholine; Cho, choline; f2, forward (opening) rate constant; b2obs, observed backward (closing) rate constant; b2cor, backward rate constant corrected for channel block; E2, diliganded gating equilibrium constant (calculated as f2/b2cor); n, number of patches. Normalized values are mutant/WT. No single-channel currents were observed with ϵR3′C, ϵR3′D, ϵR3′E, and ϵR3′V mutants.
| Construct | Agonista | f2 | b2obs | b2cor | E2 (f2/b2cor) | Normalized f2 (mutant/WT) | Normalized E2 (mutant/WT) | n |
|---|---|---|---|---|---|---|---|---|
| s−1 | s−1 | s−1 | ||||||
| WTb | ACh | 48,000 | 1750 | 28.00 | 1.00 | 1.00 | ||
| WT | Cho | 120 | 2583 | 0.05 | 1.00 | 1.00 | ||
| ϵR2′A | Cho | 104 ± 10 | 783 ± 88 | 2934 ± 329 | 0.04 ± 0.002 | 0.86 | 0.76 | 3 |
| ϵR2′C | Cho | 139 ± 24 | 933 ± 202 | 3232 ± 700 | 0.05 ± 0.02 | 1.16 | 0.97 | 3 |
| ϵR2′D | Cho | 95 ± 15 | 869 ± 60 | 3372 ± 235 | 0.03 ± 0.01 | 0.79 | 0.61 | 3 |
| ϵR2′N | Cho | 90 ± 17 | 849 ± 217 | 3586 ± 918 | 0.03 ± 0.01 | 0.75 | 0.56 | 3 |
| ϵR2′W | Cho | 131 ± 24 | 1247 ± 209 | 3891 ± 652 | 0.04 ± 0.01 | 1.09 | 0.76 | 3 |
| ϵR2′V | Cho | 129 ± 10 | 896 ± 109 | 3719 ± 454 | 0.04 ± 0.01 | 1.08 | 0.76 | 3 |
| ϵR3′A | ACh | 991 ± 171 | 4397 ± 826 | 6572 ± 1234 | 0.15 ± 0.02 | 0.02 | 0.005 | 3 |
| ϵR3′H | ACh | 9019 ± 1907 | 2623 ± 648 | 6194 ± 1530 | 1.56 ± 0.63 | 0.19 | 0.055 | 3 |
| ϵR3′K | ACh | 6363 ± 856 | 2579 ± 261 | 4627 ± 468 | 1.40 ± 0.29 | 0.13 | 0.050 | 4 |
| ϵR3′N | ACh | 1559 ± 159 | 6813 ± 980 | 10,311 ± 1484 | 0.15 ± 0.04 | 0.03 | 0.006 | 3 |
| ϵR3′Q | ACh | 1446 ± 35 | 3180 ± 840 | 5726 ± 1513 | 0.27 ± 0.07 | 0.03 | 0.009 | 4 |
| ϵK4′A | Cho | 325 ± 56 | 431 ± 76 | 1634 ± 286 | 0.20 ± 0.03 | 2.71 | 4.31 | 3 |
| ϵK4′C | Cho | 300 ± 60 | 712 ± 251 | 2327 ± 820 | 0.15 ± 0.08 | 2.50 | 3.14 | 3 |
| ϵK4′D | Cho | 221 ± 20 | 593 ± 46 | 2260 ± 174 | 0.10 ± 0.01 | 1.84 | 2.11 | 3 |
| ϵK4′N | Cho | 111 ± 8 | 525 ± 141 | 2213 ± 596 | 0.05 ± 0.01 | 0.93 | 1.12 | 3 |
| ϵK4′W | Cho | 351 ± 36 | 351 ± 25 | 1099 ± 79 | 0.32 ± 0.03 | 2.92 | 6.87 | 3 |
| ϵK4′V | Cho | 135 ± 20 | 706 ± 43 | 2600 ± 158 | 0.05 ± 0.01 | 1.12 | 1.12 | 3 |
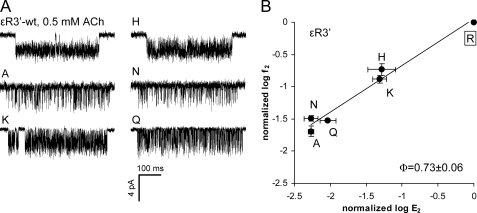
Nine substitutions of the central position ϵArg3′ were examined, but only Ala, His, Lys, Asn, and Gln expressed functional channels. We were unable to observe single-channel currents from the Cys, Asp, Glu, and Val mutants (3–10 patches per mutant, recording for 15–25 min/patch). In this respect, position ϵ3′ behaves similarly to its homolog in the α-subunit (14) and to α1Arg220 in the GABA receptor channel (17), where mutations also reduce the expression of functional channels. Mutations of ϵArg3′ had substantial effects on E2 (Table 2). The Ala, His, Lys, Asn, and Gln substitutions all decreased E2 compared with the WT (Fig. 3). The Ala and Asn substitutions had the largest effect and decreased E2 by ∼180-fold. The range energy for ϵArg3′ was ∼3.1 kcal/mol, which is about the median value for two α-subunit mutations (10, 30). The R/E plot for ϵArg3′ had a slope (Φ-value) of 0.73 ± 0.06 (Fig. 3B), which indicates that the change in E2 was caused mainly by a reduction in the forward channel-opening rate constant (f2). This Φ-value is the same as for αArg3′ (14), which suggests that the central Arg residues experience a change in energy at about the same time in the gating reaction in the α- and ϵ-subunits.
