Abstract
Background
In Huntington’s disease (HD), the cerebral cortex is involved early in the disease process. The study of cortical excitability can therefore contribute to understanding HD pathophysiology.
Methods
Using transcranial magnetic stimulation (TMS) we examined motor cortex excitability in 8 premanifest HD gene carriers, 8 very early symptomatic HD patients and 22 healthy controls. Electrophysiological measures were correlated with the clinical stage of HD to identify motor cortical dysfunction prior to overt clinical disease onset.
Results
Premanifest and early manifest HD patients had higher resting and active motor cortex thresholds than controls (p=0.024). At rest, recruitment of motor evoked potentials was more gradual in both patient groups than in controls (p=0.001). When active, recruitment and the duration of the cortical silent period were similar in all groups. There was a tendency for short latency intra-cortical inhibition (SICI) to have a higher threshold in all patients taken together but not in each group separately. Short latency afferent inhibition (SAI) was reduced in early manifest patients compared with controls and premanifest patients (p<0.001) and in contrast to all other measures was inversely associated with predicted years to onset of HD signs (p=0.013, adjusted R2=0.32) and the UHDRS motor score (p=0.001, adjusted R2=0.5). A combination of age, CAG repeat length, and SAI strongly predicted the UHDRS motor score (p=0.001, adjusted R2=0.68).
Conclusions
Since reduced excitatory and inhibitory corticospinal thresholds and MEP recruitment in patients at rest do not correlate with symptom severity, they may be a consequence of carrying the HD mutation. In contrast, SAI correlated with the severity of motor signs and may reflect the disease state.
Keywords: transcranial magnetic stimulation, UHDRS, Huntington’s disease, corticospinal system, short latency afferent inhibition
Introduction
Huntington’s disease (HD), a movement disorder with neuropsychiatric symptoms and cognitive impairment, is caused by a CAG repeat expansion in the IT15 gene. HD is inevitably fatal with a mean age of onset of 40 years and a mean survival of 15 to 20 years and has no currently effective treatment. In recent years, yeast, fly, worm and mouse models of HD have substantially advanced our understanding of disease mechanisms at the molecular level (1). Transcranial magnetic stimulation (TMS) is a safe, non-invasive and painless tool to examine the excitability of the motor cortex in vivo. This provides insight into the electrophysiological properties of corticospinal neurones and the trans-synaptic regulation of inhibitory and facilitatory circuits within the motor cortex. In HD, the study of cortical excitability is important since there is no doubt that the cerebral cortex is involved in the disease process. Structural imaging studies indicate cortical degeneration occurs early (2, 3), and microarray analysis of human HD revealed more pronounced changes in HD motor cortex compared to the pre-frontal cortex and cerebellum (4). In the disease process cortical dysfunction may precede striatal abnormalities since depletion of cortical BDNF results in striatal degeneration in a pattern closely similar to human HD (5).
To date, the assessment of cortical function using TMS in manifest HD patients has not been able to delineate a clear picture of what is normal or abnormal in HD (6-11). Various different abnormalities have been reported in heterogeneous mainly manifest patient populations including prolonged cortical silent periods (CSP), reduced short interval intra-cortical inhibition (SICI), enhanced or normal intra-cortical facilitation. The first aim of the present study was therefore to investigate whether methodological reasons may account for some of the discrepancies. For instance, SICI was measured using a paired-pulse TMS paradigm with only one intensity of conditioning stimulus (8, 11). This could produce misleading results since the amount of SICI depends on the intensity of the conditioning stimulus (12-14). If the relationship between intensity and amount of SICI differs in patients and controls, a single measure can give an erroneous estimate of the maximum sensitivity of SICI. Therefore, in this study, we measured SICI at a range of conditioning intensities. Another measure of intra-cortical inhibition, the CSP, can also be difficult to interpret if it is not corrected for the size of the preceding motor evoked potential (MEP) (15). The second aim of this study was to extend the measures to another form of cortical inhibition. It is well known that somatosensory evoked potentials become abnormal as HD progresses (16). This may reflect a disease related influence on the sensory input part of sensory-motor integration pathways. One such pathway that can be tested easily in humans is short latency afferent inhibition (SAI (17)) in which a transient sensory input leads to a rapid and short lasting inhibition of the motor cortex.
The third aim of the study was to test whether any electrophysiological measures were associated with the clinical stage of HD and whether we could detect cortical dysfunction prior to overt clinical disease onset.
Material and methods
Patients and control subjects
Sixteen patients (13 women, mean age 41.6 years, range 28-64) with a molecular genetic diagnosis of HD (for CAG repeat length see table 1) and 22 control subjects (9 women, mean age 36.1 years, range 28-58) were recruited. The same clinician (SJT) with long standing experience in HD examined patients clinically; the Unified Huntington’s Disease Rating Scale (UHDRS) motor scale was used to score motor signs (18). 8 patients were clinically premanifest according to the UHDRS (diagnostic confidence score of less than 4), and 8 patients were early manifest (Clinical stage 1 (19)). The time to symptom onset was estimated according to Langbehn et al (20). Patients and controls were unmedicated at the time of the study.Subjects gave informed written consent according to the Declaration of Helsinki, and the local ethics board approved the study protocol.
Table 1.
