Abstract
Proper folding of the Na,K-ATPase β subunits followed by assembly with the α subunits is necessary for their export from the endoplasmic reticulum (ER). Here we examine roles of the ER lectin chaperone, calnexin, and non-lectin chaperone, BiP, in folding and quality control of the β1 and β2 subunits in Madin-Darby canine kidney cells. Short term prevention of glycan-calnexin interactions by castanospermine slightly increases ER retention of β1, suggesting minor involvement of calnexin in subunit folding. However, both prolonged incubation with castanospermine and removal of N-glycosylation sites do not affect the α1-assembly or trafficking of β1 but increase the amount of the β1-bound BiP, showing that BiP can compensate for calnexin in assisting β1 folding. In contrast to β1, prevention of either N-glycosylation or glycan-calnexin interactions abolishes the α1-assembly and export of β2 from the ER despite increased β2-BiP binding. Mutations in the α1-interacting regions of β1 and β2 subunits impair α1 assembly but do not affect folding of the β subunits tested by their sensitivity to trypsin. At the same time, these mutations increase the amount of β-bound BiP but not of β-bound calnexin and increase ER retention of both β-isoforms. BiP, therefore, prevents the ER export of folded but α1-unassembled β subunits. These α1-unassembled β subunits are degraded faster than α1-bound β subunits, preventing ER overload. In conclusion, folding of the β1 and β2 subunits is assisted predominantly by BiP and calnexin, respectively. Folded β1 and β2 either assemble with α1 or bind BiP. The α1-bound β subunits traffic to the Golgi, whereas BiP-bound β subunits are retained and degraded in the ER.
Keywords: Glycosylation; Lectin; Na,K-ATPase; Protein Folding; Trafficking; BiP; Na,K-ATPase Beta Subunits; Calnexin; Chaperones; Protein Quality Control
Introduction
Recent studies have shown that N-glycans of newly synthesized glycoproteins serve as recognition tags for the ER2 lectin chaperones, which assist maturation of these proteins (1–3). Calnexin and UGGT1 (UDP-glucose glycoprotein glucosyltransferase 1) are the two key components of the ER lectin quality control systems. Calnexin binding facilitates co-translational protein folding with help from folding enzymes (1, 3). Many proteins complete their folding program at this point. The properly folded molecules are then exported to the Golgi, whereas terminally misfolded conformers are retained and later degraded. However, other proteins require additional rounds of calnexin-assisted folding to achieve their native conformation. UGGT1 acts as a conformation sensor by reglucosylating incompletely folded glycoproteins and targeting them to additional cycles of calnexin-assisted refolding (1, 3). VIP36 (vesicular integral membrane protein) and ERGIC53 (ER-Golgi intermediate compartment) assist export of the properly folded glycoproteins from the ER to the Golgi. Other lectins, including EDEM-1 (ER degradation enhancer, mannosidase α-like 1), target terminally misfolded glycoproteins to ER-associated degradation (4).
N-Glycans are added co-translationally to the asparagines of N-glycosylation sites of nascent glycoproteins. Immediately after covalent linkage of the N-glycan, which initially contains 14-monosaccharide residues, the ER enzymes start trimming this structure by sequential removal of three glucose resides by glucosidases I and II, followed by removal of up to four mannose residues by the ER mannosidase I (2, 4). Trimming of mannose residues can be delayed by UGGT1 when it adds back a glucose residue to deglucosylated N-glycans, thus promoting repeated cycles of deglucosylation and reglucosylation of N-glycans (3, 5–7). Each step of N-glycan modification in the ER results in specific recognition of the N-glycan structure by a particular ER lectin (8). For example, the monoglucosylated N-glycans have a high affinity for calnexin, whereas deglucosylated N-glycans bind UGGT1. The coupling of each N-glycan modification reaction to recognition by a particular lectin establishes a strict order of lectin-assisted steps of protein folding and quality control. As a result, only native conformers and, in the case of proteins with several subunits, only properly assembled oligomers exit the ER and reach their final destinations.
In addition to lectin chaperones, the ER also contains variety of non-lectin chaperones, such as heat shock protein homologues (1, 9). These chaperones assist maturation of non-glycosylated proteins synthesized in the ER and also compete with lectin chaperones for binding to newly synthesized glycoproteins. A central player in the non-lectin chaperone system is GRP78 (BiP) (9, 10). This is the most abundant ER chaperone. BiP has multiple functions. It assists translocation of the nascent chains into the ER lumen, acting as a molecular ratchet (11). BiP also enables co-translational folding of nascent proteins by recruiting protein-disulfide isomerases and peptidyl-prolyl cis-trans isomerases (12). BiP binds properly folded subunits of multisubunit proteins to prevent their early exit from the ER prior to full assembly of an oligomeric complex (1, 13). BiP can also bind to terminally misfolded proteins and facilitate their disposal (1). Thus, BiP binds to nascent, incompletely folded, properly folded, and terminally misfolded proteins. The specificity of BiP binding to a particular form is determined by accessory co-chaperones, such as ERdjs (9, 14, 15).
The Na,K-ATPase is an essential transport enzyme expressed in all animal tissues, where it generates ion gradients to maintain membrane potential and drive transport of other solutes. In addition to pumping ions, the Na,K-ATPase is important in signal transduction (16, 17), intercellular adhesion and cell migration (18–22). The minimal functional unit of the Na,K-ATPase consists of a catalytic α subunit and an N-glycosylated β subunit, which is required for maturation and membrane targeting of the enzyme. There are four isoforms of the Na,K-ATPase α subunit (α1, α2, α3, and α4) and three isoforms of the Na,K-ATPase β subunit (β1, β2, and β3) (23, 24). N-Glycans and their interaction with the ER lectin chaperone, calnexin, are essential for maturation of the β2 subunit, which has eight N-glycans, but not of the β1 subunit, which has only three N-glycans. Either removal of N-glycosylation sites or prevention of glycan-calnexin interactions abolishes α-assembly and export of the β2, but not of the β1 subunit, from the ER in renal MDCK cells (25).
In their proper conformation, both β1 and β2 subunits assemble with the α subunit, which allows the export of the α-β complexes from the ER to the Golgi and delivery to the plasma membrane (26–28). Various Na,K-ATPase α subunit isoforms are retained in the ER when not assembled with any of the β subunits (22, 29, 30). Also, α-unassembled β subunits are retained in the ER in amphibian and mammalian cells; the β1 subunit is retained in Xenopus oocytes and MDCK cells (26, 28); the β2 subunit is retained in MDCK cells (28); and the β3 subunit is retained in Xenopus oocytes (27). Thus, only functional α-β complexes are exported from the ER. Accordingly, the α and β subunits are present in equimolar amounts not only in purified Na,K-ATPase preparations (31–34) but also in the plasma membrane fractions (35, 36) or total microsomal membrane fractions (37–40) isolated from various cells. Further, when the ratio between newly synthesized α and β subunits is changed by overexpression of additional β subunits in MDCK cells, only the α-β complexes assembled at 1:1 stoichiometry reach the plasma membrane, whereas the α-unassembled β subunits are retained in the ER and rapidly degraded (28).
Although these data provide insight into the mechanism of maturation of the Na,K-ATPase, they also raise additional questions. First, it is not known which ER chaperone(s) are responsible for folding of the β1 subunit. The relative insignificance of N-glycosylation for maturation of the β1 subunit suggests involvement of non-lectin chaperone(s) in its folding in the ER. Second, it is not clear how the ER quality control system ensures that only the assembled α-β Na,K-ATPase is delivered to the plasma membrane. Earlier studies suggested that the ER chaperones, BiP and calnexin, are involved in the ER retention of α1, β1, and β3 subunits in Xenopus oocytes (41–43). However, the mechanism of the ER retention of unassembled Na,K-ATPase subunits in mammalian cells has not been studied.
In the present study, we used renal MDCK cells as an expression system to study interactions of YFP-linked β1 and β2 subunit isoforms of the Na,K-ATPase (YFP-β1 and YFP-β2) with calnexin, BiP and the endogenous α1 subunit by co-immunoprecipitation. We also monitored trafficking of YFP-linked subunits by confocal microscopy and analyzed the nature of N-linked glycans of the subunits to determine their localization either in the ER or in the post-ER compartments. The results show that, in contrast to the β2 subunit, which requires prolonged interaction with calnexin to achieve its α-assembly-competent conformation, the β1 subunit undergoes rapid maturation that is mainly assisted by BiP. Consistent with distinct preferences for the major ER chaperones, the two subunits differentially respond to the ER stress. Further, the data show that binding to BiP, but not to calnexin, prevents premature export of properly folded but α-unassembled β1 and β2 subunits from the ER.
EXPERIMENTAL PROCEDURES
Construction of MDCK Stable Cell Lines
The YFP-linked rat β1 or human β2 subunits of the Na,K-ATPase and their mutants were constructed as described previously (20, 25). Stable MDCK cell lines expressing wild type and mutated YFP-β1 and YFP-β2 were obtained as described previously (44).