FIGURE 3.
Mutations of ϵArg3′ (ϵArg218) decrease the gating equilibrium constant. A, example clusters elicited by 0.5 mm acetylcholine (ACh). Single-channel currents were not detected for the Cys, Asp, Glu, Val, and Trp mutants. B, R/E analysis. Each point represents the average of three to four patches with its S.D. (Table 2). ●, acetylcholine-activated. The WT is boxed. The Φ-value (lower right) was estimated as the linear slope of log f2 versus log E2 and gives the relative timing of the residue's gating energy change (1 to 0, start to end). The ϵArg3′ side chain changes its energy relatively early in the channel-opening process.
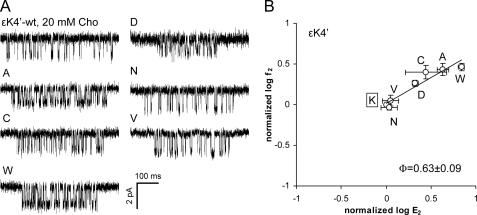
All substitutions tested at ϵLys4′ expressed functional AChRs. Ala, Cys, Asp, and Trp mutations increased E2, but only modestly (Fig. 4 and Table 2). The Trp mutant showed the largest energy change (∼1.1 kcal/mol). AChRs with an Asn or Val side chain here had WT gating properties, as did a Gln substitution (26). The Φ-value of the ϵLys4′ series was 0.63 ± 0.09, similar to that of its neighbor ϵArg3′. However, in the α-subunit, the Φ-value of αLeu4′ (Φ = 0.35) was distinctly lower than that of the adjacent residue αArg3′ (Φ = 0.72) (14).
FIGURE 4.
Mutations of ϵLys4′ (ϵLys219) modestly increase the gating equilibrium constant. A, example clusters activated by 20 mm choline (Cho). B, R/E analysis. Each point represents the average of three patches with its S.D. (Table 2). The WT is boxed. The Φ-value (lower right) was estimated as the linear slope of log f2 versus log E2.
Energetic Coupling between ϵArg3′ and ϵGlu45
In the cryo-EM structure of the Torpedo AChR (4), the positively charged side chain of αArg3′ faces the negatively charged side chain of αGlu45, and swapping charges here restores functional gating (12). We investigated the effects of five side chain substitutions at ϵGlu45 and three ϵGlu45/ϵArg3′ double-mutant combinations to explore the interactions between these ϵ-positions during gating.
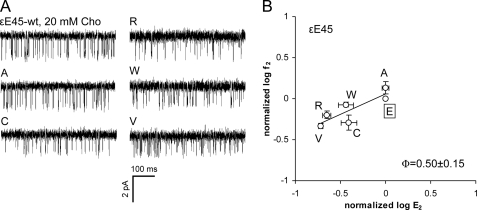
Fig. 5 and Table 3 show the effects of mutating ϵGlu45. The ϵGlu45 substitutions Cys, Arg, Trp, and Val all decreased E2, but only slightly (<1 kcal/mol). The Φ-value measured for the ϵGlu45 mutation series was 0.50 ± 0.15. Because of the near-WT gating properties of the ϵE45A mutant, we conclude that a negatively charged side chain at this position in the ϵ-subunit is not critical for efficient gating. The small range energy makes the ϵGlu45 Φ-value estimate imprecise (31), but it may be that this residue changes its energy somewhat after its α-subunit homolog (Φ = 0.80) (14).