Demographic, molecular genetic and clinical data from HD patients. Predicted symptom onset was calculated according to Langbehn et al (20). Since all manifest patients by definition had HD motor signs the calculated ‘onset’ is given in brackets. All premanifest patients had a diagnostic confidence score of less than 4 on the UHDRS.
| Patient | age | gender | CAG | UHDRS motor | Years to predicted onset |
|---|---|---|---|---|---|
| Premanifest 1 | 41 | F | 43 | 0 | 10.59 |
| Premanifest 2 | 39 | F | 42 | 2 | 16.10 |
| Premanifest 3 | 40 | M | 41 | 4 | 19.10 |
| Premanifest 4 | 32 | M | 40 | 0 | 33.54 |
| Premanifest 5 | 28 | F | 47 | 7 | 10.50 |
| Premanifest 6 | 38 | F | 40 | 2 | 27.68 |
| Premanifest 7 | 38 | F | 40 | 1 | 27.68 |
| Premanifest 8 | 53 | F | 41 | 5 | 9.44 |
| Manifest 1 | 44 | F | 43 | 8 | (8.60) |
| Manifest 2 | 48 | M | 47 | 16 | (3.53) |
| Manifest 3 | 43 | F | 43 | 15 | (9.22) |
| Manifest 4 | 33 | F | 44 | 13 | (13.82) |
| Manifest 5 | 48 | F | 46 | 23 | (3.79) |
| Manifest 6 | 37 | F | 43 | 8 | (13.80) |
| Manifest 7 | 64 | F | 44 | 30 | (4.09) |
| Manifest 8 | 40 | F | 46 | 30 | (4.98) |
Electromyography recordings
Surface electromyograms (EMG) were recorded from the right first dorsal interossoeus (FDI) muscle using silver/silver-chloride disc surface electrodes (1 cm diameter) in a belly tendon montage. The EMG signal was amplified and analogue filtered (30Hz to 1kHz) with a Digitimer D150 amplifier (Digitimer Ltd., Welwyn Garden City, UK). Data (sampling rate 4kHz) was digitised for off-line analysis using Signal software (Cambridge Electronic Devices, Cambridge, UK).
Transcranial Magnetic Stimulation
Patients and controls were seated in a comfortable chair. They were asked to relax as much as possible. Magnetic stimuli were given with a hand-held figure-of-eight coil (outer winding diameter 9cm) connected to a High Power Magstim 200 stimulator (Magstim Co., Whitland, Dyfed, UK). This stimulator generates a magnetic pulse with monophasic waveform and induces a current in the brain with posterior-anterior flow when the coil handle is positioned at an angle of 45° pointing backwards. The optimal spot for right FDI stimulation was marked with a felt pen.
Motor thresholds, input-output (I/O) curves at rest
Resting motor threshold (RMT) was defined as the minimum intensity needed to evoke an MEP of >50μV in 5 out of 10 consecutive trials in the relaxed FDI. Active motor threshold (AMT) was defined as the minimum intensity (in % of maximum stimulator output) needed to evoke a MEP of >200μV in 5 out of 10 trials in the tonically active FDI (~20% of maximal contraction as assessed visually on an oscilloscope). Thresholds were approached from above threshold in steps of 1% stimulator output. Once no MEPs could be elicited the intensity was increased in steps of 1% stimulator output until a minimal MEP was observed. This intensity was taken as motor threshold.
Input-output (I/O) curves were examined by measuring MEP size of MEPs elicited at stimulus intensities of 110, 125 and 150%RMT. Ten trials were recorded, and the average MEP area was taken as MEP size.
Cortical silent periods (CSP)
CSPs were recorded from the tonically active right FDI with the subjects squeezing an object between the thumb and index finger at around 20-30% of maximum force output. Ten trials at fixed test stimulus intensities of 130, 150 and 175% AMT were collected in each subject with an interval of 4 seconds between trials. In each individual trial the duration of the silent period was measured from the visually identified beginning of the MEP evoked by the test stimulus to the resumption of (any level of) sustained EMG activity. In addition, the area under the MEP was determined and a ratio of silent period duration/MEP area calculated because silent period duration and the size of the preceding MEP correlate so that a ratio represents an additional measure of the inhibitory circuits underlying the CSP (15). The gain of the recordings was set to 1mV/V in order to measure the end of the silent period, and in a second channel was set to 10mV/V in order to measure the size of the MEP. Gain settings were the same for all experiments.
Paired pulse paradigm
In each individual, an intensity of the unconditioned TMS pulse was chosen that elicited a MEP of 0.5 to 1.5mV amplitude. The conditioning pulse intensity was varied (60, 70, 80, 90 or 100% of AMT) resulting in 5 different experimental blocks. With each conditioning pulse intensity, and in a randomised order, the 2 and 3ms interstimulus intervals (ISI) were examined. This examines short interval intra-cortical inhibition (SICI). With an interval of 4 seconds between trials, 10 conditioned MEPs were collected for each ISI, and in each experimental block a total of 20 unconditioned test stimulus MEPs were recorded. The order of data collection for each conditioning pulse intensity was randomised between subjects. Trials recorded while the patients contracted the hand muscles were excluded on-line. No trials were excluded in the off-line analysis. The average of the amplitudes of each conditioned MEP was expressed as a percentage of the average test stimulus MEP amplitude in the same session. SICI thresholds were determined as described previously (13). In brief, in each subject the %SICI was plotted against the absolute intensity of the conditioning stimulus and the data was fitted with a second order polynomial function. The theoretical threshold was defined as the value where the function crossed the x axis, thus the conditioning stimulus intensity where the net amount of inhibition was zero. This conditioning stimulus intensity was then related to AMT in each individual.