Cell Culture
Cells were grown in DMEM medium (Cellgro Mediatech) containing 4.5 g/liter glucose, 2 mm l-glutamine, 8 mg/liter phenol red, 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 10% FBS unless specified otherwise.
Confocal Microscopy
Confocal microscopy images were acquired using the Zeiss LSM 510 laser-scanning confocal microscope and LSM 510 software, version 3.2.
Primary Antibodies
The following monoclonal antibodies were used for immunoprecipitation and/or Western blot analysis: against the Na,K-ATPase α1 subunit, clone C464.6 (Millipore); against GFP, clones 7.1 and 13.1, which also recognizes YFP (Roche Applied Science); and against the Na,K-ATPase β1 subunit, clone M17-P5-F11 (Affinity Bioreagents). Also, polyclonal antibodies against the Na,K-ATPase α1 subunit (Cell Signaling), against calnexin (Abcam), against BiP (Abcam), and against GFP, which also recognizes YFP (Clontech), were used.
Immunofluorescent Staining of MDCK Cells
Cells were fixed by incubation with 3.75% formaldehyde, which was prepared by diluting formalin in PBS, for 15 min at 37 °C. After three consecutive washes in PBS, 5 min each, cells were permeabilized by incubation with 0.1% Triton X-100 for 5 min. Then cells were incubated with Dako protein block serum-free solution (Dako Corp.) for 30 min. Immunostaining of the Na,K-ATPase α1 subunit was performed by 1-h incubation with the monoclonal antibody against the Na,K-ATPase α1 subunit followed by a 1-h incubation with Alexa633-conjugated anti-mouse (Invitrogen).
Transient Transfection of MDCK Cells with the Fluorescent Marker of the ER
Cells grown on glass bottom microwell dishes (MatTek Corp.) were transfected with the plasmid encoding a fusion protein between Discosoma sp. red fluorescent protein and the marker of the ER, DsRed2-ER (Clontech) using the Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's instructions. Confocal microscopy images of transfected cells were acquired 24–48 h after transfection.
Immunoprecipitation
Monolayers of MDCK cells grown in two or three 35-mm2 wells of a 6-well plate were rinsed twice with ice-cold PBS and lysed by incubation with 200 μl/well 150 mm NaCl in 50 mm Tris, pH 7.5, containing 1% Nonidet P-40, 0.5% sodium deoxycholate, and Complete protease inhibitor mixture (1 tablet/50 ml) (Roche Applied Science). Cell extracts were clarified by centrifugation (15,000 × g, 10 min) at 4 °C. Then the cell extracts were incubated with 50 μl of the protein A-agarose or protein G-agarose suspension (Roche Applied Science) in a total volume of 1 ml of the lysis buffer at 4 °C with continuous rotation for at least 3 h (or overnight) to remove the components that nonspecifically bind to protein A or G. Protein A was used when immunoprecipitation was performed by using polyclonal antibodies against the Na,K-ATPase α1 subunit (Cell Signaling), whereas protein G was used when immunoprecipitation was performed by using monoclonal antibodies against GFP/YFP (Roche Applied Science) or against the Na,K-ATPase α1 subunit (Millipore). Precleared supernatant was mixed with 20 μl of the polyclonal antibodies against the Na,K-ATPase α1 subunit, 2 μl of the monoclonal antibodies against the Na,K-ATPase α1 subunit, or 3 μl of the monoclonal antibodies against GFP/YFP and incubated with continuous rotation at 4 °C for 60 min. After the addition of 50 μl of the protein A-agarose or protein G-agarose suspension, the mixture was incubated at 4 °C with continuous rotation overnight. The bead-adherent complexes were washed on the beads first with the lysis buffer and then with 500 mm NaCl in 50 mm Tris, pH 7.5, containing 0.1% Nonidet P40 and 0.05% sodium deoxycholate and finally with 10 mm Tris, pH 7.5, containing 0.1% Nonidet P-40 and 0.05% sodium deoxycholate. The bead-adherent complexes obtained from two or three wells were collected in one tube, and then proteins were eluted from the beads by incubation in 80–120 μl of SDS-PAGE sample buffer (4% SDS, 0.05% bromphenol blue, 20% glycerol, 1% β-mercaptoethanol in 0.1 m Tris, pH 6.8) for 5 min at 80 °C. Proteins eluted from the beads were separated by SDS-PAGE and analyzed by Western blot to detect immunoprecipitated and co-immunoprecipitated proteins. Proteins immunoprecipitated by the rabbit polyclonal antibody against the Na,K-ATPase α1 subunit were analyzed by using mouse monoclonal antibodies against the Na,K-ATPase α1 subunit and against GFP/YFP. Proteins immunoprecipitated by the mouse monoclonal antibodies against the Na,K-ATPase α1 subunit were analyzed by using rabbit polyclonal antibodies against BiP. Proteins immunoprecipitated by the mouse monoclonal antibodies against GFP/YFP were analyzed by using rabbit polyclonal antibodies against GFP/YFP, against the Na,K-ATPase α1 subunit, against BiP, and against calnexin.
Western Blot Analysis of the Total and Immunoprecipitated Proteins of MDCK Cells
Samples containing 5–20 μl of the MDCK cell extract mixed with the equal volume of SDS-PAGE sample buffer or 10–30 μl of proteins eluted from the protein A/G-conjugated agarose beads were loaded onto 4–12% gradient SDS-polyacrylamide gels (Invitrogen). Proteins were separated by SDS-PAGE using MES/SDS running buffer (0.05 m MES, 0.05 m Tris base, 0.1% SDS, and 1 mm EDTA, pH 7.3), transferred onto a nitrocellulose membrane (Bio-Rad), and detected by Western blot analysis using the appropriate primary antibody and the anti-mouse or anti-rabbit secondary antibody conjugated to alkaline phosphatase (Promega) or horseradish peroxidase (American Qualex). Alkaline phosphatase was detected using nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate in alkaline phosphatase buffer (150 mm NaCl, 1 mm MgCl2 in 10 mm Tris-HCl, pH 9.0). Horseradish peroxidase was detected by using a chemiluminescent Western blotting substrate (Pierce). Immunoblots were quantified by densitometry using Zeiss LSM 510 software, version 3.2.
Trypsin Digestion
Susceptibility of the mutated and wild type Na,K-ATPase β subunits to limited tryptic digestion was used to assess the effect of mutations on correct folding of the subunit. In order to prevent heterogeneity in N-glycan sizes, cells were treated with 100 μg/ml deoxymannojirimycin, an α-mannosidase 1 inhibitor, for 48 h prior to the trypsin digest experiment. Deoxymannojirimycin prevents synthesis of both complex- and hybrid type N-linked oligosaccharide chains; as a result, all newly synthesized N-glycans become high mannose type. Incubation with the inhibitor for 48 h was found to be sufficient to replace the vast majority of the existing YFP-linked β subunits by the newly synthesized high mannose type glycosylated forms of the subunits.
The monolayers of deoxymannojirimycin-treated cells were rinsed twice with ice-cold PBS and lysed by incubation with 200 μl of 150 mm NaCl in 50 mm Tris, pH 7.5, containing 1% Nonidet P-40 and 0.5% sodium deoxycholate. Protein concentration in cell lysates was determined using the BCA assay kit (Pierce) and adjusted to 3 mg/ml. Cell lysates were incubated with trypsin, which was added in the range of 0–3 μg/ml as indicated at 37 °C for 30 min. The reaction was stopped by the addition of 20 μg/ml trypsin inhibitor (Sigma-Aldrich) and incubation of samples on ice for 10 min. Then samples containing 20 μl of reaction mixture combined with 20 μl of SDS-PAGE sample buffer were incubated at 80 °C for 5 min, subjected to 4–12% SDS-PAGE, and analyzed by Western blotting using a monoclonal anti-YFP antibody.
Biotinylation of Basolateral Plasma Membrane Proteins of MDCK Cells to Determine the Rate of Degradation of the Mature Na,K-ATPase Subunits
Cells were maintained for 6 days after becoming confluent in transwell inserts. Biotinylation of surface proteins was performed according to procedures described previously (45, 46). Cell monolayers were biotinylated with EZ-LinkTM sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate (Pierce), which was added into the well only (basolateral surface of the tight cell monolayers). After quenching the biotinylation reaction, cells were washed on ice, incubated at 37 °C for the indicated periods of time, and then lysed by incubation with 200 μl of 0.15 m NaCl in 15 mm Tris, pH 8.0, with 1% Triton X-100 and 4 mm EGTA. Cell extracts were clarified by centrifugation (15,000 × g, 10 min) at 4 °C. To isolate surface-biotinylated proteins, the cell extract was incubated with 100 μl of streptavidin-agarose beads (Sigma-Aldrich) in a total volume of 1 ml of 0.15 m NaCl in 15 mm Tris, pH 8.0, with 0.5% Triton X-100 and 4 mm EGTA at 4 °C with continuous rotation for 60 min. The bead-adherent complexes were washed three times on the beads, and then proteins were eluted from the beads by incubation in 40 μl of SDS-PAGE sample buffer for 5 min at 80 °C and further analyzed by SDS-PAGE and immunoblotting.