TABLE 3.
Kinetic parameters for ϵGlu45 mutants
See the legend to Table 2. No single-channel currents were observed with the ϵR3′E/ϵE45R double mutant. Cho, choline; ACh, acetylcholine.
| Construct | Agonist | f2 | b2obs | b2cor | E2 (f2/b2cor) | Normalized E2 (mutant/WT) |
ΔΔG | n | |
|---|---|---|---|---|---|---|---|---|---|
| Observed | Predicted | ||||||||
| s−1 | s−1 | s−1 | kcal/mol | ||||||
| ϵE45A | Cho | 164 ± 27 | 1238 ± 112 | 3519 ± 319 | 0.05 ± 0.004 | 1.01 | 3 | ||
| ϵE45C | Cho | 61 ± 13 | 1041 ± 26 | 3365 ± 83 | 0.02 ± 0.004 | 0.40 | 3 | ||
| ϵE45R | Cho | 56 ± 4 | 1551 ± 74 | 6321 ± 301 | 0.01 ± 0.001 | 0.19 | 3 | ||
| ϵE45W | Cho | 102 ± 7 | 1156 ± 141 | 6032 ± 733 | 0.02 ± 0.003 | 0.37 | 2 | ||
| ϵE45V | Cho | 76 ± 8 | 2443 ± 1 | 7404 ± 1 | 0.01 ± 0.001 | 0.22 | 2 | ||
| ϵR3′Q/ϵE45A | ACh | 2258 ± 382 | 3219 ± 521 | 5631 ± 911 | 0.41 ± 0.10 | 0.015 | 0.009 | −0.30 | 5 |
| ϵR3′K/ϵE45A | Cho | 38 ± 12 | 1076 ± 181 | 3986 ± 669 | 0.01 ± 0.01 | 0.216 | 0.050 | −0.86 | 4 |
FIGURE 5.
Mutations of ϵGlu45 have only a small effect on the gating equilibrium constant. A, example clusters activated by 20 mm choline (Cho). B, R/E analysis. Each point represents the average of two to three patches with its S.D. (Table 3). The WT is boxed. The Φ-value (lower right) was estimated as the linear slope of log f2 versus log E2.
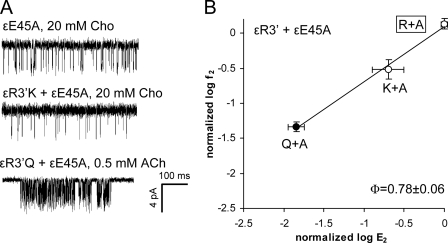
We created three ϵGlu45/ϵArg3′ double-mutant constructs: Arg/Glu, Ala/Gln, and Ala/Lys. In the α-subunit, the side chains of αGlu45 influence the expression of αArg3′ mutants (14). The ϵR3′E construct alone did not express functional AChRs, and when we expressed this along with ϵE45R (a charge swap), we still did not observe the expression of functional AChRs currents. The other two double-mutant pairs (Ala/Gln and Ala/Lys) did express functional channels and produced AChRs having slightly larger E2 values than predicted assuming independence (Fig. 6 and Table 3). The Ala/Gln and Ala/Lys double mutants exhibited very small coupling energies (−0.3 and −0.9 kcal/mol, respectively). The R/E plot for ϵArg3′ on the ϵE45A background yielded a Φ-value of 0.78 ± 0.06 (Fig. 6B), which is similar to its value on the WT background (Fig. 3).
FIGURE 6.
Energy coupling between ϵArg3′ and ϵGlu45. A, example clusters activated by different agonists. B, R/E analysis. Each point represents the average of three to five patches with its S.D. (Table 4). ●, acetylcholine (ACh); ○, choline (Cho). The WT is boxed. The Φ-value (lower right) was estimated as the linear slope of log f2 versus log E2.