Short latency afferent inhibition (SAI) by somatosensory input from the median nerve
SAI of the motor cortex was examined as previously described (17). In brief, a MEP of ~1mV peak-to-peak amplitude was elicited in the FDI by TMS. A paired pulse paradigm examined the influence on MEP size of a supra-threshold electrical stimulus given to the median nerve through bipolar electrodes. The electrical stimulus to the median nerve was delivered at an intensity just above the threshold to elicit a visible contraction in the thenar muscles and preceded the TMS pulse to the FDI hot spot by 14, 18, 20, 22, 24, 26 or 29ms. Twenty trials of the MEP elicited by TMS alone and 10 trials of conditioned MEPs for each ISI were collected. The amplitude of the MEP in the FDI was measured with in-house software. The average amplitude of the conditioned MEP was expressed in percent of the average amplitude of the test MEP alone. Trials recorded while the patients contracted the hand muscles were excluded on-line. No trials were excluded in the off-line analysis.
Data analysis
Data were collected without knowledge about the clinical assessment of patients. Peak to peak amplitude of MEP, the area under the curve of the MEP and the silent period duration were measured with in-house software. We have previously shown that under normal conditions MEP area and amplitude give equivalent results (Orth et al, 2003), but since some patients had polyphasic EMG responses to some of the stimuli, we employed MEP area in the correlation analyses. The slopes of the silent period recruitment, SICI recruitment and I/O curves at rest and during activity were fitted with linear regression in each subject. We examined whether there was a main effect of ‘intensity’ (130, 150 or 175% AMT for CSP, and 110, 125 or 150% RMT for MEP recruitment, respectively) on CSP duration, MEP area with pre-activation or MEP area at rest. Similarly, in the paired pulse paradigms we investigated whether there was a main effect of ISI on MEP size or conditioning stimulus intensity (60, 70, 80, 90, 100%AMT) on the amount of SICI; or an effect of ISI on MEP size in the short-latency afferent inhibition paradigm. This was tested using analysis of variance (repeated measures ANOVA) statistically. To test whether patients, either premanifest or early manifest, differed from controls or from each other we introduced ‘group’ as between-subjects factor. In addition, we tested whether the slopes of the recruitment curves differed between patients and controls (interaction of ‘intensity’ and ‘group’ in the repeated measures ANOVAs).
In order to assess how TMS parameters (RMT, SICI threshold, slopes of the I/O curve for MEP size at rest, maximum SAI) were associated with the estimate of time to onset of symptoms, or the UHDRS motor score, we used backward stepwise regression analysis with ‘years to onset’ or ‘UHDRS motor score’ as the dependent variable. In a second model, we entered ‘maximum SAI’, ‘CAG repeat length’ and ‘age’ as independent parameters. A parameter was removed from the model if the probability of its contribution was less then 0.1.
A statistical difference in the ANOVAs was followed by a post-hoc paired t-test analysis. Mauchly’s test was used to test for sphericity in the repeated measures ANOVAs, and the Greenhouse-Geisser correction was applied to the DFs if necessary. Statistical significance levels were set to p=0.05. All statistical analyses were performed using SPSS 11 for Windows software package.
Results
Motor thresholds and motor cortex excitability at rest
Resting motor thresholds of all patients taken together (mean 43.4, SE 1.7, 95%CI 40-46.8) were higher than in controls (mean 38, SE 1.4, 95%CI 35.5-41) (ANOVA, main effect of ‘group’, F1,36=5.57, p=0.024). Dividing patients into the subgroups of premanifest (mean 42.6, SE 2.4, 95%CI 37.8-47.5) and early manifest patients (mean 44.1, SE 2.4, 95%CI 39.3-49) there was still a trend towards higher RMT in patients than in controls (ANOVA, main effect of ‘subgroup’, p=0.07). Thresholds with pre-activation were also higher in patients (mean 33.2, SE 1.5 95%CI 30.1-36.2) than in controls (mean 28, SE 1.2, 95%CI 25.5-30.5) (ANOVA, main effect of ‘group’, F1,36=7.04 p=0.012) even if dividing patients into premanifest (mean 32.8, SE 2.2, 95%CI 28.4-37.1) and early manifest (mean 33.6, SE 2.2, 95%CI 29.3-38) (ANOVA, main effect of ‘subgroup’, F2,36=4.74, p=0.042). Pairwise comparison revealed that the main difference was between controls and early manifest patients (p=0.03) with a trend comparing controls and premanifest patients (p=0.065).