Statistical Analysis
Statistical analysis was performed using Student's t test (GraphPad Prism 4 software and Microsoft Excel). Statistical significance is specified in the figure legends.
RESULTS
The β2 and β1 Subunits Discriminate between Different ER Chaperones
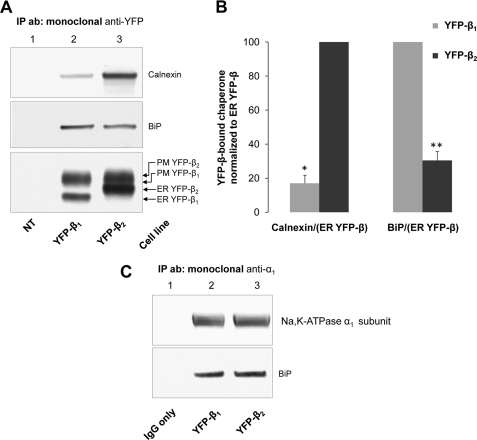
Glycan-calnexin interactions are essential for maturation of the Na,K-ATPase β2 subunit but not of the β1 subunit (25). To determine whether the β1 subunit employs a non-lectin chaperone system for its maturation in the ER, we evaluated its binding to BiP, using the co-immunoprecipitation technique. Both YFP-β1 and YFP-β2 immunoprecipitated from corresponding cell lysates using a monoclonal antibody against YFP were detected on SDS-PAGE as two bands (Fig. 1A, bottom). The lower bands represent the high mannose type glycosylated forms, which reside exclusively in the ER (28). The ER form of YFP-β1 migrated faster than the ER form of YFP-β2, consistent with the presence of three N-glycans in the β1 subunit (47) and eight N-glycans in the β2 subunit (25). The top bands represent the complex type glycosylated forms, which are formed due to the action of the Golgi-resident enzymes and are located predominantly in the plasma membrane (28). These plasma membrane forms of both YFP-β1 and YFP-β2 ran more slowly than their corresponding ER forms in SDS-PAGE, reflecting the increase in N-glycan size due to elongation and branching by the Golgi-resident glycosidases. If this increase in size were the same for each N-glycan of the two subunits, the difference in the gel mobility of the plasma membrane and ER forms would be greater for YFP-β2 (eight N-glycans) than for YFP-β1 (three N-glycans). However, this difference is even smaller in YFP-β2 than in YFP-β1 (Fig. 1), indicating that, on average, N-glycans of the β2 subunit undergo less elongation and branching in the Golgi of MDCK cells than the N-glycans of the β1 subunit.
FIGURE 1.
YFP-β1 and YFP-β2 preferentially interact with different ER chaperones. A, an anti-YFP antibody precipitates YFP-β1 and YFP-β2 (bottom) and co-precipitates BiP (middle) and calnexin (top) from the whole lysates of the YFP-β1- and YFP-β2-expressing cells (lanes 2 and 3) but not from the cell lysates of non-transfected MDCK cells (lane 1). B, densitometry quantification of the results shown in A. C, an anti-Na,K-ATPase α1 antibody precipitates the α1 subunit (top) and co-precipitates BiP (middle) from the whole lysates of the YFP-β1- and YFP-β2-expressing cells (lanes 2 and 3). No bands are detected in a negative control sample containing all of the components used for immunoprecipitation except for cell lysate (lane 1). Error bars, ± S.D. (n = 3); *, significant difference from YFP-β2; **, significant difference from YFP-β1; p < 0.001, Student's t test. IP ab, antibody used for immunoprecipitation; NT, non-transfected MDCK cells; PM, plasma membrane.
Both YFP-β1 and YFP-β2 co-precipitated BiP (Fig. 1A, bottom and middle). To compare the amounts of BiP bound to two subunits, we normalized the BiP signal to the YFP signal of the ER forms of YFP-β1 or YFP-β2 because the ER chaperone BiP can only bind to the ER forms of YFP-β. Quantification showed that the amount of YFP-β1-bound BiP was about 3-fold greater than the amount of YFP-β2-bound BiP (Fig. 1B). In agreement with previously published results (25), Western blot analysis of the same immunoprecipitated fractions using a polyclonal anti-calnexin antibody followed by the same normalizing procedure showed that the amount of YFP-β2-bound calnexin was 6-fold greater than the amount of YFP-β1-bound calnexin (Fig. 1, A (bottom and top, lanes 2 and 3) and B). BiP also co-immunoprecipitated with the Na,K-ATPase α1 subunit in both YFP-β1- and YFP-β2-expressing cells (Fig. 1C).
The Loss of N-Glycan-Calnexin Interactions Is Compensated for by Increased BiP Binding to the β1 Subunit
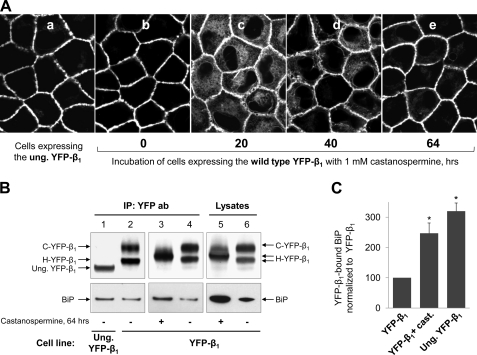
Removal of all three N-glycans from the β1 subunit did not affect its delivery to the plasma membrane in MDCK cells (20, 25, 48) (Fig. 2A, a and b). Paradoxically, cell incubation with the glucosidase inhibitor castanospermine, which blocks removal of the outermost glucose residue from N-glycans, preventing their interaction with calnexin, resulted in partial retention of the YFP-linked β1 subunit in the ER (25). Time lapse confocal microscopy of YFP-β1-expressing cells showed that the maximal ER retention of YFP-β1 was reached after 20 h of cell incubation with castanospermine (Fig. 2A, c). This intracellular retention gradually decreased after further incubation with the inhibitor (Fig. 2A, d and e). After 64 h, the majority of YFP-β1 was seen in the lateral membrane of MDCK cells, as in the control cells (Fig. 2A, b and e). After 72 h of cell incubation with castanospermine, cells started detaching from the surface (not shown).
FIGURE 2.
Both prolonged incubation of YFP-β1-expressing cells with castanospermine and the removal of N-glycosylation sites from YFP-β1 increase BiP binding to the subunit. A, confocal microscopy images of MDCK cells expressing the unglycosylated mutant of YFP-β1 (a) or the wild type YFP-β1, which were incubated in the absence (b) or in the presence (c–e) of 1 mm castanospermine for the indicated periods of time. To ensure constant inhibition by castanospermine during prolonged incubation, the cell culture medium containing fresh inhibitor was replaced every 24 h. B, the total cell lysate proteins or the proteins immunoprecipitated using the antibody against YFP were obtained from the cells expressing the wild type YFP-β1 or the unglycosylated mutant of YFP-β1 before or after 64-h cell incubation with 1 mm castanospermine. The proteins were analyzed by SDS-PAGE followed by immunoblotting using antibodies against YFP or BiP. C, densitometric quantification of the results shown in B. IP, immunoprecipitation; C-YFP-β1, complex type form of YFP-β1; H-YFP-β1, high mannose form of YFP-β1; Ung. YFP-β1, the unglycosylated mutant of YFP-β1. Error bars, ±S.D. (n = 3); *, significant difference from YFP-β1; p < 0.001, Student's t test.
Prolonged cell incubation with castanospermine up-regulated expression of a non-lectin chaperone, BiP, that increased by about 3-fold in total cell lysate (Fig. 2B, lanes 5 and 6). The amount of YFP-β1-bound BiP increased 2.5-fold (Fig. 2, B (lanes 3 and 4) and C). Similarly, a 3-fold increase in BiP immunoprecipitation was found due to mutation of all thee N-glycosylation sites in YFP-β1 (Fig. 2, B (lanes 1 and 2) and C). Thus, the lack of interaction of calnexin with the N-glycans of the β1 subunit, either due to the removal of N-glycosylation sites in the subunit or due to the presence of castanospermine, was compensated for by increased binding of BiP to the subunit.