β- and δ-Subunit Linkers
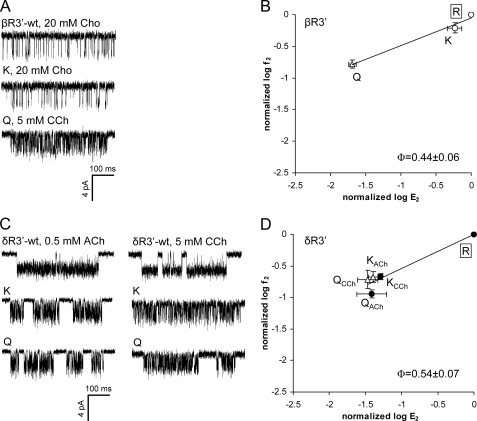
We also examined the kinetics of mutations at position 3′ in the β- and δ-subunits (Fig. 7 and Table 4). The mutation βR3′K had little effect on gating, whereas a Gln mutation here resulted in a 49-fold reduction of E2. The estimated Φ-value of position β3′ was 0.44 ± 0.06 (Fig. 7B). In the δ-subunit, Lys and Gln substitutions were assayed using two different agonists, acetylcholine and carbamylcholine. With both δArg3′ mutations, the fold-changes in E2 were similar for these ligands, even though acetylcholine is a full agonist and carbamylcholine is a partial agonist. The R/E plot for position δ3′ yielded a Φ-value of 0.54 ± 0.07 and a range energy of ∼2.0 kcal/mol.
FIGURE 7.
Mutations of βArg3′ (βArg220) and δArg3′ (δArg223) decrease the diliganded gating equilibrium constant. A and C, example clusters of position β3′ and δ3′ mutants activated by 0.5 mm acetylcholine (ACh), 5 mm carbamylcholine (CCh), or 20 mm choline (Cho). In C, the left clusters were activated by acetylcholine and the right clusters by carbamylcholine. B and D, R/E analyses of positions β3′ and δ3′ mutants. Each point represents the average of three to four patches with its S.D. (Table 4). ●, acetylcholine; ○, choline; △, carbamylcholine. The WT is boxed. The Φ-value (lower right) was estimated as the linear slope of log f2 versus log E2.
TABLE 4.
Kinetic parameters for βArg3′ and δArg3′ positions
βArg3′ and δArg3′ are numbered in the mouse AChR as βArg220 and δArg223, respectively. See the legend to Table 2. CCh, carbamylcholine; Cho, choline; ACh, acetylcholine.
| Construct | Agonist | f2 | b2obs | b2cor | E2 (f2/b2cor) | Normalized f2 (mutant/WT) | Normalized E2 (mutant/WT) | n |
|---|---|---|---|---|---|---|---|---|
| s−1 | s−1 | s−1 | ||||||
| WTa | CCh | 7721 | 1138 | 6.80 | 1.00 | 1.00 | ||
| βR3′K | Cho | 75 ± 14 | 769 ± 33 | 2709 ± 116 | 0.03 ± 0.01 | 0.62 | 0.60 | 4 |
| βR3′Q | CCh | 1290 ± 188 | 2586 ± 497 | 9300 ± 1789 | 0.14 ± 0.02 | 0.17 | 0.02 | 3 |
| δR3′K | ACh | 10,315 ± 1170 | 4668 ± 547 | 7271 ± 852 | 1.42 ± 0.10 | 0.21 | 0.05 | 4 |
| δR3′Q | ACh | 5462 ± 801 | 3341 ± 1502 | 5362 ± 2411 | 1.16 ± 0.48 | 0.11 | 0.04 | 4 |
| δR3′K | CCh | 1646 ± 318 | 1797 ± 650 | 6137 ± 2220 | 0.28 ± 0.04 | 0.21 | 0.04 | 3 |
| δR3′Q | CCh | 1553 ± 492 | 2656 ± 667 | 6644 ± 1668 | 0.24 ± 0.07 | 0.20 | 0.03 | 4 |
a WT rate constants for carbamylcholine were taken from Purohit and Auerbach (21).
DISCUSSION
In the Torpedo AChR (4) structure, the αArg3′ side chain forms a salt bridge with αGlu45 in loop 2 (and possibly with αGlu175 in loop 9). In the ECD fragment of the mouse nicotinic acetylcholine receptor α-subunit (8), αArg3′ interacts indirectly with αGlu45 via a structural water. An electrostatic contact is present between the Arg3′ and Glu31 (in loop 2) side chains in the x-ray structure of GLIC (7). ELIC lacks this salt bridge because the loop 2 residue is a Thr that faces, but does not contact, Arg3′ (which does appear to form a salt bridge with Asp122 in loop 7 and Glu159 in loop 9). Thus, the conserved pre-M1 linker Arg3′ side chain is evidently involved in protein stability through electrostatic interactions with surrounding loops. However, analyses of function suggest that a perturbation of the salt bridge between αArg3′ and αGlu45 is not a critically important event in AChR gating (14).