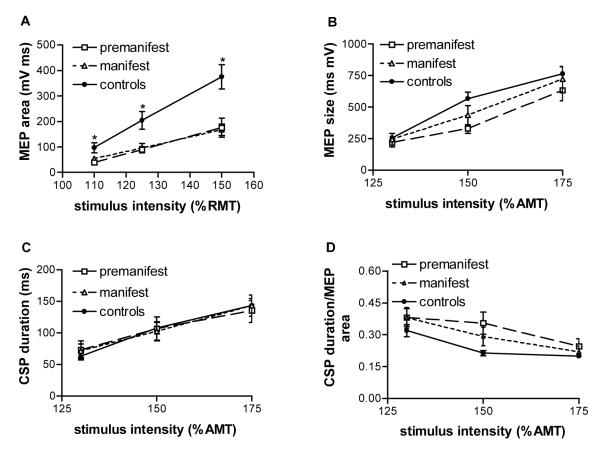
Above RMT, MEP size (area) increased with increasing stimulation intensity (repeated measures ANOVA, main effect of ‘stimulation intensity’, F2,72=55.4, p<0.0001). However, patients recruitment slopes were flatter than those of controls (repeated measures ANOVA, interaction ‘intensity*group’, F2,72=7.9, p=0.001) even with two patients subgroups (repeated measures ANOVA, interaction ‘intensity*group’, F2,72=3.9, p=0.006, Figure 1A). Post-hoc pairwise comparisons showed that the slope of both premanifest (p=0.013) and early manifest patients (p=0.017) was flatter than in controls (Figure 1A) whereas the slopes of both patient subgroups were similar.
Figure 1.
cortico-spinal system excitability. A. MEP size (area) recorded from relaxed FDI after TMS shock to the M1 hand area with 110%, 125% or 150% of RMT. Patients recruitment slopes were flatter than those of controls (repeated measures ANOVA, interaction ‘intensity*group’, F2,72=3.9, p=0.006). Post-hoc pairwise comparisons showed that the slope of both premanifest (0=0.013) and early manifest patients (p=0.017) was flatter than in controls whereas the slopes of both patient subgroups were similar. B-D. MEP size and CSP duration recorded from active FDI after TMS shock to the M1 hand area with 130%, 150% or 175% of AMT. MEP area (B), CSP duration (C) and the ratio of CSP duration and MEP area (D) are similar in controls and HD patients. Values are means ±SEM, n=16 for HD patients (n=8 premanifest, n=8 early manifest), n=22 for controls.
Motor cortex excitability with pre-activation and cortical silent periods
MEP size (area) increased significantly with increasing stimulation intensity in both the controls and the patients (repeated measures ANOVA, F2,70=97.76, p<0.001, Figure 1B). This increase in MEP size was similar in patients and controls. Cortical silent period duration also increased with increasing stimulation intensity (repeated measures ANOVA, main effect of ‘stimulation intensity’, F2, 70=136.3, p<0.001, Figure 1C) without major differences between patients and controls. The same was true for the ratios of cortical silent period duration and MEP area (Figure 1D).
Short interval intra-cortical inhibition
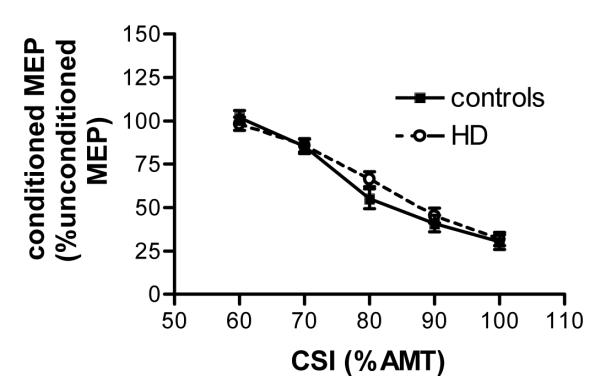
First we determined the threshold for SICI as described previously (13). For technical reasons this was not possible in one control and in two premanifest patients. SICI thresholds were lower in controls (mean 17.9%, SE 0.79, 95%CI 16.25-19.49) than in patients (mean 20.8, SE 1, 95%CI 18.7-22.9) (ANOVA, main effect of ‘group’, F2,34=5, p=0.032). This effect was lost when patients were divided into the premanifest and early manifest subgroups.
Above threshold increasing the conditioning stimulus intensities reduced conditioned MEP amplitude size (repeated measures ANOVA, main effect of ‘conditioning stimulus intensity’, F2.56, 95.8=90.15, p<0.001, Figure 2). The recruitment slope was similar in patients and controls.
Figure 2.
short intra-cortical inhibition. In controls and patients increasing intensity of the conditioning stimulus reduced the size of the conditioned MEP (amplitude) in a similar way. Values are means ±SEM, n=14 for HD patients, n=22 for controls.
Short latency afferent inhibition
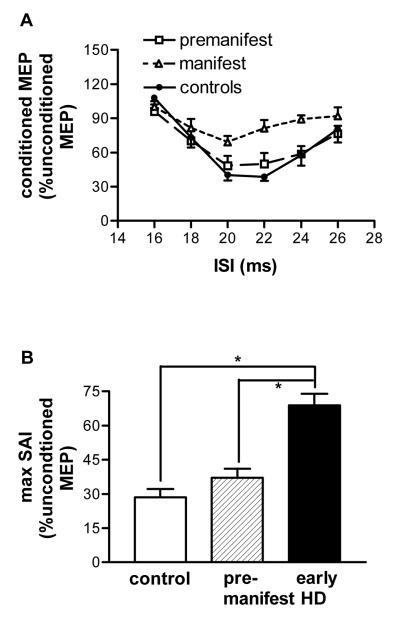
In controls and patients, a supra-threshold electrical stimulus to the median nerve at the wrist before the TMS pulse to the FDI hot-spot reduced the mean amplitude of the test stimulus predominantly at ISIs of 20, 22 and 24ms (repeated measures ANOVA, main effect of ‘ISI’, F2.34,84.31=17.28, p<0.001, Figure 3A). Since the early period of inhibition is more likely to have a partly cortical origin than later timings (17) we assessed the maximum amount of afferent inhibition in each individual. Maximal SAI was greatest in controls followed by premanifest patients and early manifest patients (ANOVA, main effect of ‘group’, F2,35=19.7, p<0.001, Figure 3B). Post-hoc pairwise comparisons revealed that early manifest patients (mean 68.8, SE 5.5, 95%CI 57.6-80) differed from controls (mean 28.5, SE 3.3, 95%CI 21.8-35.3, p<0.001) and premanifest patients (mean 37.15, SE 5.5, 95%CI 26-48.3, p<0.001, Figure 3B). Premanifest patients and controls were similar.