The ER Residence Time of the β2 Subunit Is Longer than the ER Residence Time of the β1 Subunit
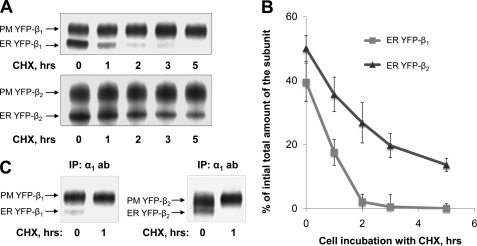
To compare the rates of degradation of YFP-β1 and YFP-β2, we determined how the abundance of the fusion proteins changed during cell incubation with CHX. The amount of the ER form of YFP-β2 decreased more slowly than the amount of the ER-resident YFP-β1 (Fig. 3A, ER YFP-β1 and ER YFP-β2 bands). The amount of the ER forms of YFP-β1 and YFP-β2 could decrease not only due to their degradation but also due to their trafficking to the Golgi followed by their transformation to the mature fully glycosylated forms. Only α1-assembled β subunits can be exported to the Golgi (28). Hence, to distinguish between degradation and trafficking to the Golgi, we determined what fraction of YFP-β1 and YFP-β2 molecules in the ER were assembled with the α1 subunit. The percentage of the ER form of YFP-β1 in the α1-immunoprecipitated fraction (Fig. 3C, left panel, left lane) was significantly less than the percentage of the ER form in the total fraction of YFP-β1 (Fig. 3A, top panel, left lane), consistent with previously published results (28). Therefore, the majority of the ER-resident YFP-β1 molecules were not assembled with the α1 subunit. The majority of the ER-resident YFP-β2 molecules were also not assembled with the α1 subunit as seen from the significantly lower percentage of the ER form of YFP-β2 in the α1-immunoprecipitated fraction (Fig. 3C, right panel, left lane) as compared with the percentage of the ER YFP-β2 form in the total cell lysate (Fig. 3B, bottom panel, left lane). The α1-assembled ER forms of both YFP-β1 and YFP-β2 were not detected after 1 h of cell incubation with CHX (Fig. 3C). Therefore, in the absence of protein synthesis, these α1-assembled β subunits were rapidly exported to the Golgi, where they were converted to the complex type forms. Also, the results show that after 1 h of cell incubation with CHX, there were no spare α1 subunits left in the ER to form new α-β complexes.
FIGURE 3.
The ER-resident YFP-β2 is degraded more slowly than ER-resident YFP-β1. A, cells expressing YFP-β1 or YFP-β2 were incubated in the presence of 20 μg/ml CHX for the indicated time periods and lysed. To separate the high mannose form of YFP-β2 from its complex type form on SDS-PAGE, immunoprecipitated YFP-β2 was treated with endoglycosidase H (EndoH) to transform the high mannose form to the deglycosylated form prior to SDS-PAGE. B, densitometry of the results presented in A. C, the proteins immunoprecipitated using the antibody against the Na,K-ATPase α1 subunit were obtained from the YFP-β1- and YFP-β2-expressing cells before or after a 1-h cell incubation with 20 μg/ml CHX and then were analyzed by SDS-PAGE followed by immunoblotting using the antibodies against YFP. Error bars, ± S.D. (n = 3); IP, immunoprecipitation; PM, plasma membrane.
Therefore, the α1-unassembled β subunits represented the vast majority of the ER forms before cell incubation with CHX and all of the ER forms after 1 h of cell incubation with CHX. Accordingly, the decrease in the content of the ER forms of the β subunits in the 5 h of cell incubation with CHX mainly reflected degradation of these unassembled subunits. Thus, the α1-unassembled YFP-β1 was degraded more rapidly than the α1-unassembled YFP-β2 (Fig. 3, A (ER YFP-β1 and ER YFP-β2 bands) and B).
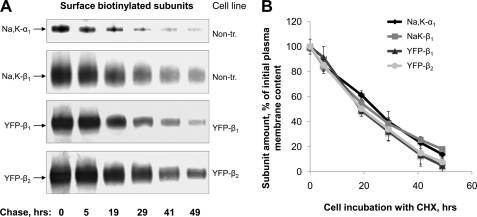
In contrast, the amount of the plasma membrane-resident forms of YFP-β1 and YFP-β2 was hardly changed after 5 h of incubation with CHX (Fig. 3A, PM YFP-β1 and PM YFP-β2 bands). Because longer incubation with CHX could result in toxic effects, we applied a different approach to determine the degradation rates of the plasma membrane forms. YFP-β1 and YFP-β2, as well as endogenous Na,K-ATPase subunits present on the basolateral surface, were labeled with a membrane-impermeable biotinylation reagent. After cell incubation at 37 °C for the indicated periods of time, the biotinylated proteins were isolated and analyzed by SDS-PAGE followed by immunoblotting (Fig. 4A). Quantification of biotinylated proteins showed that the amounts of the endogenous α1 and β1 subunits of the Na,K-ATPase and expressed YFP-β1 and YFP-β2 each decreased at a similar rate (Fig. 4B).
FIGURE 4.
The plasma membrane-resident Na,K-ATPase subunits are degraded at a similar rate. A, cell monolayers of non-transfected, YFP-β1-expressing, and YFP-β2-expressing MDCK cells were biotinylated from the basolateral side using a membrane-impermeable biotinylation reagent and then were chased at 37 °C for the indicated time periods. After cell lysis, the biotinylated proteins were isolated and analyzed by SDS-PAGE followed by immunoblotting using the antibodies against the α1 and β1 subunits of the Na,K-ATPase and against YFP. B, densitometry of the results presented in A. Error bars, ±S.D. (n = 3); PM, plasma membrane; Na,K-α1 and Na,K-β1, the endogenous α1 and β1 subunits of the Na,K-ATPase; Non-tr., non-transfected MDCK cells.
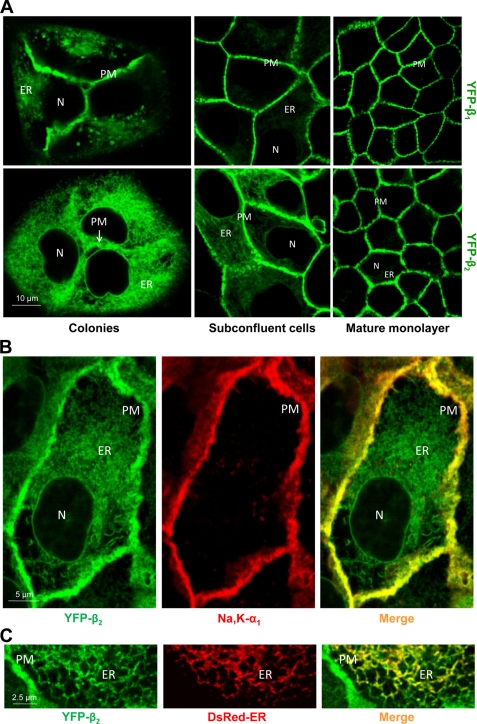
The ER retention of YFP-β2 was much more prominent than ER retention of YFP-β1 in all stages of cell monolayer development (Fig. 5A). ER retention of YFP-β1 and YFP-β2 was particularly evident in small colonies and also in subconfluent monolayers (Fig. 5A, left and middle). Only the plasma membrane fraction of YFP-β2 was co-localized with the α1 subunit, whereas the intracellular fractions of both YFP-β1 and YFP-β2 were co-localized with a marker of the ER (only YFP-β2 is shown; Fig. 5, B and D), consistent with the export of only α1-assembled β subunits from the ER. During formation of the mature cell monolayer, the ER retention gradually decreased, so that, in the mature cell monolayers, both subunits were mainly located in the lateral membranes of MDCK cells (Fig. 5A, right panels).
FIGURE 5.
The unassembled YFP-linked β2 subunit, but not β1 subunit, is largely retained in the ER in MDCK cells grown at low density. A, confocal microscopy images of YFP-β1- and YFP-β2-expressing cells showing that intracellular retention of both fusion proteins, but especially of YFP-β2, gradually decreases during development of the mature cell monolayers. B, YFP-β2 (green) is co-localized with the endogenous α1 subunit (red) in the lateral membranes but not inside the cells as detected by immunostaining of subconfluent YFP-β2-expressing cells using the monoclonal antibody against the Na,K-ATPase α1 subunit. C, the intracellular fraction of YFP-β2 (green) co-localized with the ER (red) as detected by transient expression of the fluorescent ER marker DsRed2-ER in subconfluent YFP-β2-expressing cells. N, nucleus; PM, plasma membrane; Na,K-α1, the endogenous Na,K-ATPase α1 subunit.