One of our goals was to probe the degree of functional symmetry between subunits with regard to residues in the pre-M1 linker and loop 2. The non-α-linker contains three positive residues, whereas the α-linker contains only one. Recall that in the α-subunit linker, (i) many mutations at position 3′ reduce expression, (ii) only mutations at positions 3′ and 4′ affect E2, and (iii) loop 2 residue αGlu45 experiences a very large range energy change (∼5 kcal/mol) early in the channel-opening process (Φ ∼ 0.8) (14). We can compare this basic pattern in the α-subunit with that we have found in the ϵ-subunit and, to a lesser extent, in the β- and δ-subunits.
Position 2′ is isoenergetic in both the α- and ϵ-subunits. All mutants tested here in both subunits expressed functional AChRs that had WT-like currents. It appears that in the mouse AChR, this position in the pre-M1 linker in the α- and ϵ-subunits is not essential for folding, expression, conductance, gating, or desensitization.
Position 3′ is much more interesting. In both the α- and ϵ-subunits, many substitutions here prevented the expression of functional AChRs. Single-channel currents were observed only with the Arg, Gln, His, and Lys side chains in both the α- and ϵ-subunits and also with Ala and Asn only in the ϵ-subunit. This difference in expression may simply reflect the fact that each AChR has two α-subunits but only one ϵ-subunit. The R3′Q substitution resulted in a reduction of E2 in both α-subunits and all non-α-subunits, but the gating energy change was only moderate. The range energy (per subunit) was slightly larger in the ϵ-subunit (∼3 kcal/mol) compared with the α-subunit (∼2 kcal/mol); this result was influenced by the inclusion of the Ala and Asn substitutions only in the ϵ-subunit. Limiting this estimate to the Arg, Gln, His, and Lys side chains, the range energies were 1.8 and 1.0 kcal/mol/subunit for the α- and ϵ-subunits, respectively. The Φ-value for position 3′ was the same in the α- and ϵ-subunits, but those in the β- and δ-subunits were somewhat smaller. Overall, this pattern for the central pre-M1 linker position suggests that this region is approximately symmetric between subunits with regard to protein expression and the magnitude and relative timing of the gating energy changes. However, a Lys substitution at Arg3′ resulted in an E2 increase in the α-subunits but a decrease in the δ- and ϵ-subunits and no change in the β-subunit, suggesting that the α-subunit may have a unique chemical environment near the pre-M1 linker.
Position 4′ is not conserved between the α- and ϵ-subunits (Leu versus Lys), and this residue behaved quite differently in these two subunits. The αL4′K mutation increased E2 by ∼50-fold, whereas the ϵK4′V mutation (Leu was not tested) had no effect. Indeed, only small changes were observed for all tested mutations of ϵLys4′, whereas all mutations in the α-subunit modestly increased E2 (range energy of ∼2.3 kcal/mol). An even more interesting difference is the distinct Φ-values for position 4′ in the α-subunit versus the ϵ-subunit (0.35 versus 0.63). This suggests that in the α-subunit, but not the ϵ-subunit, there is a boundary between pre-M1 linker positions 3′ and 4′ that defines the relative timing of the gating movements of the ECD and TMD.
The gating behavior of loop 2 residue Glu45 was also very different in the α-subunit compared with the ϵ-subunit. Substitutions in the ϵ-subunit decreased E2 very slightly, whereas those in the α-subunit either increased or decreased E2 substantially. Indeed, the αGlu45 range energy (∼5 kcal/mol, His to Ile) is the third largest measured so far in the ECD (after αAla96 and αTyr127) (30). An important and common feature shared by Glu45 in the α- and ϵ-subunits is that double mutants of Arg3′ and Glu45 in both subunits suggest weak energetic interactions between these side chains in gating.
In summary, the pre-M1 linker is mostly symmetrical between subunits at positions 2′ and 3′ with regard to expression, gating, and interactions with Glu45 in loop 2. Asymmetry between subunits was apparent at pre-M1 linker position 4′ and Glu45, where only the α-subunit plays a major role. It is of interest to identify the chemical details that define the distinct subunit environments at the ECD-TMD interface and to further explore how energy spreads between subunits in the AChR gating isomerization.
Acknowledgments
We thank Mary Merritt for technical assistance and Archana Jha and Prasad Purohit for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant NS-23513.