Figure 3.
short latency afferent inhibition. A. The SAI curve was flatter for manifest HD patients compared with controls or premanifest patients. B. Maximal SAI was greatest in controls followed by premanifest patients and early manifest patients (ANOVA, main effect of ‘group’, F2,35=19.7, p<0.001,). Post-hoc pairwise comparisons revealed that controls and premanifest patients had more SAI than early manifest patients (*p<0.001). Values are means ±SEM, n=16 for HD patients (n=8 premanifest, n=8 early manifest), n=22 for controls.
Correlation of electrophysiological parameters and clinical measures
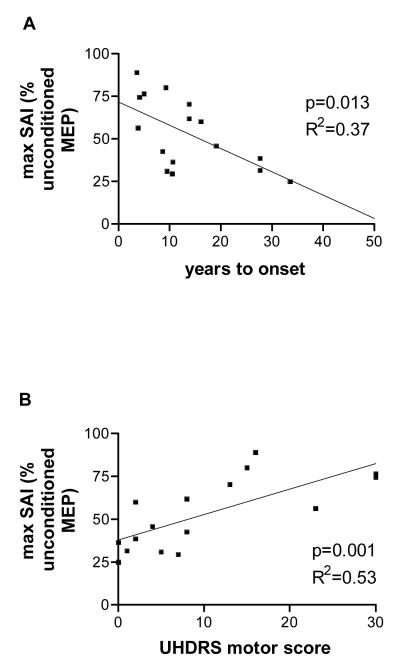
We examined whether any of the electrophysiological parameters were associated with the presumed disease state. Only maximum SAI served as a predictor for estimated years to motor onset (backward stepwise regression analysis with ‘estimated years to onset’ as dependent variable, ANOVA, F1,13=8.2, p=0.013, R=0.61, R2=0.37, adjusted R2=0.32, Figure 4A).
Figure 4.
backward stepwise regression analysis. There was a significant correlation of max SAI with predicted years to onset of symptoms (A) and with the UHDRS motor score (B). Data are from 16 Huntington patients.
We then correlated clinical severity (UHDRS motor score) with electrophysiological parameters. Again, maximum SAI was the only predictor of UHDRS motor score (backward stepwise regression analysis with ‘UHDRS motor score’ as dependent variable, ANOVA, F1,13=15.63, p=0.001, R=0.73, R2=0.53, adjusted R2=0.5, Figure 4B). Next, we examined for correlations of clinical severity with CAG repeat length, age and maximum SAI. This model strongly predicted the UHDRS motor score (ANOVA, F3,12=11.4, p=0.001, R=0.86, R2=0.74, adjusted R2=0.68).
Discussion
In the present study we show that patients with HD, both premanifest and early manifest, have higher motor cortex thresholds both at rest and in a pre-activated state. Recruitment of MEPs above threshold in the resting state is more gradual in both patient groups than in controls. In contrast, when subjects actively contract the target muscle recruitment is similar in controls and patients as is the duration of the cortical silent period. There was a tendency for SICI to have a higher threshold in all patients taken together but not in each group separately. SAI, a measure of sensory-motor integration, is reduced in early manifest patients compared with controls and premanifest patients. In addition, SAI is inversely associated with predicted years to onset of HD signs and the UHDRS motor score, and a combination of age, CAG repeat length, and SAI strongly predicted the UHDRS motor score.
The electrophysiology of Huntington’s disease
At threshold intensities, TMS to the motor cortex activates axons of cortical neurones that synaptically excite pyramidal tract neurones (I-wave inputs). These conduct impulses to spinal cord where they synaptically activate alpha motoneurones in the ventral horn. Threshold thus depends on the excitability of axon membranes at the site of stimulation and the membrane potential of postsynaptic neurones in motor cortex and spinal cord. If the latter is depolarised then excitatory inputs are more likely to cause the postsynaptic cell to discharge than if the membrane potential is hyperpolarised. During active contraction, synaptic excitability is high so that changes in threshold usually are thought to reflect changes in axonal excitability. The fact that active threshold was higher in HD thus suggests that axonal excitability was reduced. At rest, threshold will also depend on postsynaptic membrane potential. Whether this additionally contributes to reduced resting thresholds in HD is uncertain, although the reduced slope of the resting recruitment curve would be compatible with additional synaptic effects (see below).
Above threshold, recruitment of MEPs depends on the distribution of excitability (both axonal and postsynaptic) in the corticospinal system. If there were little difference between the excitability of the most and least excitable members of this population of corticospinal neurones, then a small increase in stimulus intensity would recruit many additional connections and create a large MEP: the gain of the input-output relation would be steep. In contrast, if the distribution were more widespread, then the same change in intensity would recruit only a small number of extra connections and the MEP would be small: the input-output relation would be shallow. Since HD patients recruited MEPs similarly to normal when active, we conclude that the distribution of axonal thresholds was similar to normal. In contrast, the recruitment curve was more gradual than normal in patients at rest. This suggests that in the resting state, the distribution of post-synaptic excitability was more widespread than normal, but that it can be normalised by voluntary contraction.