BiP Binds the Properly Folded α1-Unassembled β Subunits and Prevents Their Premature Export from the ER to the Golgi
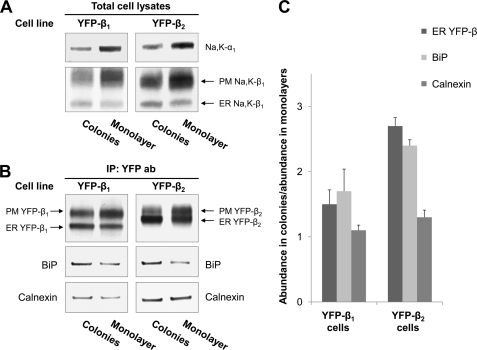
To test whether a gradual decrease in the ER retention of YFP-β1 and YFP-β2 during development of the cell monolayer (Fig. 5) was due to the changes in the abundance of the endogenous Na,K-ATPase α1 and β1 subunits, cell lysates isolated from small colonies were compared with those isolated from mature cell monolayers. The amount of endogenous α1 and β1 subunits was significantly less in colonies than in the mature monolayers in both YFP-β-expressing cell lines (Fig. 6A). As expected, the total amount of the expressed YFP-linked β subunits, which are under the control of the unregulated promoter, did not change (Fig. 6B). As a result, in colonies, the smaller fraction of YFP-linked β subunits was assembled with α1 subunits and delivered to the plasma membrane, and, accordingly, more β subunits were retained in the ER. Thus, a decrease in the α1 subunits in colonies as compared with monolayers resulted in a decrease in the amount of the plasma membrane forms of YFP-linked β subunits (Fig. 6B, top panels). A similar pattern was observed for the endogenous β1 subunit; its amount was decreased in the plasma membrane, whereas it increased in the ER in colonies as compared with monolayers (Fig. 6A, lower panels).
FIGURE 6.
The degree of the ER retention of YFP-linked β1 and β2 subunits correlates with BiP binding to the subunits. A, equal amounts of total lysates of YFP-β1- and YFP-β2-expressing cells (6 μg of protein) obtained from small colonies or from mature cell monolayers were analyzed by SDS-PAGE followed by immunoblotting using the antibodies against α1 and β1 subunits of the Na,K-ATPase. B, equal amounts of total lysates of YFP-β1- and YFP-β2-expressing cells (600 μg of protein) obtained from small colonies or from mature cell monolayers were used for immunoprecipitation of YFP-linked subunits. Immunoprecipitated YFP-β1 and YFP-β2, as well as co-immunoprecipitated BiP and calnexin, were analyzed by immunoblotting. C, densitometry quantification of the results shown in B. IP, immunoprecipitation; PM, plasma membrane.
To evaluate the possible involvement of BiP and/or calnexin in ER retention of unassembled β1 and β2 subunits, we compared the amounts of YFP-β-bound chaperones in colonies and mature monolayers. In both YFP-β1- and YFP-β2-expressing cells, the relative amount of the ER form of YFP-β was greater in colonies than in monolayers, which correlated with the greater amount of YFP-β-bound BiP (Fig. 6, B (top and middle panels) and C). On the other hand, the amount of YFP-β-bound calnexin was similar in colonies and monolayers in either cell line (Fig. 6, B (bottom) and C). Therefore, the ER retention of the α1-unassembled β subunits is due to their binding to BiP but not to calnexin.
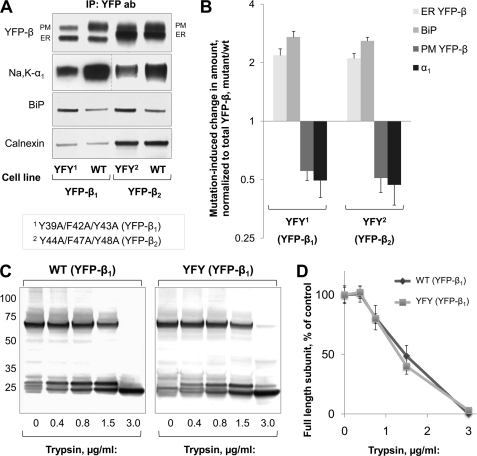
If BiP is involved in the ER retention of unassembled β1 and β2 subunits, the mutagenic impairment of α-β association should stimulate binding of the β subunits to this chaperone. In both YFP-β1 and YFP-β2, triple mutations in the α1-interacting regions of their transmembrane domains resulted in partial retention of the subunits in the ER as seen from the mutation-induced increase in the relative content of the ER-resident forms of YFP-β (Fig. 7A, YFP-β panel, ER bands), consistent with previously published data for YFP-β2 (28). Mutations also resulted in a significant decrease in co-immunoprecipitation of the α1 subunit with YFP-β (Fig. 7A, Na,K-α1 panel), indicating the impairment of α-β association. A loss in co-immunoprecipitation of the α1 subunit correlated with a decrease in the relative content of the plasma membrane forms of the β subunits (Fig. 7, A (YFP-β panel, PM bands) and B). This impairment of α-β association by mutations was not a result of global misfolding of the subunits because mutations did not change susceptibility to limited tryptic digestion of YFP-β2 (28) or YFP-β1 (Fig. 7, C and D).
FIGURE 7.
Impairment of α-β association due to point mutations in the α-interacting regions of the β1 and β2 subunits correlates with increased BIP binding to both β1 and β2 subunits. A, alanine substitutions of Tyr39, Phe42, and Tyr43 in YFP-linked rat β1 subunit produced a triple mutant Y39A/F42A/Y43A (YFY1) of YFP-β1. The residues homologous to Tyr39, Phe42, and Tyr43 were also mutated in YFP-linked human β2 subunit, producing a triple mutant, Y44A/F47A/Y48A (YFY2) of YFP-β2. The proteins were immunoprecipitated from lysates of the cells expressing the wild type YFP-β1 or YFP-β2 (WT) or their mutants (YFY) using the antibody against YFP and were analyzed by SDS-PAGE followed by immunoblotting using the antibodies against YFP, the Na,K-ATPase α1 subunit, BiP, and calnexin. B, densitometry quantification of the results shown in A. C, the products of tryptic digestion of the whole lysates of the cells expressing the wild type or mutated YFP-β1 were analyzed by immunoblotting using the antibody against YFP as described under “Experimental Procedures.” D, densitometry quantification of the results shown in C. Error bars, ±S.D. (n = 3); PM, plasma membrane.
The amount of BiP that co-precipitated with the β subunits was significantly increased by these mutations (Fig. 7A, BiP panel). This increase correlated with the increase in the relative content of the ER-resident forms of the β subunits in the mutants (Fig. 7B). On the other hand, the amount of calnexin that was co-immunoprecipitated with YFP-β1 and YFP-β2 was not changed by these mutations (Fig. 7A, calnexin panel). Thus, impairment of association with the α1 subunit increased association of the β subunits with BiP but not with calnexin. These results are consistent with involvement of BiP, but not of calnexin, in ER retention of properly folded but α1-unassembled β subunits.
Maturation and Trafficking of the Na,K-ATPase β1 and β2 Subunits during Cd2+-induced ER Stress
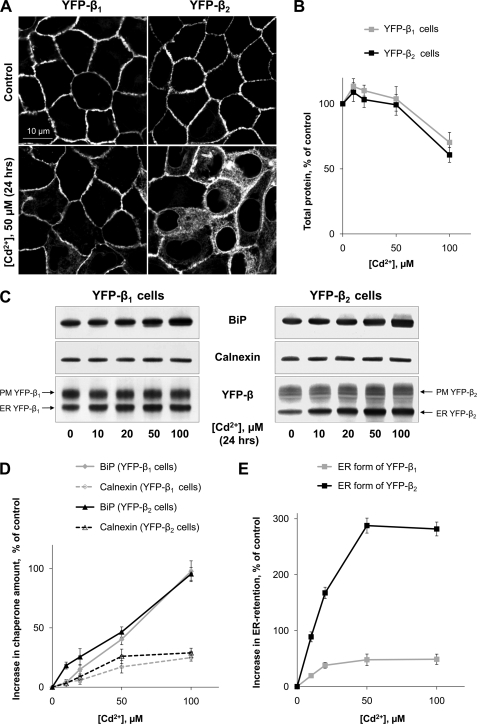
Distinct preferences of the β1 and β2 subunits to BiP and calnexin might suggest differential resistance of two subunits to the ER stress. Cd2+ is a nephrotoxic agent, which is known to induce ER stress in vivo and in cultured cells (49, 50). Exposure of YFP-β1- and YFP-β2-expressing MDCK cells to 50 μm Cd2+ for 24 h resulted in a significant and minor ER retention of YFP-β2 and YFP-β1, respectively, as detected by confocal microscopy (Fig. 8A). The greater ER retention of YFP-β2 than YFP-β1 was also evident from a Western blot analysis of both subunits in total lysates of cells exposed to various Cd2+ concentrations (Fig. 8C). The degree of retention of both fusion proteins gradually increased with an increase in concentration of Cd2+ in the culture medium and reached its maximum at 50 μm (Fig. 8E). The amount of total protein did not change with increasing Cd2+ concentrations up to 50 μm and was decreased by 30–40% in both cell types at 100 μm of Cd2+ (Fig. 8B). The decrease in the protein amount at 100 μm Cd2+ was apparently due to increased cell death, as confirmed by confocal microscopy (not shown). In both YFP-β1- and YFP-β2-expressing cells, Cd2+ increased the total amount of BiP in a dose-dependent manner, doubling the amount of this chaperone at 100 μm (Fig. 8, C and D). The amount of calnexin only slightly increased with increasing concentrations of Cd2+ in both cell types (Fig. 8, C and D). Therefore, exposure of both YFP-β1-and YFP-β2-expressing cells to Cd2+ resulted in significant up-regulation of BiP but not of calnexin. In these conditions, YFP-β2, but not YFP-β1, was largely retained in the ER. This finding shows that the Na,K-ATPase β1 subunit is more resistant to Cd2+-induced ER stress than the β2 subunit. In addition, these results confirm that BiP is important for proper folding of the β1 rather than β2 subunit.