- AChR
- acetylcholine receptor channel
- ECD
- extracellular domain
- TMD
- transmembrane domain.
REFERENCES
- 1.Hille B. (2001) Ion Channels of Excitable Membranes, 3rd Ed., Sinauer Associates, Inc., Sunderland, MA [Google Scholar]
- 2.Engel A. G., Shen X. M., Selcen D., Sine S. M. (2010) J. Mol. Neurosci. 40, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unwin N. (1998) J. Struct. Biol. 121, 181–190 [DOI] [PubMed] [Google Scholar]
- 4.Unwin N. (2005) J. Mol. Biol. 346, 967–989 [DOI] [PubMed] [Google Scholar]
- 5.Hilf R. J., Dutzler R. (2008) Nature 452, 375–379 [DOI] [PubMed] [Google Scholar]
- 6.Bocquet N., Nury H., Baaden M., Le Poupon C., Changeux J. P., Delarue M., Corringer P. J. (2009) Nature 457, 111–114 [DOI] [PubMed] [Google Scholar]
- 7.Hilf R. J., Dutzler R. (2009) Nature 457, 115–118 [DOI] [PubMed] [Google Scholar]
- 8.Dellisanti C. D., Yao Y., Stroud J. C., Wang Z. Z., Chen L. (2007) Nat. Neurosci. 10, 953–962 [DOI] [PubMed] [Google Scholar]
- 9.Brejc K., van Dijk W. J., Klaassen R. V., Schuurmans M., van Der Oost J., Smit A. B., Sixma T. K. (2001) Nature 411, 269–276 [DOI] [PubMed] [Google Scholar]
- 10.Auerbach A. (2010) J. Physiol. 588, 573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamamizu S., Todd A. P., McNamee M. G. (1995) Cell. Mol. Neurobiol. 15, 427–438 [DOI] [PubMed] [Google Scholar]
- 12.Lee W. Y., Sine S. M. (2005) Nature 438, 243–247 [DOI] [PubMed] [Google Scholar]
- 13.Xiu X., Hanek A. P., Wang J., Lester H. A., Dougherty D. A. (2005) J. Biol. Chem. 280, 41655–41666 [DOI] [PubMed] [Google Scholar]
- 14.Purohit P., Auerbach A. (2007) J. Gen. Physiol. 130, 559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X. Q., Zhang L., Stewart R. R., Weight F. F. (2003) J. Biol. Chem. 278, 46583–46589 [DOI] [PubMed] [Google Scholar]
- 16.Kash T. L., Dizon M. J., Trudell J. R., Harrison N. L. (2004) J. Biol. Chem. 279, 4887–4893 [DOI] [PubMed] [Google Scholar]
- 17.Mercado J., Czajkowski C. (2006) J. Neurosci. 26, 2031–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castaldo P., Stefanoni P., Miceli F., Coppola G., Del Giudice E. M., Bellini G., Pascotto A., Trudell J. R., Harrison N. L., Annunziato L., Taglialatela M. (2004) J. Biol. Chem. 279, 25598–25604 [DOI] [PubMed] [Google Scholar]
- 19.Mukhtasimova N., Sine S. M. (2007) J. Neurosci. 27, 4110–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakrapani S., Bailey T. D., Auerbach A. (2003) J. Gen. Physiol. 122, 521–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purohit P., Auerbach A. (2007) J. Gen. Physiol. 130, 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosman C., Salamone F. N., Sine S. M., Auerbach A. (2000) J. Gen. Physiol. 116, 327–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bafna P. A., Purohit P. G., Auerbach A. (2008) PLoS One 3, e2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitra A., Cymes G. D., Auerbach A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha A., Purohit P., Auerbach A. (2009) Biophys. J. 96, 4075–4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee W. Y., Free C. R., Sine S. M. (2009) J. Neurosci. 29, 3189–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jha A., Cadugan D. J., Purohit P., Auerbach A. (2007) J. Gen. Physiol. 130, 547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auerbach A., Sigurdson W., Chen J., Akk G. (1996) J. Physiol. 494, 155–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin F., Auerbach A., Sachs F. (1996) Biophys. J. 70, 264–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadugan D. J., Auerbach A. (2010) Biophys. J. 99, 798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cymes G. D., Grosman C., Auerbach A. (2002) Biochemistry 41, 5548–5555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakrapani S., Auerbach A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]