SICI experiments examined the excitability of intra-cortical inter-neurones. We distinguished between the threshold intensity needed to produce SICI and the amount of SICI at suprathreshold intensities of conditioning shock (13). Threshold of SICI was higher in patients whereas the above threshold I/O curves were the same as in controls. It is thought that at threshold TMS pulses recruit SICI by exciting axons leading to secondary synaptic release of inhibitory neurotransmitters. Thus, as with MEP threshold, changes in SICI thresholds could be due to axonal or synaptic effects. Although we cannot distinguish which of these may be more important, the similarity in recruitment slopes suggests that the distribution of excitability in the SICI system is the same as normal. If so, it would be compatible with the idea, that like MEP thresholds, much of the increase of SICI thresholds in HD was due to changes in axonal threshold.
The silent period had the same duration as normal whether measured in terms of absolute duration or when normalised to MEP amplitude. This contrasts with the reports of prolonged CSP in many previous papers (e.g. Tegentoff et al, 1996; Cantello, 2002), and could conceivably be due to the fact that we elicited CSP with a lower intensity of stimulation than used by some other groups. However, as noted by Modugno et al (2001), the increased duration of the CSP is likely caused by the difficulty that patients have in maintaining a constant contraction and resuming if after a period of silence. It therefore seems more likely that the normal CSP seen here is due to the fact that our patients were relatively mild and could maintain a constant level of muscle contraction without difficulty. As with SICI, the intensity of stimulation used to evoke the CSP was measured relative to active motor threshold. Since AMT was raised, the implication is that the absolute threshold of the axons activated by silent period stimulation has risen in parallel with that of the excitatory I-wave inputs to corticospinal neurones. Indeed as suggested by Orth and Rothwell (15) this would be the expected outcome if the CSP were caused by activity in the recurrent collaterals of corticospinal neurones.
We conclude that there is good evidence that the threshold for stimulation of intra-cortical axons in the I-wave circuit, axons of neurones involved in the CSP and probably also in axons of the GABAergic neurones of the SICI circuit is higher than normal in HD patients. In addition, the resting distribution of corticospinal excitability is lower than normal, but can be normalised during active contraction. However, none of these electrophysiological parameters was associated with the severity of patients’ motor signs. This suggests that motoneurones and their modulation by inhibitory inter-neurones, i.e. the quality and shaping of the motor command, may not necessarily change as HD advances from the premanifest to the early manifest stage.
In contrast to these threshold changes, the electrophysiological measure of inhibitory interactions of sensory input and motor output, SAI, was related to clinical signs. It was reduced in early manifest patients but not in premanifest patients and showed an inverse relationship to UHDRS motor scores. These abnormalities may well be associated with the known reduction in amplitude of somatosensory evoked potentials (SEP) in manifesting individuals (16). The advantage of SAI compared with SEPs is that it examines a complete circuit linking sensory input and motor output. Since we were interested in the sensory-motor integration part of the circuit we adjusted the intensity of the unconditioned TMS shock to account for any differences in motor cortex excitability. Thus reduced SAI, just like reduced SEP amplitudes, probably reflects changes within the somatosensory-motor pathways including the somatosensory cortex of HD patients. However, one limitation of our study is that we did not correlate SAI with changes in SEPs. We therefore cannot say whether SAI is a more reliable measure than SEPs to detect change in HD. This, and the changes in both SEP and SAI over time, needs to be the subject of future studies.
Implications for the pathophysiology of Huntington’s disease
Our results suggest a general theme of increased axonal thresholds in HD patients together with a reduced excitability of the corticospinal output at rest. However, there was no difference between early manifest and premanifest patients. Changes in the basal ganglia and various cortical areas occur before symptom onset. These include the formation of neuropil aggregates (21), oligodendrocytes (22), focal cortical thinning and pyramidal tract white matter abnormalities (2, 23). Increases in axonal thresholds can therefore be added to this list, and may also be an intrinsic reaction of the brain to the presence of the mutated huntingtin protein. Mutated huntingtin probably confers not only a toxic gain of function but also a loss of function (1). Huntingtin plays an important role in neuronal development (24-26). Thus life-long expression of mutant huntingtin may give rise to inherent abnormalities in the development of the HD brain including, as our data and imaging data suggest, inputs to the corticospinal axons (27, 28).
In contrast, our measure of sensory-motor integration, SAI, was normal until patients developed symptoms. It evolved relative to the severity of motor signs such that together with the patient’s age and CAG repeat length the level of SAI predicted symptom severity. Pharmacological studies suggest that the SAI paradigm used in our study, and the cortical component of SEPs, to some extent involves cholinergic trans-synaptic pathways (17, 29, 30). Cholinergic abnormalities have been described in post-mortem striatal tissue in HD (31) and transgenic mice (32). The striatum and cortex degenerate most in the course of HD (33, 34). Recent evidence indicates cortical cholinergic changes precede those in the striatum (35). In premanifest patients this includes the pre-frontal cortex, an area relevant for sensory-motor integration (36). Thus our data would be compatible with a continuous decline of cholinergic function in sensory inputs as the disease progresses similar to Alzheimer’s disease (37). A functional decline in cortical cholinergic function may be due to a loss of cholinergic synapses without neuronal cell loss (35, 38) and contribute to cognitive symptoms in HD patients and animals before motor onset (35). Cholinergic changes may be restricted to the cortex because the nucleus basalis of Meynert as the provider of most of the cholinergic cortical input does not degenerate in HD (39).