FIGURE 8.
The Na,K-ATPase β1 subunit is more resistant to Cd2+-induced ER stress than the Na,K-ATPase β2 subunit. Confluent monolayers of MDCK cells expressing either YFP-β1 or YFP-β2 grown in glass bottom dishes were incubated in DMEM in the absence or presence of 10, 20, 50, or 100 μm CdCl2 for 24 h. The cells were analyzed by confocal microscopy and then lysed to determine the amount of total protein, BiP, calnexin, and YFP-β1 or YFP-β2 as described under “Experimental Procedures.” A, confocal microscopy images of MDCK cell monolayers showing that incubation with 50 μm Cd2+ for 24 h resulted in significant ER accumulation of YFP-β2 but not of YFP-β1. B, in both YFP-β1- and YFP-β2-expressing cells, incubation with 100 μm Cd2+ for 24 h resulted in a significant decrease in total protein, whereas lower concentrations of Cd2+ had no effect. C, Western blot analysis of BiP, calnexin, and YFP-β in total lysates of YFP-β1- and YFP-β2-expressing cells incubated at various concentrations of Cd2+ for 24 h. To separate the high mannose of YFP-β2 from its complex type form on SDS-PAGE, cell lysates of YFP-β2-expressing cells were treated with endoglycosidase H to transform the high mannose form to the deglycosylated form prior to SDS-PAGE. D and E, densitometry quantification of the results presented in C. Error bars, ±S.D. (n = 3).
DISCUSSION
The β1 Subunit and β2 Subunit of the Na,K-ATPase Follow Separate Chaperone-assisted Pathways of Maturation in the ER
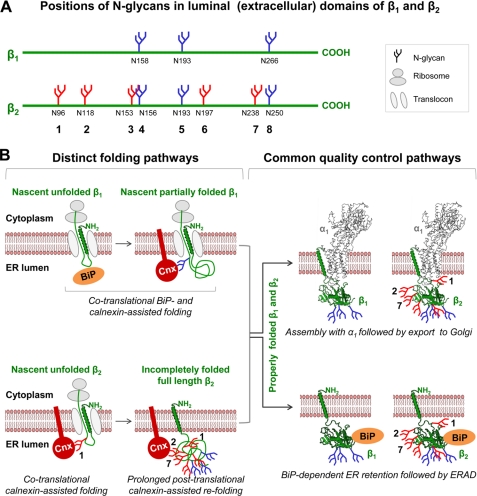
Both the β1 subunit, which has three N-glycans, and the β2 subunit, which has eight N-glycans, interact with calnexin in an N-glycan-dependent mode (25). However, the amount of calnexin that co-immunoprecipitates with YFP-β1 subunit is 6-fold less than the amount of YFP-β2-bound calnexin (25) (Fig. 1). Also, the interaction of the β2 subunit with calnexin continues for several h after completing translation, whereas the β1 subunit interacts with calnexin only transiently (25) (Fig. 9). The two N-glycans that are critical for posttranslational β2-calnexin binding are unique for this subunit (Fig. 9).
FIGURE 9.
A model illustrating differences and similarities in the maturation pathways of the Na,K-ATPase β1 and β2 subunits. A, positions of asparagine residues of N-glycosylation sites (N158, N193, etc.) and corresponding N-linked glycans (consecutively numbered 1–8) in the primary structures of the luminal domains of the Na,K-ATPase β1 and β2 subunits are compared based on the computational alignment (Vector NTI, 8.0) of the rat β1 subunit (NM_013113)and the human β2 subunit (NM_001678). The β1 subunit has three N-glycans (blue), whereas the β2 subunit has eight N-glycans, including three β1-homologous N-glycans (blue) and five β2-unique N-glycans (red). B, the Na,K-ATPase β1 and β2 subunits (both shown in green) are type II transmembrane proteins. During translation, the hydrophobic sequences of their future transmembrane domains direct insertion of the nascent polypeptide chains into the ER membrane so that the N termini stay in the cytosol, whereas the C-terminal portions of the growing chains (future extracellular domains) are extruded through the translocon into the ER lumen. The nascent β1 subunit lacking N-glycans in the beginning of its luminal domain initially binds to BiP during translocation. Later, when N-glycosylation sites are translocated into the ER lumen and occupied by N-glycans, BiP is replaced by calnexin (Cnx). In contrast, the nascent β2 subunit, which contains Asn96-linked N-glycan (1) in the very N-terminal region of its luminal domain, binds directly to calnexin during translation. The β2, but not the β1, subunit continues to bind calnexin for several h after completing translation; two of the eight N-glycans (2 and 7) are essential for persistent calnexin binding to the β2 subunit (25). The homologs of these two N-glycans are absent in the β1 subunit, consistent with the lack of posttranslational calnexin binding to this subunit. Once properly folded, the β1 and β2 subunits follow common maturation and trafficking pathways. They either assemble with the α1 to form a functional Na,K-ATPase or bind BiP. The α1-assembled β subunits are exported to the Golgi, whereas the BiP-bound β subunits are retained in the ER and eventually destroyed via ER-associated degradation. Models of the folded Na,K-ATPase subunits were created using Cn3D 4.1 based on the high resolution structure of the Na,K-ATPase for α1 and β1 (64) and on the three-dimensional homology model for β2 (25). Positions of N-glycosylation sites in the β subunits were determined based on these structures. N-Glycans linked to N-glycosylation sites, as well as unfolded luminal domains of β subunits, were drawn in arbitrary sizes and orientations.
N-Glycan-calnexin interactions are vital for correct folding of the β2 subunit (25). Both removal of N-glycosylation sites and prevention of glycan-calnexin interaction by castanospermine resulted in full ER retention of the β2 subunit (25). In contrast, the prevention of N-glycan-calnexin interactions by castanospermine led to only minor ER retention of YFP-β1. Moreover, this retention gradually decreased down to control levels with prolonged incubation with the inhibitor (Fig. 2A). Therefore, glycan-calnexin interactions play a minor role in normal maturation of the β1 subunit. On the other hand, the YFP-β1 subunit binds to BiP 3-fold more avidly than YFP-β2 (Fig. 1), suggesting that folding of the β1 subunit is assisted by this non-lectin chaperone rather than by calnexin.
BiP is the first chaperone to bind the nascent chains of virtually all non-glycosylated proteins synthesized in the ER. BiP also binds initially to most glycosylated proteins with the exception of the glycoproteins that have N-glycans within about 50 N-terminal luminal amino acid residues (1, 51). These glycoproteins bind to calnexin during translocation without prior BiP binding (1, 51). The N-glycan 1 of the β2 subunit is located within 30 N-terminal residues of its luminal domain, whereas the β1 subunit lacks N-glycans within 95 N-terminal residues of its luminal domain (Fig. 9A). Therefore, the nascent β2 subunit binds directly to calnexin, whereas the nascent β1 subunit binds initially to BiP, which is replaced by calnexin in later stages of translation after N-glycans are added to emerging N-glycosylation sites of the growing polypeptide (Fig. 9B).
In the absence of N-glycans, the β1 subunit was able to achieve normal conformation without any involvement of calnexin. The unglycosylated mutant of the β1 subunit produced an active enzyme after assembly with the α1 subunit (41, 48, 52) and was delivered to the plasma membrane (Fig. 2A, a) despite its inability to bind calnexin (25). These results show that N-glycans are not essential for folding of the β1 subunit. The amount of YFP-β1-bound BiP increased 3-fold with mutation of N-glycosylation sites, suggesting that BiP binds to the nascent chain of the mutant at an early stage of its translocation into the ER lumen and stays associated with the mutant until translation and proper folding are completed, compensating for calnexin assistance. BiP apparently also initiates co-translational folding of normally glycosylated nascent β1 subunits. However, the addition of N-glycans later during translation probably interferes with BiP binding, resulting in replacement of BiP by calnexin (Fig. 9). The N-glycan-mediated calnexin binding then assists the completion of the folding process.