Taken together, we have shown abnormalities in corticospinal output and intra-cortical pathways in HD patients. Reduced corticospinal excitability differentiated HD patients from controls but not premanifest from early manifest patients. Further study with larger patient numbers (and in several different centres) needs to clarify whether this is a result of being an HD mutation carrier per se rather than relating to the clinical disease stage. These studies should also control more rigorously for other potential confounding factors such as gender and levels of education. In contrast, our electrophysiological marker of sensory-motor integration, SAI, changed in association with the severity of motor signs. As SAI relies in part on cholinergic trans-synaptic cortical pathways we provide further evidence for cortical abnormalities in very early manifest HD. Longitudinal studies will be helpful to determine when and how SAI changes in the transition from premanifest to various manifest disease stages.
Acknowledgements
We thank all our patients, and the controls, for participating in this study. We are grateful to Dr Ed Wild for helping with patient recruitment and useful comments on the manuscript and to Ms Susie Henley for advice and help with patient recruitment. SJT is funded by the Medical Research Council, the Brain Research Trust, the UK Huntington’s disease association and the High Q Foundation.
Abbreviations
- AMT
active motor threshold
- ANOVA
analysis of variance
- CSP
cortical silent period
- EMG
electromyogram
- FDI
first dorsal interosseus muscle
- HD
Huntington’s disease
- I/O
input output
- MEP
motor evoked potential
- RMT
resting motor threshold
- SAI
short interval afferent inhibition
- SICI
short interval intra-cortical inhibition
- TMS
transcranial magnetic stimulation
- UHDRS
Unified Huntington’s Disease Rating Scale
Footnotes
Disclosure: The authors report no conflict of interest
References
- 1.Walker FO. Huntington’s disease. Lancet. 2007;369(9557):218–28. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 2.Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, et al. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: Selective white matter pathology and its relationship to clinical measures. Mov Disord. 2006;21(9):1317–25. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- 3.Henley SM, Frost C, MacManus DG, Warner TT, Fox NC, Tabrizi SJ. Increased rate of whole-brain atrophy over 6 months in early Huntington disease. Neurology. 2006;67(4):694–6. doi: 10.1212/01.wnl.0000230149.36635.c8. [DOI] [PubMed] [Google Scholar]
- 4.Hodges A, Strand AD, Aragaki AK, Kuhn A, Sengstag T, Hughes G, et al. Regional and cellular gene expression changes in human Huntington’s disease brain. Hum Mol Genet. 2006;15(6):965–77. doi: 10.1093/hmg/ddl013. [DOI] [PubMed] [Google Scholar]
- 5.Strand AD, Baquet ZC, Aragaki AK, Holmans P, Yang L, Cleren C, et al. Expression profiling of Huntington’s disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci. 2007;27(43):11758–68. doi: 10.1523/JNEUROSCI.2461-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisen A, Bohlega S, Bloch M, Hayden M. Silent periods, long-latency reflexes and cortical MEPs in Huntington’s disease and at-risk relatives. Electroencephalogr Clin Neurophysiol. 1989;74(6):444–9. doi: 10.1016/0168-5597(89)90034-8. [DOI] [PubMed] [Google Scholar]
- 7.Hanajima R, Ugawa Y, Terao Y, Furubayashi T, Machii K, Shiio Y, et al. Intracortical inhibition of the motor cortex is normal in chorea. J Neurol Neurosurg Psychiatr. 1999;66(6):783–6. doi: 10.1136/jnnp.66.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbruzzese G, Buccolieri A, Marchese R, Trompetto C, Mandich P, Schieppati M. Intracortical inhibition and facilitation are abnormal in Huntington’s disease: a paired magnetic stimulation study. Neurosci Lett. 1997;228(2):87–90. doi: 10.1016/s0304-3940(97)00363-7. [DOI] [PubMed] [Google Scholar]
- 9.Priori A, Polidori L, Rona S, Manfredi M, Berardelli A. Spinal and cortical inhibition in Huntington’s chorea. Mov Disord. 2000;15(5):938–46. doi: 10.1002/1531-8257(200009)15:5<938::aid-mds1026>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Modugno N, Curra A, Giovannelli M, Priori A, Squitieri F, Ruggieri S, et al. The prolonged cortical silent period in patients with Huntington’s disease. Clin Neurophysiol. 2001;112(8):1470–4. doi: 10.1016/s1388-2457(01)00599-5. [DOI] [PubMed] [Google Scholar]
- 11.Nardone R, Lochner P, Marth R, Ausserer H, Bratti A, Tezzon F. Abnormal intracortical facilitation in early-stage Huntington’s disease. Clin Neurophysiol. 2007;118(5):1149–54. doi: 10.1016/j.clinph.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–81. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- 13.Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114:2362–9. doi: 10.1016/s1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- 14.Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–67. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol. 2004;115(5):1076–82. doi: 10.1016/j.clinph.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Abbruzzese G, Dall’Agata D, Morena M, Reni L, Favale E. Abnormalities of parietal and prerolandic somatosensory evoked potentials in Huntington’s disease. Electroencephalogr Clin Neurophysiol. 1990;77(5):340–6. doi: 10.1016/0168-5597(90)90055-i. [DOI] [PubMed] [Google Scholar]