Thus, when N-glycans were present, but their interaction with calnexin was prevented by castanospermine, the proper conformation of the β1 subunit was not achieved, and, hence, a fraction of these β1 subunits was retained in the ER (Fig. 2A, c and d). Prolonged cell incubation with castanospermine resulted in an ER stress response followed by up-regulation of BiP (Fig. 2B). As a result, the amount of the YFP-β1-bound BiP was increased (Fig. 2B), facilitating successful maturation of the β1 subunit (Fig. 2A, e). Therefore, BiP and perhaps other non-lectin chaperones can compensate for the lack of calnexin-glycan interactions and successfully mediate maturation of the β1 subunit. Such compensation did not happen with the β2 subunit; it was retained in the ER during prolonged cell incubation with castanospermine despite increased β2-BIP binding (not shown). Also, in contrast to the unglycosylated β1 subunit, the unglycosylated β2 subunit was fully retained in the ER (25). Therefore, the data strongly suggest involvement of BiP in folding of the β1 subunit but not of the β2 subunit (Fig. 9). Conversely, calnexin binding is essential for maturation of the β2 subunit but not of the β1 subunit (25).
Maturation of the β1 Subunit Is More Rapid than Maturation of the β2 Subunit
YFP-β2 spends more time in the ER as compared with YFP-β1 (Fig. 3A, ER YFP-β2 and ER YFP-β1 bands), resulting in a greater accumulation of YFP-β2 than YFP-β1 in the ER at steady state (Fig. 1A, bottom). These differences in the ER residence time are consistent with rapid BiP- and calnexin-assisted folding of YFP-β1 and prolonged calnexin-assisted folding of YFP-β2, respectively (Fig. 9).
It is possible that prolonged interaction with calnexin of the β2 subunit in the ER of MDCK cells is due to the lower binding affinity of the α1 subunit to the β2 subunit than to the β1 subunit. MDCK cells naturally express only α1 and β1 subunits. It is not clear whether there is preferential association of particular α subunit isoforms with particular β subunit isoforms in tissues with multiple Na,K-ATPase subunit expression, such as brain, heart, and muscle (23, 53–56). However, if the α2 subunit rather than the α1 subunit is a preferred partner of the β2 subunit, prolonged interaction of the β2 subunit with calnexin might be required prior to its assembly with the α1 but not with the α2 subunit. Thus, these differences in duration of interaction with calnexin might facilitate formation of the α2-β2 heterodimers rather than α1-β2 heterodimers in the tissues that express various Na,K-ATPase subunits. This hypothesis could be tested by expressing α1, α2, β1, and β2 subunits in the same cell.
The Na,K-ATPase β1 Subunit Is More Resistant to Cd2+-induced ER Stress than the β2 Subunit
The ER-resident protein folding and quality control machinery is highly sensitive to various types of stress. Numerous cytotoxic agents or pathophysiological conditions can induce accumulation of unfolded and misfolded proteins in the ER, which is referred to as ER stress. To compensate for the impairment of folding and quality control machinery, a cell turns on specific signaling pathways that collectively are called the unfolded protein response (UPR). UPR results in inhibition of translation, activation of ER-associated degradation, and induction of expression of BiP and other ER chaperones (57, 58). When the stress is beyond the compensatory capacity of a cell, UPR triggers proapoptotic signaling. ER stress and UPR are involved in a diverse range of diseases. ER stress plays a critical role in cadmium intoxication, which results from its intake of contaminated water, food, air, and cigarette smoke (59). Cadmium accumulates in different organs, especially in the kidney, and causes a wide range of pathological effects, including renal dysfunction, osteoporosis/bone fractures, and development of cancer (60). In many ER stress-related diseases, including cadmium intoxication, abnormalities in ion-pumping activity of the Na,K-ATPase have been reported (61–63). However, there is a lack of information regarding effects of the ER stress on maturation, quality control, and degradation of the enzyme. Also, there are almost no reports on the effect of the ER stress-inducing agents, particularly of cadmium, on expression of lectin chaperones. The results presented here indicate that two β isoforms of the Na,K-ATPase, β1 and β2, differentially respond to the ER stress induced by cadmium. The β2 subunit was largely retained in the ER in Cd2+-exposed cells in a dose-dependent manner (Fig. 8, A, C, and E). In contrast, the β1 subunit was only slightly retained in the ER (Fig. 8, A, C, and D), even at the high Cd2+ concentration that caused cell death as seen from a significant decrease in total protein (Fig. 8B). In parallel, Cd2+ greatly induced expression of BiP but only slightly increased expression of calnexin. The results suggest that the β1 subunit, but not the β2 subunit, was rescued from misfolding by its preferred binding to BiP during co-translation folding. The ubiquitously expressed α1-β1 Na,K-ATPase is vital for maintenance of membrane potential and, hence, for the survival of the vast majority of animal cells. Therefore, high resistance of the β1 subunit to the ER stress apparently plays an important role in UPR-mediated cell adaptation to various pathological conditions.
Role of BiP in the Quality Control of the Na,K-ATPase
In MDCK cells, α-β assembly occurs after successful folding of individual subunits (25) and is required for the export of both α and β subunits from the ER (28). Unassembled subunits are retained in the ER (28). These unassembled subunits do not overload the ER because they are degraded much more rapidly than the mature α-β complexes in the plasma membrane (28) (Fig. 3).
Two lines of evidence presented here indicate that BiP is responsible for the ER retention of unassembled β subunits. First, the degree of the ER retention of YFP-β1 and YFP-β2 in cells grown at low and high density correlated with the amount of BiP bound to YFP-linked subunits and inversely correlated with the level of expression of the endogenous Na,K-ATPase α1 subunit (Fig. 6). In contrast, the amount of YFP-β-bound calnexin was similar in colonies and cell monolayers (Fig. 6). Second, the mutations in YFP-β1 and YFP-β2, which specifically impaired association of the β subunits with the α1 subunit, also increased the amount of the subunit-bound BiP (Fig. 7, A and B). This correlated with the degree of the ER retention of the YFP-linked β subunits (Fig. 7, A (ER bands) and B), whereas the amount of the YFP-β-bound calnexin did not change due to these mutations (Fig. 7A). Importantly, these mutations did not impair folding of the subunits as deduced from the unchanged sensitivity to limited trypsin digestion of both the YFP-β2 mutant (28) and the YFP-β1 mutant (Fig. 7C). Therefore, BiP, but not calnexin, is involved in the ER retention of properly folded, but α1-unassembled, β1 and β2 subunits of the Na,K-ATPase in MDCK cells (Fig. 9).
The preferential binding of BiP to unassembled α1 subunits rather than to β-bound α1 subunits demonstrated in Xenopus oocytes suggested involvement of BiP in ER retention of unassembled α1 subunits (42, 43). BiP also interacted with the endogenous α1 subunit in MDCK cells (Fig. 1C), suggesting a role for BiP in folding and quality control of the α1 subunit also in these cells.
These data illuminate the mechanisms whereby only α-β heterodimers of the Na,K-ATPase that are properly folded and assembled at 1:1 stoichiometry are delivered to the plasma membrane in mammalian cells. Both lectin and non-lectin ER chaperones contribute to maturation of the Na,K-ATPase. A lectin chaperone, calnexin, is required for folding of the β2 but not of the β1 subunit (25). In contrast, a non-lectin chaperone, BiP, mainly assists folding of the β1 subunit. When β1 and β2 subunits acquire their proper conformation, BiP binding prevents their premature export from the ER to the Golgi prior to their assembly with the α1 subunit. The results show that the β2 subunit is highly sensitive, whereas the β1 subunit is resistant to the ER stress, proving the pathophysiological significance of diverse chaperone-dependent maturation pathways for various Na,K-ATPase subunit isoforms.
This work was supported, in whole or in part, by National Institutes of Health Grants DK077149 and DK058333.
- ER
- endoplasmic reticulum
- CHX
- cycloheximide
- UPR
- unfolded protein response
- YFP
- yellow fluorescent protein
- MDCK
- Madin-Darby canine kidney.