- 17.Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523(Pt 2):503–13. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huntington Study Group Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 19.Shoulson I, Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29(1):1–3. doi: 10.1212/wnl.29.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65(4):267–77. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 21.Gutekunst CA, Li SH, Yi H, Mulroy JS, Kuemmerle S, Jones R, et al. Nuclear and neuropil aggregates in Huntington’s disease: relationship to neuropathology. J Neurosci. 1999;19(7):2522–34. doi: 10.1523/JNEUROSCI.19-07-02522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Tortosa E, MacDonald ME, Friend JC, Taylor SA, Weiler LJ, Cupples LA, et al. Quantitative neuropathological changes in presymptomatic Huntington’s disease. Ann Neurol. 2001;49(1):29–34. [PubMed] [Google Scholar]
- 23.Rosas HD, Koroshetz WJ, Chen YI, Skeuse C, Vangel M, Cudkowicz ME, et al. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60(10):1615–20. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- 24.Zeitlin S, Liu JP, Chapman DL, Papaioannou VE, Efstratiadis A. Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington’s disease gene homologue. Nat Genet. 1995;11(2):155–63. doi: 10.1038/ng1095-155. [DOI] [PubMed] [Google Scholar]
- 25.Nasir J, Floresco SB, O’Kusky JR, Diewert VM, Richman JM, Zeisler J, et al. Targeted disruption of the Huntington’s disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell. 1995;81(5):811–23. doi: 10.1016/0092-8674(95)90542-1. [DOI] [PubMed] [Google Scholar]
- 26.Duyao MP, Auerbach AB, Ryan A, Persichetti F, Barnes GT, McNeil SM, et al. Inactivation of the mouse Huntington’s disease gene homolog Hdh. Science. 1995;269(5222):407–10. doi: 10.1126/science.7618107. [DOI] [PubMed] [Google Scholar]
- 27.Paulsen JS, Magnotta VA, Mikos AE, Paulson HL, Penziner E, Andreasen NC, et al. Brain structure in preclinical Huntington’s disease. Biol Psychiatry. 2006;59(1):57–63. doi: 10.1016/j.biopsych.2005.06.003. Epub 2005 Aug 22. [DOI] [PubMed] [Google Scholar]
- 28.Nopoulos P, Magnotta VA, Mikos A, Paulson H, Andreasen NC, Paulsen JS. Morphology of the cerebral cortex in preclinical Huntington’s disease. Am J Psychiatry. 2007;164(9):1428–34. doi: 10.1176/appi.ajp.2007.06081266. [DOI] [PubMed] [Google Scholar]
- 29.Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, et al. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000;135:455–61. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- 30.Restuccia D, Della Marca G, Valeriani M, Rubino M, Paciello N, Vollono C, et al. Influence of cholinergic circuitries in generation of high-frequency somatosensory evoked potentials. Clin Neurophysiol. 2003;114(8):1538–48. doi: 10.1016/s1388-2457(03)00138-x. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M, Desmond TJ, Albin RL, Frey KA. Vesicular neurotransmitter transporters in Huntington’s disease: initial observations and comparison with traditional synaptic markers. Synapse. 2001;41(4):329–36. doi: 10.1002/syn.1089. [DOI] [PubMed] [Google Scholar]
- 32.Vetter JM, Jehle T, Heinemeyer J, Franz P, Behrens PF, Jackisch R, et al. Mice transgenic for exon 1 of Huntington’s disease: properties of cholinergic and dopaminergic pre-synaptic function in the striatum. J Neurochem. 2003;85(4):1054–63. doi: 10.1046/j.1471-4159.2003.01704.x. [DOI] [PubMed] [Google Scholar]
- 33.Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57(5):369–84. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44(6):559–77. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Smith R, Chung H, Rundquist S, Maat-Schieman ML, Colgan L, Englund E, et al. Cholinergic neuronal defect without cell loss in Huntington’s disease. Hum Mol Genet. 2006;15(21):3119–31. doi: 10.1093/hmg/ddl252. [DOI] [PubMed] [Google Scholar]
- 36.Gomez-Anson B, Alegret M, Munoz E, Sainz A, Monte GC, Tolosa E. Decreased frontal choline and neuropsychological performance in preclinical Huntington disease. Neurology. 2007;68(12):906–10. doi: 10.1212/01.wnl.0000257090.01107.2f. [DOI] [PubMed] [Google Scholar]
- 37.Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, et al. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology. 2002;59(3):392–7. doi: 10.1212/wnl.59.3.392. [DOI] [PubMed] [Google Scholar]
- 38.Ferrante RJ, Beal MF, Kowall NW, Richardson EP, Jr., Martin JB. Sparing of acetylcholinesterase-containing striatal neurons in Huntington’s disease. Brain Res. 1987;411(1):162–6. doi: 10.1016/0006-8993(87)90694-9. [DOI] [PubMed] [Google Scholar]
- 39.Clark AW, Parhad IM, Folstein SE, Whitehouse PJ, Hedreen JC, Price DL, et al. The nucleus basalis in Huntington’s disease. Neurology. 1983;33(10):1262–7. doi: 10.1212/wnl.33.10.1262. [DOI] [PubMed] [Google Scholar]