REFERENCES
- 1.Hebert D. N., Molinari M. (2007) Physiol. Rev. 87, 1377–1408 [DOI] [PubMed] [Google Scholar]
- 2.Hebert D. N., Bernasconi R., Molinari M. (2010) Semin Cell. Dev. Biol. 21, 526–532 [DOI] [PubMed] [Google Scholar]
- 3.Caramelo J. J., Parodi A. J. (2008) J. Biol. Chem. 283, 10221–10225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura T., Sunryd J. C., Hebert D. N. (2010) Mol. Membr. Biol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helenius A., Aebi M. (2004) Annu. Rev. Biochem. 73, 1019–1049 [DOI] [PubMed] [Google Scholar]
- 6.Ritter C., Quirin K., Kowarik M., Helenius A. (2005) EMBO J. 24, 1730–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caramelo J. J., Parodi A. J. (2007) Semin. Cell. Dev. Biol. 18, 732–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebert D. N., Garman S. C., Molinari M. (2005) Trends Cell. Biol. 15, 364–370 [DOI] [PubMed] [Google Scholar]
- 9.Määttänen P., Gehring K., Bergeron J. J., Thomas D. Y. (2010) Semin. Cell. Dev. Biol. 21, 500–511 [DOI] [PubMed] [Google Scholar]
- 10.Hendershot L. M. (2004) Mt. Sinai J. Med. 71, 289–297 [PubMed] [Google Scholar]
- 11.Matlack K. E., Misselwitz B., Plath K., Rapoport T. A. (1999) Cell 97, 553–564 [DOI] [PubMed] [Google Scholar]
- 12.Meunier L., Usherwood Y. K., Chung K. T., Hendershot L. M. (2002) Mol. Biol. Cell 13, 4456–4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forsayeth J. R., Gu Y., Hall Z. W. (1992) J. Cell. Biol. 117, 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otero J. H., Lizák B., Hendershot L. M. (2010) Semin. Cell. Dev. Biol. 21, 472–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong M., Bridges J. P., Apsley K., Xu Y., Weaver T. E. (2008) Mol. Biol. Cell. 19, 2620–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Z. (2003) Ann. N.Y. Acad. Sci. 986, 497–503 [DOI] [PubMed] [Google Scholar]
- 17.Tian J., Xie Z. J. (2008) Physiology 23, 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gloor S., Antonicek H., Sweadner K. J., Pagliusi S., Frank R., Moos M., Schachner M. (1990) J. Cell. Biol. 110, 165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajasekaran S. A., Barwe S. P., Gopal J., Ryazantsev S., Schneeberger E. E., Rajasekaran A. K. (2007) Am. J. Physiol. Gastrointest. Liver Physiol. 292, G124–G133 [DOI] [PubMed] [Google Scholar]
- 20.Vagin O., Tokhtaeva E., Sachs G. (2006) J. Biol. Chem. 281, 39573–39587 [DOI] [PubMed] [Google Scholar]
- 21.Vagin O., Tokhtaeva E., Yakubov I., Shevchenko E., Sachs G. (2008) J. Biol. Chem. 283, 2192–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geering K. (2008) Curr. Opin. Nephrol. Hypertens. 17, 526–532 [DOI] [PubMed] [Google Scholar]
- 23.Blanco G., Mercer R. W. (1998) Am. J. Physiol. 275, F633–F650 [DOI] [PubMed] [Google Scholar]
- 24.Crambert G., Hasler U., Beggah A. T., Yu C., Modyanov N. N., Horisberger J. D., Lelièvre L., Geering K. (2000) J. Biol. Chem. 275, 1976–1986 [DOI] [PubMed] [Google Scholar]
- 25.Tokhtaeva E., Munson K., Sachs G., Vagin O. (2010) Biochemistry 49, 3116–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackermann U., Geering K. (1990) FEBS Lett. 269, 105–108 [DOI] [PubMed] [Google Scholar]
- 27.Jaunin P., Horisberger J. D., Richter K., Good P. J., Rossier B. C., Geering K. (1992) J. Biol. Chem. 267, 577–585 [PubMed] [Google Scholar]
- 28.Tokhtaeva E., Sachs G., Vagin O. (2009) Biochemistry 48, 11421–11431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi S., Mishina M., Kawamura M., Numa S. (1987) FEBS Lett. 225, 27–32 [DOI] [PubMed] [Google Scholar]
- 30.Geering K. (2001) J. Bioenerg. Biomembr. 33, 425–438 [DOI] [PubMed] [Google Scholar]
- 31.Craig W. S., Kyte J. (1980) J. Biol. Chem. 255, 6262–6269 [PubMed] [Google Scholar]
- 32.Peterson G. L., Hokin L. E. (1981) J. Biol. Chem. 256, 3751–3761 [PubMed] [Google Scholar]
- 33.Brotherus J. R., Jacobsen L., Jørgensen P. L. (1983) Biochim. Biophys. Acta 731, 290–303 [DOI] [PubMed] [Google Scholar]
- 34.Kyte J. (1971) J. Biol. Chem. 246, 4157–4165 [PubMed] [Google Scholar]
- 35.Geering K., Theulaz I., Verrey F., Häuptle M. T., Rossier B. C. (1989) Am. J. Physiol. 257, C851–C858 [DOI] [PubMed] [Google Scholar]
- 36.Liu L., Askari A. (2006) Am. J. Physiol. Cell. Physiol. 291, C569–C578 [DOI] [PubMed] [Google Scholar]
- 37.Shanbaky N. M., Pressley T. A. (1995) Biochem. Cell Biol. 73, 261–268 [DOI] [PubMed] [Google Scholar]
- 38.Therien A. G., Nestor N. B., Ball W. J., Blostein R. (1996) J. Biol. Chem. 271, 7104–7112 [DOI] [PubMed] [Google Scholar]
- 39.Sun Y., Ball W. J., Jr. (1992) Am. J. Physiol. 262, C1491–C1499 [DOI] [PubMed] [Google Scholar]
- 40.Jørgensen P. L. (1980) Physiol. Rev. 60, 864–917 [DOI] [PubMed] [Google Scholar]
- 41.Beggah A. T., Jaunin P., Geering K. (1997) J. Biol. Chem. 272, 10318–10326 [DOI] [PubMed] [Google Scholar]
- 42.Beggah A. T., Geering K. (1997) Ann. N.Y. Acad. Sci. 834, 537–539 [DOI] [PubMed] [Google Scholar]
- 43.Beggah A., Mathews P., Beguin P., Geering K. (1996) J. Biol. Chem. 271, 20895–20902 [DOI] [PubMed] [Google Scholar]
- 44.Vagin O., Turdikulova S., Sachs G. (2005) J. Biol. Chem. 280, 43159–43167 [DOI] [PubMed] [Google Scholar]
- 45.Gottardi C. J., Dunbar L. A., Caplan M. J. (1995) Am. J. Physiol. 268, F285–F295 [DOI] [PubMed] [Google Scholar]
- 46.Kroepfl J. F., Gardinier M. V. (2001) J. Neurochem. 77, 1301–1309 [DOI] [PubMed] [Google Scholar]
- 47.Miller R. P., Farley R. A. (1988) Biochim. Biophys. Acta 954, 50–57 [DOI] [PubMed] [Google Scholar]
- 48.Laughery M. D., Todd M. L., Kaplan J. H. (2003) J. Biol. Chem. 278, 34794–34803 [DOI] [PubMed] [Google Scholar]
- 49.Hamada T., Nakano S., Iwai S., Tanimoto A., Ariyoshi K., Koide O. (1991) Toxicol. Pathol. 19, 138–147 [DOI] [PubMed] [Google Scholar]
- 50.Yokouchi M., Hiramatsu N., Hayakawa K., Kasai A., Takano Y., Yao J., Kitamura M. (2007) Cell Death Differ. 14, 1467–1474 [DOI] [PubMed] [Google Scholar]
- 51.Molinari M., Helenius A. (2000) Science 288, 331–333 [DOI] [PubMed] [Google Scholar]
- 52.Takeda K., Noguchi S., Sugino A., Kawamura M. (1988) FEBS Lett. 238, 201–204 [DOI] [PubMed] [Google Scholar]
- 53.Blanco G. (2005) Semin. Nephrol. 25, 292–303 [DOI] [PubMed] [Google Scholar]
- 54.Cameron R., Klein L., Shyjan A. W., Rakic P., Levenson R. (1994) Brain Res. Mol. Brain Res. 21, 333–343 [DOI] [PubMed] [Google Scholar]
- 55.Peng L., Martin-Vasallo P., Sweadner K. J. (1997) J. Neurosci. 17, 3488–3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cougnon M. H., Moseley A. E., Radzyukevich T. L., Lingrel J. B., Heiny J. A. (2002) Pflugers Arch. 445, 123–131 [DOI] [PubMed] [Google Scholar]
- 57.Zhang K., Kaufman R. J. (2008) Nature 454, 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitamura M. (2008) Am. J. Physiol. Renal Physiol. 295, F323–F334 [DOI] [PubMed] [Google Scholar]
- 59.Kitamura M., Hiramatsu N. (2010) Biometal 5, 941–950 [DOI] [PubMed] [Google Scholar]
- 60.Järup L., Akesson A. (2009) Toxicol. Appl. Pharmacol. 238, 201–208 [DOI] [PubMed] [Google Scholar]
- 61.Karthikeyan J., Bavani G. (2009) J. Environ. Biol. 30, 895–898 [PubMed] [Google Scholar]
- 62.Eriyamremu G. E., Asagba S. O., Onyeneke E. C., Adaikpoh M. A. (2005) Biometals 18, 1–6 [DOI] [PubMed] [Google Scholar]
- 63.Bae E. H., Lee K. S., Lee J., Ma S. K., Kim N. H., Choi K. C., Frøkiaer J., Nielsen S., Kim S. Y., Kim S. Z., Kim S. H., Kim S. W. (2008) Am. J. Physiol. Renal Physiol. 294, F272–F280 [DOI] [PubMed] [Google Scholar]
- 64.Shinoda T., Ogawa H., Cornelius F., Toyoshima C. (2009) Nature 459, 446–450 [DOI] [PubMed] [Google Scholar]